Abstract
Inflammation of the arterial wall is critical to atherosclerosis pathogenesis. The switch of vascular smooth muscle cells (VSMCs) to macrophage-like cells is essential in the exacerbation of vascular inflammation. Platonin, a cyanine photosensitizing dye, exhibits protective effects in sepsis, trauma, and acute ischemic stroke through its anti-inflammatory capacity in macrophages. The present study investigated the effects and underlying mechanisms of platonin in inflammatory VSMCs. Pretreatment with platonin suppressed the expression of inducible nitric oxide synthetase and mature interleukin-1β but not that of monocyte chemoattractant protein-1 (MCP-1) in VSMCs stimulated by a combination of lipopolysaccharide and interferon-γ (LPS/IFN-γ). Furthermore, platonin inhibited LPS/IFN-γ-induced Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation though the direct reduction of p65Ser536 phosphorylation but not the restoration of Inhibitor of nuclear factor kappa B (IκBα) degradation in VSMCs. However, platonin inhibited Oxidized low-density lipoprotein (ox-LDL)-induced MCP-1 production, possibly through the attenuation of Activator protein 1 (AP-1) binding activity and C-Jun N-terminal kinases ½ (JNK1/2) phosphorylation. Platonin also lowered lipid drop accumulation in VSMCs in Oil red O staining assay. The results collectively indicated that platonin has a vascular protective property with potent anti-inflammatory effects in VSMCs. In conclusion, platonin should be a potential for treating vascular inflammatory diseases such as atherosclerosis.
1. Introduction
Chronic inflammation of the arterial wall contributes critically to the formation of atherosclerosis [1]. Atherosclerosis features gradual lipids and accumulation of immune cells in the arterial intima, which can lead to acute clinical outcomes including myocardial infarction and ischemic stroke [2]. Lipid deposition at specific arterial wall sites initiates atheroma pathogenesis. As the disease progresses, the intimal layer undergoes multiple stages of inflammation, necrosis, fibrosis, and calcification and results in plaque formation [3]. The formation of cholesterol-laden macrophage-derived foam cells is critical in these stages [4]. Foam cells have lower mobility and produce proinflammatory cytokines and chemokines to recruit additional monocytes, T cells, and neutrophils, thus amplifying the inflammatory cascade [5]. In addition to immune cells, vascular smooth muscle cells (VSMCs) are pivotal in this vascular inflammatory disease.
VSMCs are usually quiescent and differentiated in the “contractile” phenotype, where they maintain hemodynamics and vascular wall structure [6]. However, VSMCs also exhibit high dedifferentiation in response to stimulators such as inflammatory mediators, growth factors, extracellular matrix, and lipoproteins [7]. Dedifferentiated VSMCs, referred to as the “synthetic” phenotype, can proliferate, migrate, and express macrophage markers and thus demonstrate inflammatory properties, such as cytokine or chemoattractant production [8]. Lipid accumulation may be critical in the switch of VSMCs into macrophage-like cells. Cholesterol loading to VSMCs was observed to stimulate many proinflammatory genes and reduce the expression of VSMC marker genes [9]. These VSMC-derived macrophage-like cells are unlike typical immune cells: They have low phagocytic capacity but promote atherosclerosis pathogenesis by exacerbating vascular inflammation [10].
Oxidized low-density lipoprotein (ox-LDL), considered as a main cause of atherosclerosis [11,12], contributes to lipid accumulation in the intimal layer, which leads to the subsequent atherosclerosis event [13]. Lectin-like ox-LDL receptor 1 (LOX-1) is the major scavenger receptor of ox-LDL in endothelial cells [13]. However, it is not identified only in endothelial cells but also in macrophages and VSMCs [13]. Furthermore, the binding of ox-LDL to LOX-1 enhances the expression of monocyte chemoattractant protein-1 (MCP-1) [14], which is critical in the pathogenesis of inflammatory diseases, including cancer and atherosclerosis [15]. Together with proinflammatory cytokines, ox-LDL thus contributes to atherosclerosis pathogenesis. Therefore, the inhibition of proinflammatory factors (and the subsequent inflammatory cascade) may be effective in atherosclerosis therapy.
Platonin (Figure 1A), a cyanine photosensitizing dye with immunomodulating activity, is currently used in clinics to treat immune diseases such as rheumatoid arthritis [16]. Platonin exhibits free radical scavenging activity and can inhibit the production of inflammatory mediators including interleukin (IL)-6, IL-1β, and tumor necrosis factor α, and the expression of inducible nitric oxide synthase (iNOS) in lipopolysaccharide (LPS)-activated macrophages [17,18]. Platonin can also diminish circulatory failure and increase the survival rate of septic rats [19]. A recent study reported that platonin can prevent injury due to macrophage-mediated inflammatory responses in the brain [20]. Considering the critical role of inflammatory VSMCs in atherosclerosis pathogenesis, the anti-inflammatory effects and possible underlying mechanisms of platonin in VSMCs treated with different stimulators were determined in the present study. The treatment of platonin may be a potential for treating vascular diseases in clinical application due to its possible anti-inflammatory capacity in VSMCs.
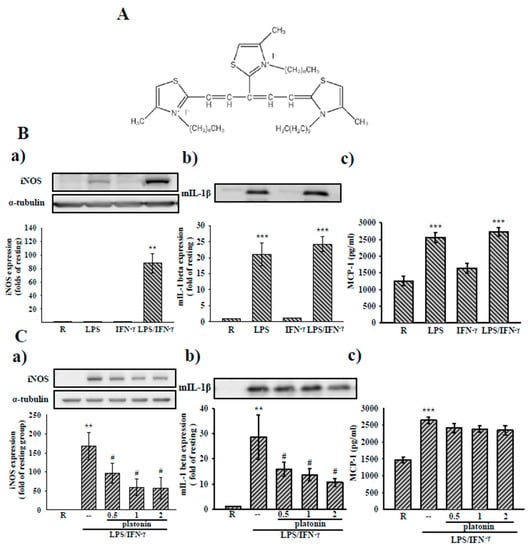
Figure 1.
Effects of platonin on Lipopolysaccharides/Interferon gamma (LPS/IFN-γ)-induced inducible nitric oxide synthase (iNOS) expression, and production of mature Interleukin 1β(IL-1β) (mIL-1β) and monocyte chemoattractant protein-1 (MCP-1) production in vascular smooth muscle cells (VSMCs). (A) The chemical structure of platonin. (B,C) VSMCs were pretreated without (B) or with (C) platonin (0.5–2 M) for 30 min and then stimulated with LPS, IFN-γ, or the combination of LPS (50 μg/mL) and IFN-γ (100 units/mL, LPS/IFN-γ) for 24 h. Western blotting assay was used to determine the cytosolic expression of iNOS and mIL-1β content in the medium. MCP-1 production was determined using the ELISA kit described in “Materials and Methods.” Data are presented as mean ± structural equation modeling (S.E.M.) (n = 3); ** p < 0.01, *** p < 0.001 compared to the resting (R) group; # p < 0.05 compared with the positive control group.
2. Materials and Methods
2.1. Materials
Platonin was provided from Gwo Chyang Pharmaceuticals (Tainan, Taiwan). It was then dissolved in phosphate-buffered saline (PBS) and stored at 4 °C until used. Penicillin/streptomycin/L-glutamine, Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and trypsin (0.25%) were from Invitrogen (Waltham, MA, USA). LPS was obtained from Sigma-Aldrich (St. Louis, MO, USA), human high oxidized low density lipoprotein (770,252-4) was purchased from Kalen Biomedical LLC (Montgomery Village, MD, USA), okadaic acid (>98% purity) from Merck Millipore (Billerica, MA, USA), and recombinant murine interferon gamma (IFN-γ) from Peprotech (Rocky Hill, NJ, USA). Moreover, we procured anti-iNOS polyclonal antibody (pAb) from Santa Cruz Biotechnology (Dallas, TX, USA); anti-IL-1β pAb from BioVision (Milpitas, CA, USA); anti-phospho-p65 (Ser536), anti-phospho-c-JNK (Thr183/Tyr185), and anti-IκBα monoclonal antibodies (mAbs) from Cell Signaling (St. Louis, MO, USA); and anti-α-tubulin mAb from Thermo Fisher Scientific (Waltham, MA, USA). The secondary antibodies including horseradish peroxidase–conjugated Immunoglobulin G (IgG) of donkey anti-rabbit and sheep anti-mouse were purchased from Amersham (Buckinghamshire, UK). The reporter plasmid nuclear factor-κB (NF-κB)-Luc and activator protein 1 (AP-1)-Luc were purchased from Signosis (Santa Clara, CA, USA). A Dual-Luciferase Reporter (DLR) Assay System was procured from Promega (Madison, WI, USA). The enhanced chemiluminescent (ECL) detection kit and hybond-P polyvinylidene difluoride (PVDF) membranes were obtained from GE Healthcare Life Sciences (Waukesha, WI, USA).
2.2. Cell Cultivation
A murine VSMC line was ordered from American Type Culture Collection (ATCC) (Manassas, VA, USA; ATCC number: CRL-2797). The cells were cultured in DMEM supplemented with penicillin G (100 units/mL)/streptomycin (100 mg/mL)/glutamine (2 mM) and 10% FBS in a humidified atmosphere of 5% CO2/95% air at 37 °C.
2.3. Content of MCP-1 Analysis
The VSMCs were treated with or without platonin and then administered with LPS/IFN-γ or ox-LDL for 24 h or 48 h, respectively. To measure MCP-1 secreted in the culture supernatant, an appropriate ELISA kit, Mouse MCP-1 ELISA MAX Deluxe Set (432,704; BioLegend, San Diego, CA, USA) was used according to the manufacturer’s protocols. The optical density was spectrophotometrically measured at 450 nm using a microplate reader (Biotek Instruments, Winooski, VT, USA).
2.4. Western Blotting Assay
The VSMCs were pretreated with or without platonin and then administered with LPS/IFN-γ or ox-LDL for 24 h or 48 h, respectively. The cell lysates and conditioned medium were subjected to Western blotting assay. The protein samples were separated by using sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then transferred onto a PVDF membrane by a semi-dry transfer machine from Bio-Rad (Hercules, CA, USA). After blocking with 5% skimmed milk, which dissolved in the buffer containing 10 mM Tris-base, 100 mM NaCl, and 0.01% Tween 20 for 1 h, the membranes were then recognized with different primary antibodies for 1 h and followed incubation with the secondary antibody for another 1 h. ECL system was utilized to detect the immune-reactive bands, and the densitometric analysis was performed by Visionworks Windows Application (Visionworks of America, San Antonio, TX, USA).
2.5. Transfection and Luciferase Assays
The cells (1.5 × 106/well) were transfected with NF-κB-luc or AP-1-luc plasmid (Signosis) plus Renilla-luc plasmid (Promega) by using lipofectamine reagent (Invitrogen). These transfected cells were treated with or without platonin and then administered with LPS/IFN-γ or ox-LDL for the indicated times (6 h or 12 h). Luciferase activity was evaluated with a DLR Assay System kit and normalized by basal Renilla luciferase activity. The levels of activity were compared through the ratio of different groups.
2.6. Oil Red O Staining
The cultured VSMCs were plated on six-well plates and administered with or without platonin and following treatment with ox-LDL for 48 h. The cells were then washed twice with PBS, fixed for 30 min in 4% paraformaldehyde, and then incubated with 0.3% Oil red O solution for 10 min. The samples were then washed five times with Distillation-Distillation H2O (ddH2O) and photographed by a microscope at 400× magnification (Carl Zeiss Microscopy GmbH, Jean, Germany). The images are typical of those obtained in three separate experiments demonstrating the accumulation of cholesterol. The deep purple dots indicated cholesterol-laden positive cells. The percentage of positive cells was counted in four microscope fields of each experiment, and the mean was subjected to the statistical analysis.
2.7. Statistical Analysis
The data are shown as the mean ± standard error of the mean (S.E.M.) and the number of independent experiments (n). In the first, results were analyzed using one-way analysis of variance (ANOVA). If one-way ANOVA revealed significant differences among the group means, a following comparison by the Newman–Keuls method was conducted. A P of <0.05 was considered statistically significant.
3. Results
3.1. Effects of Platonin on LPS/IFN-γ-Induced Expression of Inflammatory Mediators in VSMCs
Studies have indicated that the expression of inflammatory mediators such as iNOS is upregulated in VSMCs after LPS treatment, and the combination with one or more cytokines is an additive [21]. As Figure 1 suggests, we examined the inhibition of platonin on iNOS, IL-1β, and MCP-1 expressions in VSMCs stimulated by the combination of LPS (50 μg/mL) and IFN-γ (100 units/mL (LPS/IFN-γ) to determine the protective effects of platonin in the vascular inflammatory microenvironment. As can be seen in Figure 1B(a–c), the treatment with LPS/IFN-γ for 24 h significantly increased iNOS protein levels and mature IL-1β (mIL-1β) and MCP-1 production in the VSMCs. The administration of platonin (0.5–2 μM) concentration-dependently inhibited the upregulation of iNOS and mature form IL-1β (mIL-1β) in LPS/IFN-γ-stimulated VSMCs (Figure 1C(a,b)). However, LPS/IFN-γ-induced MCP-1 production in VSMCs was unaffected by the incubation with platonin (Figure 1C(c)).
3.2. Platonin Attenuates Ox-LDL-Stimulated MCP-1 Production in VSMCs
In the present study, we also measured the anti-inflammatory effect of platonin against ox-LDL, a crucial effector in atherosclerosis [22], -treated VSMCs. As is shown in Figure 2A, the administration of ox-LDL (10–40 μg/mL) for 48 h did not induce the expression of iNOS and mIL-1β but substantially increased MCP-1 production in the VSMCs (Figure 2B). In addition, the treatment of platonin (0.5–2 μM) remarkably suppressed the production of MCP-1 in ox-LDL-stimulated VSMCs (Figure 2C).
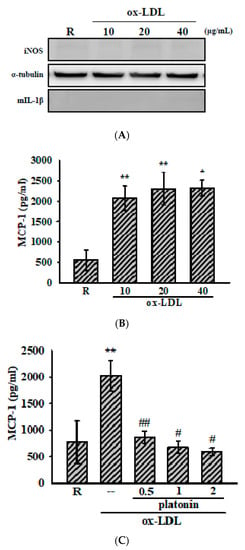
Figure 2.
Platonin suppresses ox-LDL-induced production of monocyte chemoattractant protein-1 (MCP-1) in vascular smooth muscle cells (VSMCs). VSMCs were pretreated without (A,B) or with (C) platonin (0.5–2 μM) for 30 min and then stimulated with (A,B) ox-LDL (10–40 μg/mL) or (C) ox-LDL (20 μg/mL) for 48 h. Western blotting assay was used to determine the cytosolic expression of iNOS and mIL-1β content in the medium. MCP-1 production was determined using the ELISA kit as described in “Materials and Methods.” Data are presented as mean ± S.E.M. (n = 3); * p < 0.05, ** p < 0.01, compared with the resting (R) group; # p < 0.05, ## p < 0.01 compared with the positive control group.
3.3. Effects of Platonin on NF-κB Activation in LPS/IFN-γ-Induced VSMCs
To clarify the anti-inflammatory mechanisms of platonin in LPS/IFN-γ-treated VSMCs, we determined the effect of platonin on the NF-κB signaling cascade, a critical transcriptional factor for driving the expression of inflammatory genes such as iNOS and mIL-1β [23]. An NF-κB luciferase reporter assay was employed to evaluate the effect of platonin on NF-κB activity. As may be observed in Figure 3A(a), treatment with LPS/IFN-γ for 6 h significantly stimulated NF-κB activity in the VSMCs. The administration of platonin (2 μM) substantially attenuated NF-κB activation in LPS/IFN-γ-stimulated VSMCs (Figure 3A(b)). We next determined the effect of platonin on the degradation of the IκB protein, which we observed to be considerably reduced after exposure to LPS/IFN-γ for 30 min (Figure 3B(a)). Nevertheless, pretreatment with platonin (0.5–2 μM) did not reverse the degradation of IκBα in the LPS/IFN-γ-stimulated VSMCs (Figure 3B(b)). We then determined whether NF-κB/p65 contributed to the inhibitory effect of platonin on LPS/IFN-γ-stimulated NF-κB activation. As is indicated by Figure 3C(a), p65Ser536 phosphorylation was remarkably increased in VSMCs after incubation with LPS/IFN-γ for 60 min. The LPS/IFN-γ-induced p65Ser536 phosphorylation was significantly suppressed by pretreatment with platonin (2 μM; Figure 3C(b)). Our previous study indicated that p65 phosphorylation regulated by protein phosphatase 2A (PP2A) may modulate NF-κB activation in an IκBα -independent pathway [24]. Thus, we confirmed the role of PP2A in the anti-inflammatory mechanism of platonin in VSMCs by using okadaic acid, a selective inhibitor of PP2A. As is presented in Figure 3C(c), pretreatment with okadaic acid (10 nM) did not restore the downregulation of iNOS by platonin in the LPS/IFN-γ-stimulated VSMCs.
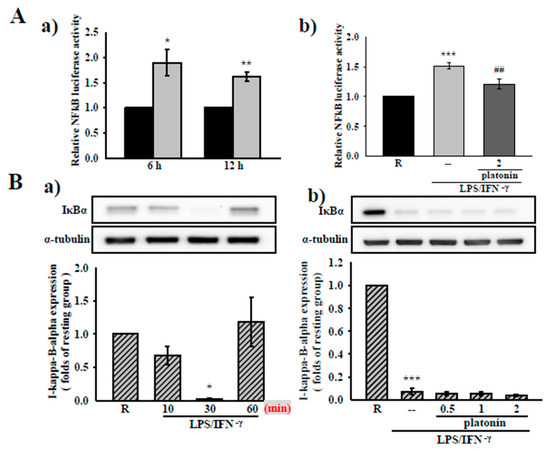
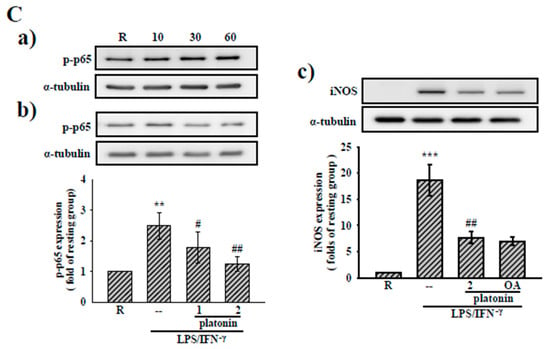
Figure 3.
Effects of platonin on the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway in vascular smooth muscle cells (VSMCs) stimulated by LPS/IFN-γ. (A) After transient transfection with NF-κB-luc and Renilla-luc plasmids for 24 h, the VSMCs were administered without (a) or with (b) platonin (2 μM) for 30 min then stimulated by the combination of LPS (50 μg/mL) and IFN-γ (100 units/mL; LPS/IFN-γ) for 6 h or 12 h (black bar: resting group; gray bar: LPS/IFN-γ-treated group; deep-gray bar: platonin + LPS/IFN-γ-treated group). A luciferase assay was conducted as described in “Materials and Methods.” (B) (a) Cells were treated with LPS/IFN-γ for the indicated period. (b) Cells were pretreated with platonin (0.5–2 μM) for 30 min before treatment with LPS/IFN-γ for another 30 min. (C) Cells were pretreated without (a) or with platonin (1 μM or 2 μM) (b) or okadaic acid (10 nM) + platonin (2 μM) (c) for 30 min, then treated with LPS/IFN-γ for the indicated time (a), 30 min (b), and 24 h (c). The extent of IκBα phosphorylated p65, and inducible nitric oxide synthetase (iNOS) were determined using Western blotting assay. Data are represented as mean ± S.E.M. (n = 3); * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the resting (R) group; # p < 0.05, ## p < 0.01 compared with the positive control group.
3.4. Platonin Suppresses AP-1 Activation and JNK1/2 Phosphorylation in Ox-LDL-Treated VSMCs
As suggests by Figure 4A(a), treatment with ox-LDL (20 μg/mL) for 6 and 12 h did not show any substantial increase in NF-κB luciferase activity in VSMCs (Figure 4A(a)). Some studies had indicated that ox-LDL could activate the DNA binding activity of AP-1 in VSMCs [25]. We therefore determined the effect of platonin on AP-1 binding activity in ox-LDL-treated VSMCs. The administration of ox-LDL (20 μg/mL) for 12 h significantly stimulated AP-1 DNA binding activity compared with the control group (Figure 4A(b)). As is depicted in Figure 4A(c), the ox-LDL-stimulated increase in AP-1 binding activity was notably suppressed in VSMCs pretreated with platonin (2 μM). Several studies have indicated that ox-LDL-induced AP-1 activation is strongly associated with JNK1/2 phosphorylation [26,27]. We found that the extent of JNK phosphorylation was apparently increased after exposure to ox-LDL (20 μg/mL) for 30 min (Figure 4B(a)). Furthermore, ox-LDL-induced JNK phosphorylation was significantly inhibited in VSMCs pretreated with 2 μM platonin (Figure 4B(b)). These results indicate that the inhibition of AP-1 binding activity may be responsible for the anti-inflammatory mechanism of platonin in ox-LDL-stimulated VSMCs.
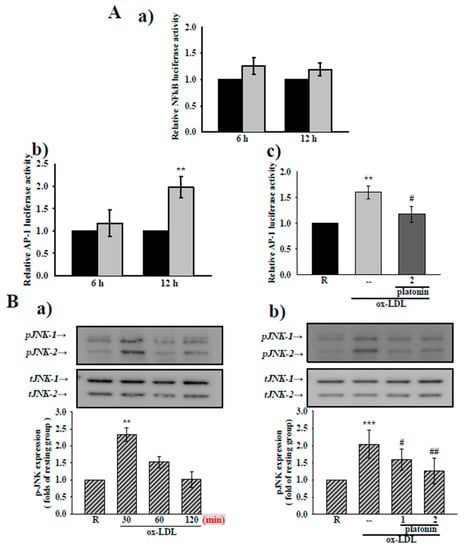
Figure 4.
Effects of platonin on AP-1 activity and JNK phosphorylation in ox-LDL-stimulated vascular smooth muscle cells (VSMCs). (A(a)) VSMCs were transiently transfected with NF-κB-luc and Renilla-luc plasmids for 24 h and then treated with ox-LDL (20 μg/mL) for 6 h or 12 h. (A(b,c)) Transient transfection with AP-1-luc and Renilla-luc plasmids were also performed in VSMCs for 24 h. After transfection, the cells were pretreated without (b) or with (c) platonin (2 μM) for 30 min then treated with the ox-LDL (20 μg/mL) for 6 h or 12 h (black bar: resting group; gray bar: ox-LDL-treated group; deep-gray bar: platonin+ ox-LDL-treated group). A luciferase assay was performed as described in “Materials and Methods.” (B) Cells were pretreated without (a) or with platonin (1 or 2 μM) (b) for 30 min, then treated with ox-LDL (20 μg/mL) for (a) the indicated time or (b) 30 min. The extent of JNK phosphorylation was then determined through Western blotting assay. Data are represented as mean ± S.E.M. (n = 3); ** p < 0.01, *** p < 0.001 compared with the resting (R) group; # p < 0.05, ## p < 0.01 compared with the positive control group.
3.5. Treatment with Platonin Attenuates Lipid Accumulation in ox-LDL-Stimulated VSMCs
Lipid accumulation plays crucial roles in the development of atherosclerosis [28]. Incubation with ox-LDL can induce lipid drop formation in VSMCs [29]. To determine the effect of platonin on ox-LDL-induced lipid drop formation, we performed Oil red O staining assay in VSMCs. As shown in Figure 5A, stimulation by ox-LDL (50 μg/mL) for 48 h remarkably increased neutral lipid droplet accumulation in VSMCs. However, pretreatment with platonin (2 μM) significantly reduced the ox-LDL-induced accumulation of lipid drops in the VSMCs (Figure 5A,B).
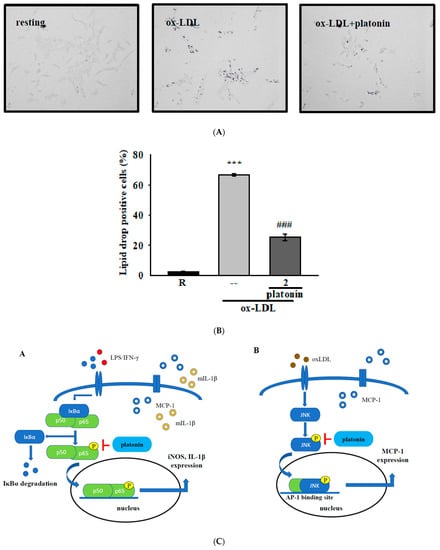
Figure 5.
Effect of platonin on lipid drop formation in ox-LDL-stimulated vascular smooth muscle cells (VSMCs). (A,B) VSMCs were treated with ox-LDL (50 μg/mL) for 48 h in the presence or absence of platonin (2 μM). Oil red O staining was performed as described in “Materials and Methods.” The deep-purple dots indicate cholesterol-laden positive cells. Data are expressed as mean ± S.E.M. (n = 3); *** p < 0.01 compared with the resting group; ### p < 0.05 compared with the ox-LDL treated group. (C) Schematic of the anti-inflammatory mechanisms of platonin in LPS/IFN-γ or ox-LDL-treated VSMCs.
4. Discussion
Atherosclerosis is an inflammatory disease with a progressive, long-term pathology, and it places heavy economic burdens on human beings. Vascular inflammation was thought to be the first incident responsible for atherosclerosis development. In previous studies, we uncovered that treatment by platonin can reduce VSMC proliferation and attenuate macrophage activation [30]. The present study further demonstrates that platonin, a cyanine photosensitizing dye, suppresses inflammatory responses in VSMCs induced by various mediators. The inhibitory property of platonin in inflammatory VSMCs may be another advantage for the treatment of atherosclerosis. Indeed, platonin was reported to reduce mortality and lung injury and to preserve blood–brain barrier integrity in septic rats [31]. The protective effect of platonin against vascular inflammation thus may also be involved in the mechanisms underlying sepsis.
Vascular inflammation is crucial in the pathogenesis of atherosclerosis and other inflammatory diseases [32]. In this study, platonin suppressed LPS/IFN-γ or ox-LDL-induced inflammation in VSMCs at 0.5–2 μM/mL. The results suggest that platonin could be a potential agent for treating VSMC inflammation-associated diseases. Vascular injury follows inflammatory processes such as the recruiting of immune cells and the releasing of cytokines/chemokines, which are crucial in atherosclerosis [33]. The recruitment of circulating monocyte-derived cells occurs in lesions of atherosclerosis [34], where they differentiate into macrophages. These macrophages yield lipid-laden foam cells by actively ingesting and accumulating lipoprotein in atherosclerotic lesions [34,35,36]. The accumulation of foam cells results in lipid storage and atherosclerotic plaque growth [34]. MCP-1, a member of chemotactic cytokine family, is a potent monocyte activator induced by oxidized lipids [37]. It is also a proinflammatory factor playing key roles in foam cell formation [38]. Ox-LDL is known to induce robust expression of MCP-1 in the lesions of atherosclerosis. This indicates that MCP-1 should be a crucial factor in the recruitment of monocytes or macrophages in the early stage of atherosclerosis [37]. Platonin has been reported to be a potent antioxidant and to suppress macrophage activation and associated inflammatory responses [20]. In the present study, platonin inhibited ox-LDL-induced MCP-1 production in VSMCs. These results indicate that platonin could also be a potential agent for treating vascular inflammatory diseases through its dual inhibition on macrophages and VSMCs.
IκBα protein degradation is a critical step in the NF-κB signaling pathway; the binding of IκBα protein modulates the entry of cytosolic NF-κB into nuclei. The dissociation of IκBα from the NF-κB subunits is modulated by the phosphorylation of IκB kinase complex and is ubiquitinated then rapidly degraded by the proteasome [39]. In the present study, the administration of platonin led to significant inhibition of NF-κB activation; however, IκBα degradation was unaffected by treatment with platonin in LPS/IFN-γ-stimulated VSMCs. Previous studies reported that the phosphorylation of crucial serine residues in p65 can regulate its dimerization, nuclear translocation, and DNA binding activity [23]. An individual phosphorylation site may be modulated by a single or multiple kinases [40]. The phosphorylation of Ser536 after LPS or TNF stimulation can increase transcriptional activity of NF-κB [41]. We, thus, examined whether the phosphorylation of p65 contributes to the inhibitory mechanism of platonin in NF-κB activation. Pretreatment by platonin indeed attenuated p65ser536 phosphorylation in VSMCs stimulated by LPS/IFN-γ. It is imaginable that platonin may directly dephosphorylate Ser536 through activating a protein phosphatase, then lead to the downregulation of NF-κB and iNOS. The previous study indicated that PP2A-modulated p65 phosphorylation regulated NF-κB activation in an IκBα-independent pathway [24]. Nevertheless, pretreatment with okadaic acid, a PP2A selective inhibitor, did not restore platonin-downregulated LPS/IFN-γ-induced iNOS expression in VSMCs. The underlying mechanism of platonin-modulated p65 dephosphorylation in VSMCs stimulated by LPS/IFN-γ requires further clarification.
Ox-LDL is considered as a well-established risk factor in pathogenesis of atherosclerosis [42]. Ox-LDL and its active lipid components have been demonstrated to induce atherothrombosis directly or by amplifying inflammation [12]. Accordingly, ox-LDL is known to activate the mitogen-activated protein kinase (MAPK) family, transcription factors such as AP-1, and several early genes including c-jun, c-fos, and c-myc and thus controls the expression of cytokines and growth factors [43]. A previous study indicated that ox-LDL-induced Matrix metalloproteinase MMP-9 expression is regulated by JNK1/2, Akt, and ERK1/2, resulting in the activation of AP-1 [27]. Activated JNKs motivate the phosphorylation of various transcription factors including c-jun, a subunit of AP-1 [44]. Ox-LDL was also shown to stimulate VSMC proliferation through the PI3K/Akt pathway and to prolong the survival of macrophage foam cell in atherosclerotic lesions [45]. In addition, like macrophages, VSMCs can transform into foam cells [46]. In this study, platonin inhibited ox-LDL-induced AP-1 binding activity by suppressing JNK phosphorylation and reducing lipid drop accumulation in VSMCs. These results indicate that platonin alleviates the pathogenesis in ox-LDL-activated VSMCs.
Here, we investigated the anti-inflammatory effects and underlying mechanisms of platonin in VSMCs. Treatment with platonin suppressed both LPS/IFN-induced and ox-LDL-induced vascular inflammation through the NF-κB and the AP-1 signaling pathways, respectively. As may be observed in Figure 5C, platonin inhibited LPS/IFN-induced iNOS and mIL-1β through the dephosphorylation of p65, which led to reduced NF-κB activity. MCP-1 production, JNK phosphorylation, and AP-1 activation were suppressed in ox-LDL-stimulated VSMCs through pretreatment with platonin. In addition, in Oil red O staining assay, the administration of platonin significantly reduced lipid drop accumulation in VSMCs. These findings collectively indicate a potent anti-inflammatory effect of platonin in VSMCs and further prove that platonin should be a potential therapeutic agent against vascular inflammatory diseases.
Author Contributions
Conceptualization, C.-Y.H. and S.-Y.H.; methodology, C.-H.Y.; investigation, C.-W.C. and J.-H.T.; data curation, C.-W.C. and J.-H.T.; writing—original draft preparation, C.-W.C.; supervision, C.-H.Y.; funding acquisition, C.-Y.H. and S.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants provided by the Ministry of Science and Technology of Taiwan (MOST-108-2320-B-038-021-MY2) and the Taipei Medical University (DP2-107-21121-N-02 and DP2-109-21121-01-N-08-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Roostalu, U.; Wong, J.K. Arterial smooth muscle dynamics in development and repair. Dev. Biol. 2018, 435, 109–121. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef]
- Ramel, D.; Gayral, S.; Sarthou, M.K.; Augé, N.; Nègre-Salvayre, A.; Laffargue, M. Immune and Smooth Muscle Cells Interactions in Atherosclerosis: How to Target a Breaking Bad Dialogue? Front. Pharmacol. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, Y.; Zhao, K.; Gao, Y.; Cui, J.; Qi, L.; Huang, L. miR-21/PTEN pathway mediates the cardioprotection of geniposide against oxidized low-density lipoprotein-induced endothelial injury via suppressing oxidative stress and inflammatory response. Int. J. Mol. Med. 2020, 45, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef] [PubMed]
- Akagi, M.; Ueda, A.; Teramura, T.; Kanata, S.; Sawamura, T.; Hamanishi, C. Oxidized LDL binding to LOX-1 enhances MCP-1 expression in cultured human articular chondrocytes. Osteoarthr. Cartil. 2009, 17, 271–275. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Motoyoshi, F.; Kondo, N.; Ono, H.; Orii, T. The effect of photosensitive dye platonin on juvenile rheumatoid arthritis. Biotherapy 1991, 3, 241–244. [Google Scholar] [CrossRef]
- Lee, J.J.; Huang, W.T.; Shao, D.Z.; Liao, J.F.; Lin, M.T. Platonin, a cyanine photosensitizing dye, inhibits pyrogen release and results in antipyresis. J. Pharm. Sci. 2003, 93, 376–380. [Google Scholar] [CrossRef]
- Chen, C.C.; Lee, J.J.; Tsai, P.S.; Lu, Y.T.; Huang, C.L.; Huang, C.J. Platonin attenuates LPS-induced CAT-2 and CAT-2B induction in stimulated murine macrophages. Acta Anaesthesiol. Scand. 2006, 50, 604–612. [Google Scholar] [CrossRef]
- Hsiao, G.; Lee, J.J.; Chou, D.S.; Fong, T.H.; Shen, M.Y.; Lin, C.H.; Sheu, J.R. Platonin, a photosensitizing dye, improves circulatory failure and mortality in rat models of endotoxemia. Biol. Pharm. Bull. 2002, 25, 995–999. [Google Scholar] [CrossRef][Green Version]
- Sheu, J.R.; Chen, Z.C.; Jayakumar, T.; Chou, D.S.; Yen, T.L.; Lee, H.N.; Pan, S.H.; Hsia, C.H.; Yang, C.H.; Hsieh, C.Y. A novel indication of platonin, a therapeutic immunomodulating medicine, on neuroprotection against ischemic stroke in mice. Sci Rep. 2017, 7, 42277. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Hsu, M.J.; Hsiao, G.; Wang, Y.H.; Huang, C.W.; Chen, S.W.; Jayakumar, T.; Chiu, P.T.; Chiu, Y.H.; Sheu, J.R. Andrographolide enhances nuclear factor-kappaB subunit p65 Ser536 dephosphorylation through activation of protein phosphatase 2A in vascular smooth muscle cells. J. Biol. Chem. 2011, 286, 5942–5955. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Hsiao, G.; Hsu, M.J.; Wang, Y.H.; Sheu, J.R. PMC, a potent hydrophilic α-tocopherol derivative, inhibits NF-κB activation via PP2A but not IκBα-dependent signals in vascular smooth muscle cells. J. Cell. Mol. Med. 2014, 18, 1278–1289. [Google Scholar] [CrossRef]
- Mazière, C.; Mazière, J.C. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic. Biol. Med. 2009, 46, 127–137. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Y.C.; Zhou, Q.; Ge, Y.Q.; Lan, Y. Oxidized LDL induces transcription factor activator protein-1 in rat mesangial cells. Cell Biochem. Funct. 2003, 21, 249–256. [Google Scholar] [CrossRef]
- Wang, H.H.; Hsieh, H.L.; Wu, C.Y.; Sun, C.C.; Yang, C.M. Oxidized low-density lipoprotein induces matrix metalloproteinase-9 expression via a p42/p44 and JNK-dependent AP-1 pathway in brain astrocytes. Glia 2009, 57, 24–38. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, L.; Chen, C.; Wang, Q.; Guo, L.; Ma, Q.; Deng, P.; Zhu, G.; Li, B.; Pi, Y.; et al. The critical role of ABCG1 and PPARγ/LXRα signaling in TLR4 mediates inflammatory responses and lipid accumulation in vascular smooth muscle cells. Cell Tissue Res. 2017, 368, 145–157. [Google Scholar] [CrossRef]
- Yin, Y.W.; Liao, S.Q.; Zhang, M.J.; Liu, Y.; Li, B.H.; Zhou, Y.; Chen, L.; Gao, C.Y.; Li, J.C.; Zhang, L.L. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression. Cell Death Dis. 2014, 5, e1574. [Google Scholar] [CrossRef]
- Chang, Y.; Uen, Y.H.; Chen, C.C.; Lin, S.C.; Tseng, S.Y.; Wang, Y.H.; Sheu, J.R.; Hsieh, C.Y. Platonin inhibited PDGF-BB-induced proliferation of rat vascular smooth muscle cells via JNK1/2-dependent signaling. Acta Pharm. Sin. 2011, 32, 1337–1344. [Google Scholar] [CrossRef]
- Yeh, C.T.; Kao, M.C.; Chen, C.H.; Huang, C.J. Platonin preserves blood-brain barrier integrity in septic rats. Acta Anaesthesiol Taiwan 2015, 53, 12–15. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Gosling, J.; Slaymaker, S.; Gu, L.; Tseng, S.; Zlot, C.H.; Young, S.G.; Rollins, B.J.; Charo., I.F. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Invest. 1999, 103, 773–778. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hsu, M.J.; Hsieh, C.Y.; Lee, L.W.; Chen, Z.C.; Sheu, J.R. Andrographolide inhibits nuclear factor-κB activation through JNK-Akt-p65 signaling cascade in tumor necrosis factor-α-stimulated vascular smooth muscle cells. Sci. World J. 2014, 2014, 130381. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mohamed, A.S.; Zhou, S.H. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. 2012, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.W.; Chien, C.S.; Hsiao, L.D.; Lin, C.H.; Yang, C.M. OxLDL induces mitogen-activated protein kinase activation mediated via PI3-kinase/Akt in vascular smooth muscle cells. J. Lipid Res. 2003, 44, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Garces de Los Fayos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef]
- Ley, K.; Miller, Y.I.; Hedrick, C.C. Monocyte and macrophage dynamics during atherogenesis. Arter. Thromb. Vasc. Biol. 2011, 31, 1506–1516. [Google Scholar] [CrossRef]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).