Abstract
Nanostructured titania (TiO2) is the most widely applied semiconducting oxide for a variety of purposes, and it is found in many commercial products. The vast majority of uses rely on its photo-activity, which, upon light irradiation, results in excited states that can be used for diverse applications. These range from catalysis, especially for energy or environmental remediation, to medicine—in particular, to attain antimicrobial surfaces and coatings for titanium implants. Clearly, the properties of titania are enhanced when working at the nanoscale, thanks to the increasingly active surface area. Nanomorphology plays a key role in the determination of the materials’ final properties. In particular, the nucleation and growth of nanosized titania onto carbon nanostructures as a support is a hot topic of investigation, as the nanocarbons not only provide structural stability but also display the ability of electronic communication with the titania, leading to enhanced photoelectronic properties of the final materials. In this concise review, we present the latest progress pertinent to the use of nanocarbons as templates to tailor nanostructured titania, and we briefly review the most promising applications and future trends of this field.
Keywords:
titania; anatase; rutile; carbon; nanotubes; nanoparticles; nanorods; nanosheets; graphene; photocatalysis 1. Introduction
1.1. Titania Properties and Uses
Titanium dioxide or titania is certainly the most studied semiconducting oxide due to its well-established photo-activity. Well-known features that render it so attractive are its low cost, negligible toxicity, high stability, easy handling, and resistance to chemical corrosion. This semiconductor has the known ability to absorb light in the ultraviolet (UV) wavelength range and generate excited charges, electrons and holes, which separate in the conduction and valence band, respectively, and are at the core of its photo-activity. This key property of TiO2 has been widely investigated firstly in catalysis and later in medicine, especially to attain antimicrobial coatings for medical implants [1]. The wide applications of titania for air and water remediation, cultural heritage preservation, and self-healing, as well as microbial inactivation and the mitigation of SARS-CoV-2 spreading onto surfaces, have been recently reviewed [2] and are thus not discussed in detail in this review.
Despite the many advantages offered by titania, this material also suffers from some drawbacks, such as a rapid recombination of the photo-excited charge carriers and the relatively wide band gap (in the range of 3.2 eV) which implies a poor utilization of solar light due to the small percentage of UV radiation of sunlight reaching the Earth’s surface. Remedies that have been developed to address these issues include the use of suitable supports [3], doping with other elements [4], embedding in composites [5], and the development of suitable nanostructures [6], also following green protocols [7]. These approaches have indeed allowed the practical widespread use of TiO2—for instance, for biomass conversion processes [8]—thanks to the improved hydrothermal stability of the catalyst [9]. In particular, the combination of titania with noble metals and carbon nanostructures has allowed the development of high-performing gas sensors [10] and photocatalysts [11]. Dye-sensitizers combined with titania allow metal-free photocatalysts to be attained for hydrogen production using visible light [12]. Hydrogen-peroxide production is another area in which titania is highly promising [13], as well as solar cells [14].
The morphology and crystallinity of the titania phase are important parameters in the determination of the resulting photo-electronic properties of the final material [15]. However, only a few studies compare the photo-activity of the three phases of titania (rutile, brookite, and anatase; Figure 1) [16], while the majority of works focus on the generally best-performing anatase phase [17]. Titania can be produced by several methods, with sol-gel [18], hydrothermal [19], and solvothermal [20] processes being the most popular, and in some cases also microwave-assisted methods [21]. However, other approaches are also often used, such as anodic oxidation [22] and atomic layer deposition [23], and others are under continuous development; for instance, the use of laser beams [24], molecular layer deposition [25], and air-plasma spraying [26].
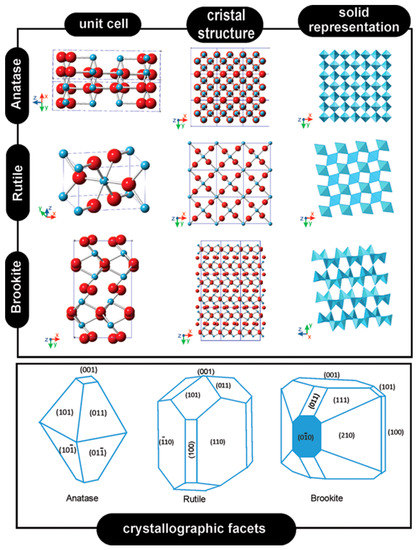
Figure 1.
Crystal structures of titania anatase, rutile, and brookite polymorphs. Reprinted from [21].
1.2. Carbon Nanostructures Properties and Uses
Carbon nanostructures represent a large family of materials based on carbon characterized by a diversity of morphologies and structures (Figure 2) [27]. Typically, they present very interesting electronic and conductive properties that arise from the extended conjugation of sp2 atoms, although exceptions exist. For instance, nanodiamonds contain mainly sp3-hybridized carbon atoms, as the name suggests [28]. Nanocarbons have been engineered to feature sp-hybridized carbon atoms as well, especially for energy conversion and storage applications [29]. In particular, sp-hybridized one-dimensional “synthetic carbon allotropes” are emerging as attractive molecular wires for advanced applications in electronics and opto-electronics [30].
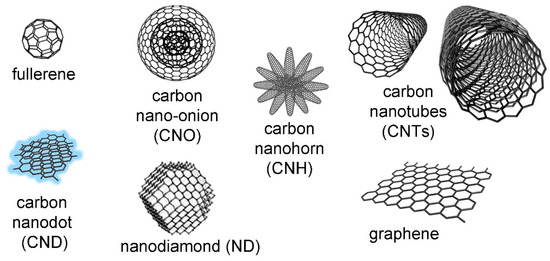
Figure 2.
Different types of well-known carbon nanostructures (not to scale). Reproduced from [31]. The nano-onion is reproduced with permission from [32], copyright © 1996, Elsevier.
Among the most studied carbon nano-allotropes are zero-dimensional fullerenes [33], which can be considered as soccer-ball shaped structures, one-dimensional carbon nanotubes (CNTs), which have a tubular morphology [34], and two-dimensional graphene-based materials [35], which generally consist of nanosheets. Other well-known examples include nano-onions (CNOs) [36], which consist of concentric fullerenes, and nanohorns (CNHs), which arise from clusters of nanocones [37]. In recent years, the class of carbon dots has attracted attention thanks to their ultrasmall structure (<10 nm), which provides them with characteristic luminescent properties [38].
Carbon nanostructures have found a wide variety of applications over the years thanks to their electronic and thermal conductivity, low density, and high mechanical strength, as well as the ability to undergo chemical functionalization to further tune their properties as needed for the intended use [39]. They are being studied especially for energy [40] and catalysis [41,42,43], including electro-catalysis [44,45] and nanozymes [46], as well as for the development of advanced electronic applications [47], including supercapacitors [48,49] and batteries [50], wearable electronics [51], electro-catalytic water-splitting [52], electromagnetic interference (EMI) shielding materials [53], molecular magnets [54], thermal-energy harvesting [55], photo-detectors [56], and electrochemical sensors [57]. In particular, in the area of sensing [58], recent developments have been made in the areas of nano-mass and nano-force sensors [59], gas sensors [60], biosensors [61], temperature sensors [62], and the growing field of touch or motion-driven sensors, or “haptics” [63]. Another area of growing interest regards environmental remediation [64], including water purification [65] and the detection of various pollutants, such as pesticides [66] and pharmaceuticals [67]. In materials science, they are well-known as nano-fillers [68,69], but also used as flame-retardants [70].
Finally, carbon nanomaterials can be applied for biomedical use [71,72,73,74], especially in oncology [75,76], theranostics [77], drug delivery [78], antimicrobials [79,80], and DNA analysis [81]. A biomedical area that is progressing at a fast pace is tissue engineering [82], especially for nerve [83], cardiac [84], and bone [85] tissues. In bioelectronics, synaptic transistors and neuromorphic computing [86] are progressing at a fast pace.
2. Carbon Nanostructures as Templates for Titania Nanomorphologies
The efficiency of titania photo-activity can be significantly improved through nanostructuring and heterostructuring with carbon nanomaterials, allowing the enhanced use of the solar spectrum and better charge separation [87]. The addition of carbon nanostructures can lead to an increase of the adsorption capacity, of the absorption of visible light, and of the lifetime of photogenerated electron–hole pairs. In particular, CNTs have been widely used as supports for the growth of nanosized titania. The advantages of interfacing TiO2 with CNTs include not only improved structural stability, but also electronic communication between the two phases, whereby charge carriers (electrons or holes) may be transferred through the phase boundaries through several proposed mechanisms. For instance, a general view is that CNTs can efficiently scavenge the titania photoexcited electrons, thus retarding the electron–hole recombination rates and resulting in enhanced photocatalytic activities [88]. It is worth noting, however, that alternative hypotheses have been advanced; for example, on the basis of transient absorption experiments, where the photogenerated holes are transferred from the TiO2 to the CNT [89], confirming the complexity of the electron transfer dynamics, which for example could depend on the level of functionalization (in particular, with oxygenated groups) of the CNT surface. Other reports have suggested that CNTs can act as photosensitizers, with the electrons excited and injected from the CNTs into the TiO2 conduction band, with subsequent electron transfer from the titania valence band into the CNTs, thus yielding a charge separation state (electron–hole) on the metal oxide. However, such sensitization effects assume a semiconducting character of the CNT, which cannot always be taken for granted. In general, the micro-structure of the CNT and its functional group distribution, doping, and method of preparation deeply affect the photochemical response. Furthermore, Ti-C and Ti-O-C bonds can be formed during calcination, thus resulting in a “doping” effect with the formation of intragap states within the titania and a consequent improved absorption of visible light [88].
The use of carbon nanomaterials to template titania with nanomorphological control was reviewed in 2016 [90]; thus, we focus here on the progresses made over the last five years. An overview of selected examples is provided in Table 1. A summary of the physicochemical properties of carbon nanostructures after decoration with titania, including the type of surface functionalization used to anchor the metal oxide, is provided in Table 2.

Table 1.
Overview of the use of different carbon nanomaterials as templates for nanostructured titania over the last five years.

Table 2.
Summary of the physicochemical properties of carbon nanostructures decorated with titania over the last five years.
Carbon nanodots are not present in the table since they are typically not used as templates; rather, they are added onto preformed titania, for instance on anodized nanotube arrays for uses in photocatalysis [91] and sensing [92]. Readers with a particular interest in graphene quantum dots incorporated in titania nanostructures are referred elsewhere [93]. Furthermore, studies that used commercially available titania nanoparticles are not included, since our focus is on the influence of carbon nanomaterials on their formation.
2.1. Fullerenes
Fullerenes have been intensively studied for photocatalytic applications, as well as in combination with other semiconductors as photocatalyst enhancers [175]. The use of fullerenes as nano-templates for titania nucleation and growth was mainly developed in previous years [88], despite a modern renaissance of their use in solar cells [176].
Zinc-functionalized fullerene was recently combined with nanostructured titania for water remediation; however, the two nanomaterials were formed separately and only later combined to make nanostructured composites [177]. The interface between fullerenes and titania has been deeply investigated. It was recently found that defect states in the band gap of titania are quenched by C70 while an interfacial state appears, showing a barrier-free extraction of charges (Figure 3) for the next generation of organic solar cells [178]. However, in studies such as this one, fullerenes are added onto preformed titania.
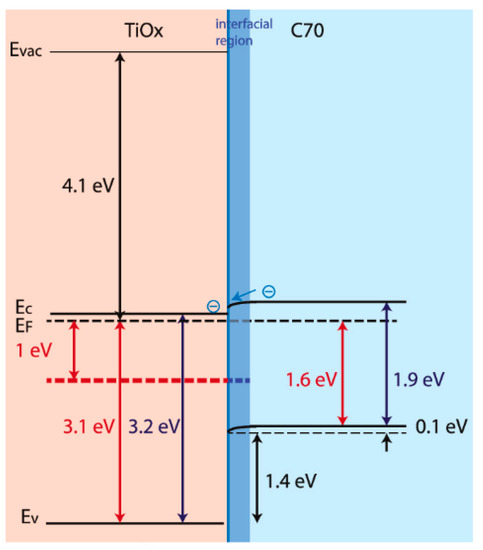
Figure 3.
C70/titania band diagrams derived from the photoemission spectra, with energy gaps based on optical absorption data. Reproduced with permission from [178], copyright © 2021, American Chemical Society.
In an interesting study, fullerenol was used as a template for titania to exploit the buckyball’s hydroxyl groups as nucleation sites for the generation of titania nanoparticles by atomic layer deposition using titanium tetraisopropoxide as a precursor [94]. Given the low temperatures used in the process, the resulting titania was amorphous, yet it surprisingly demonstrated photocatalytic activity that was ascribed to the presence of fullerene in the composite material [94].
2.2. Nano-Onions
Carbon nano-onions consist of concentric fullerenes and have been used usually to template nanostructured titania by means of sol-gel methods. X-ray diffraction analyses confirmed that the nanocarbons were anchored onto anatase, whose microscopy images suggested a certain level of control against the particle agglomeration exerted by the nano-onions, relative to a reference without the template, despite the limited morphological control over the titania particles. The nano-onions enhanced the specific surface area, average pore size, and pore volume of the composite leading to the adsorption of pollutants, as well as its visible-light absorption, overall leading to a more efficient photocatalytic dye degradation as demonstrated on rhodamine B. Finally, the paramagnetic nature of the nano-onions with a magnetic core allowed for the easy recovery of the composite through magnetic separation [95].
Carbon nano-onion/anatase hybrids have been proposed as innovative replacements for graphite anodes in lithium-ion batteries. Titania was formed with a sol-gel method onto the nanocarbon template, exerting a certain level of both morphological and size control over the nanocrystals, which appeared to be smaller than 10 nanometers in diameter. Furthermore, the presence of the conductive carbon nanostructure overall significantly enhanced the electrochemical properties of the final material relative to the reference without the nano-onions [96].
Carbon nano-onions/anatase materials were also obtained by a hydrothermal route, with various mass compositions, ranging from an excess of the nano-onion to an excess of the titania, as confirmed by thermogravimetric analyses. The best results within the series were obtained with compositions featuring approximately 80–90 wt% nano-onions. In particular, high-resolution transmission electron microscopy (HR-TEM) images (Figure 4) revealed an intimate contact between the inorganic and the carbon components, with both the graphitic walls of the nano-onions and the lattice of the anatase nanocrystals being clearly visible. These materials demonstrated the highest capacitance and an enhanced electrochemical performance overall, which was rationalized through a cooperative effect of both the electrochemical double layer capacitance from the nano-onions and non-capacitive Faradaic storage regarded as pseudocapacitance from titania [97]. Carbon nano-onions have been proposed for use in dye-sensitized solar cells, thanks to a number of advantageous features, including their limited cost, ease of dispersibility that does not require the use of binders—in contrast with other carbon nanomaterials—and the high level of optical transparency of the final device [179].
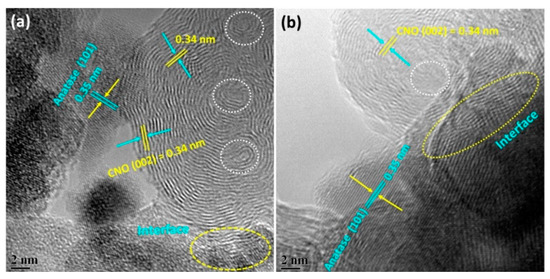
Figure 4.
HR-TEM images of carbon nano-onion/anatase nanocrystals with different ratios of the two components, consisting of either (a) 89 wt%/11 wt% or (b) 83 wt%/17 wt%, respectively. White-dotted circles highlight the nano-onion cores; yellow-dotted circles indicate the interface between the two components. The nano-onion graphitic walls area visible with the typical 0.34 nm-distance, as is the lattice spacing of 0.35 nm, corresponding to the (101) plane of anatase. Reprinted from [97], Copyright © 2017, with permission from Elsevier.
2.3. Nanocones
The cone-like morphology of carbon nanocones leads to very interesting features, such as an uncommon graphene-sheet stacking depending on the apex (cone) angle, which in turn implies the presence of a number of pentagons within the six-membered ring patterns, creating the conical geometry. In the absence of pentagons, the angle can reach 0°, thus resulting in a flat disk. Nanocones are normally produced as a mixture of these varied structures with differing geometrical parameters and containing a large excess (approximately 70%) of disks. The nanocones were oxidized to display hydroxyl groups that could act as anchor sites for the inorganic phase, which was grown as a uniform coating reproducing the nanocone morphology with high fidelity through a sol-gel process. The coating included embedded palladium nanoparticles, and the final material displayed very high photocatalytic activity under UV irradiation thanks to an increased surface area and the ability of the carbon framework to scavenge the photo-excited electrons to retard the charge recombination rates. Despite the lower conductivity relative to carbon nanotubes or graphene, nanocones provide other advantages, such as the ease of dispersibility in liquid media. The intimate contact between the organic and the inorganic phases, leading to an increased number of heterojunctions, was responsible for the higher activity in photocatalytic hydrogen production, using ethanol as an environmentally friendly sacrificial donor, which is conveniently a product of fermentation from feedstock (corn, sugarcane, etc.) [98].
2.4. Nanohorns
Nanohorns can be considered as clusters of nanocones, and they have also been used as templates for titania. The nanocarbon entanglement features a high surface area that templates a large distribution of “hard–soft” bimetallic sites, where 1.5 nm palladium nanoparticles were embedded within the titania phase while being electrically wired to an electrode by the nanohorn support. This hybrid electrocatalyst activated carbon dioxide reduction to formic acid nearly at zero overpotential in the aqueous phase, while being able to produce hydrogen thanks to a sequential formic acid reduction [99].
In another study, nanohorns templated the formation of anatase with nanoflower morphology through a solvothermal method. The photocatalytic degradation of two model dyes (i.e., methylene blue and methyl orange) was combined with the generation of hydrogen fuel upon solar-light irradiation [100].
Besides the prospective uses in solar cells [180,181], nanohorns are potentially suitable for biological uses thanks to their additional advantageous features relative to other carbon nano-allotropes, such as the ease of oxidation and the high dispersibility in buffer solutions [182]. To this end, they have been used as templates for nanostructured titania with the nanohorn morphology and applied to phosphoproteomics on cancer cell lysates for diagnostics purposes thanks to the well-known binding affinity for titania with phosphorylated peptides (Figure 5) [101].
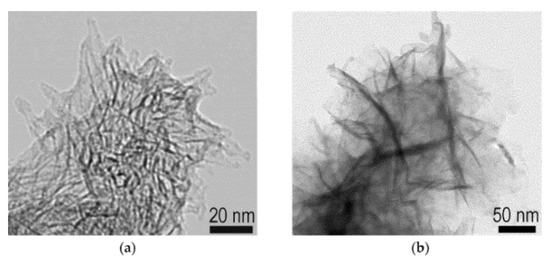
Figure 5.
TEM images of carbon nanohorns (a) without or (b) with anatase [101].
2.5. Nanodiamonds
Nanodiamonds’ high thermal conductivity, hardness and friction-resistance, high surface area, chemical inertness, non-toxicity, tunable structure, and excellent opto-mechanical properties have rendered them attractive building blocks in nanotechnology [183]. Nanodiamonds are typically produced by explosive detonation under oxygen-deficient conditions and consist of nanoparticles as small as 4–5 nm with a large portion of sp3-hybrized carbon atoms, in addition to sp2-hybridized atoms. A hydrothermal method at 70 °C was used as an easy and low-cost production method for the nucleation and growth of titania nanoparticles. Despite the limited control over the particle morphology and the presence of agglomerates, the presence of the nanodiamonds allowed for a reduced recombination rate of photogenerated charge carriers, with an improved photodegradation of bisphenol A as a model organic pollutant [102]. Similar protocols produced nanodiamonds–titania composites that were successfully tested for the photodegradation of other pollutants, thus confirming their versatility towards environmental remediation [103,104]. Further, this kind of nanomaterial has been envisaged as an anodic component for lithium batteries, thanks to the features of nanocarbon, such as its high lithium adsorption capacity, wide surface area, and chemical inertness, as well as is overall high capacitance and the long-term cycle stability of the composites [105].
2.6. Single-Walled Carbon Nanotubes (SWCNTs)
The use of SWCNTs is less common than MWCNTs due to the latter being easier to handle and disperse and presenting lower costs of production. In one study, SWCNTs successfully templated the formation of titania through a sol-gel method, and subsequent calcination at 600 °C allowed for the crystallization into anatase/rutile phases onto the tubes and the formation of an aerogel, which was tested for photocatalysis. The addition of platinum nanoparticles as co-catalysts onto the tubes, prior to titania nucleation and growth, was also studied to enhance the activity thanks to a more efficient charge carrier separation [106].
In another work, SWCNTs were first coated with a dendrofullerene (Figure 6) to provide suitable anchoring points for the nucleation and growth of titania while leading to good performance with regard to photo-catalytic H2 evolution under visible-light irradiation. Co-axial inorganic–organic nanowires were thus obtained with a high photo-activity that was rationalized in terms of the electron-extracting TiO2 layer accelerating the electron forward-transfer and the concomitant deceleration of the undesired back-transfer [107].
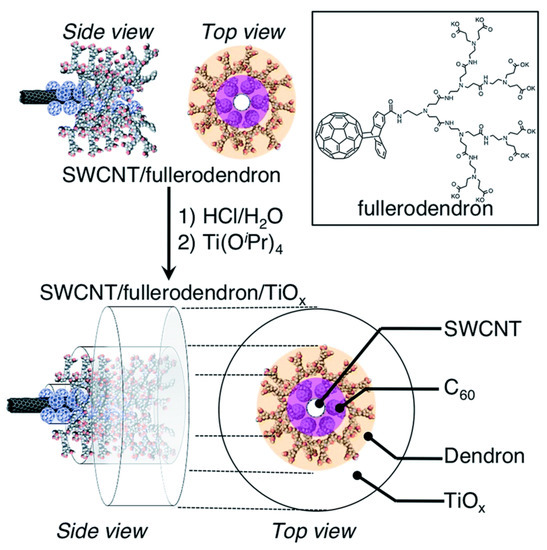
Figure 6.
Schematic illustration of the fabrication of SWCNT/fullerodendron/titania co-axial hybrid nano-wires. Reproduced from [107], published by the Royal Society of Chemistry.
2.7. Multi-Walled Carbon Nanotubes (MWCNTs)
MWCNTs have been widely studied as templates for titania nanostructures, as can be seen from the many entries in Table 1; thus, only a few studies are discussed here. With a sol-gel process in ethanol using a tetrabutyl titanate precursor, it was possible to decorate CNTs with titania nanocrystals, and the materials were used to obtain a film for potential applications as a battery anode [119]. When the same precursor was used in alkaline aqueous solutions with wider, hydroxylated CNTs (average diameter of 50 nm), nanowires were obtained instead, which entangled with CNTs in a network and were envisaged for the same application [126]. The CNT/titanium relative ratio is one of the critical parameters that determines nanomorphology. For instance, when an excess of titania precursor was used in a solvothermal method, nanoflowers were obtained, while increasing the relative amount of CNTs yielded nanowires through which the titania anatase coated the CNTs [130].
The hollow interior of MWCNTs can also be exploited for various functions. For instance, iron-filled nanotubes could harness a magnetic separation after use for the easy recovery of the photocatalytic system. The tubes were functionalized on their surface to display carboxylic acid groups as anchors for titania nucleation and growth, and a thermal treatment allowed for the formation of anatase nanocrystals on the surface of CNTs (Figure 7) [184].
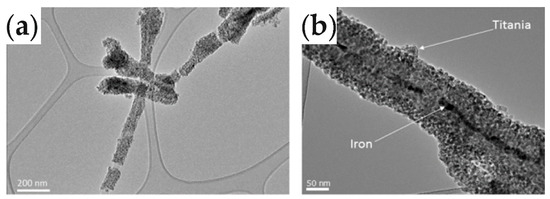
Figure 7.
TEM images at lower (a) and higher (b) magnification show MWCNTs coated with titania nanocrystals and partially filled with iron inside for the magnetic separation and recovery of the photocatalyst after use. Reprinted from [184], Copyright © 2018, with permission from Elsevier.
2.8. Graphene, Graphene Oxide (GO) and Reduced Graphene Oxide (rGO)
The combination of graphene-based materials and titania is perhaps the most investigated nanocarbon-titania combination, and the topic was reviewed in 2017 [185] and 2019 [186]. Therefore, we focus here on the progresses made in the last two years and, given the vast amount of relevant literature, only selected examples are discussed in this section, while more can be found in Table 1.
Despite the sheet morphology of graphene-based materials, they can be used also to successfully attain elongated nanofibers, as demonstrated by the use of electrospinning techniques. This technique allows continuous nanofibers to be drawn from a liquid, thanks to electrostatic forces in the liquid jet that accelerates through an electric field. The inclusion of both rGO and a titania precursor in the dispersion of reagents allowed electrospun nanofibers to be attained that after calcination revealed a shell anatase titania round a core with rGO. The nanofibrous exhibited the efficient photocatalytic degradation of a drug and a dye as model pollutants [171].
The enhanced photo-activity arises from synergistic effects, and it critically hinges on the covalent bonds between the two phases, which can be confirmed through the spectroscopic characterization of the Ti-O-C signature. To this end, it can be convenient to use titanium tetrachloride as a titania precursor, as the chloride is an excellent leaving group that can be replaced by the oxygen of the hydroxyl groups present on graphene [153].
Other important parameters that determine the performance of the final materials are clearly the crystallinity and particle size or film thickness of titania [187]. Solvothermal methods have been proven to be appropriate for attaining the complete coverage of graphene oxide flakes by highly homogeneous anatase nanocrystals (approximately 10–20 nm in size) (Figure 8a). In silico investigations confirmed that the presence of oxygen-bearing functional groups on graphene oxide acted as anchoring sites for the nucleation of anatase (Figure 8b–d), which was favored over the growth of larger crystals. Comparison with analogous composites obtained using different nnocarbons (i.e., MWCNTs, nanohorns, or graphitized carbon black), revealed that graphene-based material displayed the highest selectivity for phosphopeptides relative to non-phosphorylated peptides. The former could thus be enriched from cancer cell lysates for phosphoproteomics profiling, in a proof-of-concept study for oncological research and potentially for the early detection of cancer [101].
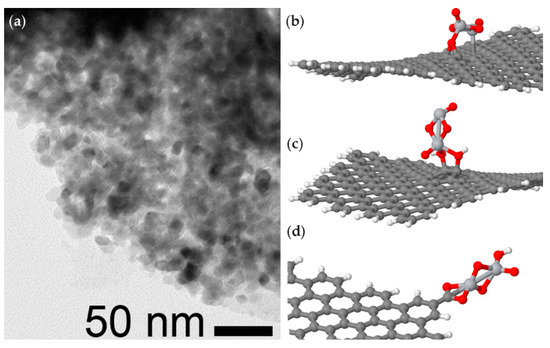
Figure 8.
(a) TEM image of a graphene oxide flake covered by anatase titania nanocrystals that are highly homogeneous in size; (b–d) in silico investigation revealed how the nanocarbon’s oxygen-bearing functional groups (i.e., (b) epoxide, (c) hydroxyl, and (d) carboxylic acid) anchored titanium species, thus favoring nucleation over the growth of the nanocrystals. Adapted by permission from Springer Nature [101], copyright © 2020.
3. Applications
As mentioned in the introduction, nanostructured titania finds a wide variety of applications in areas spanning from energy to medicine, as described below more in detail.
3.1. Photocatalysis for Energy and Synthesis
Titania is a promising candidate for photocatalytic applications related to sustainable schemes for energy and environment. For instance, titania is well-studied for the conversion of carbon dioxide into a range of useful fuels or commodities, such as methane, methanol, ethylene, formaldehyde, or formic acid [188]. For hydrogenation reactions, such as those of carbon dioxide and monoxide, titania and titanates have also been used as active supports for Rh catalysts [189]. Additionally, iridium-based catalysts have benefited from research that developed titania-based active supports for the photocatalytic synthesis of benzimidazoles, whereby rutile surpassed anatase in terms of performance, thanks to an increase in the adsorption of the reagents and in the charge transfer from the support, both of which led to higher product yields [190].
The photoelectrochemical generation of hydrogen from water has also enjoyed great prominence, where one-dimensional titania nanostructures demonstrated promising performance [191]. In silico tools are highly valuable to understand the nature of the rate-limiting steps and anticipate the better approaches to improve the efficiency of the process [192]. The further improvement of the photocatalytic performance of titania can be achieved through combination with other metal oxides (such as SnO2 [193], ZnO [194], CuO [195]), gold [196,197], as well as alkali metals and conducting polymers [198], carbon-doping [199], iron oxides to attain magnetic systems [200], gadolinium [201], various rare earths [202], and bismuth vanadate [203], amongst others. In particular, the use of 4D transition metals was shown to be effective through the production of impurity-originating intra-gap energy states, but also through the semiconductor–metal phase transition [204].
A recent promising approach consists of single-atom doping. In the case of copper, light irradiation leads to electron transfer and the protonation of Cu/titania, and a local distortion around the copper atom stabilizes the deep-trap state on the copper d-orbital and its decoupling from free charges, thus yielding high photocatalytic hydrogen generation activity [205]. Further, the photocatalytic performance of Cu/titania can be improved by spin selection, which is achieved via optical intersite spin transfer or chiral semiconductor coating [205]. Both hydrogen adsorption and spin selection processes increase charge carrier lifetimes by an order of magnitude [205].
3.2. Photocatalysis for Environmental Remediation
The photoactivity of titania has been widely applied for environmental remediation; for instance, through the generation of graphene-bearing membranes for water purification [186,206]. The mechanisms of titania-photocatalyzed dye degradation proceed through the generation of reactive oxygen species (ROS) that trigger the photooxidation of organic molecules [207]. This phenomenon can be applied to a variety of different pollutants. They include dyes, for which titania co-doping with iron and praseodymium was recently reported to significantly narrow the band gap. Moreover, it promoted the generation of oxygen vacancies, which can trap electrons and thus reduce the recombination of charge carriers [208]. Nitrogen and sulfur co-doping represents a very popular strategy to enhance the photodegradation of cationic dyes [209]. Another type of target pollutants consists of volatile organics, whose photo-degradation is heavily influenced by levels of humidity [210]. Another area that requires urgent action is the environmental pollution by drugs, especially antibiotics, whose mechanisms of photodegradation are still the subject of intense investigations [211]. Clearly, the removal of pesticides is also highly sought after, although a number of variables have to be taken into consideration as they may affect the efficiency of the process [212]. Ammonia is a critical pollutant of agricultural concern, both in gaseous and aqueous matrices, for which heterogeneous nanostructures find application as photocatalysts [213]. In fact, ammonia splitting into nitrogen and hydrogen gases is particularly attractive as it would concomitantly generate clean fuel [214]. The fundamental reaction pathways involved in the process are shown in Figure 9 [214].
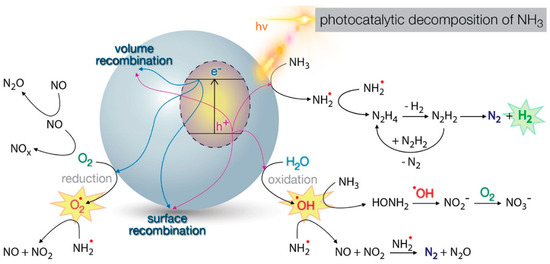
Figure 9.
Scheme of the fundamental reaction pathways of ammonia degradation photocatalyzed by titania. Reprinted with permission from [214], Copyright © 2020, American Chemical Society.
Sometimes, different photoactive catalysts are combined together to enhance the performance of the final materials [215]. Alternatively, doping can be an effective strategy; for instance, with bismuth [216], tungsten [217], nickel [218], and so on.
3.3. Biomedical Applications and Public Health
The photoactivity of titania nanoparticles has also been recently investigated for applications in medicine. The most popular uses exploit the ability of nanostructured titania to generate ROS upon light irradiation, as these species can be employed for photodynamic therapy, but also for the inactivation of bacteria that are resistant to antibiotics [219]. Another area that is gaining momentum is the development of active coatings and films for food packaging [220]. To this end, the antimicrobial activity phototriggered by nanostructured titania can be boosted upon the inclusion of silver or copper species, for applications that go beyond food packaging, including also active textiles and self-cleaning fabrics [221].
Titania nanotubes are being investigated as vehicles for local drug delivery, especially if functionalized to promote osteogenesis at the bone–implant interface, as recently reviewed [222]. Given the widespread use of titanium implants, the possibility to use nanotopography to modify their surface to render it bioactive is particularly attractive. To this end, the usefulness of a variety of potential designs, including titania nanotube arrays loaded with antibiotics (Figure 10), has been recently discussed as a potential strategy to address localized infections [223].
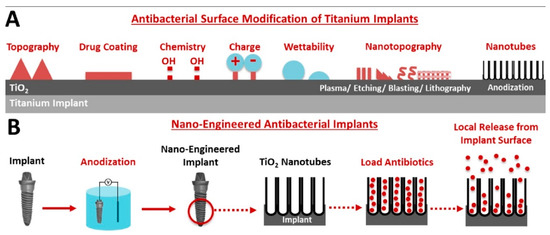
Figure 10.
Schematic representation of (A) potential titanium–implant surface modification to attain antibacterial activity, and (B) in particular, the use of electrochemically anodized titania nanotube arrays for the local release of antibiotics. Reprinted from [223], Copyright © 2021, with permission from Elsevier.
3.4. Sensing
Nanostructured titania has been investigated to develop a variety of electrochemical sensors, especially as applied to biomarkers for early disease diagnosis. Volatile organic compounds (VOCs) can be useful biomarkers for the early detection of a variety of pathological states, and therefore chemoresistive breath sensors for their detection are investigated, often using semiconducting metal oxides. To this end, various forms of nanostructured titania can be used, such as nanoparticles, nanotube arrays, and nanosheets, as recently reviewed [224]. Zinc-doped titania nanoparticles have been also proposed to develop glutamate sensors, as glutamate is a common food additive but also a useful biomarker, since abnormal levels were linked to pathologies such as epilepsy, Alzheimer’s and Parkinson’s diseases, ischemia, and amyotrophic lateral sclerosis [225].
For all these sensing applications, the inclusion of carbon nanostructures can be beneficial to enhance sensitivity and the performance of the devices generally, as recently reviewed [10]. This is true for a variety of carbon nanomorphologies. For instance, the inclusion of CNTs allowed a lower detection limit and an increased linear range of operativity for an epinephrine sensor [226]. In another recent example, an rGO/titania nanohybrid deposited on a glassy carbon electrode displayed a better performance for the electrochemical detection of a red dye relative to the sensor made with either component, thanks to a larger electroactive surface area and lower charge-transferred resistance [227]. Finally, graphene quantum dots also allowed for an enhanced photoresponse, with higher photocurrents and improved charge carrier separation efficiency, for an NO gas sensor based on titania that operated under UV-light irradiation [228].
4. Conclusions and Future Perspectives
The synergy between carbon nanostructures and titania has been extensively investigated over the years and allowed great progress in a variety of fields; in particular, for tailored applications to address urgent societal needs such as clean energy, medicine, and environmental remediation, which is now being extended to the issue of microplastics [229]. It has been undoubtedly demonstrated that the inclusion of nanocarbon into titania-based functional materials leads to the significant enhancement of the materials’ properties. In photocatalysis, for example, depending on the efficiency of the titania/carbon contact, a clear reduction in the electron–hole recombination rate has been observed, as the nanocarbon scaffold is able to scavenge the photoexcited electrons. On the other hand, in electrocatalysis, the carbon conductivity has been exploited to facilitate the electron transfer processes occurring at the TiO2-based catalytic sites, resulting in enhanced performances. Regardless of the application, it appears that the suitable interface between the carbon and the TiO2 phases is in most cases an essential parameter to gain definite advantages in performance. For this reason, we have seen that efforts have focused on the development of synthetic protocols that could guarantee adequate phase contact, giving rise to the desired synergistic effects. As an example, the production of co-axial CNT–titania nanostructures, with an intimate contact between the two phases, could be attained through the careful selection of CNT functionalization, so that the support displays suitable oxygen-bearing functional groups to effectively anchor titania, and the optimization of the thermal annealing step to seal the CNT–titania interface [230].
The main limitation of titania for use in photochemical applications, namely its relatively wide bandgap that makes it unresponsive to visible light, has been linked in recent times to a predicted slow decline in the popularity of titania-based materials for photo-chemical sustainable processes, paving the way for the exploration of new semiconductor materials with a more profitable exploitation of sunlight. However, we foresee that TiO2 will still have a long life as the leading semiconductor as many authors have shown avenues for expanding its use to incorporate the exploitation of visible light. For example, defect engineering in TiO2 polymorphs has reached a deep level of understanding, and the controlled alteration of the TiO2 structure at atomic level can lead to considerable advantages. Black titania, obtained in 2011 by an intensive H2 treatment that causes reduced Ti states by means of oxygen atom removal, is a symbolic case of a visible-light active TiO2 material [231]. As the activity under visible light is caused by intragap states, it is frequently hypothesized that combined nanocarbon–TiO2 could cause a C-doping of the titania at the phase interface, creating new midgap states.
TiO2 is being revisited in modern times as a component in more complex composites, and the interfacing with carbon nanostructures certainly brings many advantages (some of them illustrated above), in concomitance with a confinement at the nanoscale level of the material, with the expected benefits of nanomaterials. However, TiO2 junctions with other semiconductors bearing suitable electronic features could make further amends for the intrinsic shortcoming of self-standing TiO2. Z-schemes or p-n heterojunctions are the two best known examples to improve photochemical properties. Therefore, opportune multi-phase nanohybrids or nanocomposites featuring nanocarbon and TiO2 in conjunction with other components bear great potential for unlocking new multifunctionalities and directing these properties to specific purpose.
As we advance our detailed understanding of the mechanisms of interactions and reactions of nanostructured titania, further progress is set to be realized in this fascinating field. New opportunities are arising also from studies on surfaces [232]. The use of photoluminescence spectroscopy is emerging as a key tool to unravel mechanistic details of photocatalytic processes [233]. Finally, chiral nanostructures that can be attained through the templating of self-assembling molecules are also opening the door to new opportunities in non-linear optics, sensing, and photonics [234]. Examples of potential applications include circular polarizers, since titania chiral superstructures showed strong optical activity due to the difference of absorbing left and right-handed circularly polarized light [235]. Optically active films were also obtained from anatase nanocrystals that were spatially organized with long-range chiral nematic ordering, allowing for the selective reflection of circularly polarized light and iridescence [236].
Another emerging area of application lies in electronics and memory devices [114,237,238]. A recent report noted that the doping of titania with hydrogen, deuterium, and lithium led to bipolar conduction and a giant positive magnetoresistance, thus significantly expanding the properties of the materials [239]. Doping with cerium enhanced the magnetic properties of semiconductors from UV-light irradiation, thanks to the ferromagnetic orientation of spin densities near oxygen vacancies in Ce-doped titania, as opposed to the anti-ferromagnetic orientations of those found in undoped titania [240].
In conclusion, it appears that there is still a bright future ahead for the application of titania’s great ability to interact with light, especially if maximized through the synergy with other nanomaterials and advanced techniques of characterization and fabrication.
Author Contributions
Writing—original draft preparation, M.C.C.; writing—review and editing, S.P., P.F. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
Part of the described research was funded by EU H2020 NMBP-SPIRE project, grant no. 820723, and by Italian Ministry of University and Research (MIUR) PRIN2015 project number 2015TWP83Z.
Acknowledgments
The authors would like to thank Antonella Orviati and Cristina Cocever for their valuable assistance with the library services. The authors would like to acknowledge COST Action EsSENce CA19118.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef] [PubMed]
- Dell’Edera, M.; Lo Porto, C.; De Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-based coatings for environmental applications. Catal. Today 2021. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Z.; Chang, X.; Xu, J.; Wang, D.-H. Modified Nano-TiO2 Based Composites for Environmental Photocatalytic Applications. Catalysts 2020, 10, 759. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M. Titanium dioxide nanostructures as efficient photocatalyst: Progress, challenges and perspective. Int. J. Energy Res. 2021, 45, 3569–3589. [Google Scholar] [CrossRef]
- Govardhana Reddy, P.V.; Rajendra Prasad Reddy, B.; Venkata Krishna Reddy, M.; Raghava Reddy, K.; Shetti, N.P.; Saleh, T.A.; Aminabhavi, T.M. A review on multicomponent reactions catalysed by zero-dimensional/one-dimensional titanium dioxide (TiO2) nanomaterials: Promising green methodologies in organic chemistry. J. Environ. Man. 2021, 279, 111603. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Li, H.; Sagar, T.V. TiO2-Based Water-Tolerant Acid Catalysis for Biomass-Based Fuels and Chemicals. ACS Catal. 2020, 10, 9555–9584. [Google Scholar] [CrossRef]
- Huo, J.; Tessonnier, J.-P.; Shanks, B.H. Improving Hydrothermal Stability of Supported Metal Catalysts for Biomass Conversions: A Review. ACS Catal. 2021, 11, 5248–5270. [Google Scholar] [CrossRef]
- Nunes Simonetti, E.A.; Cardoso de Oliveira, T.; Enrico do Carmo Machado, Á.; Coutinho Silva, A.A.; Silva dos Santos, A.; de Simone Cividanes, L. TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity—A review. Ceram. Int. 2021, 47, 17844–17876. [Google Scholar] [CrossRef]
- Melchionna, M.; Prato, M.; Fornasiero, P. Mix and match metal oxides and nanocarbons for new photocatalytic frontiers. Catal. Today 2016, 277, 202–213. [Google Scholar] [CrossRef]
- Huang, J.-F.; Lei, Y.; Luo, T.; Liu, J.-M. Photocatalytic H2 Production from Water by Metal-free Dye-sensitized TiO2 Semiconductors: The Role and Development Process of Organic Sensitizers. ChemSusChem 2020, 13, 5863–5895. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wei, L.; Chen, L.; Zhang, H.; Su, J. Photocatalytic Production of Hydrogen Peroxide over Modified Semiconductor Materials: A Minireview. Top. Catal. 2020, 63, 895–912. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Feng, H.-L.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Recent advances in hierarchical three-dimensional titanium dioxide nanotree arrays for high-performance solar cells. J. Mater. Chem. A 2017, 5, 12699–12717. [Google Scholar] [CrossRef]
- Žerjav, G.; Pintar, A. Influence of TiO2 Morphology and Crystallinity on Visible-Light Photocatalytic Activity of TiO2-Bi2O3 Composite in AOPs. Catalysts 2020, 10, 395. [Google Scholar] [CrossRef]
- Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Abdul Latif, A.; Griebel, J.; Prager, A.; Schulze, A. Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis. Catalysts 2017, 7, 209. [Google Scholar] [CrossRef]
- Sun, J.; Sun, J.; Wang, X. Anatase TiO2 with Co-exposed (001) and (101) Surface-Based Photocatalytic Materials for Energy Conversion and Environmental Purification. Chem. Asian J. 2020, 15, 4168–4183. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Hidayat, R.; Fadillah, G.; Wahyuningsih, S. A control of TiO2 nanostructures by hydrothermal condition and their application: A short review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012031. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Nunes, D.; Pereira, S.; Martins, R.; Fortunato, E. Photocatalytic Activity of TiO2 nanostructured arrays prepared by microwave-assisted solvothermal method. In Semiconductor Photocatalysis: Materials, Mechanisms and Applications; Cao, W., Ed.; IntechOpen: London, UK, 2016; pp. 81–103. [Google Scholar]
- Nie, X.; Yin, S.; Duan, W.; Zhao, Z.; Li, L.; Zhang, Z. Recent Progress in Anodic Oxidation of TiO2 Nanotubes and Enhanced Photocatalytic Performance: A Short Review. Nano 2021, 16, 2130002. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Tech. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Siuzdak, K.; Haryński, Ł.; Wawrzyniak, J.; Grochowska, K. Review on robust laser light interaction with titania—Patterning, crystallisation and ablation processes. Prog. Solid State Chem. 2021, 62, 100297. [Google Scholar] [CrossRef]
- Jayanthi, S.; Sarkar, D.; Taffa, D.H.; Yerushalmi, R. Photoreactivity of Deep VB Titania Attained Via Molecular Layer Deposition; Interplay of Metal Oxide Thin Film Built-in Strain and Molecular Effects. Top. Catal. 2021, 64, 297–312. [Google Scholar] [CrossRef]
- Thalib Basha, G.M.; Srikanth, A.; Venkateshwarlu, B. A Critical Review on Nano structured Coatings for Alumina-Titania (Al2O3-TiO2) Deposited by Air Plasma Spraying Process (APS). Mater. Today Proc. 2020, 22, 1554–1562. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Basso, L.; Cazzanelli, M.; Orlandi, M.; Miotello, A. Nanodiamonds: Synthesis and application in sensing, catalysis, and the possible connection with some processes occurring in space. Appl. Sci. 2020, 10, 4094. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zheng, L.; Shi, X.; Zheng, H. Carbon nanomaterials with sp2 or/and sp hybridization in energy conversion and storage applications: A review. Energy Storage Mater. 2020, 26, 349–370. [Google Scholar] [CrossRef]
- Bryce, M.R. A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Adorinni, S.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Green Approaches to Carbon Nanostructure-Based Biomaterials. Appl. Sci. 2021, 11, 2490. [Google Scholar] [CrossRef]
- Ugarte, D. Onion-like graphitic particles. In Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 1996; pp. 163–167. [Google Scholar]
- Zieleniewska, A.; Lodermeyer, F.; Roth, A.; Guldi, D.M. Fullerenes—how 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef]
- Bottari, G.; Herranz, M.Á.; Wibmer, L.; Volland, M.; Rodríguez-Pérez, L.; Guldi, D.M.; Hirsch, A.; Martín, N.; D’Souza, F.; Torres, T. Chemical functionalization and characterization of graphene-based materials. Chem. Soc. Rev. 2017, 46, 4464–4500. [Google Scholar] [CrossRef]
- Dhand, V.; Yadav, M.; Kim, S.H.; Rhee, K.Y. A comprehensive review on the prospects of multi-functional carbon nano onions as an effective, high-performance energy storage material. Carbon 2021, 175, 534–575. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Wire Up on Carbon Nanostructures! How To Play a Winning Game. ACS Nano 2015, 9, 9441–9450. [Google Scholar] [CrossRef]
- Shi, C.; Owusu, K.A.; Xu, X.; Zhu, T.; Zhang, G.; Yang, W.; Mai, L. 1D Carbon-Based Nanocomposites for Electrochemical Energy Storage. Small 2019, 15, 1902348. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Qu, J.; Xiao, Y.; Zhao, S.; Chen, H.; Dai, L. Carbon Nanomaterials for Energy and Biorelated Catalysis: Recent Advances and Looking Forward. ACS Cent. Sci. 2019, 5, 389–408. [Google Scholar] [CrossRef]
- Ortiz-Medina, J.; Wang, Z.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X.; Terrones, M.; Endo, M. Defect Engineering and Surface Functionalization of Nanocarbons for Metal-Free Catalysis. Adv. Mater. 2019, 31, 1805717. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, C.-Y.; Zhang, D.; Gong, L.; Zhu, Y.; Zhao, Z.; Xu, Q.; Li, H.; Xia, Z. Guiding Principles for Designing Highly Efficient Metal-Free Carbon Catalysts. Adv. Mater. 2019, 31, 1805252. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, 180467. [Google Scholar] [CrossRef]
- Sideri, I.K.; Tagmatarchis, N. Noble-Metal-Free Doped Carbon Nanomaterial Electrocatalysts. Chem. Eur. J. 2020, 26, 15397–15415. [Google Scholar] [CrossRef]
- Ding, H.; Hu, B.; Zhang, B.; Zhang, H.; Yan, X.; Nie, G.; Liang, M. Carbon-based nanozymes for biomedical applications. Nano Res. 2021, 14, 570–583. [Google Scholar] [CrossRef]
- Park, S.-J.; Deshmukh, M.A.; Kang, B.-C.; Jeon, J.-Y.; Chen, C.; Ha, T.-J. Review—A Review of Advanced Electronic Applications Based on Carbon Nanomaterials. ECS J. Solid State Sci. Technol. 2020, 9, 071002. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Dasireddy, V.D.B.C.; Guan, X.; Wu, T.; Liu, G. Advances on Emerging Materials for Flexible Supercapacitors: Current Trends and Beyond. Adv. Funct. Mater. 2020, 30, 2002993. [Google Scholar] [CrossRef]
- Iglesias, D.; Senokos, E.; Alemán, B.; Cabana, L.; Navío, C.; Marcilla, R.; Prato, M.; Vilatela, J.J.; Marchesan, S. Gas-Phase Functionalization of Macroscopic Carbon Nanotube Fiber Assemblies: Reaction Control, Electrochemical Properties, and Use for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 5760–5770. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuang, Q.; Fan, H.J. Dual-Carbon Batteries: Materials and Mechanism. Small 2020, 16, 2002803. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-Nanomaterial-Based Flexible Batteries for Wearable Electronics. Adv. Mater. 2019, 31, 1800716. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Bhardwaj, P.A.; Honorato, A.M.B.; Varela, H. Metallic single-atoms confined in carbon nanomaterials for the electrocatalysis of oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Catal. Sci. Technol. 2020, 10, 6420–6448. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.-H. Carbon materials and their composites for electromagnetic interference shielding effectiveness in X-band. Carbon 2019, 152, 159–187. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Hu, X.; Chi, X.; Liu, J.; Chu, W.; Sun, L. Molecular Magnets Based on Graphenes and Carbon Nanotubes. Adv. Mater. 2019, 31, 1804917. [Google Scholar] [CrossRef]
- Zhang, G.; Koman, V.B.; Shikdar, T.; Oliver, R.J.; Perez-Lodeiro, N.; Strano, M.S. High Thermal Effusivity Nanocarbon Materials for Resonant Thermal Energy Harvesting. Small 2021, 2006752. [Google Scholar] [CrossRef]
- Howlader, A.H.; Li, F.; Zheng, R. Carbon Nanomaterials for Halide Perovskites-Based Hybrid Photodetectors. Adv. Mater. Technol. 2020, 5, 2000643. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sensor. Actuat. B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-X.; Lei, X.-W.; Natsuki, T. Review on Carbon Nanomaterials-Based Nano-Mass and Nano-Force Sensors by Theoretical Analysis of Vibration Behavior. Sensors 2021, 21, 1907. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Joshi, P.; Mishra, R.; Narayan, R.J. Biosensing applications of carbon-based materials. Curr. Opin. Biomed. Eng. 2021, 18, 100274. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Ma, R.; Zhang, X.; Rao, J.; Yin, Y.; Wang, X.; Yi, F. Flexible temperature sensors based on carbon nanomaterials. J. Mater. Chem. B 2021, 9, 1941–1964. [Google Scholar] [CrossRef]
- Biswas, S.; Visell, Y. Emerging Material Technologies for Haptics. Adv. Mater. Technol. 2019, 4, 1900042. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Wang, J.; James, D.K.; Narkhede, P.; Singh, S.P.; Jassby, D.; Tour, J.M.; Arnusch, C.J. Laser-induced graphene and carbon nanotubes as conductive carbon-based materials in environmental technology. Mater. Today 2020, 34, 115–131. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Su, D.; Li, H.; Yan, X.; Lin, Y.; Lu, G. Biosensors based on fluorescence carbon nanomaterials for detection of pesticides. Trends Anal. Chem. 2021, 134, 116126. [Google Scholar] [CrossRef]
- Torrinha, Á.; Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Correia, A.N.; Lima-Neto, P.; Morais, S. Application of Nanostructured Carbon-Based Electrochemical (Bio)Sensors for Screening of Emerging Pharmaceutical Pollutants in Waters and Aquatic Species: A Review. Nanomaterials 2020, 10, 1268. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.; Zhang, Z. Interface mechanics in carbon nanomaterials-based nanocomposites. Compos. Part A Appl. Sci. Manuf. 2021, 141, 106212. [Google Scholar] [CrossRef]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The glitter of carbon nanostructures in hybrid/composite hydrogels for medicinal use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent advances in carbon-based nanomaterials for flame retardant polymers and composites. Compos. Part B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon Nanostructures for Nanomedicine: Opportunities and Challenges. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Riley, P.R.; Narayan, R.J. Recent advances in carbon nanomaterials for biomedical applications: A review. Curr. Opin. Biomed. Eng. 2021, 17, 100262. [Google Scholar] [CrossRef]
- Plachá, D.; Jampilek, J. Graphenic Materials for Biomedical Applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, e1802368. [Google Scholar] [CrossRef]
- Mehra, N.K.; Jain, A.K.; Nahar, M. Carbon nanomaterials in oncology: An expanding horizon. Drug Discov. Today 2018, 23, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.-C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Sandhyarani, N. Carbon nanostructures as immobilization platform for DNA: A review on current progress in electrochemical DNA sensors. Biosens. Bioelectron. 2017, 97, 226–237. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-based nanomaterials for tissue engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Marchesan, S.; Ballerini, L.; Prato, M. Nanomaterials for stimulating nerve growth. Science 2017, 356, 1010–1011. [Google Scholar] [CrossRef]
- Marchesan, S.; Bosi, S.; Alshatwi, A.; Prato, M. Carbon nanotubes for organ regeneration: An electrifying performance. Nano Today 2016, 11, 398–401. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Ren, Y.; Qu, Z.; Gao, L.; Zhai, Y.; Han, S.-T.; Zhou, Y. Synaptic transistors and neuromorphic systems based on carbon nano-materials. Nanoscale 2021, 13, 7498–7522. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Marchesan, S.; Fornasiero, P.; Prato, M. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef]
- Saha, A.; Moya, A.; Kahnt, A.; Iglesias, D.; Marchesan, S.; Wannemacher, R.; Prato, M.; Vilatela, J.J.; Guldi, D.M. Interfacial charge transfer in functionalized multi-walled carbon nanotube@TiO2 nanofibres. Nanoscale 2017, 9, 7911–7921. [Google Scholar] [CrossRef]
- Hamandi, M.; Meksi, M.; Kochkar, H. Nanoscale Advances of Carbon-Titanium Dioxide Nanomaterials in Photocatalysis Applications. Rev. Nanosci. Nanotechnol. 2016, 4, 108–134. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Biesold-McGee, G.V.; Huang, J.; Ng, Y.H.; Sun, H.; Wang, J.; Lai, Y.; Lin, Z. Silk fibroin-derived nitrogen-doped carbon quantum dots anchored on TiO2 nanotube arrays for heterogeneous photocatalytic degradation and water splitting. Nano Energy 2020, 78, 105313. [Google Scholar] [CrossRef]
- He, C.; Peng, L.; Lv, L.; Cao, Y.; Tu, J.; Huang, W.; Zhang, K. In situ growth of carbon dots on TiO2 nanotube arrays for PEC enzyme biosensors with visible light response. RSC Adv. 2019, 9, 15084–15091. [Google Scholar] [CrossRef]
- Huo, P.; Shi, X.; Zhang, W.; Kumar, P.; Liu, B. An overview on the incorporation of graphene quantum dots on TiO2 for enhanced performances. J. Mater. Sci. 2021, 56, 6031–6051. [Google Scholar] [CrossRef]
- Justh, N.; Firkala, T.; László, K.; Lábár, J.; Szilágyi, I.M. Photocatalytic C60-amorphous TiO2 composites prepared by atomic layer deposition. Appl. Surf. Sci. 2017, 419, 497–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Yang, K.; Yang, Y.; Jia, J.; Liang, Y.; Guo, L. Carbon Nano-Onions (CNOs)/TiO2 Composite Preparation and Its Photocatalytic Performance under Visible Light Irradiation. J. Environ. Eng. 2020, 146, 04020009. [Google Scholar] [CrossRef]
- Lim, E.; Shim, H.; Fleischmann, S.; Presser, V. Fast and stable lithium-ion storage kinetics of anatase titanium dioxide/carbon onion hybrid electrodes. J. Mater. Chem. A 2018, 6, 9480–9488. [Google Scholar] [CrossRef]
- Mohapatra, D.; Parida, S.; Singh, B.K.; Sutar, D.S. Importance of microstructure and interface in designing metal oxide nanocomposites for supercapacitor electrodes. J. Electroanal. Chem. 2017, 803, 30–39. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Montini, T.; Monai, M.; Nasi, L.; Fornasiero, P.; Prato, M. Highly efficient hydrogen production through ethanol photoreforming by a carbon nanocone/Pd@TiO2 hybrid catalyst. Chem. Commun. 2016, 52, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Bracamonte, M.V.; Giuliani, A.; Nasi, L.; Montini, T.; Tavagnacco, C.; Bonchio, M.; Fornasiero, P.; Prato, M. Pd@TiO2/carbon nanohorn electrocatalysts: Reversible CO2 hydrogenation to formic acid. Energy Environ. Sci. 2018, 11, 1571–1580. [Google Scholar] [CrossRef]
- Ramesh Reddy, N.; Mamatha Kumari, M.; Shankar, M.V.; Raghava Reddy, K.; Woo Joo, S.; Aminabhavi, T.M. Photocatalytic hydrogen production from dye contaminated water and electrochemical supercapacitors using carbon nanohorns and TiO2 nanoflower heterogeneous catalysts. J. Environ. Man. 2021, 277, 111433. [Google Scholar] [CrossRef]
- Piovesana, S.; Iglesias, D.; Melle-Franco, M.; Kralj, S.; Cavaliere, C.; Melchionna, M.; Laganà, A.; Capriotti, A.L.; Marchesan, S. Carbon nanostructure morphology templates nanocomposites for phosphoproteomics. Nano Res. 2020, 13, 380–388. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination. J. Colloid Interf. Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Henych, J.; Stehlík, Š.; Mazanec, K.; Tolasz, J.; Čermák, J.; Rezek, B.; Mattsson, A.; Österlund, L. Reactive adsorption and photodegradation of soman and dimethyl methylphosphonate on TiO2/nanodiamond composites. Appl. Catal. B Environ. 2019, 259, 118097. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Silva, A.M.T.; Faria, J.L. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Appl. Surf. Sci. 2018, 458, 839–848. [Google Scholar] [CrossRef]
- Gao, X.; Sun, X.; Jiang, Z.; Wang, Q.; Gao, N.; Li, H.; Zhang, H.; Yu, K.; Su, C. Introducing nanodiamond into TiO2-based anode for improving the performance of lithium-ion batteries. New J. Chem. 2019, 43, 3907–3912. [Google Scholar] [CrossRef]
- Park, H.-A.; Liu, S.; Salvador, P.A.; Rohrer, G.S.; Islam, M.F. High visible-light photochemical activity of titania decorated on single-wall carbon nanotube aerogels. RSC Adv. 2016, 6, 22285–22294. [Google Scholar] [CrossRef]
- Kurniawan, K.; Tajima, T.; Kubo, Y.; Miyake, H.; Kurashige, W.; Negishi, Y.; Takaguchi, Y. Incorporating a TiOx shell in single-walled carbon nanotube/fullerodendron coaxial nanowires: Increasing the photocatalytic evolution of H2 from water under irradiation with visible light. RSC Adv. 2017, 7, 31767–31770. [Google Scholar] [CrossRef]
- Acauan, L.; Dias, A.C.; Pereira, M.B.; Horowitz, F.; Bergmann, C.P. Influence of Different Defects in Vertically Aligned Carbon Nanotubes on TiO2 Nanoparticle Formation through Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2016, 8, 16444–16450. [Google Scholar] [CrossRef]
- Delekar, S.D.; Dhodamani, A.G.; More, K.V.; Dongale, T.D.; Kamat, R.K.; Acquah, S.F.A.; Dalal, N.S.; Panda, D.K. Structural and Optical Properties of Nanocrystalline TiO2 with Multiwalled Carbon Nanotubes and Its Photovoltaic Studies Using Ru(II) Sensitizers. ACS Omega 2018, 3, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Park, S.-J. Effect of incorporation of multiwalled carbon nanotubes on photodegradation efficiency of mesoporous anatase TiO2 spheres. Mater. Chem. Phys. 2017, 186, 261–270. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Zhao, Z.-Y.; Zhao, H.-B.; Wang, Y.-Z. A titanium dioxide–carbon nanotube hybrid to simultaneously achieve the mechanical enhancement of natural rubber and its stability under extreme frictional conditions. Mater. Adv. 2021, 2, 2408–2418. [Google Scholar] [CrossRef]
- Huang, M.; Chu, Y.; Xi, B.; Shi, N.; Duan, B.; Zhang, C.; Chen, W.; Feng, J.; Xiong, S. TiO2-Based Heterostructures with Different Mechanism: A General Synergistic Effect toward High-Performance Sodium Storage. Small 2020, 16, 2004054. [Google Scholar] [CrossRef]
- Sharma, H.K.; Sharma, S.K.; Vemula, K.; Koirala, A.R.; Yadav, H.M.; Singh, B.P. CNT facilitated interfacial charge transfer of TiO2 nanocomposite for controlling the electron-hole recombination. Solid State Sci. 2021, 112, 106492. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Mohamed, S.K. Rutile TiO2 nanorods/MWCNT composites for enhanced simultaneous photocatalytic oxidation of organic dyes and reduction of metal ions. Mater. Res. Express 2018, 5, 015057. [Google Scholar] [CrossRef]
- Mullani, N.; Ali, I.; Dongale, T.D.; Kim, G.H.; Choi, B.J.; Basit, M.A.; Park, T.J. Improved resistive switching behavior of multiwalled carbon nanotube/TiO2 nanorods composite film by increased oxygen vacancy reservoir. Mater. Sci. Semicond. Proc. 2020, 108, 104907. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, J.; Tao, J.; Higashi, M.; Yamauchi, M.; Nakashima, N. Wrapping Multiwalled Carbon Nanotubes with Anatase Titanium Oxide for the Electrosynthesis of Glycolic Acid. ACS Appl. Nano Mater. 2019, 2, 6360–6367. [Google Scholar] [CrossRef]
- Ghosh, S.; Kiran Kumar, V.; Kumar, S.K.; Biswas, S.; Martha, S.K. An insight of sodium-ion storage, diffusivity into TiO2 nanoparticles and practical realization to sodium-ion full cell. Electrochim. Acta 2019, 316, 69–78. [Google Scholar] [CrossRef]
- Song, L.; Chen, P.; Li, Z.; Du, P.; Yang, Y.; Li, N.; Xiong, J. Flexible carbon nanotubes/TiO2/C nanofibrous film as counter electrode of flexible quasi-solid dye-sensitized solar cells. Thin Solid Film. 2020, 711, 138307. [Google Scholar] [CrossRef]
- Zhu, K.; Li, C.; Jiao, Y.; Zhu, J.; Ren, H.; Luo, Y.; Fan, S.; Liu, K. Free-standing hybrid films comprising of ultra-dispersed titania nanocrystals and hierarchical conductive network for excellent high rate performance of lithium storage. Nano Res. 2020. [Google Scholar] [CrossRef]
- Zou, M.; Ma, Z.; Wang, Q.; Yang, Y.; Wu, S.; Yang, L.; Hu, S.; Xu, W.; Han, P.; Zou, R.; et al. Coaxial TiO2–carbon nanotube sponges as compressible anodes for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 7398–7405. [Google Scholar] [CrossRef]
- Beltram, A.; Melchionna, M.; Montini, T.; Nasi, L.; Fornasiero, P.; Prato, M. Making H2 from light and biomass-derived alcohols: The outstanding activity of newly designed hierarchical MWCNT/Pd@TiO2 hybrid catalysts. Green Chem. 2017, 19, 2379–2389. [Google Scholar] [CrossRef]
- Mo, Z.; Yang, R.; Lu, D.; Yang, L.; Hu, Q.; Li, H.; Zhu, H.; Tang, Z.; Gui, X. Lightweight, three-dimensional carbon Nanotube@TiO2 sponge with enhanced microwave absorption performance. Carbon 2019, 144, 433–439. [Google Scholar] [CrossRef]
- Belkhanchi, H.; Ziat, Y.; Hammi, M.; Laghlimi, C.; Moutcine, A.; Benyounes, A.; Kzaiber, F. Nitrogen doped carbon nanotubes grafted TiO2 rutile nanofilms: Promising material for dye sensitized solar cell application. Optik 2021, 229, 166234. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Duan, B.; Zhang, M. Modified Sol-Gel Synthesis of Carbon Nanotubes Supported Titania Composites with Enhanced Visible Light Induced Photocatalytic Activity. J. Nanomater. 2016, 2016, 3967156. [Google Scholar] [CrossRef]
- Samadi, A.; Ahmadi, R.; Hosseini, S.M. Influence of TiO2-Fe3O4-MWCNT hybrid nanotubes on piezoelectric and electromagnetic wave absorption properties of electrospun PVDF nanocomposites. Org. Electron. 2019, 75, 105405. [Google Scholar] [CrossRef]
- Lu, X.; Luo, F.; Tian, Q.; Zhang, W.; Sui, Z.; Chen, J. Anatase TiO2 nanowires intertangled with CNT for conductive additive-free lithium-ion battery anodes. J. Phys. Chem. Solids 2021, 153, 110037. [Google Scholar] [CrossRef]
- Akram, M.; Khan, R.M.; Afzal, F.; Mustafa, G.M.; Ahmad, A.; Ramay, S.M.; Mahmood, A.; Ali, S.M.; Atiq, S. CNTs mediated electrochemical performance and dielectric dispersion of TiO2-based hydrothermally synthesized nanocomposites. Ionics 2021, 27, 2107–2118. [Google Scholar] [CrossRef]
- Vajda, K.; Hernádi, K.; Coteţ, C.; Kovács, G.; Pap, Z. Shape-Tailored TiO2 Photocatalysts Obtained in the Presence of Different Types of Carbon Materials. J. Nanosci. Nanotechnol. 2021, 21, 2360–2367. [Google Scholar] [CrossRef]
- Idris, N.J.; Bakar, S.A.; Mohamed, A.; Muqoyyanah, M.; Othman, M.H.D.; Mamat, M.H.; Ahmad, M.K.; Birowosuto, M.D.; Soga, T. Photocatalytic performance improvement by utilizing GO_MWCNTs hybrid solution on sand/ZnO/TiO2-based photocatalysts to degrade methylene blue dye. Environ. Sci. Pollut. Res. 2021, 28, 6966–6979. [Google Scholar] [CrossRef]
- Zhao, S.; Ding, H.; Chen, J.; Yang, C.; Xian, X. Facile synthesis of CNTs@TiO2 composites by solvothermal reaction for high-rate and long-life lithium-ion batteries. J. Phys. Chem. Solids 2021, 152, 109950. [Google Scholar] [CrossRef]
- Jiang, K.; Niu, Y.; Fang, D.; Zhang, L.; Wang, C. Sulfur Incorporation in Hierarchical TiO2 Nanosheet/Carbon Nanotube Hybrids for Improved Lithium Storage Performance. ChemElectroChem 2020, 7, 2905–2916. [Google Scholar] [CrossRef]
- Silva, M.R.F.; Lourenço, M.A.O.; Tobaldi, D.M.; da Silva, C.F.; Seabra, M.P.; Ferreira, P. Carbon-modified titanium oxide materials for photocatalytic water and air decontamination. Chem. Eng. J. 2020, 387, 124099. [Google Scholar] [CrossRef]
- Zhu, C.; Tang, Y.; Liu, L.; Sheng, R.; Li, X.; Gao, Y.; NuLi, Y. A high-performance rechargeable Mg2+/Li+ hybrid battery using CNT@TiO2 nanocables as the cathode. J. Colloid Interf. Sci. 2021, 581, 307–313. [Google Scholar] [CrossRef]
- Liang, Z.; Bai, X.; Hao, P.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H. Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B Environ. 2019, 243, 711–720. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, W.-S.; Jiang, Y.; Sun, W.; Sang, H.-Q.; He, R.-X.; Tai, Q.; Chen, B.; Liu, Y.; Zhao, X.-Z. Multi-walled carbon nanotubes induced a controllable TiO2 morphology transformation for high-rate and long-life lithium-ion batteries. RSC Adv. 2017, 7, 21988–21996. [Google Scholar] [CrossRef]
- Lo, W.-C.; Su, S.-H.; Chu, H.-J.; He, J.-L. TiO2-CNTs grown on titanium as an anode layer for lithium-ion batteries. Surf. Coat. Technol. 2018, 337, 544–551. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, S.; Wu, Z.; Zhao, S.; Piao, L. Enhanced photocatalytic activity towards degradation and H2 evolution over one dimensional TiO2@MWCNTs heterojunction. Appl. Surf. Sci. 2017, 402, 360–368. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liao, S.-Y.; Wang, C.-C.; Kei, C.-C.; Gan, J.-Y.; Perng, T.-P. Direct formation of anatase TiO2 nanoparticles on carbon nanotubes by atomic layer deposition and their photocatalytic properties. Nanotechnology 2016, 27, 405702. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Eliáš, M.; Michalička, J.; Hegemann, D.; Pytlíček, Z.; Nečas, D.; Zajíčková, L. Atomic layer deposition of titanium dioxide on multi-walled carbon nanotubes for ammonia gas sensing. Surf. Coat. Technol. 2019, 370, 235–243. [Google Scholar] [CrossRef]
- Avasthi, P.; Balakrishnan, V. Tuning the Wettability of Vertically Aligned CNT–TiO2 Hybrid Electrodes for Enhanced Supercapacitor Performance. Adv. Mater. Interfaces 2019, 6, 1801842. [Google Scholar] [CrossRef]
- Moya, A.; Kemnade, N.; Osorio, M.R.; Cherevan, A.; Granados, D.; Eder, D.; Vilatela, J.J. Large area photoelectrodes based on hybrids of CNT fibres and ALD-grown TiO2. J. Mater. Chem. A 2017, 5, 24695–24706. [Google Scholar] [CrossRef]
- Li, M.; Zu, M.; Yu, J.; Cheng, H.; Li, Q.; Li, B. Controllable synthesis of core-sheath structured aligned carbon nanotube/titanium dioxide hybrid fibers by atomic layer deposition. Carbon 2017, 123, 151–157. [Google Scholar] [CrossRef]
- Mombeshora, E.T.; Ndungu, P.G.; Jarvis, A.L.L.; Nyamori, V.O. The physical and electrochemical properties of nitrogen-doped carbon nanotube- and reduced graphene oxide-titania nanocomposites. Mater. Chem. Phys. 2018, 213, 102–112. [Google Scholar] [CrossRef]
- El Marouazi, H.; Jiménez-Calvo, P.; Breniaux, E.; Colbeau-Justin, C.; Janowska, I.; Keller, V. Few Layer Graphene/TiO2 Composites for Enhanced Solar-Driven H2 Production from Methanol. ACS Sust. Chem. Eng. 2021, 9, 3633–3646. [Google Scholar] [CrossRef]
- Siew, Q.Y.; Pang, E.L.; Loh, H.-S.; Tan, M.T.T. Highly sensitive and specific graphene/TiO2 impedimetric immunosensor based on plant-derived tetravalent envelope glycoprotein domain III (EDIII) probe antigen for dengue diagnosis. Biosens. Bioelectron. 2021, 176, 112895. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, Y.; Dong, Y.; Wu, Y.; Bao, Y.; Yan, H.; Ma, J. Fabrication and investigation on Ag nanowires/TiO2 nanosheets/graphene hybrid nanocomposite and its water treatment performance. Adv. Comp. Hybrid. Mater. 2020, 3, 402–414. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Cho, K.Y.; Oh, W.-C. Novel Micro and Nanostructure of a AgCuInS2–Graphene–TiO2 Ternary Composite for Photocatalytic CO2 Reduction for Methanol Fuel. ACS Omega 2020, 5, 26389–26401. [Google Scholar] [CrossRef]
- De Angelis, D.; Presel, F.; Jabeen, N.; Bignardi, L.; Lizzit, D.; Lacovig, P.; Lizzit, S.; Montini, T.; Fornasiero, P.; Alfè, D.; et al. Interfacial two-dimensional oxide enhances photocatalytic activity of graphene/titania via electronic structure modification. Carbon 2020, 157, 350–357. [Google Scholar] [CrossRef]
- Falak, A.; Tian, Y.; Yan, L.; Zhang, X.; Xu, L.; Song, Z.; Dong, F.; Chen, P.; Zhao, M.; Wang, H.; et al. Simultaneous achievement of superior response and full recovery of titanium dioxide/graphene hybrid FET sensors for NH3 through p- to n-mode switch. Phys. Chem. Chem. Phys. 2020, 22, 16701–16711. [Google Scholar] [CrossRef] [PubMed]
- Fornasini, L.; Scaravonati, S.; Magnani, G.; Morenghi, A.; Sidoli, M.; Bersani, D.; Bertoni, G.; Aversa, L.; Verucchi, R.; Riccò, M.; et al. In situ decoration of laser-scribed graphene with TiO2 nanoparticles for scalable high-performance micro-supercapacitors. Carbon 2021, 176, 296–306. [Google Scholar] [CrossRef]
- Cui, R.; Liu, S.; Guo, X.; Huang, H.; Wang, J.; Liu, B.; Li, Y.; Zhao, D.; Dong, J.; Sun, B. N-Doping Holey Graphene TiO2–Pt Composite as Efficient Electrocatalyst for Methanol Oxidation. ACS Appl. Energy Mater. 2020, 3, 2665–2673. [Google Scholar] [CrossRef]
- Long, B.; Zhang, J.; Luo, L.; Ouyang, G.; Balogun, M.-S.; Song, S.; Tong, Y. High pseudocapacitance boosts the performance of monolithic porous carbon cloth/closely packed TiO2 nanodots as an anode of an all-flexible sodium-ion battery. J. Mater. Chem. A 2019, 7, 2626–2635. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Saini, H.; Pandiarajan, D.; Prashanth, M.K.; Parashuram, L.; Raghu, M.S. Controllable synthesis of TiO2 chemically bonded graphene for photocatalytic hydrogen evolution and dye degradation. Catal. Today 2020, 340, 170–177. [Google Scholar] [CrossRef]
- Wei, J.; Ping, H.; Xie, J.; Zou, Z.; Wang, K.; Xie, H.; Wang, W.; Lei, L.; Fu, Z. Bioprocess-Inspired Microscale Additive Manufacturing of Multilayered TiO2/Polymer Composites with Enamel-Like Structures and High Mechanical Properties. Adv. Funct. Mater. 2020, 30, 1904880. [Google Scholar] [CrossRef]
- El-Gendy, D.M.; Abdel Ghany, N.A.; Allam, N.K. Black titania nanotubes/spongy graphene nanocomposites for high-performance supercapacitors. RSC Adv. 2019, 9, 12555–12566. [Google Scholar] [CrossRef]
- Rao, Z.; Lu, G.; Mahmood, A.; Shi, G.; Xie, X.; Sun, J. Deactivation and activation mechanism of TiO2 and rGO/Er3+-TiO2 during flowing gaseous VOCs photodegradation. Appl. Catal. B Environ. 2021, 284, 119813. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Jayaraj, M.K.; John, H. Graphene hybridized high energy faceted titanium dioxide for transparent self-cleaning coatings. Catal. Today 2020, 348, 63–71. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.-X.; He, L.; Wang, L.-Y.; Liu, X.-L.; Liu, J.-W.; Li, Y.-Z.; Tian, G.; Zhao, H.; Yang, X.-H.; et al. Interfacial co-existence of oxygen and titanium vacancies in nanostructured TiO2 for enhancement of carrier transport. Nanoscale 2020, 12, 8364–8370. [Google Scholar] [CrossRef]
- Veera Manohara Reddy, Y.; Sravani, B.; Łuczak, T.; Mallikarjuna, K.; Madhavi, G. An ultra-sensitive rifampicin electrochemical sensor based on titanium nanoparticles (TiO2) anchored reduced graphene oxide modified glassy carbon electrode. Coll. Surf. A 2021, 608, 125533. [Google Scholar] [CrossRef]
- Ye, F.; Wang, Z.; Mi, Y.; Kuang, J.; Jiang, X.; Huang, Z.; Luo, Y.; Shen, L.; Yuan, H.; Zhang, Z. Preparation of reduced graphene oxide/titanium dioxide composite materials and its application in the treatment of oily wastewater. Coll. Surf. A 2020, 586, 124251. [Google Scholar] [CrossRef]
- Maarisetty, D.; Mahanta, S.; Sahoo, A.K.; Mohapatra, P.; Baral, S.S. Steering the Charge Kinetics in Dual-Functional Photocatalysis by Surface Dipole Moments and Band Edge Modulation: A Defect Study in TiO2-ZnS-rGO Composites. ACS Appl. Mater. Interfaces 2020, 12, 11679–11692. [Google Scholar] [CrossRef]
- Li, C.; Hu, R.; Lu, X.; Bashir, S.; Liu, J.L. Efficiency enhancement of photocatalytic degradation of tetracycline using reduced graphene oxide coordinated titania nanoplatelet. Catal. Today 2020, 350, 171–183. [Google Scholar] [CrossRef]
- Carreño-Lizcano, M.I.; Gualdrón-Reyes, A.F.; Rodríguez-González, V.; Pedraza-Avella, J.A.; Niño-Gómez, M.E. Photoelectrocatalytic phenol oxidation employing nitrogen doped TiO2-rGO films as photoanodes. Catal. Today 2020, 341, 96–103. [Google Scholar] [CrossRef]
- Mokhtarifar, M.; Kaveh, R.; Bagherzadeh, M.; Lucotti, A.; Pedeferri, M.; Diamanti, M.V. Heterostructured TiO2/SiO2/γ-Fe2O3/rGO Coating with Highly Efficient Visible-Light-Induced Self-Cleaning Properties for Metallic Artifacts. ACS Appl. Mater. Interfaces 2020, 12, 29671–29683. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, H.; Garlisi, C.; Sa, J.; Lewin, E.; Al-Ali, K.; Palmisano, G. Unveiling the role of bisulfide in the photocatalytic splitting of H2S in aqueous solutions. Appl. Catal. B Environ. 2020, 270, 118886. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, M.; Hu, F.; Wu, J.; Xu, L.; Xu, G.; Jian, Y.; Peng, X. Controllable construction of hierarchical TiO2 supported on hollow rGO/P-HC heterostructure for highly efficient photocatalysis. Coll. Surf. A 2020, 598, 124831. [Google Scholar] [CrossRef]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arivanandhan, M.; Asath Bahadur, S.; Swaminathan, M. Efficient Photoreduction of Hexavalent Chromium Using the Reduced Graphene Oxide-Sm2MoO6-TiO2 Catalyst under Visible Light Illumination. ACS Omega 2020, 5, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mu, H.; Cao, X.; He, X. Polymer-supported graphene–TiO2 doped with nonmetallic elements with enhanced photocatalytic reaction under visible light. J. Mater. Sci. 2020, 55, 1577–1591. [Google Scholar] [CrossRef]
- Zhao, W.; Ci, X. TiO2 Nanoparticle/Fluorinated Reduced Graphene Oxide Nanosheet Composites for Lubrication and Wear Resistance. ACS Appl. Nano Mater. 2020, 3, 8732–8741. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Yang, X.; Zhao, G.; Zhang, D.; Huang, H.; Yang, S.; Wen, N.; Javid, M.; Fan, Z.; et al. A Facile Synthesis of Novel Amorphous TiO2 Nanorods Decorated rGO Hybrid Composites with Wide Band Microwave Absorption. Nanomaterials 2020, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, N.; Jiang, C.; Xu, C.; Yu, S.; Liang, P.; Zhang, X.; Liang, S.; Huang, X. Filtration-enhanced highly efficient photocatalytic degradation with a novel electrospun rGO@TiO2 nanofibrous membrane: Implication for improving photocatalytic efficiency. Appl. Catal. B Environ. 2020, 268, 118737. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Liu, D. Mechanism of nicotine degradation and adsorption by a nano-TiO2 engineered reduced graphene oxide composite in light variant conditions. Catal. Sci. Technol. 2020, 10, 2797–2809. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, F.; Pervez, S.A.; Rehman, S.; Pope, M.A.; Fichtner, M.; Roberts, E.P.L. A stable TiO2–graphene nanocomposite anode with high rate capability for lithium-ion batteries. RSC Adv. 2020, 10, 29975–29982. [Google Scholar] [CrossRef]
- Chen, M.; Wu, G.; Zhang, M.; Liu, J.; Zai, J.; Qian, X.; Yu, X. A highly efficient nano-graphite electron transport layer for high performance ZnO/Si solar cells. Sustain. Energy Fuels 2018, 2, 820–826. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, X.; Jiang, L.; Xiong, T.; Zhang, J. Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications. Materials 2020, 13, 2924. [Google Scholar] [CrossRef] [PubMed]
- Gatti, T.; Menna, E.; Meneghetti, M.; Maggini, M.; Petrozza, A.; Lamberti, F. The Renaissance of fullerenes with perovskite solar cells. Nano Energy 2017, 41, 84–100. [Google Scholar] [CrossRef]
- Regulska, E.; Rivera-Nazario, D.M.; Karpinska, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Zinc Porphyrin-Functionalized Fullerenes for the Sensitization of Titania as a Visible-Light Active Photocatalyst: River Waters and Wastewaters Remediation. Molecules 2019, 24, 1118. [Google Scholar] [CrossRef]
- Amelot, D.; Ahmadpour, M.; Ros, Q.; Cruguel, H.; Casaretto, N.; Cossaro, A.; Floreano, L.; Madsen, M.; Witkowski, N. Deciphering Electron Interplay at the Fullerene/Sputtered TiOx Interface: A Barrier-Free Electron Extraction for Organic Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 19460–19466. [Google Scholar] [CrossRef]
- Mohapatra, D.; Nemala, S.S.; Sayed, M.S.; Shim, J.-J.; Mallick, S.; Bhargava, P.; Parida, S. Carbon nano-onion-powered optically transparent and economical dye-sensitized solar cells. Nanoscale 2020, 12, 20621–20630. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Atienzar, P.; Vázquez, E.; Herrero, M.A.; García, H. Carbon Nanohorns Modified with Conjugated Terthienyl/Terthiophene Structures: Additives to Enhance the Performance of Dye-Sensitized Solar Cells. Nanomaterials 2017, 7, 294. [Google Scholar] [CrossRef] [PubMed]
- Lodermeyer, F.; Costa, R.D.; Guldi, D.M. Implementation of Single-Walled Carbon Nanohorns into Solar Cell Schemes. Adv. Energy Mater. 2017, 7, 1601883. [Google Scholar] [CrossRef]
- Iglesias, D.; Melle-Franco, M.; Kurbasic, M.; Melchionna, M.; Abrami, M.; Grassi, M.; Prato, M.; Marchesan, S. Oxidized nanocarbons-tripeptide supramolecular hydrogels: Shape matters! ACS Nano 2018, 12, 5530–5538. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanodiamonds: Emerging face of future nanotechnology. Carbon 2019, 143, 678–699. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Stopin, A.; Montini, T.; Lodge, R.W.; Khlobystov, A.N.; Bonifazi, D.; Prato, M.; Fornasiero, P. Magnetic shepherding of nanocatalysts through hierarchically-assembled Fe-filled CNTs hybrids. Appl. Catal. B Environ. 2018, 227, 356–365. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285, 13–28. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Ampelli, C.; Tavella, F.; Perathoner, S.; Centi, G. Engineering of photoanodes based on ordered TiO2-nanotube arrays in solar photo-electrocatalytic (PECa) cells. Chem. Eng. J. 2017, 320, 352–362. [Google Scholar] [CrossRef]
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and Surface Modification of TiO2-Based Photocatalysts for the Conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef]
- Kiss, J.; Sápi, A.; Tóth, M.; Kukovecz, Á.; Kónya, Z. Rh-induced Support Transformation and Rh Incorporation in Titanate Structures and Their Influence on Catalytic Activity. Catalysts 2020, 10, 212. [Google Scholar] [CrossRef]
- Wada, K.; Yu, H.; Feng, Q. Titania-supported iridium catalysts for dehydrogenative synthesis of benzimidazoles. Chin. Chem. Lett. 2020, 31, 605–608. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, 1903378. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, S.; Gao, X. Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review. Nanotechnol. Rev. 2020, 9, 1080–1103. [Google Scholar] [CrossRef]
- Tada, H.; Naya, S.-I. Atomic Level Interface Control of SnO2-TiO2 Nanohybrids for the Photocatalytic Activity Enhancement. Catalysts 2021, 11, 205. [Google Scholar] [CrossRef]
- Syed, M.H.; Tabassum, H.; Moeen, F.; Qasim, A.; Shafaqat, A.; Muhammad, R.; Abdullah, I.H.; Madhumita, B.R.; Shahzad, A.S.C. Emerging Aspects of Photo-catalysts (TiO2 & ZnO) Doped Zeolites and Advanced Oxidation Processes for Degradation of Azo Dyes: A Review. Curr. Anal. Chem. 2021, 17, 82–97. [Google Scholar]
- de Brito, J.F.; Tavella, F.; Genovese, C.; Ampelli, C.; Zanoni, M.V.B.; Centi, G.; Perathoner, S. Role of CuO in the modification of the photocatalytic water splitting behavior of TiO2 nanotube thin films. Appl. Catal. B Environ. 2018, 224, 136–145. [Google Scholar] [CrossRef]
- Abed, J.; Rajput, N.S.; Moutaouakil, A.E.; Jouiad, M. Recent Advances in the Design of Plasmonic Au/TiO2 Nanostructures for Enhanced Photocatalytic Water Splitting. Nanomaterials 2020, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Ampelli, C.; Tavella, F.; Genovese, C.; Perathoner, S.; Favaro, M.; Centi, G. Analysis of the factors controlling performances of Au-modified TiO2 nanotube array based photoanode in photo-electrocatalytic (PECa) cells. J. Energy Chem. 2017, 26, 284–294. [Google Scholar] [CrossRef]
- Abdelnasser, S.; Al Sakkaf, R.; Palmisano, G. Environmental and energy applications of TiO2 photoanodes modified with alkali metals and polymers. J. Environ. Chem. Eng. 2021, 9, 104873. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S. Insight into the interaction of magnetic photocatalysts with the incoming light accelerating bacterial inactivation and environmental cleaning. Appl. Catal. B Environ. 2021, 281, 119420. [Google Scholar] [CrossRef]
- Arulmozhi, S.; Ezhil Arasi, S. Size controlled synthesis of gadolinium doped titanium dioxide nanoparticles by low temperature hydrothermal method towards effective photovoltaic application. Mater. Today Proc. 2020, 8, 456. [Google Scholar]
- Li, J.; Chu, B.; Xie, Z.; Deng, Y.; Zhou, Y.; Dong, L.; Li, B.; Chen, Z. Mechanism and DFT Study of Degradation of Organic Pollutants on Rare Earth Ions Doped TiO2 Photocatalysts Prepared by Sol-Hydrothermal Synthesis. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Perini, J.A.L.; Tavella, F.; Ferreira Neto, E.P.; Zanoni, M.V.B.; Ribeiro, S.J.L.; Giusi, D.; Centi, G.; Perathoner, S.; Ampelli, C. Role of nanostructure in the behaviour of BiVO4–TiO2 nanotube photoanodes for solar water splitting in relation to operational conditions. Sol. Energy Mater. Sol. Cells 2021, 223, 110980. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Q.; Su, Q.; Yang, J.; Zhao, Y.; Wen, H.; Jiang, Z. Effects of 4d transition metals doping on the photocatalytic activities of anatase TiO2 (101) surface. Int. J. Quantum Chem. 2021, 121, e26683. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, W.-H.; Long, R.; Prezhdo, O.V. Water Splitting with a Single-Atom Cu/TiO2 Photocatalyst: Atomistic Origin of High Efficiency and Proposed Enhancement by Spin Selection. J. Am. Chem. Soc. Au 2021, 1, 550–559. [Google Scholar]
- Bhol, P.; Yadav, S.; Altaee, A.; Saxena, M.; Misra, P.K.; Samal, A.K. Graphene-Based Membranes for Water and Wastewater Treatment: A Review. ACS Appl. Nano Mater. 2021, 4, 3274–3293. [Google Scholar] [CrossRef]
- Som, I.; Roy, M.; Saha, R. Advances in Nanomaterial-based Water Treatment Approaches for Photocatalytic Degradation of Water Pollutants. ChemCatChem 2020, 12, 3409–3433. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible light active Fe-Pr co-doped TiO2 for water pollutants degradation. Catal. Today 2021. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Iqbal, M.J.; Shabir, M.; Akhter, P.; Hamayun, M.H.; Ahmed, A.; Hussain, M. Synergistic effect of NS co-doped TiO2 adsorbent for removal of cationic dyes. J. Environ. Chem. Eng. 2021, 9, 105480. [Google Scholar] [CrossRef]
- Zhang, L.; Moralejo, C.; Anderson, W.A. A review of the influence of humidity on photocatalytic decomposition of gaseous pollutants on TiO2-based catalysts. Can. J. Chem. Eng. 2020, 98, 263–273. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Hadei, M.; Mesdaghinia, A.; Nabizadeh, R.; Mahvi, A.H.; Rabbani, S.; Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. Environ. Sci. Pollut. Res. 2021, 28, 13055–13071. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Li, X.; Zhang, J.; Zang, Q.; Wang, S. Building heterogeneous nanostructures for photocatalytic ammonia decomposition. Nanoscale Adv. 2020, 2, 3610–3623. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Dong, F.; Giannakoudakis, D.A. Photocatalytic Platforms for Removal of Ammonia from Gaseous and Aqueous Matrixes: Status and Challenges. ACS Catal. 2020, 10, 8683–8716. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Gul, I.; Sayed, M.; Shah, N.S.; Rehman, F.; Khan, J.A.; Gul, S.; Bibi, N.; Iqbal, J. A novel route for catalytic activation of peroxymonosulfate by oxygen vacancies improved bismuth-doped titania for the removal of recalcitrant organic contaminant. Environ. Sci. Pollut. Res. 2021, 28, 23368–23385. [Google Scholar] [CrossRef]
- Raut, S.S.; Kamble, S.P.; Kulkarni, P.S. Improved photocatalytic efficiency of TiO2 by doping with tungsten and synthesizing in ionic liquid: Precise kinetics-mechanism and effect of oxidizing agents. Environ. Sci. Pollut. Res. 2021, 28, 17532–17545. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Orikasa, S.; Asakura, Y.; Ide, Y.; Sugahara, Y.; Ogasawara, M.; Yin, S.; Kato, S. Ni-Doped Protonated Layered Titanate/TiO2 Composite with Efficient Photocatalytic Activity for NOx Decomposition Reactions. Int. J. Photoen. 2021, 2021, 8847956. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control. 2020, 112, 107086. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Raluca, I.; Madalina Georgiana, N.; Anca, M.; Valentina, M.; Patricia, N.; Patrik, S.; Anisoara, C. Drug Delivery Systems Based on Titania Nanotubes and Active Agents for Enhanced Osseointegration of Bone Implants. Curr. Med. Chem. 2020, 27, 854–902. [Google Scholar]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021, 127, 80–101. [Google Scholar] [CrossRef]
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Review—Metal Oxides: Application in Exhaled Breath Acetone Chemiresistive Sensors. J. Electrochem. Soc. 2020, 167, 037537. [Google Scholar] [CrossRef]
- Meesombad, K.; Sato, N.; Pitiphattharabun, S.; Panomsuwan, G.; Techapiesancharoenkij, R.; Surawathanawises, K.; Wongchoosuk, C.; Boonsalee, S.; Pee, J.H.; Jongprateep, O. Zn-doped TiO2 nanoparticles for glutamate sensors. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Duan, H.; Wang, Y.; Luo, C. Ultra-sensitive determination of epinephrine based on TiO2-Au nanoclusters supported on reduced graphene oxide and carbon nanotube hybrid nanocomposites. Mater. Sci. Eng. C 2016, 64, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, J.; Jin, H.; Xia, Y.; Liu, J.; He, Q.; Chen, D. Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red. Nanomaterials 2020, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Reddeppa, M.; Reddy, C.S.; Park, S.; Chandrakalavathi, T.; Kim, M.-D.; In, I. Enhancing the Charge Carrier Separation and Transport via Nitrogen-Doped Graphene Quantum Dot-TiO2 Nanoplate Hybrid Structure for an Efficient NO Gas Sensor. ACS Appl. Mater. Interfaces 2020, 12, 13428–13436. [Google Scholar] [CrossRef]
- Vital-Grappin, A.D.; Ariza-Tarazona, M.C.; Luna-Hernández, V.M.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Siligardi, C.; Cedillo-González, E.I. The Role of the Reactive Species Involved in the Photocatalytic Degradation of HDPE Microplastics Using C,N-TiO2 Powders. Polymers 2021, 13, 999. [Google Scholar] [CrossRef]
- Valenti, G.; Boni, A.; Melchionna, M.; Cargnello, M.; Nasi, L.; Bertoni, G.; Gorte, R.J.; Marcaccio, M.; Rapino, S.; Bonchio, M.; et al. Co-axial heterostructures integrating palladium/titanium dioxide with carbon nanotubes for efficient electrocatalytic hydrogen evolution. Nat. Commun. 2016, 7, 13549. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Sun, K.; Fang, Y.; Chi, L. On-Surface Synthesis on Nonmetallic Substrates. ACS Mater. Lett. 2021, 3, 56–63. [Google Scholar] [CrossRef]
- Li, Q.; Anpo, M.; Wang, X. Application of photoluminescence spectroscopy to elucidate photocatalytic reactions at the molecular level. Res. Chem. Intermed. 2020, 46, 4325–4344. [Google Scholar] [CrossRef]
- Fan, J.; Kotov, N.A. Chiral Nanoceramics. Adv. Mater. 2020, 32, 1906738. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, S.; Duan, Y.; Huang, Z.; Che, S. Hard-templating of chiral TiO2 nanofibres with electron transition-based optical activity. Sci. Technol. Adv. Mater. 2015, 16, 054206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shopsowitz, K.E.; Stahl, A.; Hamad, W.Y.; MacLachlan, M.J. Hard Templating of Nanocrystalline Titanium Dioxide with Chiral Nematic Ordering. Angew. Chem. Int. Ed. 2012, 51, 6886–6890. [Google Scholar] [CrossRef] [PubMed]
- Bondavalli, P.; Martin, M.B.; Hamidouche, L.; Montanaro, A.; Trompeta, A.-F.; Charitidis, C.A. Nano-Graphitic based Non-Volatile Memories Fabricated by the Dynamic Spray-Gun Deposition Method. Micromachines 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, S.; Kanwal, F.; Atiq, S.; Moussa, M.; Azhar, U.; Losic, D. Dielectric Properties of Graphene/Titania/Polyvinylidene Fluoride (G/TiO2/PVDF) Nanocomposites. Materials 2020, 13, 205. [Google Scholar] [CrossRef]
- Huang, K.; Wang, T.; Jin, M.; Wu, L.; Wang, J.F.; Li, S.; Qi, D.-C.; Cheng, S.; Li, Y.; Chen, J.; et al. Bipolar Conduction and Giant Positive Magnetoresistance in Doped Metallic Titanium Oxide Heterostructures. Adv. Mater. Interfaces 2021, 8, 2002147. [Google Scholar] [CrossRef]
- Wu, T.-S.; Syu, L.-Y.; Lin, B.-H.; Weng, S.-C.; Jeng, H.-T.; Huang, Y.-S.; Soo, Y.-L. Reduction of dopant ions and enhancement of magnetic properties by UV irradiation in Ce-doped TiO2. Sci. Rep. 2021, 11, 7668. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).