Abstract
Due to the increasing risk of chemical contaminations in the application of synthetic fungicides, the use of plant essential oils and extracts has recently been increased. In the present review, the antimicrobial potential of the most active plant-food essential oils in liquid and vapor phases has been reviewed. The volatile isothiocyanates, aldehydes, and phenols, including allyl isothiocyanate, carvacrol, thymol, and eugenol, are considered to be the predominant components of essential oils, possessing significant antimicrobial activities. These components alone or in mixture can be effective. Overall, the antimicrobial activity of aroma compounds depends on the plant species, concentration, and method of application. This review provides useful information about the inhibitory application of the most common plant-foods’ essential oils in liquid and vapor phases against the growth of pathogenic microorganisms. Essential oils (EOs) are promising natural antimicrobial alternatives in food processing facilities. Although the food industry primarily uses spices and herbs to impart flavor, aroma, and pungency to foods, potent EOs represent interesting sources of natural products for food preservation.
1. Introduction
Medicinal plants are widely consumed in cuisine as foods, additives, spices, and herbal teas. Some of them, including mint, thyme, cinnamon, lavender, and savory are also used in the pharmaceutical industry due to their helpful bioactivities, such as antibacterial, antifungal, and fever- and pain-relief properties. The abovementioned plants are considered aromatic plants and are widely applied in the food industry, for instance, as spices to improve the taste of foods [1]. Essential oils (EOs) are volatile compounds extracted from plants and may have antibacterial and antifungal properties [2]. Many of the used EOs are generally recognized as safe (GRAS) by the United States Food and Drug Administration (FDA) to consume in the food industry [3]. The emergence of antibiotic-resistant microbial organisms, the spreading of foodborne diseases, and consumers’ negative attitudes toward chemical preservatives have led to widespread efforts to find natural food additives. Along with processing technologies, antimicrobial and antioxidant activities can show stability and improve the quality and storage time of perishable food [4]. The continuous application of synthetic fungicides causes resistance in different fungal strains, while resistance to plant EOs will occur more slowly due to the different combination of compounds in EOs [5]. The synergistic effect of two or more natural compounds leads to an increase in antimicrobial potential, since the pathogens cannot easily resist all essential oil constituents [6]. The composition of EOs should be considered in their use as preservatives, because the diversity of chemical compounds may affect their biological activities. In many cases, the main constituents of EOs show bioactivity, while small amounts of EOs play an important and vital role in improving their bioactivity [7]. The type of compounds in the essential oil is one of the things that should be considered in their use as a preservative, because the variability of the chemical composition may biologically affect them. Moreover, it is very difficult to detect the exacerbating effects of some bioactive components on each other because their amounts of EOs vary. In recent decades, numerous studies have investigated the chemical composition of EOs and plant extracts which possess antimicrobial potency [8,9,10,11,12,13] (Table S1). The susceptibility of different microbial species also differs correlating to the plant’s type of EO and its different concentrations [14]. Previous studies have demonstrated that EO constituents are good microbial agents, where the microbial potency decreases in the following order: phenols > aldehydes > ketones > alcohols > esters > hydrocarbons [13]. In the present study, we comprehensively reviewed the chemical composition and antimicrobial potential of EOs by searching the keyword “chemical composition and antimicrobial” in the PubMed and Web of Science databases (last search: 25 September 2020).
2. Major Essential Oil Components
Terpenes, comprising over 7000 derivatives, are considered to be major EOs compounds and are divided into several groups. Despite their wide chemical diversities, based on their hydrocarbon structure they are classified into two major groups: terpenoids and phenylpropanoids. Both groups contain phenolic compounds, which in most cases constitute the main composition of most EOs [15]. Terpenoids are divided into alcohol (e.g., geraniol, menthol, linalool, citronellol), ester, aldehyde (e.g., geranial, neral), ketone (e.g., carvone, camphor), and phenol (e.g., thymol, carvacrol) derivatives. Terpenes possess a hydrocarbon skeleton, which is classified by the number of isoprene units (C5H8), including monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20). Moreover, the major phenylpropanoids include cinnamaldehyde, cinnamyl alcohol, chavicol, eugenol, estragole, methyl eugenol, and methyl cinnamate [16,17]. In Table 1, the major volatile terpenoid compounds which are potent against fungi/bacteria pathogens are mentioned. Figure 1 exhibits the main chemical structures of the essential oil constituents.

Table 1.
Major terpene constituents of essential oils documented in the literature.
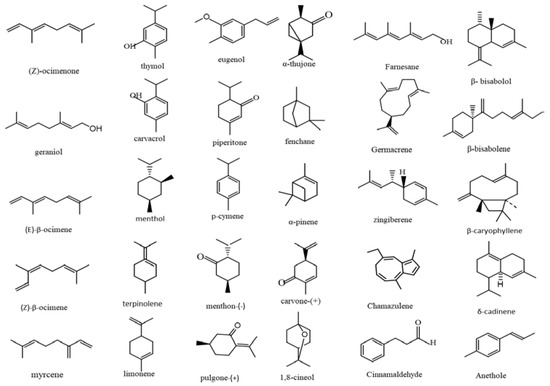
Figure 1.
Chemical structures of the main essential oil constituents.
2.1. Monoterpenes
Monoterpenes possessing 10 carbons in their structures (C10H16) are divided into three main groups, including acyclic (linear), monocyclic, and bicyclic. The most linear and mono-cyclic monoterpene structures are based on 10-carbon and para-methane structures, respectively. Bicyclic monoterpenes are very structurally diverse, mainly comprising caranes, thujanes, pinenes, camphanes, and fenchanes. Further variability can be gained by adding double bonds (oxidation) or removing them (reduction), and also by combining with oxygen in the forms of alcohol (‒OH), ketone (‒CO), aldehyde (‒CHO), and ester (‒OCO) compounds [18].
2.2. Sesquiterpenes
Sesquiterpenes have a 15-carbon skeleton (C15H24). Sesquiterpene alcohols, known as farnesols, are divided into three main groups, including linear (e.g., farnesane (E,E)), mono-cyclic (e.g., β-bisabolene, cyclofarnesane), and polycyclic (e.g., caryophyllene, humulane) derivatives [19].
2.3. Diterpenes
One of the most important terpenoid compounds is composed of four isoprene units. These terpenoids contain 20 carbons in their skeleton (C20H32). Its component is geranylgeranyl diphosphate, which is formed by the binding of an IPP (isopentenyl pyrophosphate) group to farnesyl diphosphate through a head-to-tail reaction [20].
3. The Assessment Methods of Essential Oils’ Antimicrobial Potency
The antimicrobial activity of essential oils has been previously evaluated by disc diffusion and microbroth dilution assays.
3.1. Disk-Diffusion Agar Method
This method can be designed by applying paper disks, which are placed on agar media or through wells created in agar media. The effect of essential oil on the desired microorganism is mainly evaluated through the halo zones created around the disk. This method has some drawbacks, including the volatility of the compounds during heating and the lack of the proper release of compounds with antimicrobial properties in the agar media [21].
3.2. Microbroth Dilution Method
This method has been considered as one of the most common assays for testing the antimicrobial potency of various materials, quantitatively as well. The MIC value is determined through the study of apparent growth, light density or opacity, colorimetric methods, and microorganism counting [22].
3.3. Vapor Method (Essential Oil Application in Vapor Phase)
In diffusion assays, EO components are partitioned through the agar according to their affinity with water, whilst in dilution methods a low water solubility has to be overcome by the addition of emulsifiers or solvents (such as Tween 80, DMSO, ethanol), which may alter the activity [23]. The EOs components and their relative volatilities determine the characteristics of their vapors, which in turn has an impact on the antimicrobial potential [21]. The EO in vapor phase could highly be effective against food-borne pathogens and spoilage bacteria at relatively lower concentrations than in the liquid phase, thereby causing a minimum effect on the organoleptic properties [10], though it should be noted that EO consumption in high concentrations should not affect the taste.
EO application in vapor form at low concentrations is more effective than in the liquid phase, therefore it has no unpleasant impact on the quality characteristics and taste [24]. The antimicrobial properties of EOs are usually reported in high concentrations of these compounds, which change the taste and smell of food and affect its quality properties; to reduce this impact, vapor is an alternative to the direct use of these compounds [25]. The distance application of EOs in gas form may control pathogens without having any negative influence on food quality [26]. The EO application of thyme (Thymus vulgaris), cinnamon (Cinnamomum verum), and lavender (Lavandula pubescens) in vapor phase demonstrated a higher inhibitory effect than liquid, explaining that the diminished activity of the EO in liquid media was due to the presence of detergents and lipophilic materials [27].
4. Antimicrobial Activities of Plant-Food EOs in Liquid and Vapor Phases
4.1. Antifungal Activities of EOs in Liquid and Vapor Phases
The antimicrobial power of EOs describes with parameters such as the minimum inhibitory concentration (MIC) and the minimum fungal concentration (MFC) [21] (Table S1). Plant EOs antifungal properties are correlated with their constituents. Lemongrass (Cymbopogon martinii) EO with 88% carbonyl compounds, such as geraniol (48.14%) and neral (38.32%), compared to Citrus aurantifolia and Citrus limon EOs, with 91% and 79% monoterpene hydrocarbons and possessing a much lower content of carbonyl groups, which showed more fungicidal properties against Botrytis cinerea [28,29].
Citrus sinensis EO indicated an MIC value of 1600 mg/L in direct application and 800 mg/L in vapor application against Aspergillus flavus, and the main fragrant compounds were identified as limonene, β-myrcene, α-, β-pinene, as well as (Z)-, (E)-citral. Of these, limonene represented 96.62% [10,30]. Aguilar-González et al. (2015) reported the MIC of Syzygium aromaticum EO with a 75.39% eugenol content as 92.56 μL/Lair, while for Brassica nigra EO with 98% allyl isothiocyanate it was 15.42 μL/Lair against Botrytis cinerea [31]. Mejia-Garibay et al. (2015) reported that 41.1 μL/Lair of Brassica nigra EO with 98.88 allyl isothiocyanate delayed the growth of Penicillum citrinum, Aspergillus ochraceus, and Aspergillus niger [32]. Another study reported the positive effect of Origanum dictamnus EO in preventing Botrytis cinerea growth, possessing an MIC of 100 μL/L [33]. The high sensitivity of fungal species to oregano (Lippia berlandieri), thyme (Thymus vulgaris), or mustard (Brassica nigra) EOs with an MIC of 0.012–1.0 μg/mL of air has been reported. The major volatile compounds are allyl isothiocyanate in mustard EO; p-cymene, linalool, and thymol in thyme EO; and p-cymene and carvacrol in oregano EO [34].
Raeisi et al. (2019) also showed that the antimicrobial activity of Mentha piperita and Zataria multiflora EOs is correlated with the presence of monoterpenes such as menthol (39.18%), menthone (21.64%), carvacrol (36.81%), and thymol (33.04%) [35]. The amount of monoterpene hydrocarbons in Mentha piperita EO in the vapor phase was analyzed as 62.8%, three-fold that of the liquid phase. The MIC and MFC values varied from 1.13 to 2.25 mg/mL and 2.25 to 4.5 mg/mL for Penicillium digitatum, Aspergillus flavus, Aspergillus niger, Mucor spp., and Fusarium oxysporum. The rapid solubility of these compounds in the cell membrane in the gaseous phase is due to the strong antimicrobial properties of this EO [25].
The antifungal effect of Satureja bachtiarica, S. khuzistanica, and S. rechingeri EOs was studied by Saharkhiz et al. (2016), with an MIC 0.062 μL/mL, where the main chemical constituents of EOs are identified as monoterpenoid derivatives in the majority of thymol (87.7%) and carvacrol (28%) [36]. In a similar study, the potent antifungal properties of EOs in three Satureja species (S. hortensis, S. spicigera, and S. khuzistanica) were reported in the control of fungi microorganisms Botrytis cinerea, while high concentrations of phenolic monoterpenes, including carvacrol and thymol, have been documented in those EOs. Considering that these components are produced by the metabolic pathway of the auto-oxidation of γ-terpinene and p-cymene, they are assumed as precursors of oxygenated derivatives. In this study, the MIC values of Satureja species EOs have been measured as between 1200 and 600 μL/L against Botrytis cinerea [11].
Gamma-terpinene and cumin aldehyde are reported as the predominant constituents of Cuminum cyminum EO. The fungicidal properties of C. cyminum are mostly associated with volatile phenolics such as cuminaldehyde, p-cymene, β-pinene, and α-terpinene [37]. Eucalyptus globulus EO, mainly containing the potent ingredients of 1,8-cineole, limonene, p-cymene, terpinene, α-pinene, and α-terpineol, shows a higher antifungal activity in vapor phase compared to direct application on spoilage microorganisms. The MIC amounts varied from 2.25 to 9 mg/mL for fungal strains and from 1.13 to 2.25 mg/mL for yeast strains [25].
4.2. Antibacterial Activities of EOs in Liquid and Vapor Phases
Many studies have shown that EOs possess antibacterial properties against a wide range of bacterial strains [35,36,37] (Table S1). The D-values on exposure to Mentha piperita EO were analyzed as 2.14 and 2.8 min and 1.4 and 12.8 min, against Escherichia coli and Staphylococcus aureus, respectively; in this study, the antimicrobial potency of M. piperita EO was correlated with its major constituents, such as α-terpinene (19.7%), isomenthone (10.3%), piperitone oxide (19.3%), and β-caryophyllene (7.6%). In a study carried out by Yadegarinia et al. (2006) on Origanum vulgare EO, among 22 identified compounds pulegone (31.54%), 1,8-cineole (15.89%), menthofuran (11.8%), and cis-isopulegone (9.74%) were the major constituents, and the MIC values against Staphylococcus aureus was ranged from 75 to 1200 µg/mL.
In an investigation of Lavandula tenuisecta EO, 38 components (88.6%) were characterized, where the major constituents were identified as camphor (26.9%), fenchone (22.7%), and 1,8-cineole (18.1%); the EO was effective against almost all studied bacteria, including Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Enterobacter cloacae, with MIC and MBC values ranging from 6.25 to 50 µg/mL [38]. In a study carried out on Tanacetum walteri EO, thymol (22.5%), 1,8-cineole (8.2%), umbellulone (6.9%), α-bisabolol (6.3%), and camphor (5.3%) were characterized as the principal constituents; the highest antimicrobial activity of the EO was observed against Staphylococcus aureus, Enterococcus faecalis, and Klebsiella pneumoniae with MIC values of 0.63 mg/mL [39].
Hajlaoui et al. (2010) showed that the EO of Cuminum cyminum mostly contains cuminaldehyde (39.48%), γ-terpinene (15.21%), o-cymene (11.82%), β-pinene (11.13%), 2-caren-10-al (7.93%), trans-carveol (4.49%), and myrtenal (3.5%), indicating MIC values of 0.078 mg/mL for Gram-positive bacteria and from 0.078 to 0.15 mg/mL for Gram-negative bacteria [8]. In one study, the major constituents of fennel (Foeniculum vulgare) EO were identified as trans-anethole (63.16%), l-fenchone (15.53%), estragole (6.43%), limonene (4.69%), and α-pinene (4.33%). In this experiment, the MIC amounts were determined by the microbroth dilution method and were in the range of 50–100 µL/mL, depending on the studied bacteria and the tested yeast C. albicans [40]. The antibacterial activities of Satureja species (S. hortensis, S. spicigera, and S. khuzistanica) EOs against Gram-positive and Gram-negative bacteria was studied by Saharkhiz et al. (2016), and the MIC value was determined to be 0.031 and 0.062 μL/mL, respectively [36]. The antibacterial effects of Mentha piperita EOs against Staphylococcus aureus and Escherichia coli microorganisms were in agreement with those of linalool, carvone, and p-menthan-1-ol contents, while the MIC value was analyzed as 0.1% [41].
The stronger activity of cinnamon (Cinnamomum verum) EO on Escherichia coli and Staphylococcus aureus may be due to cinnamaldehyde, the major identified constituent in its EO (92.40%). The MIC value was similar for both bacteria (1.0 mg/mL), while the MBC values were found to be 4.0 and 2.0 mg/mL against E. coli and Staphylococcus aureus, respectively [42]. Other research revealed that the EOs of garlic (Allium sativum), onion (Allium cepa), and cinnamon (Cinnamomum cassia) show an effective activity against Listeria monocytogenes. Sulfides (80%) were the most abundant compounds present in onion and garlic essential oils, while cinnamaldehyde (76.54%) is rich in cinnamon EO. In this study, the MIC values were 0.025 mg/mL for onion EO and 0.100 mg/mL for cinnamon and garlic [43].
5. EOs Mechanism of Action in Inhibiting Growth of Microorganisms
Thus far, many studies have been conducted to investigate the mechanisms of EOs in the growth inhibition of microorganisms. Since terpenes have been recognized as primary antimicrobial agents, a practical mechanism of these compounds has been attributed to the EOs [21]. The high activity of terpenes, such as thymol, carvacrol, and eugenol, is most probably correlated to their phenolic structures. The action mode of phenolic compounds is usually in the form of interference with the action of the cytoplasmic membrane, including protein stimulation and active transportation [17]. The EO inhibition capacity can be attributed to the presence of an aromatic ring attached to the polar group [44]. The widespread use of phenols and related compounds as disinfectants can be considered as evidence. For example, due to the hydroxy phenolic group in the structure of thymol, hydrogen bonding can easily be performed with active sites of enzymes; consequently, a high activity is dedicated to this substance. Borneol and geraniol have a lower inhibitory effect compared to thymol due to the lack of an aromatic ring [21].
Cinnamon (Cinnamomum verum) essential oil, possessing a high content of cinnamaldehyde, disrupts the function of proteins and inhibits the amino decarboxylase enzyme, thus the lipophilic part of the EO combines directly with the hydrophilic parts of the protein [42]. In the case of volatile aldehyde constituents, the growth inhibition of microorganisms is mainly due to the reactions of aldehydes with sulfhydryl groups that are effective in fungal growth, and in other cases sulfhydryl compounds are not effective in inhibiting aldehydes [21]. By calculating the molecular orbital energy of aldehydes, it was found that the antifungal activity of these compounds is related to the energy of the lowest molecular orbital, which is empty, thus the lower energy level leads to a higher antifungal activity [21]. These results indicated that the antifungal actions and activities of aldehydes, in addition to their reaction with the sulfhydryl group, are probably due to their ability to form charge transfer complexes with electron donors [45]. The examination of the fungicidal mechanism of Melaleuca alternifolia EOs showed that it disrupts the mitochondrial and tricarboxylic acid cycles, therefore, accumulating reactive oxygen species and disrupting the Botrytis cinerea fungal activity [46].
Lipids are one of the most important components in cell membranes and responsible for membrane stability and the regulation of membrane permeability to water-soluble substances [3]. Prashar et al. (2003) reported that the fat structures of cells are broken down by terpenes, since fat saturation is increased [47]. The destruction of the Shigella dysenteriae cell membrane by Foeniculum vulgare EO was confirmed [48].
Due to the hydrophobic properties of EOs, they are dispersed in the lipid part of the mitochondrial and cell membrane of microorganisms; subsequently to the increase in permeability, they lead to a leak of intracellular materials and the disruption of many biological activities [49]. Moreover, Prashar et al. (2003) believes that the lipid structures of cells are broken down by terpenes via their increasing saturation [47]. Damage to the cell wall and membrane can lead to macromolecular leakage and cell deterioration. Gram-positive bacteria such as Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus are more sensitive to EOs than Gram-negative bacteria such as Escherichia coli and Salmonella enteritidis [50].
In general, it is supposed that EOs possess more antimicrobial activity against Gram-positive bacteria due to the direct interaction of water-repellent chemical components with their cell membrane [51]. Gram-negative bacteria are more resistant to EOs due to their hydrophilic cell walls. This membrane prevents the penetration of hydrophobic compounds (Figure 2) [52]. Meanwhile, one study found that, after cinnamon (Cinnamomum cassia) treatment, the metabolic activity of Listeria monocytogenes was reduced by 60.26% and the secretion of extracellular polysaccharides and proteins was significantly inhibited [53]. The gaseous Citrus limon targets the Listeria monocytogenes cell wall and plasma membrane [54].
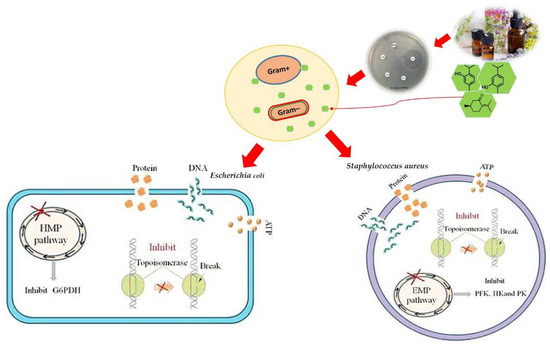
Figure 2.
Mechanism of action of essential oils on Gram-positive and Gram-negative bacteria.
The EOs are able to penetrate and disrupt the cell wall and cytoplasmic membrane of the fungus and ultimately damage the mitochondrial membrane [21]. It has been reported that carvacrol reacts with cell membranes by altering the permeability of H+/K+ channels; thus, changing an ion gradient leads to the cessation and disruption of basic cell functions [55]. Several studies have studied the effect of EOs on fungal strains. The enhancing of nucleic acid leakage by increasing the concentration of tea tree and lemongrass EOs has been reported against Botrytis cinerea [3,28]. Kalemba and Kunicka (2005) attributed the morphological changes when eugenol and carvacrol were used against Cladosporium to the action of these compounds on several cell wall enzymes, such as chitinases [56]. Furthermore, Soylu et al. (2006) attributed the morphological changes in the mycelium to a defect in the cell wall followed by plasma membrane permeability [57].
6. Conclusions and Prospective
The inhibitory activity of EOs against microorganism growth in vapor phase is mainly higher than in liquid form and highly attributed to their major constituent and strain types. Different values of MIC, MFC, and MBC are also correlated to the different assays used for the evaluation of their antimicrobial activity. In general, EOs indicate more antibacterial activity against Gram-positive bacteria due to possessing similar membrane structures (hydrophobic) and permeability effects. Regarding the performed antimicrobial investigations on EOs derived from medicinal plants, specifically agents that are promising in vapor phase, more studies should be performed to explore safe and potent volatile natural candidates as alternatives for synthetic chemicals in the food processing industry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/22/8103/s1: Table S1: Antimicrobial activities of essential oils of the main functional foods in both liquid and gas phases. References [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87] are cited in the supplementary materials.
Author Contributions
Conceptualization, A.A.; methodology, J.M., F.J.-K., K.S.; investigation, A.A.; writing—original draft preparation, A.A., F.J.-K., K.S.; review and editing, J.M., S.V.; supervision, J.M., M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; De Martino, L.; Melone, A.; De Feo, V.; Coppola, R.; Nazzaro, F. Preservation of chicken breast meat treated with thyme and balm essential oils. J. Food Sci. 2010, 75, M528–M535. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavour Fragr. J. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Mighri, H.; Noumi, E.; Snoussi, M.; Trabelsi, N.; Ksouri, R.; Bakhrouf, A. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem. Toxicol. 2010, 48, 2186–2192. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Velázquez-Nuñez, M.J.; Avila-Sosa, R.; Palou, E.; López-Malo, A. Antifungal activity of orange (Citrus sinensis var. Valencia) peel essential oil applied by direct addition or vapor contact. Food Control 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Rustaie, A.; Keshvari, R.; Samadi, N.; Khalighi-Sigaroodi, F.; Ardekani, M.R.S.; Khanavi, M. Essential oil composition and antimicrobial activity of the oil and extracts of Bunium Persicum (Boiss.) B. Fedtsch.: Wild and cultivated fruits. Pharm. Sci. 2016, 22, 296–301. [Google Scholar] [CrossRef]
- de Lira Mota, K.; de Oliveira Pereira, F.; de Oliveira, W.; Lima, I.; de Oliveira Lima, E. Antifungal Activity of Thymus vulgaris L. Essential Oil and Its Constituent Phytochemicals against Rhizopus oryzae: Interaction with Ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef] [PubMed]
- Plotto, A.; Roberts, D.D.; Roberts, R.G. Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture 628, Toronto, Canada, 11–17 August 2002; pp. 737–745. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Dehsheikh, A.B.; Sourestani, M.M.; Dehsheikh, P.B.; Mottaghipisheh, J.; Vitalini, S.; Iriti, M. Monoterpenes: Essential Oil Components with Valuable Features. Mini Rev. Med. Chem. 2020, 20, 958–974. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. Annu. Plant Rev. Online 2018, 258–303. [Google Scholar]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2002, 19, 125–132. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Bacigalupo, R.; Korsten, L. Combined application of antagonist Bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Prot. 2010, 29, 369–377. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against candida albicans: Microscopic observations and chemical characterization of cymbopogon citratus. BMC Complement. Altern. Med. 2010, 10, 65. [Google Scholar] [CrossRef]
- Inouye, S. Laboratory evaluation of gaseous essential oils (Part 1). Int. J. Aromather. 2003, 13, 95–107. [Google Scholar] [CrossRef]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botrytis cinerea on ‘Golden Delicious’, ‘Pink Lady’and ‘Granny Smith’ apples. J. Plant Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Inouye, S.; Uchida, K.; Maruyama, N.; Yamaguchi, H.; Abe, S. A Novel Method to Estimate the Contribution of the Vapor Activity of Essential Oils in Agar Diffusion Assay. Nippon Ishinkin Gakkai Zasshi 2006, 47, 91–98. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Mejia-Garibay, B.; Palou, E.; Lopez-Malo, A. Composition, diffusion, and antifungal activity of black mustard (Brassica nigra) essential oil when applied by direct addition or vapor phase contact. J. Food Prot. 2015, 78, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, A.; Loulakakis, K.; Magan, N.; Tzortzakis, N. Origanum dictamnus oil vapour suppresses the development of grey mould in eggplant fruit in vitro. Biomed Res. Int. 2014, 2014, 562679. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Raeisi, M.; Ghorbani Bidkorpeh, F.; Hashemi, M.; Tepe, B.; Moghaddam, Z.; Aman Mohammadi, M.; Noori, S.M.A. Chemical composition and antibacterial and antioxidant properties of essential oils of Zataria multiflora, Artemisia deracunculus and Mentha piperita. Med. Lab. J. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Zomorodian, K.; Taban, A.; Pakshir, K.; Heshmati, K.; Rahimi, M.J. Chemical Composition and Antimicrobial Activities of Three Satureja Species Against Food-borne Pathogens. J. Essent. Oil-Bearing Plants 2016, 19, 1984–1992. [Google Scholar] [CrossRef]
- Sekine, T.; Sugano, M.; Majid, A.; Fujii, Y. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J. Chem. Ecol. 2007, 33, 2123–2132. [Google Scholar] [CrossRef]
- Sayout, A.; Ouarhach, A.; Dilagui, I.; Soraa, N.; Romane, A. Antibacterial activity and chemical composition of essential oil from Lavandula tenuisecta Coss. ex Ball. an endemic species from Morocco. Eur. J. Integr. Med. 2020, 33, 101017. [Google Scholar] [CrossRef]
- Ghaderi, A.; Sonboli, A. Chemical composition and antimicrobial activity of the essential oil of Tanacetum walteri (Anthemideae-Asteraceae) from Iran. Nat. Prod. Res. 2019, 33, 1787–1790. [Google Scholar] [CrossRef]
- Pecarski, D.; Dragićević-Ćurić, N.; Jugović, Z. Chemical composition, antifungal and antibacterial potential of fennel (Foeniculum vulgare) and cumin (Carum carvi) essential oils (Apiaceae). Rasteniev’dni Nauk. J. Crop Sci. 2017, 54, 66–72. [Google Scholar]
- Filip, A.; Boz, I.; Dunca, S.; Ștefan, G.A.; Zamfirache, M.M. Chemical composition and antimicrobial activities of two Mentha species essential oils. Planta Med. 2019, 85, P-287. [Google Scholar]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Somrani, M.; Inglés, M.C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, onion, and cinnamon essential oil anti-biofilms’ effect against listeria monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Safaei-Ghomi, J.; Ahd, A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Kurita, N.; Miyaji, M.; Kurane, R.; Takahara, Y.; Ichimura, K. Antifungal activity and molecular orbital energies of aldehyde compounds from oils of higher plants. Agric. Biol. Chem. 1979, 43, 2365–2371. [Google Scholar]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Prashar, A.; Hili, P.; Veness, R.G.; Evans, C.S. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry 2003, 63, 569–575. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011, 19, 368–375. [Google Scholar] [CrossRef]
- Kim, S.; Kang, D.; Kim, J.; Ha, Y.; Hwang, J.Y.; Kim, T.; Lee, S. Antimicrobial activity of plant extracts against Salmonella Typhimurium, Escherichia coli O157: H7, and Listeria monocytogenes on fresh lettuce. J. Food Sci. 2011, 76, M41–M46. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Ravichandran, M.; Hettiarachchy, N.S.; Ganesh, V.; Ricke, S.C.; Singh, S. Enhancement of antimicrobial activities of naturally occurring phenolic compounds by nanoscale delivery against Listeria monocytogenes, Escherichia coli O157: H7 and Salmonella Typhimurium in broth and chicken meat system. J. Food Saf. 2011, 31, 462–471. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2005, 10, 813–829. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- Ahl, H.A.H.S.; Sarhan, A.M.; Dahab, A.; Dahab, M.A. Essential Oils of Anethum graveolens L.: Chemical Composition and Their Antimicrobial Activities at Vegetative, Flowering and Fruiting Stages of Development. Int. J. Plant Res. 2015, 1, 98–102. [Google Scholar]
- Mohammadpour, H.; Moghimipour, E.; Rasooli, I.; Fakoor, M.H.; Astaneh, S.A.; Moosaie, S.S.; Jalili, Z. Chemical composition and antifungal activity of Cuminum cyminum L. essential oil from Alborz mountain against Aspergillus species. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 50–55. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Gholipour, Z. Chemical composition, antimicrobial and antioxidant activities of essential oil from Echinophora cinerea harvested at two phenological stages. J. Essent. Oil Res. 2016, 2905, 12. [Google Scholar] [CrossRef]
- Fathi-Moghadam, E.; Shakerian, A.; Chaleshtori, R.S.; Rahimi, E. Chemical Composition and Antioxidant Properties and Antimicrobial Effects of Satureja bachtiarica Bunge and Echinophora platyloba DC. Essential Oils Against Listeria monocytogenes. J. Med. Plants By-Prod. 2020, 1, 47–58. [Google Scholar]
- Hashemi, M.; Ehsani, A.; Afshari, A.; Aminzare, M.; Raeisi, M. Chemical Composition and Antifungal Effect of Echinophora platyloba Essential Oil against Aspergillus flavus, Penicillium expansum and Fusarium graminearum. J. Chem. Health Risks 2016, 6, 91–97. [Google Scholar]
- Belabdelli, F.; Piras, A.; Bekhti, N.; Falconieri, D.; Belmokhtar, Z.; Merad, Y. Chemical Composition and Antifungal Activity of Foeniculum vulgare Mill. Chem. Afr. 2020, 3, 323–328. [Google Scholar] [CrossRef]
- Paul, S.; Dubey, R.C.; Maheswari, D.K.; Kang, S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2011, 22, 725–731. [Google Scholar] [CrossRef]
- Moein, M.R.; Zomorodian, K.; Pakshir, K.; Yavari, F.; Motamedi, M.; Zarshenas, M.M. Trachyspermum ammi (L.) Sprague: Chemical composition of essential oil and antimicrobial activities of respective fractions. J. Evid.-Based Complement. Altern. Med. 2015, 20, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Plant, A.; Sharopov, F.; Setzer, W.N. Composition and antimicrobial activity of the essential oil of Hyssopus seravschanicus growing wild in Tajikistan Composition and antimicrobial activity of the essential oil of Hyssopus seravschanicus growing wild in Tajikistan. Der Pharma Chem. 2012, 4, 961–966. [Google Scholar]
- Abdellatif, F.; Boudjella, H.; Zitouni, A.; Hassani, A. Chemical composition and antimicrobial activity of the essential oil from leaves of Algerian Melissa officinalis L. EXCLI J. 2014, 13, 772–781. [Google Scholar] [CrossRef]
- Ba-Hamdan, A.H.A.; Aly, M.M.; Bafeel, S.O. Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Ocimum basilicum, Collected from Jeddah Region, Saudi Arabia. Microbiol. Res. (Pavia) 2014, 4, 3302–3309. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Tančinová, D.; Florková, M.; Foltinová, D.; Charousová, I.; Vrbová, K.; Božik, M.; Klouček, P. Inhibitory effect of essential oils from some lamiaceae species on growth of Eurotium Spp. Isolated From Bread. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 857–862. [Google Scholar] [CrossRef]
- Moussi Imane, M.; Houda, F.; Said Amal, A.H.; Kaotar, N.; Mohammed, T.; Imane, R.; Farid, H. Phytochemical Composition and Antibacterial Activity of Moroccan Lavandula angustifolia Mill. J. Essent. Oil-Bearing Plants 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Jafari-Sales, A.; Pashazadeh, M. Study of chemical composition and antimicrobial properties of Rosemary (Rosmarinus officinalis) essential oil on Staphylococcus aureus and Escherichia coli in vitro. Int. J. Life Sci. Biotechnol. 2020, 3, 62–69. [Google Scholar] [CrossRef]
- da Silva Bomfim, N.; Kohiyama, C.Y.; Nakasugi, L.P.; Nerilo, S.B.; Mossini, S.A.G.; Romoli, J.C.Z.; Graton Mikcha, J.M.; de Abreu Filho, B.A.; Machinski, M. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D.H.; Al-Moghazy, M.; Elsayed, A.A.A. The invitro cytotoxicity, antioxidant and anti-bacterial potential of. Bioorg. Chem. 2019, 103559. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Khorram, F.; Ramezanian, A.; Saharkhiz, M.J. In vitro activity of some essential oils against penicillium digitatum. Adv. Hortic. Sci. 2018, 32, 487–494. [Google Scholar] [CrossRef]
- Izadi, Z.; AghaAlikhani, M.; Mirazi, N. Identification of chemical composition, antioxidant and antimicrobial activities of summer savory (Satureja hortensis L.) essential oil ID. Razi J. Med. Sci. 2020, 27, 35–48. [Google Scholar]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef]
- Paulus, D.; Luchesi, L.A.; Busso, C.; Frata, M.T.; Oliveira, P.J.B. de Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils of Four Species of the Lamiaceae Family. European J. Med. Plants 2020, 31, 129–140. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Darwish, N.H.E.; Sudha, T.; Bahlouli, S.; Kellou, D.; Benelmouffok, A.B.; Chader, H.; Rajabi, M.; Benali, Y.; Mousa, S.A. In vitro antifungal and topical anti-inflammatory properties of essential oil from wild-growing thymus vulgaris (Lamiaceae) used for medicinal purposes in algeria: A new source of carvacrol. Sci. Pharm. 2020, 88, 33. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Djenane, D. Antifungal, anti-aflatoxigenic, antioxidant activity and in vivo efficacy of essential oil of the aerial parts of Thymus capitatus (L.) Hoffmanns & Link. Phytotherapie 2019, 17, 299–309. [Google Scholar] [CrossRef]
- Pavel, M.; Ristić, M.; Stević, T. Essential oils of Thymus pulegioides and Thymus glabrescens from Romania: Chemical composition and antimicrobial activity. J. Serbian Chem. Soc. 2010, 75, 27–34. [Google Scholar] [CrossRef]
- Rahimi, V.; Hekmatimoghaddam, S.; Jebali, A.; Sadrabad, E.K.; Heydari, A.; Akrami Mohajeri, F. Chemical composition and antibacterial activity of Zataria multiflora essential oil. J. Essent. Oil-Bearing Plants 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Albayrak, S.; Silahtarlıoğlu, N. Determination of biological activities of essential oil and extract obtained from Achillea coarctata Poir. Adv. Tradit. Med. 2020, 20, 77–88. [Google Scholar] [CrossRef]
- Li, M.Y.; Shen, L.; Tang, S.; He, Z.Q.; Lai, P.X. Chemical Composition and Antibacterial Activity of the Essential oil of Viola lactiflora. Chem. Nat. Compd. 2020, 56, 149–151. [Google Scholar] [CrossRef]
- Oliveira de Veras, B.; Melo de Oliveira, M.B.; Granja da Silva Oliveira, F.; Queiroz dos Santos, Y.; Saturnino de Oliveira, J.R.; Lúcia de Menezes Lima, V.; Guedes da Silva Almeida, J.R.; Maria do Amaral Ferraz Navarro, D.; Ribeiro de Oliveira Farias de Aguiar, J.C.; Aguiar, J.d.S.; et al. Chemical composition and evaluation of the antinociceptive, antioxidant and antimicrobial effects of essential oil from Hymenaea cangaceira (Pinto, Mansano & Azevedo) native to Brazil: A natural medicine. J. Ethnopharmacol. 2020, 247, 112265. [Google Scholar] [CrossRef] [PubMed]
- Kiarsi, Z.; Hojjati, M.; Behbahani, B.A.; Noshad, M. In vitro antimicrobial effects of Myristica fragrans essential oil on foodborne pathogens and its influence on beef quality during refrigerated storage. J. Food Saf. 2020, 40. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).