Lipid Fraction Properties of Homemade Raw Cat Foods and Selected Commercial Cat Foods
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Self-Prepared Cat Foods
- -
- The fresh meat was ground twice;
- -
- To balance the food, additives such as hemoglobin, salmon oil, egg shell meal, etc. were added with water to ground meat in the amount determined by the BARFny kalkulator for a given mass of this meat (the addition of egg yolks, salmon oil and vitamin E was the same for all diets);
- -
- All ingredients are thoroughly mixed with each other. Each food sample was made in greater scale, portioned and frozen.
2.3. Commercial Cat Foods
2.4. Fat Extraction and Dry Matter Determination
2.5. GC Measurements
2.6. TAG Hydrolysis
2.7. PDSC Measurements
2.8. Statistical Analysis
3. Results
3.1. Fat Content and Fatty Acids Composition
3.2. Fatty Acid Distribution
3.3. Oxidative Stability
4. Discussion
4.1. Lipid Fraction Properties of Self-Prepared Cat Food
4.2. The FA Composition and Oxidative Stability of Commercial Cat Food
5. Conclusions
- The self-prepared cat foods contain high levels of EFA, which is beneficial for adult cats and essential for the cats’ growth and reproduction stage.
- For the first time the distribution of FA in TAG for cat food has been determined.
- The commercial and self-prepared cat foods have a similar ratio of omega-6 to omega-3 but self-prepared diets provide more EPA and DHA.
- It is shown that the type of meat in self-prepared cat food affects the composition and distribution of fatty acids.
- Fat samples from self-prepared cat foods containing beef are characterized by the highest oxidative stability, which can be due to a lower ratio of PUFA/SFA.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michel, K.E. Unconventional Diets for Dogs and Cats. Vet. Clin. N. Am. 2006, 36, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Remillard, R.L. Homemade Diets: Attributes, Pitfalls, and a Call for Action. Top. Companion Anim. Med. 2008, 23, 137–142. [Google Scholar] [CrossRef]
- Parr, J.M.; Remillard, R.L. Handling Alternative Dietary Requests from Pet Owners. Vet. Clin. N. Am. 2014, 44, 667–688. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.R.; Vester Boler, B.M.; Morris, C.L.; Liu, K.J.; Swanson, K.S. Apparent total tract energy and macronutrient digestibility and fecal fermentative end-product concentrations of domestic cats fed extruded, raw beef-based, and cooked beef-based diets. J. Anim. Sci. 2012, 90, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.R.; Beloshapka, A.N.; Morris, C.L.; Parsons, C.M.; Burke, S.L.; Utterback, P.L.; Swanson, K.S. Evaluation of four raw meat diets using domestic cats, captive exotic felids, and cecectomized roosters. J. Anim. Sci. 2013, 91, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamper, B.A.; Kirk, C.A.; Bartges, J.W. Apparent nutrient digestibility of two raw diets in domestic kittens. J. Feline Med. Surg. 2016, 18, 991–996. [Google Scholar] [CrossRef]
- Schlesinger, D.P.; Joffe, D.J. Raw food diets in companion animals: A critical review. Can. Vet. J. 2011, 52, 50–54. [Google Scholar]
- Freeman, L.M.; Chandler, M.L.; Hamper, B.A.; Weeth, L.P. Current knowledge about the risks and benefits of raw meat–based diets for dogs and cats. J. Am. Vet. Med. Assoc. 2013, 243, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson-Ahomaa, M.; Heikkilä, T.; Pernu, N.; Kovanen, S.; Hielm-Björkman, A.; Kivistö, R. Raw Meat-Based Diets in Dogs and Cats. Vet. Sci. 2017, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.A.; Villaverde, C.; Fascetti, A.J.; Larsen, J.A. Evaluation of the nutritional adequacy of recipes for home-prepared maintenance diets for cats. J. Am. Vet. Med. Assoc. 2019, 254, 1172–1179. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Rogers, Q.R.; Morris, J.G. Role of Linoleate as an Essential Fatty Acid for the Cat Independent of Arachidonate Synthesis. J. Nutr. 1983, 113, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G. Idiosyncratic nutrient requirements of cats appear to be diet-induced evolutionary adaptations. Nutr. Res. Rev. 2002, 15, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Rivers, J.P.W. Essential fatty acids in cats. J. Small Anim. Pract. 1982, 23, 563–576. [Google Scholar] [CrossRef]
- Lenox, C.E.; Bauer, J.E. Potential Adverse Effects of Omega-3 Fatty Acids in Dogs and Cats. J. Vet. Intern. Med. 2013, 27, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.J.E. Facilitative and functional fats in diets of cats and dogs. J. Am. Vet. Med. Assoc. 2006, 229, 680–684. [Google Scholar] [CrossRef]
- Mu, H.; Høy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Kubow, S. The influence of positional distribution of fatty acids in native, interesterified and structure-specific lipids on lipoprotein metabolism and atherogenesis. J. Nutr. Biochem. 1996, 7, 530–541. [Google Scholar] [CrossRef]
- Stroebinger, N.; Rutherfurd, S.M.; Henare, S.J.; Hernandez, J.F.P.; Moughan, P.J. Fatty Acids from Different Fat Sources and Dietary Calcium Concentration Differentially Affect Fecal Soap Formation in Growing Pigs. J. Nutr. 2021, 151, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.C.; Stockman, J.; Larsen, J.A.; Zhang, L.; Rodriguez, A.S. Evaluation of phosphorus, calcium, and magnesium content in commercially available foods formulated for healthy cats. J. Vet. Intern. Med. 2019, 34, 266–273. [Google Scholar] [CrossRef]
- Stockman, J.; Villaverde, C.; Corbee, R.J. Calcium, Phosphorus, and Vitamin D in Dogs and Cats. Vet. Clin. N. Am. 2021, 51, 623–634. [Google Scholar] [CrossRef]
- Tran, Q.D.; Hendriks, W.H.; van der Poel, A.F.B. Effects of extrusion processing on nutrients in dry pet food. J. Sci. Food Agric. 2008, 88, 1487–1493. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martínez-Monteagudo, S.I. Oxidative Stability of Fats and Oils Measured by Differential Scanning Calorimetry for Food and Industrial Applications. In Applications of Calorimetry in a Wide Context—Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; Elkordy, A.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danthine, S.; De Clercq, N.; Dewettinck, K.; Gibon, V. Monitoring batch lipase catalyzed interesterification of palm oil and fractions by differential scanning calorimetry. J. Therm. Anal. Calorim. 2014, 115, 2219–2229. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Hołda, K.; Głogowski, R. Selected quality properties of lipid fraction and oxidative stability of dry dog foods under typical storage conditions. J. Therm. Anal. Calorim. 2016, 126, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.S.; Meyer, H. Ernährung und Verhalten von Hund und Katze; Schlütersche Verlag: Hannover, Germany, 1984; ISBN 978-3877060865. [Google Scholar]
- Horzinek, M.C.; Lutz, H.; Schmidt, V. Krankheiten der Katze; Enke: Stuttgart, Germany, 1999; ISBN 978-3830410492. [Google Scholar]
- Strombeck, D.R. Home-Prepared Dog & Cat Diets: The Healthful Alternative; Wiley-Blackwell: Hoboken, NJ, USA, 1999; ISBN 978-0813821498. [Google Scholar]
- Wanner, M. Die Ernährung der Katze. Skript für Studierende der Veterinärmedizin an der Universität Zürich; University of Zurich: Zurich, Switzerland, 2004. [Google Scholar]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Mattson, F.H.; Lutton, E.S. The Specific Distribution of Fatty Acids in the Glycerides of Animal and Vegetable Fats. J. Biol. Chem. 1958, 233, 868–871. [Google Scholar] [CrossRef]
- Plantinga, E.A.; Bosch, G.; Hendriks, W.H. Estimation of the dietary nutrient profile of free-roaming feral cats: Possible implications for nutrition of domestic cats. Br. J. Nutr. 2011, 106, S35–S48. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ng, A.; Mann, N.J.; Sinclair, A.J. Contribution of meat fat to dietary arachidonic acid. Lipids 1998, 33, 437–440. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Joo, S.-T. Fatty Acid Profiles, Meat Quality, and Sensory Palatability of Grain-fed and Grass-fed Beef from Hanwoo, American, and Australian Crossbred Cattle. J. Food Sci. Anim. Resour. 2017, 37, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Biagi, G.; Mordenti, A.L.; Cocchi, M. The role of dietary omega-3 and omega-6 essential fatty acids in the nutrition of dogs and cats: A review. Prog. Nutr. 2004, 6, 97–107. [Google Scholar]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bunglavan, S.J.; Pratheesh, M.D.; Anoopraj, R.; Harish, C.; Davis, J. Therapeutic uses of omega fatty acids in cats. Indian Pet J. 2011, 12, 1–2. [Google Scholar]
- Filburn, C.R.; Griffin, D. Effects of supplementation with a docosahexaenoic acid-enriched salmon oil on total plasma and plasma phospholipid fatty acid composition in the cat. Int. J. Appl. Res. Vet. Med. 2005, 3, 116–123. [Google Scholar]
- Jokic, S.; Sudar, R.; Svilović, S.; Vidovic, S.; Bilić, M.; Velic, D.; Jurkovič, V. Fatty acid composition of oil obtained from soybeans by extraction with supercritical carbon dioxide. Czech J. Food Sci. 2013, 31, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; De Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective Effect of Borage Seed Oil and Gamma Linolenic Acid on DNA: In Vivo and In Vitro Studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [Green Version]
- Gibson, M.; Alavi, S. Pet Food Processing—Understanding Transformations in Starch during Extrusion and Baking. Cereal Foods World 2013, 58, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.R.; Kappen, K.L.; Garner, L.M.; Swanson, K.S. Commercially Available Avian and Mammalian Whole Prey Diet Items Targeted for Consumption by Managed Exotic and Domestic Pet Felines: Macronutrient, Mineral, and Long-Chain Fatty Acid Composition. Zoo Biol. 2014, 33, 327–335. [Google Scholar] [CrossRef] [PubMed]
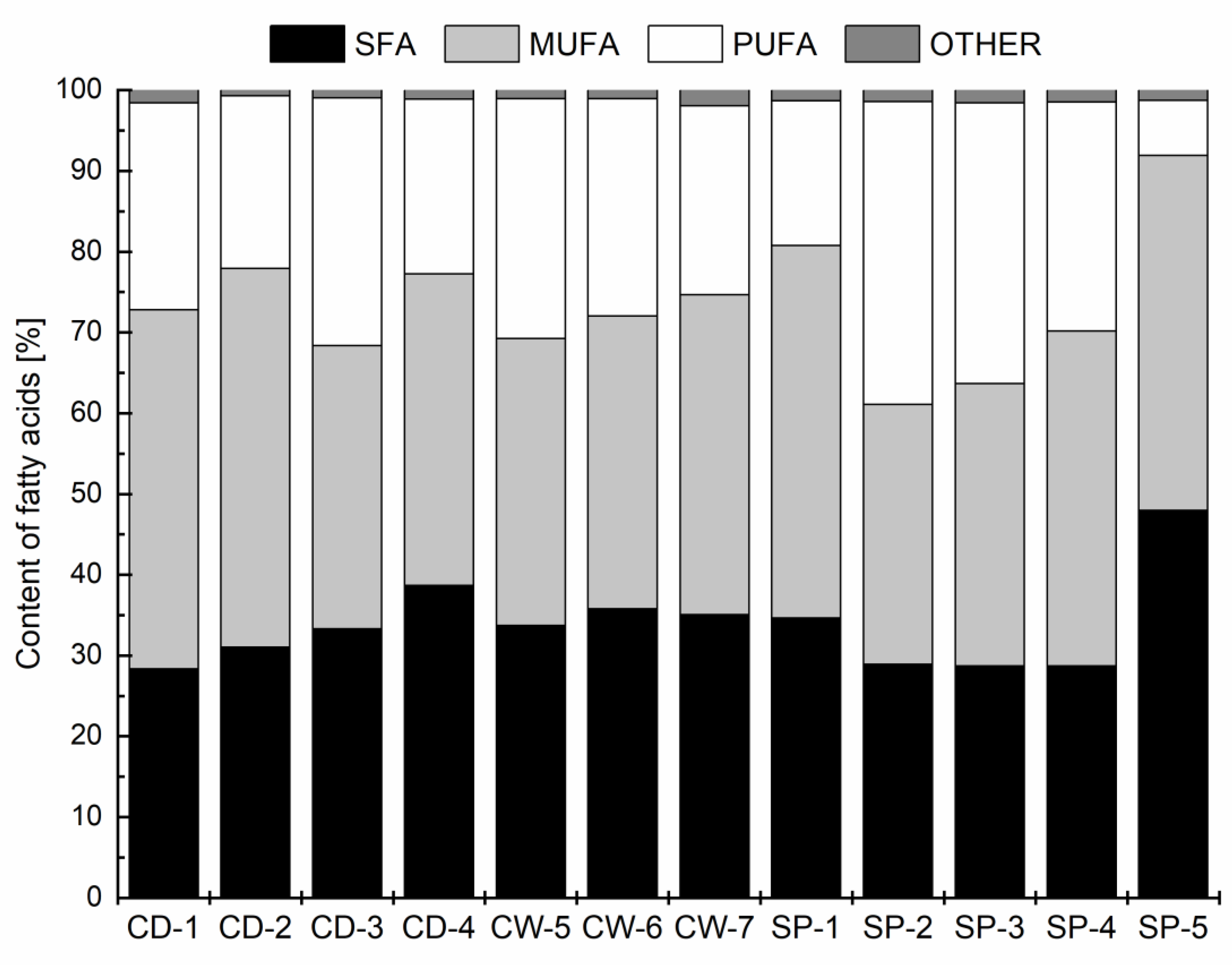

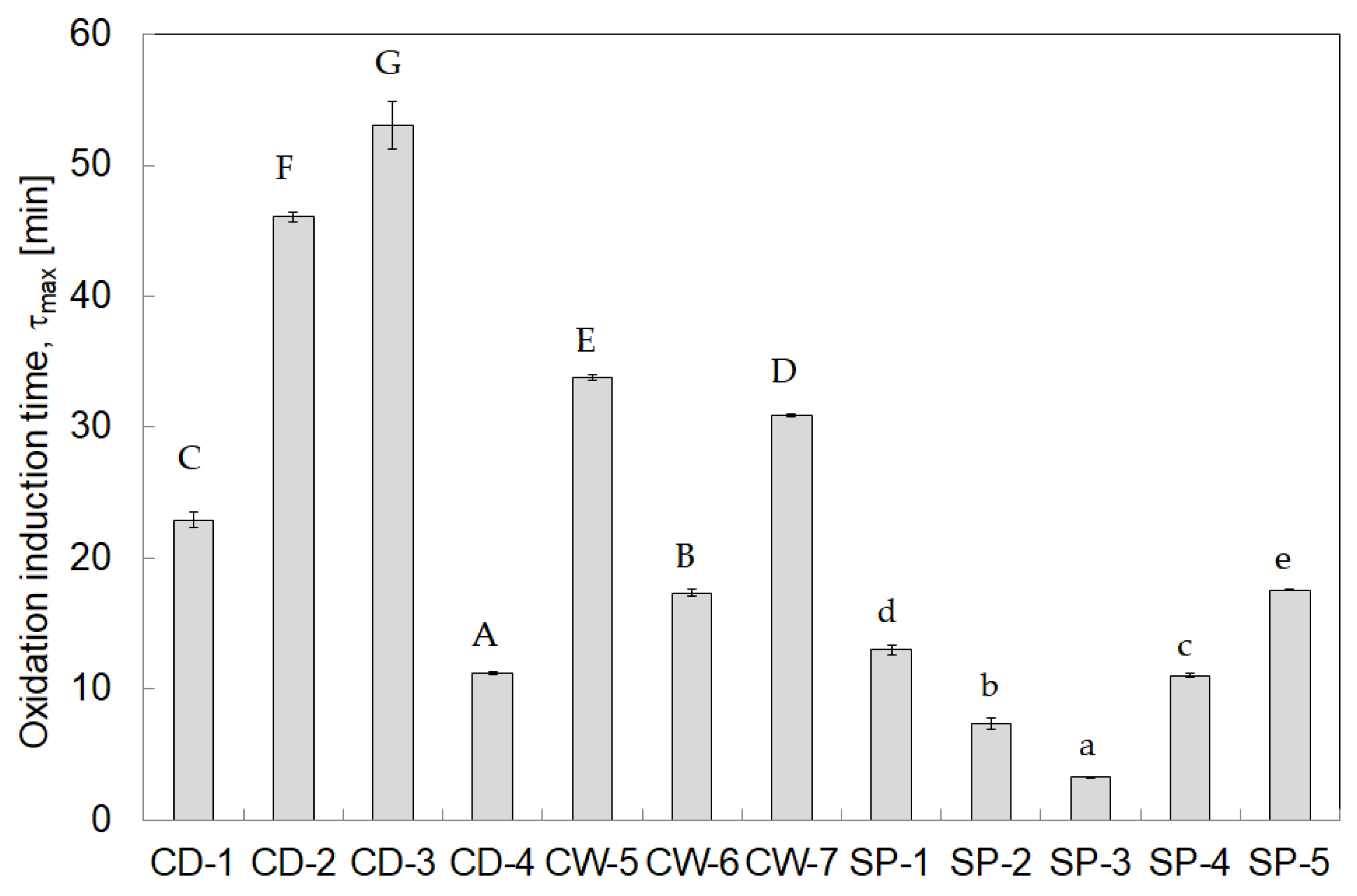
| Product Designation | SP-1 | SP-2 | SP-3 | SP-4 | SP-5 |
|---|---|---|---|---|---|
| Main Animal Ingredients | Chicken Meat, Beef Meat | Turkey Meat | Chicken Meat, Turkey Meat | Chicken Meat, Duck Meat | Beef Meat |
| DM, % † | 25.13 | 25.14 | 25.12 | 25.10 | 25.12 |
| DM, % | 24.02 ± 0.94 b | 22.80 ± 1.58 ab | 23.53 ± 2.08 b | 20.47 ± 1.81 a | 27.73 ± 1.81 c |
| Fat in DM, % † | 32.11 | 33.48 | 33.59 | 27.87 | 29.45 |
| Fat in DM, % | 35.48 ± 2.63 d | 25.14 ± 0.97 a | 28.58 ± 0.44 b | 32.00 ± 1.11 c | 36.30 ± 1.27 d |
| C10:0 | 0.04 ± 0.01 ab | 0.13 ± 0.02 d | 0.08 ± 0.02 c | 0.03 ± 0.01 a | 0.05 ± 0.01 bc |
| C12:0 | 0.33 ± 0.01 b | 1.95 ± 0.17 d | 1.34 ± 0.08 c | 0.37 ± 0.03 b | 0.08 ± 0.01 a |
| C14:0 | 2.13 ± 0.04 b | 2.44 ± 0.16 c | 2.10 ± 0.04 b | 1.28 ± 0.04 a | 3.00 ± 0.13 d |
| C14:1 | 0.59 ± 0.06 b | 0.20 ± 0.02 a | 0.17 ± 0.03 a | 0.13 ± 0.02 a | 0.79 ± 0.15 c |
| C15:0 | 0.25 ± 0.01 c | 0.18 ± 0.01 b | 0.17 ± 0.01 b | 0.13 ± 0.01 a | 0.60 ± 0.01 d |
| C16:0 | 20.39 ± 0.15 b | 17.36 ± 0.52 a | 17.43 ± 0.23 a | 19.87 ± 0.03 b | 24.24 ± 0.94 c |
| C16:1 | 4.48 ± 0.01 d | 3.14 ± 0.11 a | 3.28 ± 0.02 b | 4.00 ± 0.03 c | 3.24 ± 0.05 b |
| C17:0 | 0.69 ± 0.01 c | 0.40 ± 0.01 b | 0.39 ± 0.02 b | 0.36 ± 0.01 a | 1.29 ± 0.02 d |
| C17:1 | 0.56 ± 0.01 b | 0.12 ± 0.02 a | 0.12 ± 0.01 a | 0.14 ± 0.01 a | 0.77 ± 0.01 c |
| C18:0 | 10.81 ± 0.01 c | 6.86 ± 0.15 ab | 7.18 ± 0.09 b | 6.68 ± 0.07 a | 18.71 ± 0.48 d |
| C18:1 n-9 | 39.72 ± 0.01 e | 27.86 ± 0.24 a | 30.53 ± 0.39 b | 36.41 ± 0.10 c | 38.60 ± 1.04 d |
| C18:2 n-6 | 12.88 ± 0.02 b | 29.14 ± 0.22 e | 27.15 ± 0.15 d | 22.65 ± 0.12 c | 3.12 ± 0.07 a |
| C18:3 n-3 | 0.87 ± 0.02 b | 3.40 ± 0.05 e | 2.66 ± 0.07 d | 1.30 ± 0.03 c | 0.69 ± 0.02 a |
| C20:1 n-9 | 0.79 ± 0.05 bc | 0.71 ± 0.11 b | 0.85 ± 0.05 c | 0.81 ± 0.01 bc | 0.55 ± 0.08 a |
| C20:2 n-6 | 0.23 ± 0.01 b | 0.33 ± 0.03 c | 0.38 ± 0.02 d | 0.31 ± 0.01 c | 0.08 ± 0.05 a |
| C20:3 n-6 | 1.11 ± 0.01 b | 1.66 ± 0.11 d | 1.47 ± 0.09 c | 1.51 ± 0.01 c | 0.52 ± 0.03 a |
| C20:4 n-6 | 1.48 ± 0.02 b | 1.41 ± 0.07 ab | 1.66 ± 0.10 c | 1.32 ± 0.02 a | 1.31 ± 0.53 a |
| C20:5 n-3 | 0.12 ± 0.0 1b | 0.14 ± 0.01 c | 0.14 ± 0.02 c | 0.16 ± 0.01 c | 0.05 ± 0.01 a |
| C22:6 n-3 | 1.22 ± 0.05 bc | 1.19 ± 0.12 abc | 1.34 ± 0.18 c | 1.13 ± 0.01 ab | 1.02 ± 0.07 a |
| Σ other | 1.37 ± 0.02 ab | 1.39 ± 0.10 ab | 1.57 ± 0.07 c | 1.47 ± 0.01 bc | 1.27 ± 0.13 a |
| MUFA/PUFA | 2.58 | 0.86 | 1.00 | 1.46 | 6.47 |
| MUFA/SFA | 1.33 | 1.09 | 1.22 | 1.44 | 0.92 |
| PUFA/SFA | 0.52 | 1.27 | 1.21 | 0.99 | 0.14 |
| Omega-6/omega-3 | 7.12 | 6.88 | 7.41 | 9.97 | 2.86 |
| Product Designation | CD-1 | CD-2 | CD-3 | CD-4 | CW-5 | CW-6 | CW-7 |
|---|---|---|---|---|---|---|---|
| DM, % † | 90 | 90 | 92 | 90 | 20 | 18 | 19 |
| DM, % | 94.35 ± 0.37 d | 94.10 ± 0.28 cd | 94.28 ± 0.19 cd | 93.46 ± 0.42 c | 20.18 ± 0.63 b | 18.93 ± 0.89 a | 19.17 ± 0.13 a |
| Fat in DM, % † | 22.22 | 20.00 | 17.39 | 17.77 | 12.50 | 25.00 | 14.21 |
| Fat in DM, % | 16.31 ± 0.57 cd | 17.39 ± 2.15 d | 13.16 ± 3.42 bc | 14.78 ± 1.32 cd | 10.11 ± 2.59 ab | 10.38 ± 1.73 ab | 8.89 ± 0.67 a |
| C10:0 | 0.08 ± 0.03 ab | 0.02 ± 0.01 a | 0.20 ± 0.01 d | 0.16 ± 0.05 cd | 0.12 ± 0.07 bc | 0.06 ± 0.01 ab | 0.08 ± 0.04 ab |
| C12:0 | 0.40 ± 0.10 ab | 0.07 ± 0.01 a | 1.85 ± 0.14 c | 0.45 ± 0.05 ab | 1.98 ± 1.03 c | 1.07 ± 0.24 b | 0.29 ± 0.17 a |
| C14:0 | 1.36 ± 0.21 b | 0.73 ± 0.02 a | 2.72 ± 0.09 d | 2.19 ± 0.10 ad | 2.09 ± 0.75 c | 1.37 ± 0.24 b | 1.22 ± 0.36 ab |
| C14:1 | 0.17 ± 0.02 a | 0.19 ± 0.01 a | 0.30 ± 0.02 b | 0.23 ± 0.02 ab | 0.25 ± 0.10 ab | 0.20 ± 0.04 a | 0.17 ± 0.59 a |
| C15:0 | 0.15 ± 0.02 ab | 0.11 ± 0.01 a | 0.21 ± 0.01 c | 0.19 ± 0.01 bc | 0.16 ± 0.05 bc | 0.15 ± 0.02 ab | 0.19 ± 0.04 bc |
| C16:0 | 20.08 ± 1.10 a | 22.15 ± 0.23 ab | 22.52 ± 0.03 ab | 26.27 ± 0.67 c | 22.41 ± 3.09 ab | 23.03 ± 1.83 ab | 23.91 ± 2.82 bc |
| C16:1 | 4.46 ± 0.35 ab | 5.72 ± 0.10 d | 5.15 ± 0.10 cd | 4.89 ± 0.05 bc | 3.95 ± 0.62 a | 3.94 ± 0.35 a | 3.95 ± 0.51 a |
| C17:0 | 0.37 ± 0.01 ab | 0.34 ± 0.03 a | 0.37 ± 0.01 ab | 0.41 ± 0.01 bc | 0.35 ± 0.04 ab | 0.40 ± 0.04 abc | 0.45 ± 0.07 c |
| C17:1 | 0.13 ± 0.01 bc | 0.11 ± 0.01 ab | 0.14 ± 0.01 c | 0.18 ± 0.01 d | 0.13 ± 0.02 abc | 0.11 ± 0.01 a | 0.15 ± 0.02 c |
| C18:0 | 6.52 ± 0.37 b | 7.62 ± 0.07 c | 5.42 ± 0.04 a | 8.99 ± 0.04 d | 6.58 ± 0.63 b | 9.69 ± 0.18 e | 8.89 ± 0.57 d |
| C18:1 n-9 | 38.05 ± 1.08 d | 40.33 ± 0.09 d | 29.08 ± 0.18 a | 32.70 ± 0.76 bc | 30.41 ± 2.95 ab | 31.68 ± 1.76 ab | 34.90 ± 2.34 c |
| C18:2 n-6 | 20.48 ± 0.23 c | 18.38 ± 0.09 b | 25.58 ± 0.24 e | 17.12 ± 0.02 a | 25.18 ± 1.06 e | 21.83 ± 0.51 d | 17.60 ± 0.46 ab |
| C18:3 n-3 | 2.56 ± 0.06 c | 1.83 ± 0.04 a | 2.31 ± 0.04 b | 3.12 ± 0.09 d | 2.51 ± 0.20 c | 1.89 ± 0.06 a | 2.59 ± 0.11 c |
| C20:1 n-9 | 1.19 ± 0.13 d | 0.52 ± 0.03 ab | 0.44 ± 0.02 ab | 0.61 ± 0.07 bc | 0.80 ± 0.22 c | 0.34 ± 0.015 a | 0.44 ± 0.11 ab |
| C20:2 n-6 | 0.39 ± 0.05 b | 0.19 ± 0.01 a | 0.20 ± 0.03 a | 0.22 ± 0.06 a | 0.26 ± 0.07 a | 0.23 ± 0.03 a | 0.43 ± 0.06 b |
| C20:3 n-6 | 0.34 ± 0.03 a | 0.61 ± 0.04 bc | 0.42 ± 0.01 ab | 0.64 ± 0.01 bc | 0.78 ± 0.17 c | 2.30 ± 0.12 d | 2.29 ± 0.34 d |
| C20:4 n-6 | 0.63 ± 0.05 d | 0.11 ± 0.01 a | 1.32 ± 0.06 e | 0.23 ± 0.03 b | 0.34 ± 0.06 c | 0.13 ± 0.03 a | 0.15 ± 0.04 ab |
| C20:5 n-3 | 0.08 ± 0.10 a | 0.06 ± 0.04 a | 0.08 ± 0.01 a | 0.05 ± 0.02 a | 0.08 ± 0.03 a | 0.16 ± 0.02 b | 0.07 ± 0.03 a |
| C22:6 n-3 | 1.03 ± 0.09 d | 0.19 ± 0.01 a | 0.77 ± 0.03 c | 0.29 ± 0.03 a | 0.53 ± 0.23 b | 0.37 ± 0.07 ab | 0.26 ± 0.07 a |
| Σ other | 1.53 ± 0.08 c | 0.71 ± 0.03 a | 0.97 ± 0.03 b | 1.12 ± 0.02 b | 1.09 ± 0.20 b | 1.11 ± 0.01 b | 1.98 ± 0.21 d |
| MUFA/PUFA | 1.73 | 2.19 | 1.14 | 1.78 | 1.20 | 1.35 | 1.69 |
| MUFA/SFA | 1.52 | 1.51 | 1.05 | 1.00 | 1.05 | 1.01 | 1.13 |
| PUFA/SFA | 0.88 | 0.69 | 0.92 | 0.56 | 0.88 | 0.75 | 0.67 |
| Omega-6/omega-3 | 5.96 | 9.26 | 8.72 | 5.27 | 8.53 | 10.13 | 7.01 |
| Self-Prepared Raw Meat Food | Whole Prey Items ‡ | Recommendations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAFCO | FEDIAF | ||||||||||||
| Fat in DM, % | SP-1 | SP-2 | SP-3 | SP-4 | SP-5 | Mouse | Rat | Rabbit | Quail | Adult | GR † | Adult | GR † |
| 35.48 | 25.14 | 28.58 | 32.00 | 36.30 | 29.92 | 26.35 | 22.05 | 19.24 | 9 | 9 | 9 | 9 | |
| LA | 45.67 | 73.26 | 77.60 | 72.47 | 11.31 | 27.03 | 33.47 | 10.28 | 18.76 | 6 | 6 | 5 | 5.5 |
| ALA | 3.09 | 8.55 | 7.60 | 4.14 | 2.52 | 0.46 | 1.13 | 0.47 | 0.71 | - | 0.2 | - | 0.2 |
| AA | 5.25 | 3.54 | 4.73 | 4.21 | 4.76 | 2.18 | 6.86 | 0.34 | 2.75 | 0.2 | 0.2 | 0.06 | 0.2 |
| EPA | 0.43 | 0.35 | 0.40 | 0.51 | 0.18 | 0.02 | 0.22 | 0.01 | 0.04 | - | - | - | - |
| DHA | 4.31 | 2.99 | 3.83 | 3.62 | 3.69 | 0.79 | 2.28 | 0.03 | 0.72 | - | - | - | - |
| EPA + DHA | 4.74 | 3.34 | 4.23 | 4.13 | 3.87 | 0.81 | 2.5 | 0.04 | 0.76 | - | 0.12 | - | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, A.; Mańko-Jurkowska, D.; Bryś, J.; Górska, A. Lipid Fraction Properties of Homemade Raw Cat Foods and Selected Commercial Cat Foods. Appl. Sci. 2021, 11, 10905. https://doi.org/10.3390/app112210905
Górska A, Mańko-Jurkowska D, Bryś J, Górska A. Lipid Fraction Properties of Homemade Raw Cat Foods and Selected Commercial Cat Foods. Applied Sciences. 2021; 11(22):10905. https://doi.org/10.3390/app112210905
Chicago/Turabian StyleGórska, Agnieszka, Diana Mańko-Jurkowska, Joanna Bryś, and Agata Górska. 2021. "Lipid Fraction Properties of Homemade Raw Cat Foods and Selected Commercial Cat Foods" Applied Sciences 11, no. 22: 10905. https://doi.org/10.3390/app112210905
APA StyleGórska, A., Mańko-Jurkowska, D., Bryś, J., & Górska, A. (2021). Lipid Fraction Properties of Homemade Raw Cat Foods and Selected Commercial Cat Foods. Applied Sciences, 11(22), 10905. https://doi.org/10.3390/app112210905









