Abstract
Contemporary farming practices and rapid industrialization over the last few decades, have raised significant soil and water pollution with extreme toxic effects to humans and ecosystems. The widespread and inefficient use of pesticides, which surpass the soil’s self purification capability, has accelerated soil pollution. In this study, wheat straw biochar was obtained using the traditional pyrolysis technique and its characterization; in addition, the adsorption efficiency of metribuzin was investigated. Biochars’ physical and chemical characteristics were qualified using scanning electron microscopy and Fourier transform infrared spectroscopy. A batch sorption test and liquid chromatography coupled with mass spectrometry were also used to assess the biochar efficiency. SEM and FTIR confirmed the highly reactive surfaces of biochar, establishing efficient biomass conversion in low-oxygen conditions. The adsorption process showed best fit with pseudo second-order kinetic and Langmuir models, suggesting a chemisorption procedure and monolayer-type removal. Regarding its environmental and agricultural application, wheat straw biochar can be advanced as a recommendation solution for further research, which is fundamental for soil rehabilitation and the immobilization of contaminations.
1. Introduction
Considerable and unprofessional pesticide use to manage weeds and diseases precede soil pollution and linked ecosystems [1,2,3]. As toxic chemicals, pesticides can cause a number of health problems to humans, who are in imminent risk of being poisoned [4,5]. Pesticides long term statement effects include cytotoxic conversion of body organs with human endocrine system or hormonal dysfunction [6,7]. The theory of risk assessment due to food and soil pollution by a variety of contaminants has received appreciable attention at a global scale.
Atrazine, carbamates, chlorpyrifos, DDT, lindane and other organophosphate compounds are representative anthropogenic chemicals, which have been forbidden owing to their intense toxicity and corroborated health risk [8]. Among different categories of pesticides, herbicides had the maximum ration (40%) followed by insecticides (18%) and fungicides (10%). According to FAO-STAS, China, the United States, Brazil and Argentina are the word’s most pesticide-using areas, with reported pesticide use of 1,763,000, 407,779, 377,176, 207,706 tons in 2016 [1].
Soil polluted by pesticide can promote fluctuations and deteriorating soil quality [9]. Due to hazardous features, these substances are capable of perturbing soil enzymatic processes or bacterial species, which are index keys of soil tolerance to pollutants [10,11]. Therefore, pesticide-based soil pollution may have a detrimental impact on the break down and decomposition processes associated with microbial activities [12].
A wide range of alternatives can be used for remediation of pesticide contaminated soil, such us chemical oxidation/reduction, washing with extractants and bioremediation [13,14,15]. Some of these techniques are effective but limited by their applicability at the macro level in the agriculture field, with major issues that could arise as a result of their use. Focused on the adsorption theory, application of amendments is frequently regarded as a cost-effective strategy for pesticide-polluted soil remediation [16]. These remediation technologies include various modifications capable of converting and immobilizing pesticide, such us rice husk, fruit peel, straw wastes or biochar [1].
Amongst certain amendments, biochar has arisen as promising material that is eco-friendly, renewable, cheaper, and easily available, and will solve the problem of soil remediation [14,17,18].
Currently, biochar has gained popularity as a result of its numerous agricultural conveniences as well as performance. Biochar is the carbon-rich material, produced during the process of biomass pyrolysis under oxygen-limiting conditions [19,20]. Its basic composition (carbon, nitrogen, potassium and magnesium) can provide nutrients and increase crop yields, thereby reducing fertilizer requirements. The application of biochar as a soil amendment leads to improvement of the physico-chemical quality of the soil [21,22,23].
The increased water-holding capacity of soil following biochar treatment may be one of the major aspects for crop improvement [24]. The available soil water capacity is increased over 22%, due to high total porosity, which can retain water molecules in the small-pore structure of biochar [25]. The soil aggregation capacity is increased between 8 and 36% after the application of rice husk biochar [26]. Specific experiments performed at various scales, reveal that in the 0–15 cm soil depth, total porosity is increased by a minimum of 10%, whereas bulk density is decreased according to the same ratio [27]. Enhanced physical properties of the soil, such as water-holding and aggregation capacities or bulk density can improve both nutrient and water storage and crop productivity.
The use of biochar raises the pH of the soil. According to [28], soil reactivity (pH) is increased from 7.1 to 8.1 when different types of biochar were applied to soil. At higher biochar doses (50 t·ha−1), significant improvements in soil quality were observed, including electrical conductivity (124.6%) and cation exchange capacity by 20% [29,30]. Cation exchange capacity is an indicator of soil’s ability to maintain nutrients and water. By increasing soil CEC and stimulating the rate of microorganism growth, biochar can help to minimize nutrient leaching [31,32]. In addition, it has been reported that biochar has great potential of nutrient availability and could release high quantities of P (46–664 mg/kg−1) and N (23–635 mg kg−1) [33].
Moreover, due to its highly porous structure and the presence of carboxylic and phenolic groups in its structure, biochar has the ability to sorb and retain organic and inorganic pollutants from varied environmental matrices [34,35]. In liquid media (wastewater and water), its remediation efficiency was explored for various inorganic pollutants, copper, zinc, nickel, cadmium, mercury, etc. [36]. Additionally, biochar has the potential to proceed as a catalyst in biodiesel, syngas and energy production, tar removal or waste management [37]. The successful adsorption capacity of biochar was also reported for analysis of synthetic dyes, phenols and medicines [38,39,40].
In solid matrices (soil), the addition of biochar obtained from different straw immobilized diuron, simazine and atrazine herbicide [41,42]. For instance, [43] noticed that 80–86% of bromoxynil and diuron and 70% of ametryn were retained as a consequence of the addition of 1% wheat biochar. The potential binding technique of biochar with pesticides comprise cation, anion, non-polar and polar attraction, which govern the processes of sorption, desorption, leaching and hydrolysis of pesticides [1].
Therefore, the novelty of this research was to assess the pesticides adsorption capacity of biochar-based wheat in order to comprehend the biochar amendment consequence on pesticide behavior through soil. The pesticide studied was metribuzin, which is used in soybean crops and their environmental appearance is of agricultural interest. Metribuzin (4-amino-6-tert-butyl-3-methylsulfanyl-1,2,4-triazin-5-one) is one of the most used herbicides to control certain broadleaf weeds and grassy weed species. The activity of metribuzin is due to interference with photosystem II electron transport in plant chloroplasts. Almost all of the current reports establish that metribuzin exhibits slow sorption in soil due to the octanol-water coefficient Kow (1.70), which shows relatively high mobility. Consequently, the possible risk of leaching and pollution of ground water with this pesticide is very high and is therefore frequently detected in ground and surface water [44,45].
2. Materials and Methods
2.1. Materials
Analytical grade metribuzin (95%) was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Acetone and methanol gradient grade for liquid chromatography were provided by Merk, Germany. Stock solution (1000 mg L−1) was prepared in acetone, while the working solutions were formulated with ultrapure water. A Milli-Q water purification device was used to purify the water (Millipore, Burlington, MA, USA). The solutions for batch experiments were calcium chloride (CaCl2), which had 0.01 M concentration.
Wheat straw (WS) raw materials were used as the precursor for biochar production. Wheat straw plants were supplied by the Experimental Farm of the Agricultural University of Iasi, Romania. The samples were washed with ultra-pure water cut into small pieces and dried at 70 °C until a constant mass was reached.
2.2. Preparation and Characterization
The wheat straw biomass was transformed into biochar through slow pyrolysis in an electrical furnace at a heating rate of 10 °C/min and held 2 h at 800 °C. Based on preliminary studies, biochar is obtained in an oxygen limited conditions under different temperatures ranging from 200 °C to 800 °C [46,47]. Previous literature indicates that the pyrolysis temperature is one of the key factors determining the physicochemical properties of the biochar [48,49]. Superior temperatures, similar or upper to 500 °C, generate biochar with a high specific surface area, pH and C content and less volatile matter [50]. The pyrolyzed specimens were then removed from the furnace and allowed to cool. To remove residual inorganic matters, the obtained biochar was treated with HCl 0.1 n and then washed with ultra-pure water. The wheat straw biochar samples (WSB) were ground and sieved to obtain particles with a size of 500–700 nm. The chemical composition of WSB and WS were determined as follows. By oven drying at 110 °C, the moisture content was obtained until a constant weight was reached [51]. In the muffle furnace, the ash content was measured by combustion at 750 °C for 8 h, while the volatile matter content was obtained by pyrolysis at 900 °C for 5 min.
The morphological features of wheat straw and biochar were performed via scanning electron microscopy (SEM) (FEI—Field Electron and Ion Company) having an energy dispersive X-ray (EDX) unit. The surface functional groups were identified from FTIR spectra (FTIR spectrometer Interspec 200-X, spectral domain = 400–4000 cm−1, resolution = 4 cm−1, KBr pellet technique).
2.3. Adsorption Experiments
Adsorption experiments were performed in batch systems. In each experiment, 0.2 g biochar and 20 mL metribuzin aqueous solution with the desired initial concentration (5, 50 and 100 mg L−1) were added to a set of 100 mL Erlenmeyer flasks. The experiments were performed in triplicate and the averages values were calculated. The mixture was shacked on an orbital shaker, at 350 rpm, at room temperature. The influence of the contact time was explored at various concentrations (1–100 mgL−1). After shaking, the liquid samples were collected. using PVDF filters at various preset times (10, 20, 60, 80, 240 and 24 h). The removal efficiency and the adsorbed amount q (mg g−1) were evaluated by the following:
where C (mg L−1) and Co (mg L−1) are the residual and initial concentrations of metribuzin. The agri-waste/biochar mass and the volume solution are W (g) and V (L), respectively.
To investigate the adsorption isotherms, the equilibrium experimental data were fitted by Langmuir and Freundlich models as follows:
Langmuir isotherm:
Freundlich isotherm:
where qmax is the maximum adsorption capacity (mg g−1); Ce is the concentration of metribuzin at equilibrium (mg L−1); KL (mg g−1), KF (mg g−1) and n are the Langmuir and Freundlich constants.
qe = KFCe1/n
The kinetic experimental results were obtained in the time range of 0–24 h, and were modeled using pseudo-first order [52] and pseudo-second order [53] models, as follows:
Pseudo-first order:
qt = qe(1-e−k1t)
Pseudo-second order:
where qe (mg g−1) and qt (mg g−1) are the metribuzin adsorbed at equilibrium and time t, respectively; k1 and k2 (mg·g−1·min−1) are the rate parameters of both models.
2.4. Analytical Determination of Metribuzin
All analytical measurements were performed on a Thermo Scientific LC-MS coupled with an Orbitrap Q-Exactive analyzer. The retention time of metribuzin was 5.35 min, using methanol/water (60:40 v/v) as the liquid phase at a flow rate of 1 mL min−1. A pH meter equipped with a combined with glass electrode was used to measure the pH of all the solutions.
3. Results and Discussion
3.1. Biochar Properties
The remediation efficiency of mesoporous carbon materials is hardly affected by its morphological, composition and structural properties [54]. Table 1 states the characteristics of the WS and WSB. The elemental composition of biochar particularly covers carbon, hydrogen, oxygen, nitrogen and sulfur [55,56]. The molar ratios of these elements, expressed as O/C and H/C or (O + N)/C, reveal the aromaticity and polarity of the biochar [57]. During the thermal analysis, three events were noticed [58]. The first process at 120 °C was linked to the release of moisture and adsorbed water from the material surface, while the succeeding event at 550 °C, was related with cellulose and hemicellulose volatilization. At higher temperatures (800 °C) which is the final stage, the lignin decomposition took place. In our study after pyrolysis, the carbon content increased from 47.15% to 73.25%, while the oxygen and hydrogen content decreased from 43.93% to 19.67% and 6.56% to 2.61% respectively.

Table 1.
Elemental composition of WS and WSB.
The O/C and H/C WSB ratios were lower as the WS was raw due to the high carbonization with the development of the extra aromatic and minor hydrophilic WSB structure [59]. The low H/C ratio indicates that the obtained biochar has a high stability, which is a measure of its resistance to microbial and chemical degradation [60,61]. Similarly, a low O/C ratio shows the aromatic ring’s structural arrangement. [62]. Because of the reduced polarity index (O + N)/C, the biochar surface active groups were eliminated, which may enhance metribuzin adsorption [63]. These observations also confirm the results reported by [64] and [65], which sustain that a low O/C ratio denotes a high level of stability of biochar with a half-life higher than 1000 years.
Biochar’s basic components include ash, volatile matter, fixed carbon and moisture percentage [66]. The amount of solid residues left after the sample is completely burned is referred to as the ash content. A high quantity of ash is unsuitable because of the presence of additional minerals, which may obstruct biochar pores and minimize the number of active sites [65]. Based on the low ash content of 5.68% and relatively higher carbon content, the WSB might be suitable for pollutant attraction, Biochar derived from animal manures, for example, contains more ash and less carbon content, making it unsuitable for soil amendment and the removal of toxins and pollutants from the soil and aqueous environment [64].
3.2. Morphology Analysis
The morphologies and structures of WS and WSB were identified by SEM as shown in Figure 1. The raw WS preserves the structural organization of the vegetal cell wall with a lamellar structure on the surface [67]. In opposition, the WSB had a surface with spherical structures, suggesting a carbon-based structure from the raw material biological capillary structures.

Figure 1.
SEM images of (a) raw WS (b) WS biochar.
The volatiles are eliminated during the pyrolysis treatment, which improves the porosity of the WSB and affects its adsorption capacity [68,69,70]. As shown by the studies of [46,71], the stem structure of wheat straw is destroyed and the micropore configuration is progressively created after pyrolysis.
The structural analysis of WS and WSB were established by FTIR spectrometry as presented in Figure 2. Wheat straw biochar shows low intensity bands in regions of 2000–3500 nm−1, which indicate that the material underwent complete pyrolysis with a loss of oxygen-containing species [19].
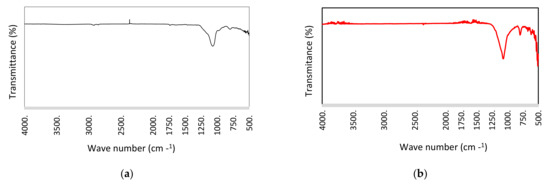
Figure 2.
FTIR spectra of (a) raw WS (b) WS biochar.
The WSB had relatively high intensity bands in regions of 3500–4000 nm−1, 1500–1750 nm−1, 1250–1000 nm−1 and 1000–500 nm−1. Peaks around 1750–1689 nm−1 were attributed to the carbonyl C=O groups, while the peaks at 1850 nm−1 correspond to C–N stretching in aromatic amines [51]. Peaks around 1189 nm−1 and 1127 nm−1 were assigned to C–O and O–C–O stretching vibrations. The bands at 839–729 nm−1 were characteristic of aromatic C–H groups, while the bands at 1543 nm−1 and 1435 nm−1 were representative of the aromatic C–C structure [72]. Being the prevalent groups on the surface of WSB, C–C and C–H gives a hydrophobic configuration with low oxygen-containing functional groups, which maximizes the adsorption mechanism. The carbon content and aromatic structures of biochar manage their rate and sorption capacity [65]. Based on this, the aromatic structures have a strong influence on the biochar’s hydrophobic properties and π–π electron interaction can appear. Triazine herbicides can behave as a π-electron donor, whereas aromatic carbon from the biochar surface can serve as an electron acceptor, indicating that a π–π electron donor–acceptor interaction between metribuzin and WSB surface is feasible [14].
3.3. Adsorption Kinetic Modeling
Any adsorbent unit’s design and optimization are controlled by accurate and relevant kinetic data. The kinetic data were examined to predict the extent of adsorption and to distinguish whether the adsorption process was chemical or physical sorption [63].
The amount of adsorbed metribuzin per unit mass of the biochar and wheat straw (qt) is plotted versus time, which shows the time dependence of metribuzin removal by WS and WSB (Figure 3). Through both cases (pseudo second order and first order model), a fast removal rate is noticed in the early stage, followed by a slower pattern before equilibrium is reached [73].
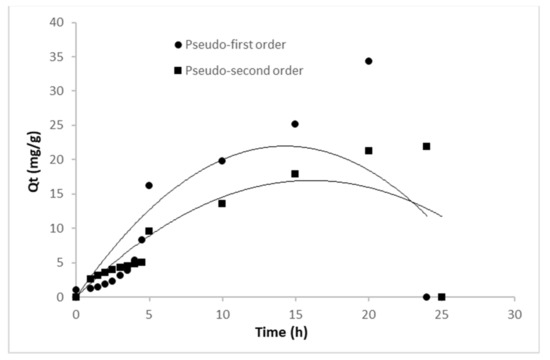
Figure 3.
Kinetic models of metribuzin adsorption.
In terms of determination coefficients—R2, the pseudo second order model was more accurate for fitting the experimental data than the pseudo first order model, according to the parameters obtained (Table 2).

Table 2.
Kinetic model parameters for metribuzin and WS/WSB system.
The pseudo second order model exhibited higher R2 values within the range of 0.996–0.998 compared to the R2 of 0.531–0.726 for the first order model. These observations are also consistent with previous research on biochar mediated metribuzin adsorption [74,75]. According to the parameters of the pseudo second order model, the values indicate adsorption of the chemisorption type. The increase in metribuzin concentration from 5 mg L−1 to 100 mg L−1 enhanced the predicted metribuzin uptake from 1.453 to 1.565 mg g−1. The same increase in metribuzin concentration decreased the K2 values from 0.956 to 0.735. Due to the greater number of metribuzin molecules and fewer actives sites, a decrease in the adsorption rate was noticed. The pseudo second order model is frequently superior to the pseudo first order model in explaining the kinetics of adsorption phenomena and fits better with the experimental data, according to the majority of adsorption studies in the literature [76].
Based on FTIR data, the abundance of biochar functional groups (OH, COO−, R-O−) and the possible adsorption rate of metribuzin were mostly linked to ion exchange or surface complexation of biochar functional groups and metribuzin molecules. Furthermore, the mesoporous structure of wheat straw biochar stimulates metribuzin retention in the pore channel by physicochemical adsorption. According to [77], within advantageous circumstances, physisorption and chemisorption processes may concomitantly or alternatively take place. These results indicate that the adsorption rate depends more on the available active sites in the biochar than on the concentration of metribuzin.
3.4. Adsorption Performance
3.4.1. The Effect of Metribuzin Concentration and Time
We assess the wheat straw biochar for its adsorption properties, using metribuzin pesticide, suggesting a greater affinity between metribuzin and biochar surface. Figure 4 presents the influence of different initial concentrations (Co) of metribuzin on the adsorption performance at equilibrium (qe) of the biochar at room temperature. A reduction in metribuzin concentration was noticed with the increment of time until equilibrium was attained within 3 and 5 h for the initial metribuzin concentrations of 5 mg·L−1 and 100 mg·L−1, respectively. The increase in time required to reach the equilibrium with the increase of initial metribuzin concentration indicates that chemical interactions between pesticide molecules and superficial functional groups of adsorbents are predominantly involved in the studied adsorption process [78].
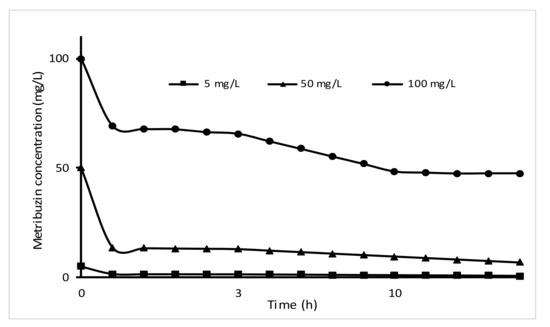
Figure 4.
Influence of initial metribuzin concentration on WSB adsorption.
Overall, metribuzin molecules were adsorbed fast at the initial stage and then slowed down gradually. The initially high adsorption rate in the concentration of metribuzin may be caused by the WSB available binding sites’ existence. On the other hand, as these sites were occupied, a slow metribuzin adsorption rate was observed over time, establishing and consolidating the equilibrium state (Figure 5).
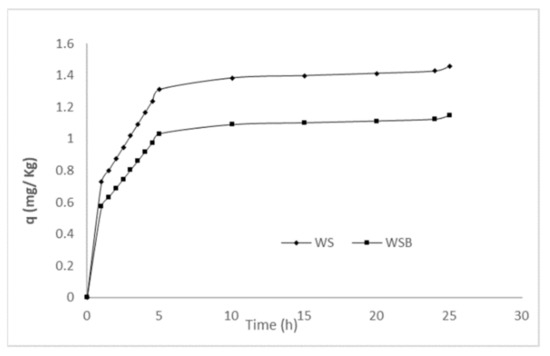
Figure 5.
Adsorption capacity of WS and WSB in batch experiments.
As the initial metribuzin concentration increased from 5 to 50 mg L−1 a decrease with 89 and 86% in the metribuzin adsorption rate was associated. For 100 mg L−1 metribuzin solution, this reduction decreased to 65% due to the high percentage of metribuzin molecules compared to the available active sites from the surface of WSB [59]. At a higher metribuzin concentration, the available superficial groups of WSB are limited and held; hence, the diffusion of metribuzin molecules on the functional groups are limited.
3.4.2. Isotherm Studies
Isotherms are a valuable approach for assessing an adsorbent potential to remove xenobiotic compounds. Adsorption equilibrium data were simulated by current isotherm models, Freundlich and Langmuir, characteristics of which can provide essential knowledge about the adsorption nature and maximum adsorption extent. Experimental equilibrium results matched the Langmuir model better with R2 values (0.994–0.996), which were higher than the Freundlich isotherm model values (0.84–0.97) (Figure 6 and Table 3).
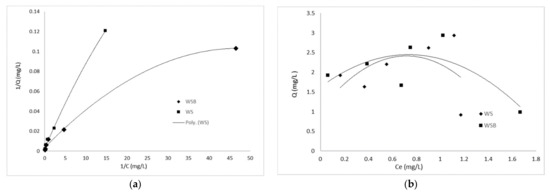
Figure 6.
Langmuir (a) and Freundlich (b) isotherms of metribuzin.

Table 3.
Adsorption isotherm parameters for metribuzin on WS and WSB.
The Langmuir model indicates a single layer chemical adsorption between WS and WSB and metribuzin molecules [79]. Further, the higher value of adsorption equilibrium constant (KL) establishes strong chemical interaction between metribuzin and superficial groups of the WSB. The linearized Langmuir adsorption model is a consequence of two or more adsorption mechanisms, including external surface adsorption, diffusion into the pores of the adsorbent and chemical interactions. According to [80], the increased adsorption capacity of biochars was induced due to a larger surface area with more porous sites produced at high temperatures. A similar isotherm behavior was related for the carbofuran, carbendazim and tebuconazole pesticide adsorption on the activated carbon derived from coconut and peanut shells [3,81].
Table 4 presents the characteristics and reported adsorption capacities of the WSB toward metribuzin with those of other pesticides and crop straw. These results indicate that, when compared to biochars from other agri-wastes, WSB can be a suitable adsorbent material with a high adsorption potential for metribuzin molecules. Reference [14] obtained sustainable soybean biochar with a higher atrazine removal capacity. This property is assigned to the pore volume of biochar and pH. Reference [82] studied the mechanism of imidacloprid, isoproturon and atrazine adsorption by biochar from rice and wheat straw and found a possible interaction between soil components and biochar and/or the consequence of biochar oxidation. Pleurotus mutilus based biochar was used to remove metribuzin from contaminated water, where at an acidic pH, the negatively charged active sites are protonated, thereby restricting metribuzin sorption [83]. Metribuzin sorption kinetics were designed onto electro-activated granular carbon, where the activation process speeds up three times the sorption capacity [84]. Moreover, the adsorption on WSB is advantageous toward chemical oxidation/reduction, bioremediation or the washing with extractants technique, all of which are considered expensive methods and not acceptable in large agricultural fields [85,86].

Table 4.
Comparison of pesticide capacities on various adsorbents.
4. Conclusions
The suitability and effectiveness of the biochar for environmental remediation were assessed in various features. Wheat straw yielded mesoporous carbon rich materials as confirmed by XRF, FTIR, SEM analyses. The results showed that the present biochar has interesting properties with highly reactive surfaces. Due to the modified superficial groups on the biochar surface, the WSB indicate good adsorption performance. The adsorption capacity data indicate that the adsorption was endothermic, while the kinetic parameters suggest that the predominant adsorption process could be chemisorption. Finally, the cumulative experiments indicate the WSB yielded good adsorption capacity, being a promising material for soil remediation. Furthermore, biochar-based wheat is suitable for environmental management applications, as the sorbent shows strong sorption for anthropogenic contamination, including pesticides and heavy metals. These results suggest the achievable and realistic availability of this waste product and its possibility to subscribe to the dynamic economy’s fundamental concepts.
Author Contributions
Conceptualization, I.G.C. and D.T.; formal analysis, M.F.; methodology, I.G.C., L.B. and D.T.; software, L.B. and L.R.; supervision, L.B., L.R. and G.J.; validation, L.B. and G.J.; writing–original draft, I.G.C. and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Competitiveness Operational Programme (COP) 2014–2020. The funding source does not involve study design.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors acknowledge the logistic support from Competitiveness Operational Programme (COP) 2014–2020, under the project number 4/AXA1/1.2.3. G/05.06.2018, SMIS2014+ code 119611, with the title “Establishing and implementing knowledge transfer partnerships between the Institute of Research for Agriculture and Environment-IASI and agricultural economic environment”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khalid, S.; Muhammad, S.; Behzard, M.; Irshad, B.N.; Muhannad, A.N.; Nabeel, K.N. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef] [PubMed]
- Minuț, M.; Roșca, M.; Hlihor, R.-M.; Cozma, P.; Gavrilescu, M. Modelling of Health Risk associated with the intake of Pesticides from Romanian fruits and vegetables. Sustainability 2020, 12, 10035. [Google Scholar] [CrossRef]
- Lee, Y.G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Kim, G.Y.; Lee, C.H.; Chon, K. Enhanced adsorption capacities of fungicides using peanuts shell biochar via successive chemical modification with KMnO4 and KOH. Separations 2021, 8, 52. [Google Scholar] [CrossRef]
- Hamsan, H.; Ho, Y.B.; Zaidon, S.Z.; Hashim, Z.; Saari, N.; Karami, A. Occurrence of commonly used pesticides in personal air samples and their associated health risk among paddy farmers. Sci. Total Environ. 2017, 603, 381–389. [Google Scholar] [CrossRef]
- Lee, K.M.; Park, S.-Y.; Lee, K.; Oh, S.-S.; Ko, S.B. Pesticide metabolites and oxidative stress in male farmers exposed to pesticides. Ann. Occupat. Environ. Med. 2017, 29, 5. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Swelam, E.S.; Mohafrash, S.M. Sub-chronic exposure to npronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015, 2, 775–784. [Google Scholar] [CrossRef]
- Reeves, W.R.; McGuire, M.K.; Stokes, M.; Vicini, J.L. Assessing the safety of pesticides in food: How current regulations protect human health. Adv. Nutr. 2019, 10, 80–88. [Google Scholar] [CrossRef]
- Gautam, K.R.; Goswami, M.; Tech, M.; Mishra, R.K.; Chaturvedi, P.; Awashthi, M.K.; Singh, R.S.; Giri, B.S.; Pandey, A. Biochar for remediation of agrochemicals and synthetic organic dyes from environmental samples: A review. Chemosphere 2021, 272, 129917. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Poll. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S.; Sahni, D.; Sehgal, M.; Srivastava, D.; Singh, A. Pesticide use and its effect on soil bacteria and fungal populations, microbial biomass carbon and enzymatic activity. Curr. Med. Sci. 2019, 116, 643–649. [Google Scholar] [CrossRef]
- Ali, N.; Khan, S.; Yao, H.; Wang, J. Biochar reduced the bioaccessibillity and (bio) uptake of orhanochlorine pesticides and changed the microbial community dynamics in agricultural soils. Chemosphere 2019, 224, 805–815. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Effects of biochars on the earthworm (Wisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci. Total Environ. 2019, 671, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Pi, F.W.; Wang, Y.F.; Xu, H.; Zhang, Y.Z.; Sun, X.L. Photocatalytic degradation of acephate, amethoate, and methyl parathion by Fe3O4 SiO2 TiO2 nanomicrospheres. J. Hazard. Mater. 2016, 315, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Charru, A.B.; Weng, C.H.; Yuan, X.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Cacioli, P.; Bultler, P.; Kyratzis, I.L. Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzym. Microb. Technol. 2014, 54, 38–44. [Google Scholar] [CrossRef]
- Garcia-Jaramillo, M.; Cox, L.; Cornejo, J.; Hermosin, M.C. Effect of soil organic amendments on the behavior of bentazone and tricyclazole. Sci. Total Environ. 2014, 466–467, 906–913. [Google Scholar] [CrossRef]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Kang, S.-W.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–87. [Google Scholar] [CrossRef]
- Ignat, M.; Sacarescu, L.; Fortuna, M.E.; Cool, P.; Harabagiu, V. Effect of synthesis parameters on sorptive properties of glycol-derived mesoporous carbon. Environ. Eng. Manag. J. 2019, 18, 59–69. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolized wood and rice rusks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. J. Sci. Technol. 2015, 45, 5580–5586. [Google Scholar]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm studies of Ni2+ and Pb2+Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability 2021, 13, 3785. [Google Scholar] [CrossRef]
- Drake, J.A.; Carrucan, A.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Biochar application during reforestation alters species present and soil chemistry. Sci. Total Environ. 2015, 514, 359–365. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Yang, S.; Wang, Y. Impact of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. Field Crop. Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent advances in biochar application in agricultural soils: Benefits and environmental implication. Clean Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Van der Velde, M.; Bastos, A.C. A quantitative review of the effect of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Peake, L.R.; Reid, B.J.; Tang, X. Quantifying the influence of biochar on the physical and hydrological properties of dissimilars soils. Geoderma 2014, 235, 182–190. [Google Scholar] [CrossRef]
- Lu, S.G.; Sun, F.f.; Zong, Y.T. Effect of rice husk biochar and coal fly ash on some physical properties of expansive clayey soil (Vertisol). Catena 2014, 114, 37–44. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 84, 687–711. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, A.K. The role of biochar and biochar compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Oguntunde, P.; Fosu, M.; Ajayi, A.; Giesen, N. Effect of charcoal production in maize yield, chemical properties and texture of soil. Biol. Fertil. Soils 2004, 39, 295–299. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Jien, S.H.; Chen, W.C.; Ok, Y.S.; Awad, Y.M.; Liao, C.S. Short-term biochar application induced variations in C and N mineralization in compost-amended tropical soil. Environ. Sci. Poll. Res. 2017, 25, 25715–25725. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyvaluoma, J. How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenerg. 2018, 119, 346–353. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 1–18. [Google Scholar] [CrossRef]
- Nemeth, J.; Sebestyen, V.; Juzsakova, T.; Cretescu, I.; Domokos, E.; Redey, A. Study of the glyphosate-amine pesticide mineralization in wastewater by ozonation treatment. Environ. Eng. Manag. J. 2019, 9, 1867–1873. [Google Scholar] [CrossRef]
- Singh, R.; Nidheesh, P.V.; Sivasankar, T. Integrating ultrasound with activated carbon prepared from mangosteen fruit peel for reactive black 5 removal. Environ. Eng. Manag. J. 2019, 18, 2335–2342. [Google Scholar]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Remediation of Lead-contamined water by virgin coniferous wood biochar adsorbent: Batch and column application. Water Air Soil Pollut. 2020, 231, 171. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore water concentrations of polycyclic aromatic hydrocarbons (pahs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Subratti, A.; Vidal, J.L.; Lalgee, L.J.; Kerton, F.M.; Jalsa, N.K. Preparation and characteriazation of biochar derived from the fruit seed of Cedrela odorata L and evaluation of its adsorption capacity with methylene blue. Sustain. Chem. Pharm. 2021, 21, 100421. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameeda, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixedbed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameeda, B.H. Insight into co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Wang, D.; Mukome, F.N.; Yan, D.; Wang, H.; Scow, K.M.; Parikh, S.J. Phenylurea herbicide sorption to biochars and agricultural soils. J. Environ. Sci. Health. Part B 2015, 50, 544–551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soil on sorption, desorption and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Yang, Y.; Huang, M.; Yang, K. Influence of pH on pesticide sorption by soil containing wheat residues-derived char. Environ. Pollut. 2005, 134, 457–463. [Google Scholar] [CrossRef]
- Lopez-Pineiro, A.; Pena, D.; Albarran, A.; Becerra, D.; Sanchez-Llerena, J. Sorption, leaching and persistence of metribuzin in Mediterranean soil amended with olive mill waste of different degrees of organic matter maturity. J. Environ. Manag. 2013, 122, 76–84. [Google Scholar] [CrossRef]
- Borja-Urzola, A.; Garcia-Gomez, R.S.; Bernal-Gonzales, M.; Duran-Dominguez-de-Bazua, M. Chitosan-calcite from shrimp residues: A low cost adsorbent for three triazines removal from aqueous media. Mater. Today Comunn. 2021, 26, 102131. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and suitable adsorbent—Critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Yi, S.; Gao, B.; Sun, Y.; Wu, J.; Shi, X.; Wu, B.; Hu, X. Removal of levofloxacin from aqueous solution using rice husk and wood chip biochars. Chemosphere 2016, 150, 694–701. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y. Effects of feedstock type, production methods and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.W.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Siedt, M.; Schaffer, A.; Smith, K.E.C.; Nabel, M.; Rob-Nickoll, M.; van Dongen, J.T. Comparing straw, compost and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Guo, W.; Hua, S.; Feng, J.; Lu, X. Adsorption of perfluorooctane sulfonate (PFOS) on corn straw derived biochar prepared at different pyrolitic temperatures. J. Taiwan Instit. Chem. Eng. 2017, 78, 265–271. [Google Scholar] [CrossRef]
- Lagergren, S. Lagergren Zur theorie der sogenannten adsorption gelöster stroffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Khanday, W.A.; Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. Single step pyrolysis of phosphoric acid activated chitin for efficient adsorption of cephalexin antibiotic. Bioresour. Technol. 2019, 280, 255–259. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.-S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Han, Q.; Yang, Y.; Wang, R.; Zhang, K.; Liu, N.; Hong, M. Biochar derived from agricultural wastes as a means of facilitating the degradation of azo dyes by sulfides. Catalysts 2021, 11, 434. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Galvis, E.A.S.; Porras, J.; Florez, E.; Torres-Palma, R.A.; Acelas, N. Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 2021, 326, 124753. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments of biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Nufida, B.A.; Ismail, N.M.; Zakaria, Z.Y.; Jagaba, A.H.; Ghaleb, A.A.S.; Al-Dhawi, B.N.S. Modeling and optimization of biochar based adsorbent derived from Kenaf using response surface methodology on adsorption of Cd2+. Water 2021, 13, 999. [Google Scholar] [CrossRef]
- Manya, J.J.; Ortigosa, M.A.; Laguarta, S.; Manso, J.A. Experimetal study on the effect of pyrolysis pressure, peak temperature, and particle size on the potential stability of vine shoots-derived biochar. Fuel 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameeda, B.H. Adsorption behaviour of salicylic acid on biochar as derived from the thermal pyrolysis of barley straw. J. Clean. Prod. 2018, 195, 1162–1169. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameeda, B.H. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Biores. Tech. 2019, 278, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature. A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Moslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy metals ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Kim, J.E.; Song, H.J.; Oh, K.B.; Jo, J.W.; Yang, Y.-H.; Lee, S.H.; Kang, G.; Kim, H.J.; Choi, Y.K. Assessment of adsorptive behaviors and properties of grape pomace-derived biochar as adsorbent for removal of cymoxanil pesticide. Environ. Technol. Innov. 2021, 21, 101242. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef]
- Li, Q.; Yu, W.; Guo, L.; Wang, Y.; Zhao, S.; Zhou, L.; Jiang, X. Sorption of Sulfamethoxazole on Inorganic acid solution etched Biochar derived from Alfalfa. Materials 2021, 14, 1033. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.-H.; Wang, X.-M. Adsorption behaviour and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; Gonzalez-Delgado, A.; Herrera, A.; De la Voz, A.V. Efficient sulfate on Modified Adsorbents prepared from Zea Mays Stems. Appl. Sci. 2021, 11, 1596. [Google Scholar] [CrossRef]
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar and vermicompost. J. Environ. Sci. Health Part B Pest Food Contamin. Agric. Waste 2019, 54, 728–735. [Google Scholar] [CrossRef]
- Bettayeb, A.; Reguig, B.A.; Mouchaal, Y.; Yahiaoui, A.; Chehimi, M.M.; Berredjem, Y. Adsorption of metribuzin herbicide on raw maghnite and acid treated maghnite in aqueous solutions. Desalin. Water Treat. 2019, 145, 262–272. [Google Scholar] [CrossRef]
- Salimi, M.; Salehi, Z.; Heidari, H.; Vahabzadeh, F. Production of activated biochar from Luffa cylindrica and its application for adsorption of 4-Nitrophenol. J. Environ. Chem. Eng. 2021, 9, 105403. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Singh, N. Biocompost from sugar distillery effluent: Effect on metribuzin degradation, sorption and mobility. Pest. Manag. Sci. 2008, 46, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Suo, F.; Liu, X.; Li, C.; Yuan, M.; Zhang, B.; Wang, J.; Ma, Y.; Lai, Z.; Mingshan, J. Mesoporous activated carbon from starch for superior rapid pesticides removal. Int. J. Biol. Macromol. 2019, 121, 806–813. [Google Scholar] [CrossRef]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of tetracycline and sulfamethoxazole on crop residues derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Njoku, V.O.; Azharul Islam, M.; Asif, M.; Hameed, B.H. Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. J. Anal. Appl. Pyrol. 2014, 110, 172–180. [Google Scholar] [CrossRef]
- Jin, J.; Kang, M.; Sun, K.; Pan, Z.; Wu, F.; Xing, B. Properties of biochar amended soils and their sorption of imidacloprid, isoproturon and atrazine. Sci. Total Environ. 2016, 550, 504–513. [Google Scholar] [CrossRef]
- Ighalo, J.; Adeniyi, A.G.; Adelodun, A. Recent advances on the adsorption of herbicides and pesticides from plluted waters: Performance evaluation via physical attributes. J. Ind. Eng. Chem. 2021, 93, 117–137. [Google Scholar] [CrossRef]
- Kitous, O.; Abdi, H.; Lounici, H.; Grib, H.; Drouiche, N.; Benyoussef, E.; Mameri, N. Modeling of the adsorption of metribuzin pesticide onto electro-activated granular carbon. Desalin. Water Treat. 2016, 57, 1865. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.L.; Liu, W.T.; Zhou, Q.X. Biochar: An effective amedment for remediating contaminated soil. Rev. Environ. Contam. Toxicol. 2014, 228, 83–99. [Google Scholar] [PubMed]
- Zhao, K.; Ouyang, W.; Hao, F.; Lin, C.; Wang, F.; Han, S.; Geng, X. Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour. Technol. 2013, 147, 338–344. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).