Abstract
Fish liver ethoxyresorufin-O-deethylase (EROD) activity is widely used as biomarker of exposure to chemicals such as polycyclic aromatic hydrocarbons (PAHs). It is known that endocrine system plays a major role in fish stress mechanism. Despite the considerable scientific information about steroid hormone’s response, namely cortisol and 17ß-estradiol (E2), to stress situations, little is known about the influence of these hormones on enzymes involved on the biotransformation process. Thus, this study aimed to assess the in vitro effects of environmentally relevant concentrations of benzo[a]pyrene (B[a]P) (0.1, 0.3, 0.9, and 2.7 µM) and of two steroid hormones (cortisol and 17ß-estradiol) in a physiologically relevant concentration (5.997 ng/mL), alone or in combination, on Anguilla anguilla liver microsomal EROD activity, previously induced by 4 mg/kg β-naphthoflavone intraperitoneal injection. Hepatic microsomes in vitro exposure to the tested B[a]P concentrations induced a dose response inhibition of EROD activity, whereas exposure to cortisol significantly induced the activity of this enzyme. The steroid hormones were able to decrease the inhibitory effects of B[a]P on microsomal EROD activity, thus revealing a protective effect of these hormones over enzyme activity inhibited by contaminants.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are one class of persistent organic contaminants present in the environment [1] mainly due to anthropogenic inputs, although their environmental release may also be related to natural sources (e.g., forest fires, volcanic emissions, natural oil seeps, coal deposits, plant debris) [2]. PAHs are of environmental concern mostly due to their persistence and bioaccumulation as well as toxicity often associated with carcinogenic and mutagenic properties [3]. Thus, evaluating the presence of PAHs in aquatic systems is considered very important [4]. Benzo[a]pyrene (B[a]P) is a nearly ubiquitous PAH contaminant categorized in the highest human carcinogenic risk level, posing risk to human health and ecosystems. As such, it is often used as a surrogate for general PAH contamination [5].
Aquatic organisms can be exposed to contaminated sediments and waters where PAHs are taken up via various pathways [6] and then converted into sets of metabolites by biotransformation phase I enzymes, namely cytochrome CYP1A dependent monooxygenase enzymes, such as ethoxyresorufin-O-deethylase (EROD) [7,8]. The liver is generally considered as the major organ involved on xenobiotic metabolism [9]. Thus, EROD activity is frequently used as a biomarker of exposure to PAHs and structurally related compounds, such as ß-naphthoflavone (BNF) [8,10]. Across most laboratory studies, intraperitoneal (i.p.) injection with naphthoflavone (BNF) consistently resulted in the highest EROD fold induction (often higher than 20-fold relative to control fish) and is therefore a reliable positive control for EROD activity investigations [8].
In the natural environment, the existence of several confounding factors such as non-chemical exogenous parameters (e.g., season) and individual-related factors (e.g., hormone levels, the physiological condition of the organism) can sometimes mask the effects of contaminant-induced stress signals, leading to misinterpretation of biomarker results. Thus, careful consideration of such parameters has been considered relevant when using EROD activity as a biomarker for environmental monitoring studies [8,11].
Exposure to a polluted environment would typically result in an endocrine stress response that promotes survival and helps restore homeostasis. Cortisol is the main corticosteroid in fish and the most abundant and active. It is widely used as a stress biomarker, since it is known that an elevation in plasmatic cortisol levels occurs in fish after short-term exposure to several types of stress-inducing conditions like exposure to contaminants [12,13]. On the other hand, 17ß-estradiol (E2), the most potent of the estrogens, plays a major role in various aspects of growth, development, and morphologic differentiation, as well as in the development and regulation of sexual and reproductive behaviors and cycles [14]. Nonetheless, despite the existence of considerable scientific information about the response of these two steroid hormones to contaminant-induced stress in several species of fish [1,15,16,17,18], there is a considerable lack of information about how they influence the response/activity of enzymes, such as the biotransformation enzyme EROD. Such knowledge may be vital towards a better understanding of the potential linkage between endocrine regulation and stress response to pollutants.
The use of in vitro toxicity assays allows the assessment of a biotransformation reaction under controlled experimental conditions [19], together with the ease in screening the toxic potencies of individual compounds (e.g., PAHs) and complex mixtures, simplifying risk assessment [20]. These assays can also be considered more environmentally friendly as a smaller amount of chemicals is needed for testing, and tests can be performed in shorter periods of time [21]. Furthermore, they allow the reduction of the number of animals, which is in line with the European Directive 2010/63/EU, for the protection of animals used for scientific purposes.
The aim of this study was to assess the in vitro effects of (i) environmentally relevant concentrations of B[a]P (0.1, 0.3, 0.9, and 2.7 µM); (ii) physiologically relevant concentration of cortisol and E2 (5.997 ng/mL); (iii) combined exposure to B[a]P (0.1, 0.3, 0.9, and 2.7 µM) with 5.997 ng/mL of cortisol or E2, on liver microsomal EROD activity of A. Anguilla.
2. Materials and Methods
2.1. Chemicals
Beta-naphthoflavone (BNF), dimethyl sulfoxide (DMSO), resorufin, benzo[a]pyrene (B[a]P), and 17ß-estradiol (E2) were all purchased at Sigma-Aldrich. 7-ethoxyresorufin-O-deethylase and NADPH were obtained from Roche and cortisol was acquired from Merck.
2.2. Test Organisms
Four (4) adult female eels—Anguilla anguilla L.—caught at the Aveiro lagoon, weighing approximately 500 ± 23 (mean ± SD) g, were transported to the laboratory facilities, and acclimatized to laboratory conditions for 2 weeks prior to experimentation under standard conditions. Briefly, fish were kept at room temperature and natural photoperiod, in aerated–dissolved oxygen: 8.7 ± 0.5 (mean ± SD) mg/L, filtered, dechlorinated, and recirculating tap water, with 7.4 ± 0.2 (mean ± SD) pH. Fish were neither fed during recovery nor during the experimental period.
2.3. Preparation of Biological Material for In Vitro Assays
All experimental procedures involving fish were carried out according to the legislation for the protection of animals used for scientific purposes (European directive 2010/63/EU). After acclimatization, the eels were intraperitoneally injected with 4 mg ß-naphthoflavone (prepared in DMSO) per kg of eel body weight. After 24 h, the eels were sacrificed. The liver was then removed, frozen in liquid nitrogen, and stored at −80 °C until homogenization. Liver microsomes were obtained according to previous reports [22,23], adapted by a previous study [24].
2.4. Microsomal In Vitro Exposure Conditions
Exposures were conducted in a quartz cuvette, with a total volume of 1mL and 3 min of incubation period. Stock solutions of benzo[a]pyrene (B[a]P) were prepared in DMSO. Cortisol (C) and 17β-estradiol (E2) were directly prepared in assay buffer, Tris-HCl 0.1 M pH 7.4 with KCl 0.15 M and 20% glycerol already containing substrate, 0.5 µM ethoxyresorufin (Supplementary Materials Table S1). The final concentration of cortisol and E2, in the cuvette, was 5.997 ng/mL, which was based in previously reported fish plasmatic E2 levels (that may reach 22.9 ng/mL [17,25,26]) and plasmatic cortisol levels (that may reach 80 ng/mL [1,9,17]).
For each condition, 5 µL of hepatic microsomes were used. The description of the in vitro exposure protocol is presented in Supplementary Materials Table S2.
2.4.1. In Vitro Effects of Cortisol, 17β-Estradiol, and B[a]P
The assays’ protocol consisted of the addition of: (a) 1090 µL of buffer substrate solution (BS) and 5 µL of buffer solution (B), used as control, (b) 1090 µL of BS and 5 µL of dimethyl sulfoxide (DMSO), used as DMSO control, for each exposure, (c) 1090 µL of buffer substrate solution with cortisol (at a final concentration of 5.997 ng/mL) (BSC) and 5 µL of DMSO, (d) 1090 µL of buffer substrate solution with 17β-estradiol (at a final concentration of 5.997 ng/mL) (BSE2) and 5 µL of DMSO, and (e) 1090 µL of BS and 5 µL of benzo[a]pyrene (B[a]P) at concentrations 0.1, 0.3, 0.9, and 2.7 µM (Table S1).
2.4.2. In Vitro Effects of B[a]P after Microsomal Pre-Exposure to Steroid Hormones
For this assessment, to 5 µL of hepatic microsome suspension was added (a) 1090 µL of buffer substrate solution with cortisol (at a final concentration of 5.997 ng/mL) (BSC) and then 5 µL of B[a]P at a final concentration of 0.1, 0.3, 0.9l and 2.7 µM; (b) 1090 µL of buffer substrate solution with 17β-estradiol (at a final concentration of 5.997 ng/mL) (BSE2) and then 5 µL of each B[a]P concentration (0.1, 0.3, 0.9, and 2.7 µM) (Table S2).
2.5. EROD Activity Determination
EROD activity was determined as described by Burke and Meyer (1974) and expressed as pmol/min/mg of microsomal protein. The reaction was carried out at 25 °C and initiated by adding 10 µL of NADPH (10 mM final concentration). The progressive increase in fluorescence, resulting resorufin formation, was measured for 3 min (excitation wavelength 530 nm; emission wavelength 585 nm), with a Jasco FP 750 spectrofluorometer. Liver microsomal protein concentrations were determined according to the Biuret method [27], using bovine serum albumin as a standard.
2.6. Statistical Analysis
Results are expressed as means ± SE (standard error), corresponding to experimental groups of four fish (n = 4). Sigmastat 2.03 software was used for statistical analysis. The experimental data were tested first for normality and homogeneity of variance to meet statistical demand. One way ANOVA, followed by post-hoc Tukey test, was performed to assess significant effects between the different groups [28]. Student t test was used to compare the results of control with control solvent (DMSO). Differences between means were considered significant at p < 0.05.
3. Results
Anguilla anguilla exposed (in vivo) to 4 mg/kg BNF had a liver microsomal EROD activity of 9.65 pmol/min/mg of protein. The addition of 5 µL of DMSO did not affect liver microsomal EROD activity (p > 0.05, student t test). On the other hand, the addition of 0.1, 0.3, 0.9, and 2.7 µM of B[a]P significantly inhibited EROD activity by 53%, 15%, 9%, and 6%, respectively (Figure 1 and Figure 2).
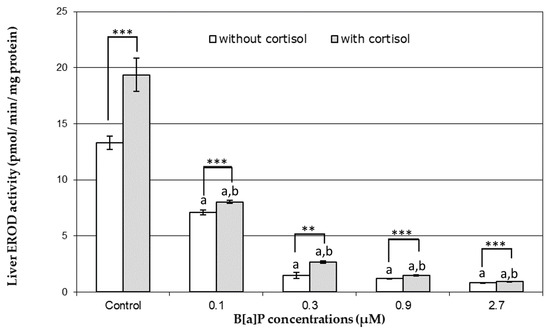
Figure 1.
In vitro effects of benzo[a]pyrene (B[a]P) (0.1, 0.3, 0.9, and 2.7 µM) without and with liver microsomal pre-exposure to 5.997 ng/mL of cortisol. Bars represent mean ± SE (n = 4). Asterisks (*) indicate differences between presence and absence of cortisol (* p < 0.05; ** p < 0.01; *** p < 0.001); the letters “a” and “b” indicate differences with control without and with cortisol, respectively.
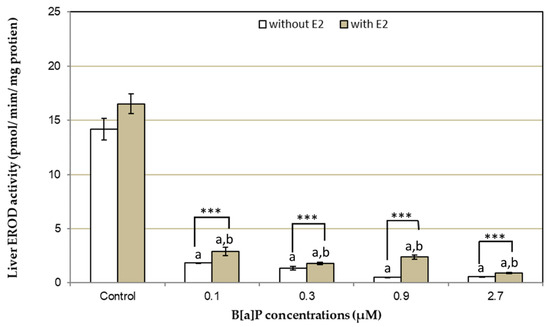
Figure 2.
In vitro effects of benzo[a]pyrene (B[a]P) (0.1, 0.3, 0.9, and 2.7 µM) without and with liver microsomal pre-exposure to 5.997 ng/mL of E2. Bars represent mean ± SE (n = 4). Asterisks (*) indicate differences between presence and absence of cortisol (* p < 0.05; ** p < 0.01; *** p < 0.001); the letters “a” and “b” indicate differences with control without and with E2, respectively.
Exposure of hepatic microsomes to cortisol (DMSO+C), presented a significant increase of EROD activity when compared to its respective control (DMSO Control) (Figure 1). Furthermore, the presence of cortisol provided a protective effect over EROD activity inhibition by B[a]P. All tested B[a]P concentrations showed a significant increase in EROD activity in the presence of cortisol, when compared to B[a]P individual exposure, with the highest increase observed for 0.1 µM B[a]P. However, increments did not correspond to a complete recovery to control activity levels. The efficiency of recovery decreased with increasing B[a]P concentrations (Figure 1).
The assessment of the effects of E2, alone or in combination with B[a]P, shows that in vitro exposure of hepatic microsomes to 5.997 ng/mL of E2 (DMSO+E2) leads to a non significant increase of EROD activity, when compared to DMSO control (absence of E2) (Figure 2). Similar to cortisol, E2 was also able to partially revert liver microsomal EROD activity inhibited by B[a]P. Indeed, all tested B[a]P concentrations also showed a mild but significant increment in EROD activity in the presence of E2; however, like cortisol, recoveries did not reach control activity levels (Figure 2). On the other hand, recovery efficiency did not decrease with B[a]P concentration increase. Here, the highest recovery was observed at 0.9 µM B[a]P. All differences with control were p < 0.001.
4. Discussion
The presented study aimed to assess if environmentally relevant concentrations of B[a]P (0.1, 0.3, 0.9, and 2.7 µM), as well as a physiologically relevant concentration of cortisol and E2 (5.997 ng/mL), alone or in combination, had a significant impact on EROD activity. This issue is of considerable relevance based on the importance given to this biomarker to assess the presence of environmental contaminants that induce Phase I biotransformation. Since BNF is recognized as a strong inducer of hepatic EROD [8,29,30], it was used in this study as a reliable in vivo positive control for EROD activity. Although EROD activity is known to be induced in the presence of PAHs, including B[a]P [31], in vivo inhibition of hepatic EROD activity by B[a]P has previously been reported [32], which suggests inducers may become inhibitors, since B[a]P, as well as other inducers of CYP1A, may also act either by competitive inhibition or by a mechanism-based inactivation, in which the inhibitor is metabolized by the P450 into a product that covalently modifies the active site and thereby inactivates the enzyme [33]. In vitro inhibition of this enzymatic activity has also been reported in A. anguilla, after exposure to retene, abietic acid [30], and heavy metals (e.g., copper, zinc, chromium) [31]. The EROD activity inhibition observed in the present study, with concentrations of B[a]P of 0.1, 0.3, 0.9, and 2.7 μM, can be considered of high environmental relevance, since it falls within the levels detected in natural waters (up to 8.2 μM) [34]. In this perspective, this study proves that EROD activity can be compromised by environmentally relevant levels of known inducers.
It is known that fish EROD activity can also be affected by individual-related factors (e.g., reproductive status, hormonal levels) [8] that in turn influence the physiological condition of the fish itself. Limited information is available concerning the effects of cortisol on the activity of CYP1A enzymes. Some of the performed studies have shown an increase of the plasmatic cortisol level as well as of the EROD activity after exposure to contaminants [16,35], namely PAHs [15]. However, information about the potential modulatory effect of EROD activity by this steroid hormone remains unclear. In this context, the in vitro assays can provide highly relevant information in order to clarify this linkage. In this study, the microsomal in vitro exposure to cortisol demonstrated the ability of this hormone to increase EROD activity and decrease the inhibition induced by B[a]P. Previous in vitro studies had also reported the increment of EROD activity in A. anguilla liver organ culture, when cortisol was added to culture medium [36].
Unlike the in vivo studies that have been performed concerning the effects of E2 on EROD activity [17,18,35,37,38], there is a considerable lack of information concerning in vitro studies. On the other hand, most of the performed studies have focused on the toxicity assessment of this natural estrogen itself as a potential aquatic contaminant, reporting, in this context, E2 as a suppressor of the constitutive CYP1A-associated EROD activity [17,38]. The results of the present study showed that in vitro liver microsomal exposure to E2 did not significantly affect EROD activity, which has been previously observed by Teles et al. (2006) [18] with D. labrax, in an in vivo study testing similar concentrations of E2. Our results revealed that E2 had a protective effect on hepatic EROD activity inhibited by B[a]P.
The protective effects of steroid hormones (cortisol and E2) over EROD activity inhibited by contaminants has been demonstrated for the first time in this work. Gravato and Santos (2002) [32] also reported that EROD activity inhibition by B[a]P could be partially reverted, in this case, by another PAH, naphthalene. However, neither hormone was able to fully recover the inhibitory effect caused by B[a]P.
The present study demonstrates the importance of considering endocrine/hormonal individual status when assessing contamination-induced EROD activity and the need for controlling the influence of this individual-related factor to allow comparisons. Further studies are needed to provide a better understanding about the underlying mechanisms of the role of cortisol and 17β-estradiol in terms of modulation and protection over biotransformation enzymes. Taking into account the importance of the endocrine system in the fitness of the aquatic organisms, a more complex approach should be taken addressing more physiologically relevant conditions (e.g., the effects of mixtures of hormones). Furthermore, the ability of these hormones to revert enzymatic inhibitions induced by other contaminants (e.g., metals) should also be studied.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/6/2533/s1, Table S1: Buffers, solvents and solutions used during Experimental Setup; Table S2: Experimental liver microsomal EROD activity assay procedures to assess effects of cortisol (C), 17β-estradiol (E2) and benzo[a]pyrene (B[a]P), alone or in combination. The numbers (1, 2, 3 and 4) represent the sequence of compounds added to the cuvette [Tris-HCl 0.1 M pH 7.4 with KCl 0.15 M 20% glycerol (B) with 0.5 µM ethoxyresorufin (BS); BS with cortisol 5.997 ng/mL (BSC); BS with E2 5.997 ng/mL (BSE2), dimethyl sulfoxide (DMSO), benzo[a]pyrene (B[a]P)].
Author Contributions
Conceptualization, supervision, project administration, resources, methodology, and funding acquisition, M.A.S., M.P., and M.O.; Investigation, data curation, software, visualization, and writing—original draft preparation, C.S.S.F.; Formal analysis, C.S.S.F., and M.O.; Validation, C.S.S.F., M.A.S., M.P., and M.O.; Writing—review and editing, M.A.S., M.P., and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CESAM—Centre for Environmental and Marine Studies and FCT—Foundation for Science and Technology, through national funds (UIDP/50017/2020+UIDB/50017/2020).
Institutional Review Board Statement
All experimental procedures followed International Guiding Principles for Biomedical Research Involving Animals (EU 2010/63) and were previously approved by the ethics committee and the responsible national legal authority “Direção Geral de Alimentação e Veterinária” (authorization N421/2013).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Thanks are due to FCT for the financial support to CESAM (UIDP/50017/2020+UIDB/50017/2020), through national funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bo, J.; Gopalakrishnan, J.; Chen, F.; Wang, K. Benzo[a]pyrene modulates the biotransformation, DNA damage and cortisol level of red sea bream challenged with lipopolysaccharide. Mar. Pollut. Bull. 2014, 85, 463–470. [Google Scholar] [CrossRef]
- Wolska, L.; Mechlińska, A.; Rogowska, J.; Namieśnik, J. Sources and fate of PAHs and PCBs in the marine environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1172–1189. [Google Scholar] [CrossRef]
- Liao, K.; Yu, J.Z. Abundance and sources of benzo[a]pyrene and other PAHs in ambient air in Hong Kong: A review of 20-year measurements (1997–2016). Chemosphere 2020, 259, 127518. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Souza, M.; Junior, A.; Soares, L.; Frena, M.; Alexandre, M. Polycyclic aromatic hydrocarbons (PAH) in superficial water from a tropical estuarine system: Distribution, seasonal variations, sources and ecological risk assessment. Mar. Pollut. Bull. 2018, 127, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Knecht, A.; Truong, L.; Simonich, M.; Tanguay, R. Developmental benzo[a]pyrene (B[a]P) exposure impacts larval behavior and impairs adult learning in zebrafish. Neurotoxicol. Teratol. 2017, 59, 27–34. [Google Scholar] [CrossRef]
- Logan, D.T. Perspective on ecotoxicology of PAHs to fish. Hum. Ecol. Risk Assess 2007, 13, 302–316. [Google Scholar] [CrossRef]
- Boehler, S.; Lörracher, A.; Schubert, J.; Braunbeck, T. Comparative live-imaging of in vivo EROD (ethoxyresorufin-O-deethylase) induction in zebrafish (Danio rerio) and fathead minnow (Pimephales promelas) embryos after exposure to PAHs and river sediment extracts. Sci. Total Environ. 2018, 621, 827–838. [Google Scholar] [CrossRef]
- Gagnon, M.; Rawson, C. Bioindicator species for EROD activity measurements: A review with Australian fish as a case study. Ecol. Indic. 2017, 73, 166–180. [Google Scholar] [CrossRef]
- Oliveira, M.; Pacheco, M.; Santos, M.A. Organ specific antioxidant responses in golden grey mullet (Liza aurata) following a short-term exposure to phenanthrene. Sci. Total Environ. 2008, 396, 70–78. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Fent, K. Induction of cytocrome P450 as a biomarker for environmental contamination in aquatic ecosystems. Crit. Rev. Environ. Sci. Technol. 1995, 25, 201–268. [Google Scholar] [CrossRef]
- Wunderlich, A.; Silva, R.; Zica, E.; Rebelo, M.; Parente, T.; Martínez, V. The influence of seasonality, fish size and reproductive status on EROD activity in Plagioscion squamosissimus: Implications for biomonitoring of tropical/subtropical reservoirs. Ecol. Indic. 2015, 58, 267–276. [Google Scholar] [CrossRef]
- Oliveira, M.; Pacheco, M.; Santos, M.A. Fish thyroidal and stress responses in contamination monitoring—An integrated biomarker approach. Ecotoxicol. Environ. Saf. 2011, 74, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Reddam, A.; Magera, E.; Grosella, M.; McDonald, M. The impact of acute PAH exposure on the toadfish glucocorticoid stress response. Aquat. Toxicol. 2017, 192, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.; Burggren, W.W.; French, K. Glands and Hormones—Physiological effects of hormones. In Animal Physiology—Mechanisms and Adaptations, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2001; Chapter 9; pp. 338–347. [Google Scholar]
- Oliveira, M.; Pacheco, M.; Santos, M.A. Cytochrome P4501A, genotoxic and stress responses in golden grey mullet (Liza aurata) following short-term exposure to phenanthrene. Chemosphere 2007, 66, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.; Santos, M.A.; Pacheco, M. Responses of European eel (Anguilla anguilla L.) in two polluted environments: In situ experiments. Ecotoxicol. Environ. Saf. 2004, 58, 373–378. [Google Scholar] [CrossRef]
- Teles, M.; Pacheco, M.; Santos, M.A. Sparus aurata L. liver EROD and GST activities, plasma cortisol, lactate, glucose and erythrocytic nuclear anomalies after short-term exposure either to 17ß-estradiol (E2) or E2 combined with 4-nonylphenol. Sci. Total Environ. 2005, 336, 57–69. [Google Scholar] [CrossRef]
- Teles, M.; Santos, M.A.; Pacheco, M. Biotransformation, stress and genotoxic effects of 17β-estradiol in juvenile sea bass (Dicentrarchus labrax L.). Environ. Int. 2006, 32, 470–477. [Google Scholar] [CrossRef]
- Barreto, A.; Carvalho, A.; Silva, D.; Pinto, E.; Almeida, A.; Paíga, P.; Correia-Sá, L.; Delerue-Matos, C.; Trindade, T.; Soares, A.M.V.M.; et al. Effects of single and combined exposures to gold (nano versus ionic form) and gemfibrozil ina liver organ culture of Sparus aurata. Mar. Pollut. Bull. 2020, 160, 111665. [Google Scholar] [CrossRef]
- Billiard, S.M.; Bols, N.C.; Hodson, P.V. In vitro and in vivo comparisons of fish-specific CYP1A induction relative potency factors for selected polycyclic aromatic hydrocarbons. Ecotoxicol. Environ. Saf. 2004, 59, 292–299. [Google Scholar] [CrossRef]
- Jain, A.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A. Chapter 3—Models and Methods for In Vitro Toxicity. In In Vitro Toxicology; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65. [Google Scholar]
- Lange, U.; Danischewski, D.; Siebers, D. Regional variability and sexual differences in ethoxyresorufin-O-deethylase activities and cytochrome P450 concentration in the liver of mature dab (Limanda limanda L.) in the German Bight. In Variability of EROD and Cytochrome P450 in North Sea Dab; Braumbeck, T., Hanke, W., Segner, H., Eds.; VCH, Verlag Chemie: New York, NY, USA, 1993. [Google Scholar]
- Monod, G.; Vindimian, E. Effect of storage conditions and subcellular fractionation of fish and cytochrome P-450-dependent enzymatic activities used for the monitoring of water pollution. Water Res. 1991, 25, 173–177. [Google Scholar] [CrossRef]
- Pacheco, M.; Santos, M.A. Induction of liver EROD and erythrocytic nuclear abnormalities by cyclophosphamide and PAH’s in Anguilla anguilla L. Ecotoxicol. Environ. Saf. 1998, 40, 71–76. [Google Scholar] [CrossRef]
- Hyeon, J.; Hur, S.; Kim, B.; Byun, J.; Kim, E.; Lim, B.; Lee, B.; Kim, S.; Takemura, A.; Kim, S. Involvement of Estrogen and Its Receptors in Morphological Changes in the Eyes of the Japanese Eel, Anguilla japonica, in the Process of Artificially-Induced Maturation. Cells 2019, 8, 310. [Google Scholar] [CrossRef]
- Tirado, J.; Valladares, L.; Muñoz, D.; Caza, J.; Manjunatha, B.; Kundapur, R. Levels of 17β-estradiol, vitellogenin, and prostaglandins during the reproductive cycle of Oreochromis niloticus. Lat. Am. J. Aquat. Res. 2017, 45, 930–936. [Google Scholar] [CrossRef]
- Gornall, A.C.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Oliveira, M.; Santos, M.A.; Pacheco, M. Heavy metal inhibition of Anguilla anguilla L. liver microsomal EROD activity and thiol protection. Fresen. Environ. Bull. 2005, 14, 59–64. [Google Scholar]
- Santos, M.A.; Maria, V.L. Abietic, Dehydroabietic acids and retene in vitro effets on Anguilla anguilla L. liver microsomal EROD activity. Fresen. Environ. Bull. 2005, 14, 698–702. [Google Scholar]
- Oliveira, M.; Santos, M.A.; Gravato, C.; Pacheco, M. Chromium effects on Anguilla anguilla liver organ culture. Fresen. Environ. Bull. 2003, 12, 349–352. [Google Scholar]
- Gravato, C.; Santos, M.A. Juvenile sea bass liver P450 EROD induction and erythrocytic genotoxic responses to PAH and PAH-like compounds. Ecotoxicol. Environ. Saf. 2002, 51, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Maria, V.L.; Gravato, C.; Correia, A.C.; Santos, M.A. Biotransformation and genotoxicity responses to PAHs in two teleost species. Fresen. Environ. Bull. 2002, 11, 609–615. [Google Scholar]
- Anyakora, C.; Ogbeche, A.; Palmer, P.; Coker, H. Determination of polynuclear aromatic hydrocarbons in marine samples of Siokolo Fishing Settlement. J. Chromatogr. A 2005, 1073, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.; Pacheco, M.; Santos, M.A. Endocrine and metabolic responses of A. anguilla L. caged in a freshwater-wetland (Pateira de Fermentelos—Portugal). Sci. Total Environ. 2007, 372, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Pacheco, M.; Magalhães, I. Anguilla anguilla L. liver organ culture as a toxicological model: In vivo and in vitro EROD induction by β-naphthoflavone. Fresen. Environ. Bull. 2000, 9, 527–534. [Google Scholar]
- Teles, M.; Gravato, C.; Pacheco, M.; Santos, M.A. Juvenile sea bass biotransformation, genotoxic and endocrine responses to β-naphthoflanove, 4-nonylphenol and 17ß-estradiol individual and combined exposures. Chemosphere 2004, 57, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, E.; Meucci, V.; Intorre, L.; Soldani, G.; Di Bello, D.; Longo, V.; Gervasi, P.G.; Pretti, C. Effects of 17ß-estradiol, 4-nonylphenol and PCB 126 on the estrogenic activity and phase I and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2005, 75, 293–305. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).