In Vitro Estimation of Relative Compliance during High-Frequency Oscillatory Ventilation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
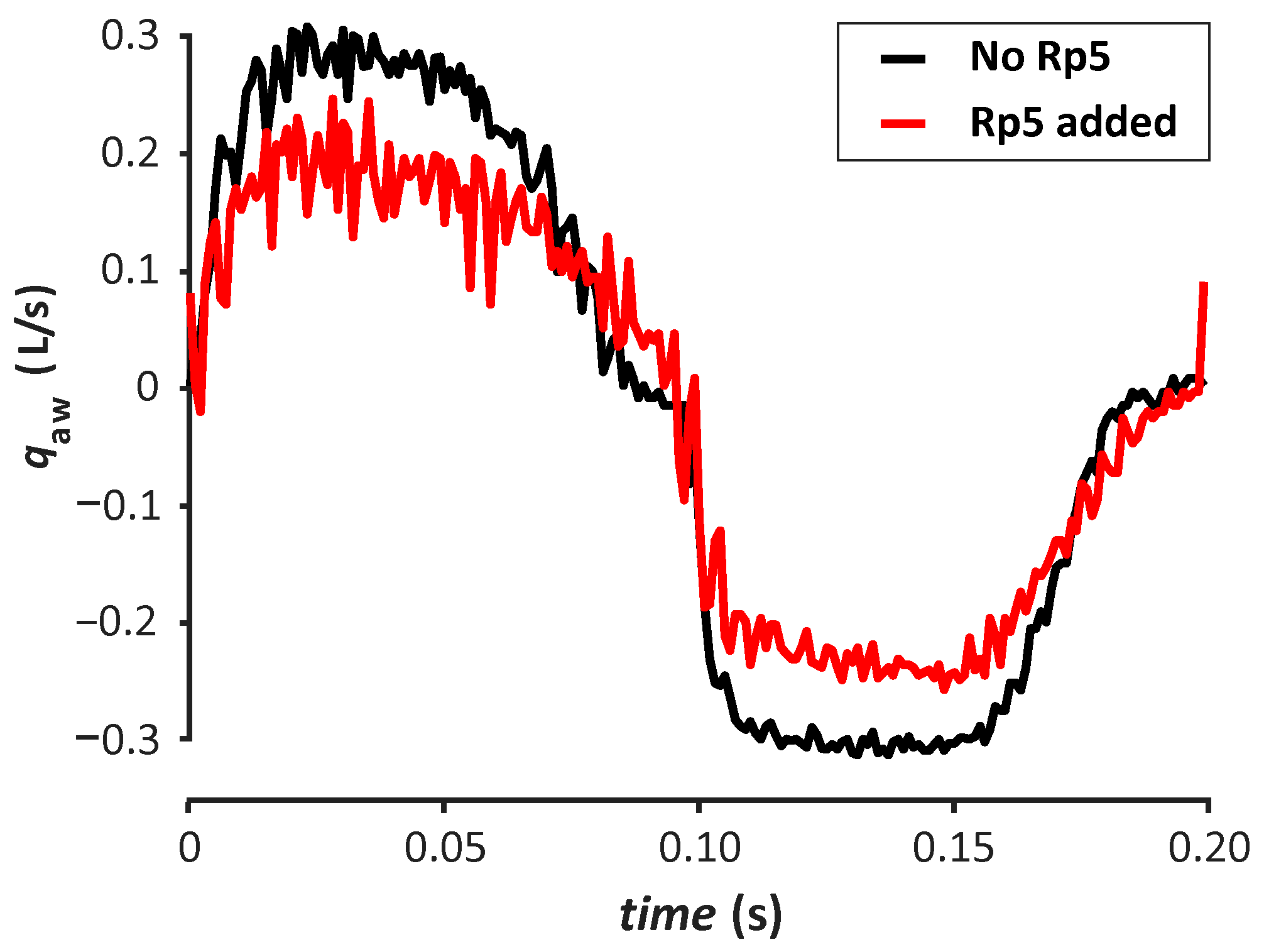
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meade, M.O.; Young, D.; Hanna, S.; Zhou, Q.; Bachman, T.E.; Bollen, C.; Slutsky, A.S.; Lamb, S.E.; Adhikari, N.K.; Mentzelopoulos, S.D.; et al. Severity of Hypoxemia and Effect of High-Frequency Oscillatory Ventilation in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 727–733. [Google Scholar] [CrossRef]
- Sud, S.; Sud, M.; Friedrich, J.O.; Meade, M.O.; Ferguson, N.D.; Wunsch, H.; Adhikari, N.K. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): Systematic review and meta-analysis. BMJ 2010, 340, c2327. [Google Scholar] [CrossRef]
- Sklar, M.C.; Fan, E.; Goligher, E.C. High-Frequency Oscillatory Ventilation in Adults With ARDS: Past, Present, and Future. Chest 2017, 152, 1306–1317. [Google Scholar] [CrossRef]
- Ng, J.; Ferguson, N.D. High-frequency oscillatory ventilation: Still a role? Curr. Opin. Crit. Care 2017, 23, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Munshi, L.; Adhikari, N.K.; Meade, M.O.; Hodgson, C.L.; Wunsch, H.; Uleryk, E.; Gajic, O.; Amato, M.P.; Ferguson, N.D.; et al. High-Frequency Oscillation for Adult Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S289–S296. [Google Scholar] [CrossRef]
- Wong, J.J.; Liu, S.; Dang, H.; Anantasit, N.; Phan, P.H.; Phumeetham, S.; Qian, S.; Ong, J.S.; Gan, C.S.; Chor, Y.K.; et al. The impact of high frequency oscillatory ventilation on mortality in paediatric acute respiratory distress syndrome. Crit. Care 2020, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Angriman, F.; Ferreyro, B.L.; Donaldson, L.; Cuthbertson, B.H.; Ferguson, N.D.; Bollen, C.W.; Bachman, T.E.; Lamontagne, F.; Adhikari, N.K. The harm of high-frequency oscillatory ventilation (HFOV) in ARDS is not related to a high baseline risk of acute cor pulmonale or short-term changes in hemodynamics. Intensive Care Med. 2020, 46, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Liang, L.; Lyu, Y.; Yu, Y.; Li, B. The effect of high-frequency oscillatory ventilation or airway pressure release ventilation on children with acute respiratory distress syndrome as a rescue therapy. Transl. Pediatr. 2020, 9, 213–220. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Cook, D.J.; Guyatt, G.H.; Mehta, S.; Hand, L.; Austin, P.; Zhou, Q.; Matte, A.; Walter, S.D.; Lamontagne, F.; et al. High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 795–805. [Google Scholar] [CrossRef]
- Young, D.; Lamb, S.E.; Shah, S.; MacKenzie, I.; Tunnicliffe, W.; Lall, R.; Rowan, K.; Cuthbertson, B.H. High-frequency oscillation for acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 806–813. [Google Scholar] [CrossRef]
- Gu, X.L.; Wu, G.N.; Yao, Y.W.; Shi, D.H.; Song, Y. Is high-frequency oscillatory ventilation more effective and safer than conventional protective ventilation in adult acute respiratory distress syndrome patients? A meta-analysis of randomized controlled trials. Crit. Care 2014, 18, R111. [Google Scholar] [CrossRef]
- Maitra, S.; Bhattacharjee, S.; Khanna, P.; Baidya, D.K. High-frequency ventilation does not provide mortality benefit in comparison with conventional lung-protective ventilation in acute respiratory distress syndrome: A meta-analysis of the randomized controlled trials. Anesthesiology 2014, 122, 841–851. [Google Scholar] [CrossRef] [PubMed]
- de Jager, P.; Kamp, T.; Dijkstra, S.K.; Burgerhof, J.G.; Markhorst, D.G.; Curley, M.A.; Cheifetz, I.M.; Kneyber, M.C. Feasibility of an alternative, physiologic, individualized open-lung approach to high-frequency oscillatory ventilation in children. Ann. Intensive Care 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- de Jager, P.; Burgerhof, J.G.; Koopman, A.A.; Markhorst, D.G.; Kneyber, M.C. Physiologic responses to a staircase lung volume optimization maneuver in pediatric high-frequency oscillatory ventilation. Ann. Intensive Care 2020, 10, 153. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Z.; Tan, L.; Wang, L.; Möller, K.; Frerichs, I.; Yu, T.; Huang, Y.; Pan, C.; Yang, Y.; et al. Optimal mean airway pressure during high-frequency oscillatory ventilation in an experimental model of acute respiratory distress syndrome: EIT-based method. Ann. Intensive Care 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Lista, G.; Bresesti, I.; Cavigioli, F.; Castoldi, F.; Lupo, E.; LoMauro, A.; Aliverti, A. Efficacy of lung volume optimization maneuver monitored by optoelectronic pletismography in the management of congenital diaphragmatic hernia. Respir. Med. Case Rep. 2017, 22, 133–136. [Google Scholar] [CrossRef]
- Zannin, E.; Dellaca, R.L.; Dognini, G.; Marconi, L.; Perego, M.; Pillow, J.J.; Tagliabue, P.E.; Ventura, M.L. Effect of frequency on pressure cost of ventilation and gas exchange in newborns receiving high-frequency oscillatory ventilation. Pediatr. Res. 2017, 82, 994–999. [Google Scholar] [CrossRef]
- Kneyber, M.C.; Markhorst, D.G. Do We Really Know How to Use High-Frequency Oscillatory Ventilation in Critically Ill Children? Am. J. Respir. Crit. Care Med. 2016, 193, 1067–1068. [Google Scholar] [CrossRef]
- Kneyber, M.C.; Markhorst, D.G. Any trial can (almost) kill a good technique. Intensive Care Med. 2016, 42, 1092–1093. [Google Scholar] [CrossRef]
- Dellacà, R.L.; Zannin, E.; Ventura, M.L.; Sancini, G.; Pedotti, A.; Tagliabue, P.; Miserocchi, G. Assessment of dynamic mechanical properties of the respiratory system during high-frequency oscillatory ventilation. Crit. Care Med. 2013, 41, 2502–2511. [Google Scholar] [CrossRef]
- Casserly, B.; McCool, F.D.; Sethi, J.M.; Kawar, E.; Read, R.; Levy, M.M. A method for determining optimal mean airway pressure in high-frequency oscillatory ventilation. Lung 2013, 191, 69–76. [Google Scholar] [CrossRef] [PubMed]
- van Genderingen, H.R.; van Vught, J.A.; Jansen, J.R.; Duval, E.L.; Markhorst, D.G.; Versprille, A. Oxygenation index, an indicator of optimal distending pressure during high-frequency oscillatory ventilation? Intensive Care Med. 2002, 28, 1151–1156. [Google Scholar] [CrossRef]
- Goddon, S.; Fujino, Y.; Hromi, J.M.; Kacmarek, R.M. Optimal mean airway pressure during high-frequency oscillation: Predicted by the pressure-volume curve. Anesthesiology 2001, 94, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.H.; Pyon, K.H.; Courtney, S.E. Optimal high-frequency oscillatory ventilation settings by nonlinear lung mechanics analysis. Am. J. Respir. Crit. Care Med. 2002, 166, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Klapsing, P.; Moerer, O.; Wende, C.; Herrmann, P.; Quintel, M.; Bleckmann, A.; Heuer, J.F. High-frequency oscillatory ventilation guided by transpulmonary pressure in acute respiratory syndrome: An experimental study in pigs. Crit. Care 2018, 22, 121. [Google Scholar] [CrossRef] [PubMed]
- Klapsing, P.; Moerer, O.; Wende, C.; Herrmann, P.; Quintel, M.; Bleckmann, A.; Heuer, J.F. Setting mean airway pressure during high-frequency oscillatory ventilation according to the static pressure-volume curve in surfactant-deficient lung injury: A computed tomography study. Anesthesiology 2003, 99, 1313–1322. [Google Scholar] [CrossRef]
- Tingay, D.G.; Mills, J.F.; Morley, C.J.; Pellicano, A.; Dargaville, P.A. Indicators of optimal lung volume during high-frequency oscillatory ventilation in infants. Crit. Care Med. 2013, 41, 237–244. [Google Scholar] [CrossRef]
- Zannin, E.; Ventura, M.L.; Dellacà, R.L.; Natile, M.; Tagliabue, P.; Perkins, E.J.; Sourial, M.; Bhatia, R.; Dargaville, P.A.; Tingay, D.G. Optimal mean airway pressure during high-frequency oscillatory ventilation determined by measurement of respiratory system reactance. Pediatr. Res. 2014, 75, 493–499. [Google Scholar] [CrossRef][Green Version]
- Miedema, M.; de Jongh, F.H.; Frerichs, I.; van Veenendaal, M.B.; van Kaam, A.H. The effect of airway pressure and oscillation amplitude on ventilation in pre-term infants. Eur. Respir. J. 2012, 40, 479–484. [Google Scholar] [CrossRef]
- Pillow, J.J. Tidal volume, recruitment and compliance in HFOV: Same principles, different frequency. Eur. Respir. J. 2012, 40, 291–293. [Google Scholar] [CrossRef]
- Kung, S.C.; Hung, Y.L.; Chen, W.L.; Wang, C.M.; Chang, H.C.; Liu, W.L. Effects of Stepwise Lung Recruitment Maneuvers in Patients with Early Acute Respiratory Distress Syndrome: A Prospective, Randomized, Controlled Trial. J. Clin. Med. 2019, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.H. The Role of Airway Shunt Elastance on the Compartmentalization of Respiratory System Impedance. J. Eng. Sci. Med. Diagn. Ther. 2019, 2, 110011–110018. [Google Scholar] [CrossRef] [PubMed]
- Brashier, B.; Salvi, S. Measuring lung function using sound waves: Role of the forced oscillation technique and impulse oscillometry system. Breathe 2015, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Obase, Y.; Nagasaka, Y.; Kishikawa, R.; Mukae, H.; Iwanaga, T. Peripheral bronchial obstruction evaluation in patients with asthma by lung sound analysis and impulse oscillometry. Allergol. Int. 2017, 66, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, D.; Hesselstrand, R.; Bozovic, G.; Wuttge, D.M.; Tufvesson, E. Airway resistance and reactance are affected in systemic sclerosis. Eur. Clin. Respir. J. 2015, 2, 28667. [Google Scholar] [CrossRef]
- Bhattarai, P.; Myers, S.; Chia, C.; Weber, H.C.; Young, S.; Williams, A.D.; Sohal, S.S. Clinical Application of Forced Oscillation Technique (FOT) in Early Detection of Airway Changes in Smokers. J. Clin. Med. 2020, 9, 2778. [Google Scholar] [CrossRef]
- Fessler, H.E.; Derdak, S.; Ferguson, N.D.; Hager, D.N.; Kacmarek, R.M.; Thompson, B.T.; Brower, R.G. A protocol for high-frequency oscillatory ventilation in adults: Results from a roundtable discussion. Crit. Care Med. 2007, 35, 1649–1654. [Google Scholar] [CrossRef]
- Roubik, K. Measuring and evaluating system designed for high-frequency oscillatory ventilation monitoring. Biomed. Tech. 2014. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Michaelson, E.D.; Grassman, E.D.; Wendell, R.P. Pulmonary mechanics by spectral analysis of forced random noise. J. Clin. Invest. 1975, 56, 1210–1230. [Google Scholar] [CrossRef]
- Rožánek, M.; Horáková, Z.; Čadek, O.; Kučera, M.; Roubík, K. Damping of the dynamic pressure amplitude in the ventilatory circuit during high-frequency oscillatory ventilation. Biomed. Eng. Biomed. Tech. 2012, 57, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Laviola, M.; Rafl, J.; Rozanek, M.; Kudrna, P.; Roubik, K. Models of PaO2 response to the continuous distending pressure maneuver during high frequency oscillatory ventilation in healthy and ARDS lung model pigs. Exp. Lung Res. 2016, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Suter, P.M.; Fairley, H.B.; Isenberg, M.D. Optimum endexpiratory airway pressure in patients with acute pulmonary failure. N. Engl. J. Med. 1975, 292, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, P.A.; Rimensberger, P.C.; Frerichs, I. Regional tidal ventilation and compliance during a stepwise vital capacity manoeuvre. Intensive Care Med. 2010, 36, 1953–1961. [Google Scholar] [CrossRef]
- Dellaca, R.L.; Veneroni, C. Trends in mechanical ventilation: Are we ventilating our patients in the best possible way? Breathe 2017, 13, 84–98. [Google Scholar] [CrossRef]
- Roubík, K.; Ráfl, J.; van Heerde, M.; Markhorst, D.G. Design and control of a demand flow system assuring spontaneous breathing of a patient connected to an HFO ventilator. IEEE Trans. Biomed. Eng. 2011, 58, 3225–3233. [Google Scholar] [CrossRef]
- Van Heerde, M.; Roubik, K.; Kopelent, V.; Plötz, F.B.; Markhorst, D.G. Demand flow facilitates spontaneous breathing during high-frequency oscillatory ventilation in a pig model. Crit. Care Med. 2009, 37, 1068–1073. [Google Scholar] [CrossRef]
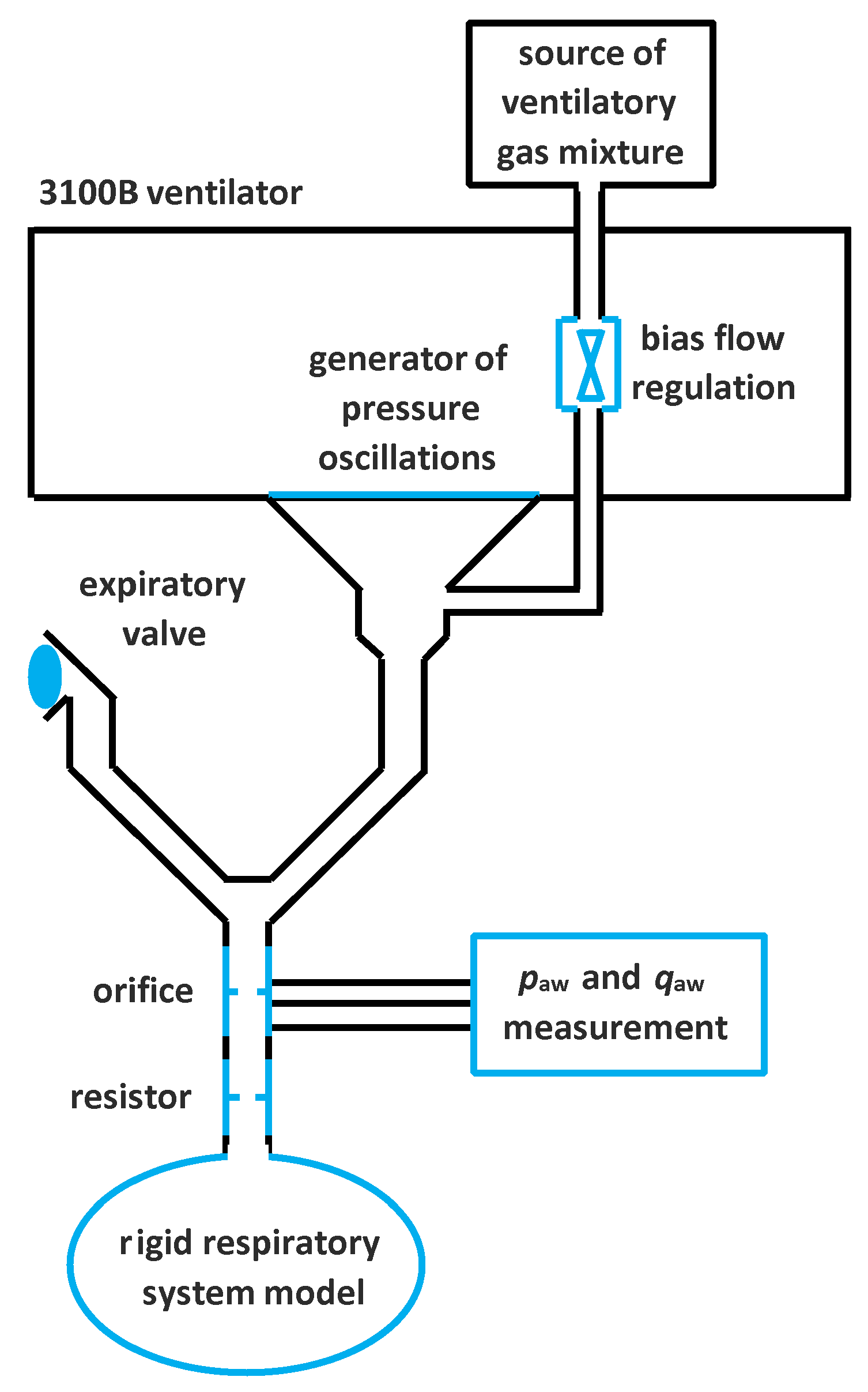
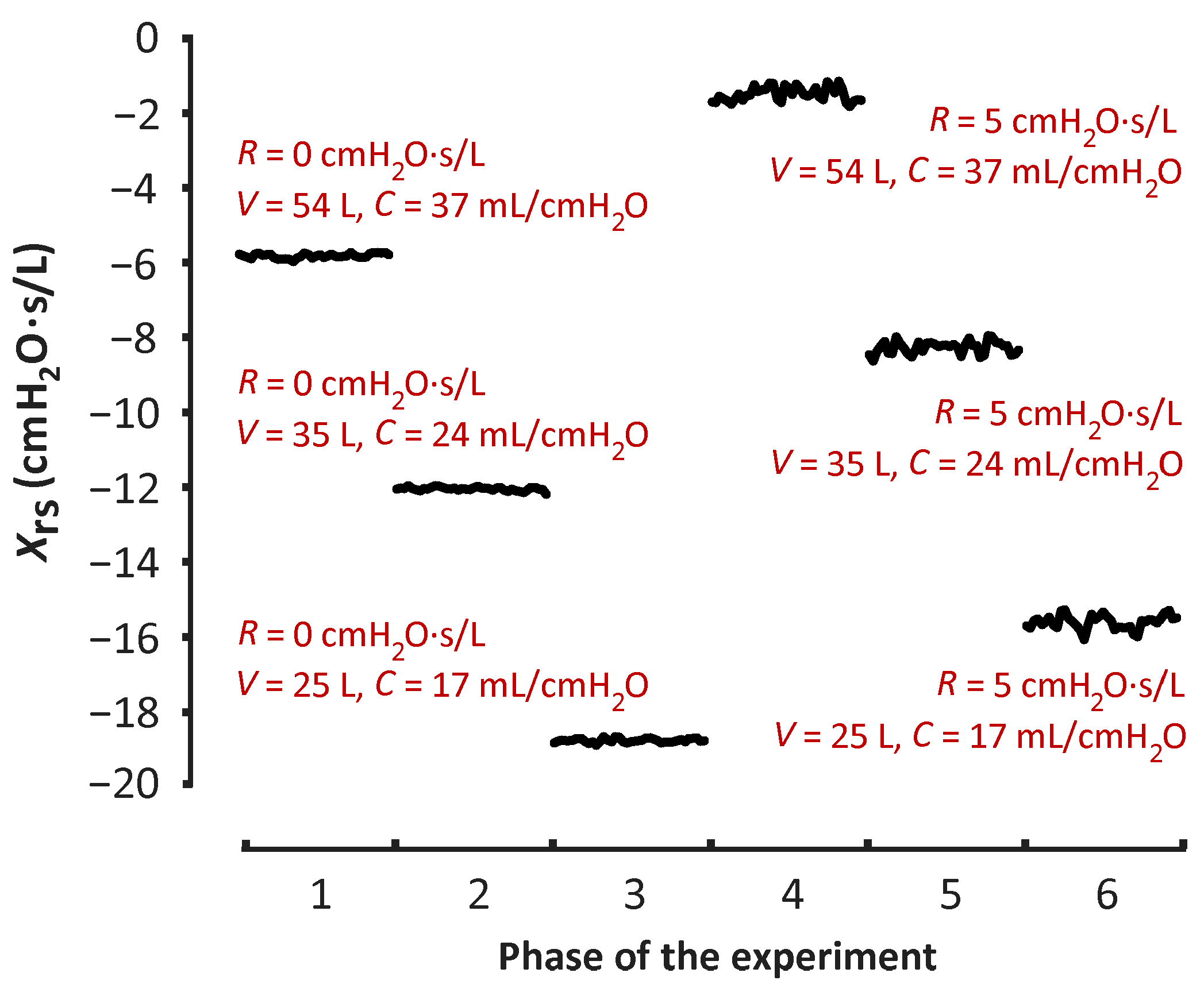
| V (L) | Xrs with No Resistor | Xrs with Added Resistor | ||
|---|---|---|---|---|
| Mean | SD 1 | Mean | SD 1 | |
| 54 | −5.82 | 0.06 | −1.56 | 0.17 |
| 35 | −12.07 | 0.05 | −8.26 | 0.17 |
| 25 | −18.76 | 0.06 | −15.62 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matejka, J.; Rozanek, M.; Rafl, J.; Kudrna, P.; Roubik, K. In Vitro Estimation of Relative Compliance during High-Frequency Oscillatory Ventilation. Appl. Sci. 2021, 11, 899. https://doi.org/10.3390/app11030899
Matejka J, Rozanek M, Rafl J, Kudrna P, Roubik K. In Vitro Estimation of Relative Compliance during High-Frequency Oscillatory Ventilation. Applied Sciences. 2021; 11(3):899. https://doi.org/10.3390/app11030899
Chicago/Turabian StyleMatejka, Jan, Martin Rozanek, Jakub Rafl, Petr Kudrna, and Karel Roubik. 2021. "In Vitro Estimation of Relative Compliance during High-Frequency Oscillatory Ventilation" Applied Sciences 11, no. 3: 899. https://doi.org/10.3390/app11030899
APA StyleMatejka, J., Rozanek, M., Rafl, J., Kudrna, P., & Roubik, K. (2021). In Vitro Estimation of Relative Compliance during High-Frequency Oscillatory Ventilation. Applied Sciences, 11(3), 899. https://doi.org/10.3390/app11030899





