Abstract
Ammonia (NH3) concentrations in summer were continuously monitored from three typical laying hen houses of CC (conventional cage), AV (aviary), and NM (natural mating colony cage) with manure belt systems in North China Plain to quantify their emission levels, to characterize the diurnal variations, and to investigate the impact of environmental factors. Diurnal profiles were acquired by hourly measurements, and the effect of environmental factors on NH3 emissions was presented by correlation analysis. The results showed that house-level NH3 emissions in summer were the highest in the NM at 27.16 ± 13.12 mg/h·hen, followed by the AV at 4.08 ± 3.23 mg/h·hen and the CC at 3.43 ± 1.46 mg/h·hen within a complete manure removal cycle, which were significantly affected by manure accumulation inside the houses. After manure removal, NH3 concentrations were reduced by 64.29%, 28.57%, and 35.71% in CC, AV, and NM, and consequently their emissions were lowered by 67.12%, 71.36%, and 55.69%, respectively. It was suggested that the manure should not be stored on the belt for more than 4 days in NM. A positive impact of indoor and outdoor temperature and ventilation rate on NH3 emissions from AV and NM were found, while indoor and outdoor relative humidity had a negative effect. However, the above five factors did not significantly affect the emissions from CC.
1. Introduction
Livestock production is one of the major sources of NH3 to the atmosphere, which is estimated to contribute 34–40% of global NH3 emissions [1]. NH3 can irritate the eyes and respiratory membranes, and develop chronic stress, which is a threat to workers and animals and has been classified as a hazardous substance under EPCRA [2]. Besides, NH3 can be a precursor of secondary particulate matter [3,4] and is also the key substance and indicator of malodor from livestock facilities [5]. Consequently, NH3 emission is considered as an issue of political and scientific concerns because of its serious influence on health, environment, and ecosystem [6,7].
Current research has revealed that about 20% of global NH3 came from China [8], which was mainly from livestock activities [9]. China is the No.1 layer producer in the world, with a share of approximately 36% of the total bird flocks. As a main production area, North China Plain (NCP), which is located at 32°–40° N, 114°–120° E with a monsoon climate of medium latitudes, alone yields about 42% of national layer eggs in 2018 [10]. The NH3 emissions from poultry farms are as high as 2–6 times compared with dairy farms and swine farms [11]. Hence, the intensive NH3 emissions from laying hen farms in the NCP cannot be ignored, especially in summer, during which most nitrogen content in manure volatilized in NH3 because of the high ambient temperature [12] and high ventilation rates of the houses.
Many researchers have previously reported NH3 emissions from layer houses [13,14]. To collect suitable NH3 emission data from animal feeding operations (AFOs) for making mitigation options and regulatory decisions, the US implemented a two-year National Air Emissions Monitoring Study (NAEMS) in 2006 to determine the baseline of NH3 emission data from different livestock and poultry farms. The results showed that NH3 was one of the main contaminated gases emitted from laying hen houses [2], and the factors such as exhaust air temperature, manure accumulation time, as well as population and activity of the birds significantly affected hen-specific NH3 emissions [15]. Average NH3 emissions from laying hen houses in California, North Carolina, and Indiana in the US were 0.95 g/d·hen, 1.08 g/d·hen, and 0.59 g/d·hen, respectively [2,16,17], which varied due to the differences in environmental parameters, house management, and production systems. Meanwhile, Huang and Guo [18] reported the NH3 emission rate from cage-layer barns was 0.33 g/d·hen in Northern Saskatoon, Canada. Thus, the NH3 emission baseline obtained from NAEMS or a particular AFO could not be generalized to other facilities. It is still essential to measure more NH3 emission data from laying hen farms under different climates, housing conditions, and management practices [2].
Due to the complex influence factors on NH3 emissions, such as the housing system, manure storage, and environmental control technique, it is hard to collect representative data [19,20]. Zhang et al. [21] used the RAINS model methodology to estimate the NH3 emission from laying hen farms in the NCP, which was 0.43 g/d·hen. This result could not fully reflect the diurnal variation and housing system differences to understand the basic NH3 emission and develop cost-effective mitigation strategies or techniques. Besides, with the development of manure cleaning technology in China, the manure belt has gradually replaced the slurry scraper to remove manure outside, which can greatly reduce NH3 concentration inside the house [22]. However, the studies concerning NH3 concentrations and emissions from laying hen houses with manure belts are still very limited in China [12,23,24].
Conventional cages (CC) are commonly used in the laying hen industry of China. In recent years, different designs of laying hen housing systems have been developed to improve the animal welfare and productivities, such as the aviary system (AV, for laying hens) and the natural mating colony cage (NM, for layer breeding), which may greatly affect NH3 emissions [19,20]. The AV, a welfare-friendly cage-free system, allows the hens to freely access wire-mesh floors at different heights and perform more natural behaviors inside. It is typically equipped with a manure belt beneath each wire-mesh floor to timely remove manure out of the house with the purpose of improving indoor air quality. The NM is an alternative of CC, or single floor system, for breeder birds. Compared with CC with artificial insemination, NM has a bigger activity space per bird and a lower stocking density for the breed hen and cock in the same cage, which allows the natural mating of the birds and thus could substantially save labor. Currently, studies on indoor air quality and emissions from the hen houses installed with different systems can rarely be found in China. It is still highly needed to study NH3 emissions from different housing systems to provide a base for the emission abatement.
This study measured NH3 concentrations and calculated the emissions from CC, AV, and NM housing systems for laying hens in the NCP during summer with the following objectives: (1) to qualify their NH3 emission levels in summer and characterize their diurnal variations; (2) to investigate the impact of the ventilation rate (VR), temperature, relative humidity (RH) and manure removal on NH3 concentrations and emissions; and (3) to analyze the differences of NH3 concentrations and emissions among the three types of laying hen houses.
2. Materials and Methods
2.1. Description of the Monitoring Sites
The studied sites included a CC, an AV, and a NM house located in the NCP. The basic information of the three laying hen houses can be founded in Table 1.

Table 1.
Description of the three laying hen houses studied.
2.2. Sampling and Measurement Methods
Gas concentrations, temperature, and RH were continuously measured in and out of CC, AV, and NM houses from 20 September 2019 to 24 September 2019, 7 June 2020 to 12 June 2020, and 10 July 2019 to 31 July 2019, respectively. NH3 concentration was monitored by using a portable monitoring unit (PMU) configured with an NH3 sensor (ME3-NH3, with a measurement range of 0–50 ppm; Winsen, China, every 20 min. CO2 concentration was detected in the AV at an interval of 20 min, with a CO2 sensor (MH-Z14, with a measurement range of 0–5000 ppm, accuracy ±100 ppm; Winsen, China) installed in the PMU. Two PMUs were set in CC and NM at the height of 1.5 m, which were fixed close to the air inlet and central exhaust fan. In the AV, three PMUs were located at a height of 1.5 m, were close to the air inlet and cage walls of two aviary cages near the exhaust fans. Temperature and RH were measured by T/RH data loggers (U23-001, HOBO, Bourne, USA), with a measurement ranges of −40 °C to 70 °C and 0–100%, and accuracies of ±0.18 °C and ±2.5%, respectively. Three T/RH data loggers were located in the center of the three houses at the height of 1.5 m, and another three loggers were set outside, which were 2.0 m away from the head of laying hen houses. Temperature and RH were continuously monitored by the data loggers.
Before the experiment, the air velocity of each fan for CC and NM was measured by a hot-wire anemometer (model KA41 L, Kanomax, Osaka, Japan). When measuring, the fan was divided into 4 equal parts by cross-marking, and 2 of them were tested randomly. Each part was evenly distributed with 16 sampling points (Figure 1). The probe of an anemometer was attached to the protective net in front of the fan to measure the air velocity of every sampling point. When the air velocity at a sampling point was comparatively stable, 20 values were recorded continuously by an anemometer, and the average of all sampling velocities was used as the average air velocity through the fan.
Figure 1.
Diagram of sampling points (a) to measure air velocity and the real fan (b) installed in laying hen house.
2.3. Ventilation Rate and Emission Rate Calculation
For the CC and NM houses equipped with exhaust fans, the ventilation rate (VR) was calculated as follows:
where VR was the ventilation rate of the house, m3/h; Vi was the average air velocity of the fan i, m/h; Si was the area of the fan i, m2; n was the number of the operating exhaust fans.
For the AV house, its VR was estimated by a CO2 mass balance method recommended by CIGR [25], and the equations were listed below:
where (CO2)P was CO2 production per heat production unit based on a 24-h period, 0.185 m3/h; (CO2)iand (CO2)o were CO2 concentrations inside and outside of the tested house, mg/m3; øtot was the total heat production of the birds under 20 °C, W; a was a constant to express the amplitude with respect to the constant 1, 0.61; h was the hours after midnight; hmin was the hours after midnight with minimum activity, −0.1; Tiwas the temperature inside the house, °C.
The NH3 emission rate was calculated as follows:
where ER was NH3 emission rate, mg/h; ΔC was NH3 concentration difference between the air outlets (at the exhaust fan end) and inlets of the tested laying hen houses, mg/m3.
2.4. Statistical Analysis
To study the diurnal NH3 concentrations and emissions, the data at the same stage of the manure removal cycle was pooled together to calculate the mean value and analyzed by least significant differences using repeated measurement analyses of variance (ANOVAs) with a significance level of 0.05 in SPSS 21.0 (IBM, New York, NY, USA). When comparing the difference of diurnal NH3 concentration and emission rate during a complete manure removal cycle, the average of the whole day data was used. The correlation impact of temperature and VR on NH3 emission was developed by SPSS 21.0, where p ≤ 0.05 means significant correlation, and p ≤ 0.01 means a very significant correlation.
3. Results and Discussion
3.1. Bird Performance and Diurnal Air Temperature and RH
Age, feed intake, and crude protein (CP) of laying hens in different houses during the experiment were listed in Table 2. Feed intake in AV was the highest because of the higher energy requirement with more activities. The CP content in the three houses was similar.

Table 2.
Age, average daily feed intake, and feed CP content of different houses.
Figure 2 shows the variations of both outdoor and indoor temperature and RH in the three laying hen houses. The outdoor temperature varied greatly from 14.8 °C to 35.9 °C, with the overall means of 21.2 °C, 28.4 °C, and 27.7 °C in CC, AV, and NM, respectively. Outdoor RH of CC, AV, and NM fluctuated from 43.3% to 93.9%, 32.4% to 73.7%, and 47.6% to 93.1%, respectively. Meanwhile, the indoor temperature and RH of three houses was controlled within a narrow range. The average indoor temperature of the three laying hen houses was stable at 21.1–29.0 °C (Figure 2c); the average RH was the highest in NM (68.4–81.6%), which was about 20.0% higher than that in CC and AV (Figure 2d).
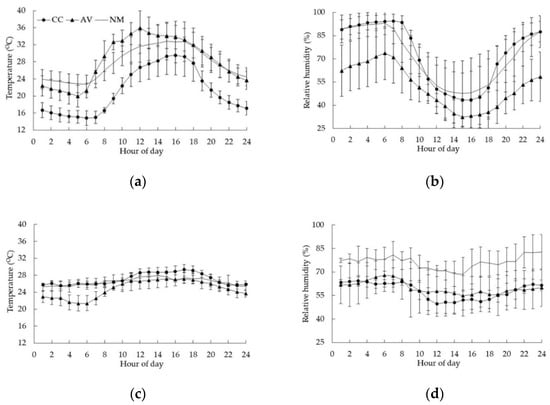
Figure 2.
Outdoor and indoor diurnal temperature and RH profiles in CC, AV, and NM houses. (a) Outdoor temperature; (b) Outdoor RH; (c) Indoor temperature; (d) Indoor RH.
3.2. Diurnal Concentration and Emission Profiles
3.2.1. CC and AV
The diurnal patterns of average VR, NH3 concentrations, and emissions in CC and AV, were plotted in Figure 3. The VR ranged from 1.21 to 5.15 m3/h·hen for CC, and 0.84 to 12.16 m3/h·hen for AV. In CC and AV, VR presented a variation pattern following that of outdoor temperature, which showed a peak within 9:00 to 20:00 in both days of a complete manure removal cycle, because of the high temperature, and it did not change greatly at the time of manure removal (11:00–12:00, and 16:00–17:00).
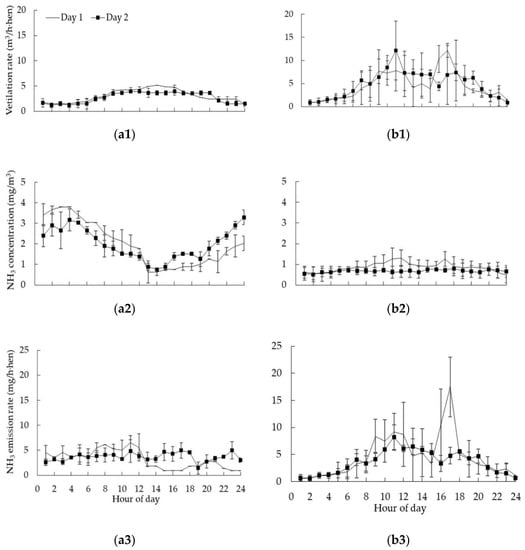
Figure 3.
Diurnal patterns average VR, NH3 concentration, and emissions in CC (a) and AV (b). (Day 1 means the day with manure removal; Day 2 means the day after manure removal. Error bar was plotted by using standard deviation.)
Figure 3(a2,b2) depicted the temporal variations in NH3 concentrations during manure removal (day 1) and the day after manure removal (day 2). On both days, concentrations of NH3 in CC were higher during the night, due to cooler temperature and lower VR, which has been supported by many studies with the same production system [12,18,26]. NH3 concentrations in the CC ranged from 0.63 to 3.79 mg/m3 (2.01 ± 0.93 mg/m3 in average), which were comparable with the average NH3 concentration in similar facilities with the ambient temperature over 25 °C and stock density of 516 cm2/hen in the US midwest (near to 38° N) [27,28].
It could be seen that NH3 concentrations kept stable on the second day after manure removal in AV. Before 10:00, the VR increased gradually as the indoor temperature increasing to emit the NH3 out of the house to keep a stable indoor NH3 concentration. The phenomenon occurred again after 20:00. As known, the hens were more active comparatively during the daytime, while, the indoor temperature, VR, and NH3 concentration in AV remained fairly constant during 12:00–20:00 on the second day, which illustrated that animal activity did not have a significant effect on NH3 concentration after separating the manure from hens kept inside the aviary units since the manure was the main NH3 source [28,29]. NH3 concentrations in AV ranged from 0.54 to 1.30 mg/m3 (0.77 ± 0.19 mg/m3 in average), which were significantly lower than that in summer (about 2.12 mg/m3) in a similar type of house with lower VR (maximal VR of 0.75 m3/h·hen) and longer manure accumulation (3 to 4 days) inside the operation that Shepherd et al. [27] studied.
On the day after manure removal in CC and AV, NH3 emission rates were higher in the daytime and lower in the nighttime, which agreed with Wang-Li et al. [16] and Alberdi et al. [30]. It was likely influenced by the house temperature and VR. High temperatures can accelerate microbiological degradation of manure to promote bigger generation rates of NH3 [16]. Liang et al. [14] also observed that VR had a significant effect on NH3 emissions and caused higher emission rates during the daytime. After manure removal, NH3 emission rates in both houses sharply dropped down. The NH3 emission rates within a whole manure removal cycle ranged from 0.92 to 6.52 mg/h·hen (3.43 ± 1.46 mg/h·hen in average) in CC, which was comparable with reported values of 4.04 and 2.83 mg/h·hen in laying hen houses with CC and similar management in the U.S. Midwest [27] and 3.60 mg/h·hen for a traditional cage with manure belt-natural drying system with a 492 cm2/hen stocking density in the laboratory [31]. The average NH3 emissions from laying hen houses in California (36.46° N) with a stocking density of 332 cm2/hen [2] and North Carolina (35.26° N) with a 400 cm2/hen density [16] in the U.S. were 39.58 mg/h·hen, and 45.00 mg/h·hen, respectively, which showed similar operational information and environmental variables having similar NH3 emission characteristics. NH3 emission rates in AV averaged at 4.08 ± 3.23 mg/h·hen, ranging from 0.66 to 17.50 mg/h·hen, which was comparatively lower than the value reported by Shepherd et al. [27]. They documented an average rate in a similar facility with the house temperature of 20–25 °C was 6.29 mg/h·hen. Meanwhile, the VR (maximal VR of 0.75 m3/h·hen) was lower and the manure accumulation (3 to 4 days) was longer than this study.
3.2.2. NM
The diurnal average VR in NM ranged from 11.70 to 20.80 m3/h·hen (Figure 4b), which was significantly higher than that in CC and AV (p < 0.05). The daily VR change trend in NM was similar to CC, and the peak of VR occurred from 9:00 to 18:00 because of the high temperature. It was seen that the NH3 concentration gradually increased with the manure accumulation extending (Figure 4a). When the manure was accumulated for 1–3 days, the change in NH3 concentration was contrary to that of VR, and the higher concentrations of NH3 in NM occurred during the night, which was similar to the situation of CC. While it changed reversely from the fourth day, NH3 concentration did not decrease with the increasing VR. In this case, after the manure accumulated on the belt for 4 days, NH3 volatilized from manure with the temperature increasing was more than that exhausted by fans. As shown in Figure 5, the NH3 concentration on day 7 was 3 times higher than that on the second day after manure removal. Mendes et al. [32] reported NH3 concentration at air outlet with manure accumulated for 6 days was about 21 times higher than that for 2 days storage in a laboratory with the hen age of 23 to 36 weeks. Thus, manure accumulation significantly affected NH3 emission rates. It was recommended that the manure should not be stored on the belt for more than 4 days in NM, which was consistent with the findings of Mendes et al. [32]. Otherwise, higher VR was demanded to maintain a good inner environment. NH3 concentrations in NM ranged from 0.93 to 5.65 mg/m3 with an average of 3.06 ± 1.29 mg/m3, which was substantially higher than those in CC and AV (p <0.05) mainly due to much longer manure accumulation time inside the house.
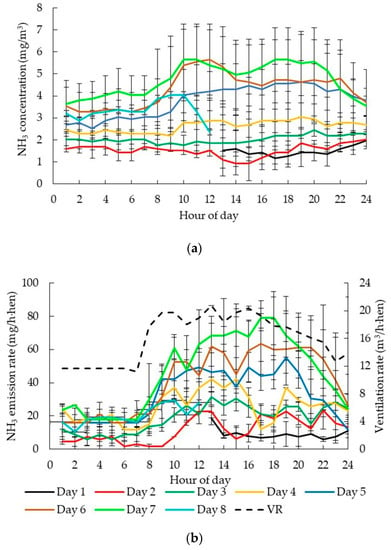
Figure 4.
Diurnal patterns of average NH3 concentration (a), VR and NH3 emissions (b) in NM under different manure accumulated time. (Day 1 means the day of manure removal; Day 2 to Day 8 means the manure accumulated for 2 days to 8 days.).
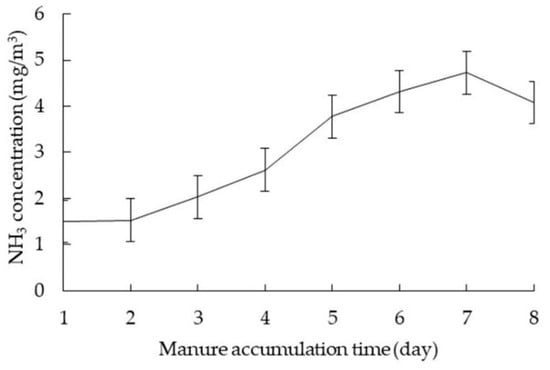
Figure 5.
NH3 concentration in NM with the manure accumulation time extending.
NH3 emission rates in NM gradually increased with manure accumulation, which agreed with the report by Ning [33]. It also varied with time, similar to that of CC and AV, in which relatively higher NH3 emission rates occurred during the day and lower emission rates occurred at night [16]. NH3 emission rates in NM ranged from 1.48 to 78.93 mg/h·hen (27.16 ± 13.12 mg/h·hen in average), which was similar to the value of 20.83 mg/h·hen reported by Hayes et al. [34]. The NH3 emission rates in NM were much higher than previously reported value (2.68 mg/h·hen in average) from a caged-hen house with a 620 cm2/hen stocking density in the laboratory, inside which the manure was accumulated for 6 days [32]. In Mendes et al. [32], the minimum VR of laying hen house was regulated based on the maximum allowed indoor CO2 concentration level of 4000 ppmv. The average VR inside NM was 16.03 m3/h·hen, highest among the three studied laying hen houses, which had a significant and positive impact on NH3 emissions [14,16], and it was also mentioned in CC and AV houses. Besides, differences also occurred in manure belt or manure pan cleanliness between the two studies. In this study, there was inevitably some manure residue on the belt even after manure removal, which contributed to higher NH3 emissions. In Mendes et al. [32], all the facilities were cleaned and the existing manure pans were replaced with new ones after each trial.
NH3 concentrations in CC, AV, and NM with manure belt systems were well controlled, and all were under the recommended threshold of 15 mg/m3 to maintain a good environmental quality given by the Ministry of Agriculture and Rural Affairs of the People’s Republic of China [35]. Furthermore, it was found that NH3 concentrations and emission rates were sharply dropped down after manure removal. In CC, AV, and NM, NH3 concentrations were reduced by 64.29%, 28.57%, and 35.71% after manure clearance, and consequently, their emissions were lowered by 67.12%, 71.36%, and 55.69%, respectively. It is suggested that the manure belt could efficiently reduce NH3 concentrations and the emissions as well by thoroughly and timely removing manure outside of the laying hen houses, which was also supported by Huang and Guo [18] and Nicholson et al. [36].
3.3. Influence of Environmental Factors on NH3 Emission Rate
The correlation coefficients for the NH3 emission rate in summer against environmental factors are listed in Table 3. It was found that temperature, RH, and VR did not significantly correlate NH3 emission rate in CC (p > 0.05), which might attribute to the indoor temperature and RH were controlled well within narrow ranges, and NH3 emission rates in CC during the day changed slightly (Figure 3(a3)). This agreed with the results of Huang and Guo [18]. Outdoor and indoor temperatures and VR had significantly positive correlations with the emissions in AV and NM (p < 0.01). A significant and negative effect of outdoor and indoor RH on NH3 emission rate was also revealed for AV (Outdoor RH: p < 0.01, indoor RH: p < 0.05) and NM (p < 0.01), which aligned with the findings of Huang and Guo [18]. While it was contrasted to the results reported by Wang-Li et al. [16], who found the RH had significant positive impact on NH3 emissions. In Wang-Li et al. [16], the correlations between NH3 emission rates and RH were analyzed using the data from 24 September 2007 to 31 December 2009, making no distinction between seasons. High RH would increase NH3 emissions by promoting manure fermentation [37], especially in cold seasons with low VR, which might cause the difference between the two studies. Besides, the manure dropped directly into the manure pit, and was cleaned out annually to make the moisture in manure volatilized difficultly.

Table 3.
Correlations between NH3 emission rates and environmental factors.
Table 4 illustrated the average NH3 emission rates on the first and second days of manure accumulation in CC, AV, and NM. On these two days of manure accumulation, the NH3 emission rate in NM was greatly higher than that in CC and AV (p < 0.05). The RH of NM was the highest compared with that of CC and AV, which could cause higher moisture content in the manure of NM to improve microbial activity to produce more NH3 [27,37]. Besides, this discrepancy might also be caused by the different levels of manure residue on the belts. Additionally, the highest VR inside NM would also promote higher NH3 emissions.

Table 4.
Average NH3 emission rates on the first and second day of manure accumulation in CC, AV, and NM (mg/h·hen) 1.
4. Conclusions
Strong diurnal variations of NH3 concentrations and emissions in summer were identified for three typical laying hen houses (CC, AV, and NM) in NCP. The impact of environmental factors on NH3 emissions was also analyzed. The following findings were summarized:
- Timely manure removal by the belt system and high VR of the houses in summer could help maintain relatively stable and low NH3 concentrations in CC, AV, and NM, which also could efficiently reduce NH3 emissions. NH3 mitigation efforts should focus on manure accumulation time inside and storage methods outside in the future.
- House-level average daily NH3 emissions were highest in the NM at 27.16 ± 13.12, followed by the AV at 4.08 ± 3.23 and the CC at 3.43 ± 1.46 mg/h·hen in summer. A clear diurnal pattern of NH3 emission was observed.
- Manure accumulation significantly affected NH3 emission rates, and it was strongly recommended to store manure on the belt for less than 4 days in NM.
- Laying hen houses with similar operational information and environmental variables had similar NH3 emission rates. A positive impact of temperature and VR, and a negative impact of RH on NH3 emissions in AV and NM were indicated, while the environmental factors did not significantly affect the NH3 emissions in CC.
It should be mentioned that the data collected for analysis were only in warm weather periods (June, July, and September). It is expected the emission would be varied due to the change of weather and, consequently, ventilation airflow rate for indoor climate control. Therefore, studies on possible seasonal effects should be conducted in the future.
Author Contributions
Conceptualization, C.W.; methodology, C.W., G.Z., and L.R.; formal analysis, Y.L., Z.L., and S.W.; writing-original draft preparation, Y.L.; writing—review and editing, C.W., G.Z., and L.R.; project administration, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (grant number: 2017YFD0701602).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmithausen, A.; Schiefler, I.; Trimborn, M.; Gerlach, K.; Südekum, K.H.; Pries, M.; Büscher, W. Quantification of methane and ammonia emissions in a naturally ventilated barn by using defined criteria to calculate emission rates. Animals 2018, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Cortus, E.L.; Zhang, R.; Jiang, S.; Heber, A.J. Ammonia, hydrogen sulfide, carbon dioxide and particulate matter emissions from California high-rise layer houses. Atmos. Environ. 2012, 46, 81–91. [Google Scholar] [CrossRef]
- Yao, L.; Garmash, O.; Bianchi, F.; Zheng, J.; Yan, C.; Kontkanen, J.; Junninen, H.; Mazon, S.B.; Ehn, M.; Paasonen, P.; et al. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science 2018, 361, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Gates, R.S.; Green, A.R.; Mitloehner, F.M.; Moore, P.A., Jr.; Wathes, C.M. Environmental impacts and sustainability of egg production systems. Poult. Sci. 2011, 90, 263–277. [Google Scholar] [CrossRef]
- Dunlop, M.W.; Blackall, P.J.; Stuetz, R.M. Odour emissions from poultry litter: A review litter properties, odour formation and odorant emissions from porous materials. J. Environ. Manage. 2016, 177, 306–319. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Anna, M.B.; Armin, M.; Johannes, B.; Volker, M.; Markus, Q. Ammonia emissions in Europe, part II: How ammonia emission abatement strategies affect secondary aerosols. Atmos. Environ. 2016, 126, 153–161. [Google Scholar] [CrossRef]
- Yamaji, K.; Ohara, T.; Akimoto, H. Regional-specific emission inventory for NH3, N2O and CH4 via livestock farming in South, Southeast, and East Asia. Atmos. Environ. 2004, 38, 7111–7121. [Google Scholar] [CrossRef]
- Wang, X.; Mauzerall, D.L.; Hu, Y.; Russell, A.G.; Larson, E.D.; Woo, J.; Streets, D.G.; Guenther, A. A high-resolution emission inventory for eastern China in 2000 and three scenarios for 2020. Atmos. Environ. 2005, 39, 5917–5933. [Google Scholar] [CrossRef][Green Version]
- Complied by National Bureau of Statistics of China. Chapter 12: Agriculture. In China Statistical Yearbook-2019; China Statistics Press: Beijing, China, 2019. Available online: http://www.stats.gov.cn/tjsj/ndsj/2019/indexch.htm (accessed on 24 September 2019).
- Koerkamp, P.W.G.G.; Metz, J.H.M.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.P.; Hartung, J.; Seedorf, J.; et al. Concentrations and emissions of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Zhu, Z.; Dong, H.; Zhou, Z.; Xin, H.; Chen, Y. Ammonia and greenhouse gases concentrations and emissions of a naturally ventilated laying hen house in Northeast China. Trans. ASABE 2011, 54, 1085–1091. [Google Scholar] [CrossRef]
- Gay, S.W.; Schmidt, D.R.; Clanton, C.J.; Janni, K.A.; Jacobson, L.D.; Weisberg, S. Odor, total reduced sulfur, and ammonia emissions from animal housing facilities and manure storage units in Minnesota. Appl. Eng. Agric. 2003, 19, 347–360. [Google Scholar] [CrossRef]
- Liang, Y.; Xin, H.; Wheeler, E.F.; Gates, R.S.; Li, H.; Zajaczkowski, J.S.; Topper, P.A.; Casey, K.D.; Behrends, B.R.; Burnham, D.J.; et al. Ammonia emissions from U.S. laying hen houses in Iowa and Pennsylvania. Trans. ASABE. 2005, 48, 1927–1941. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Darr, M.J.; Lim, T.T.; Diehl, C.A.; Bogan, B.W. Air quality monitoring and on-site computer system for livestock and poultry environment study. Trans. ASABE 2009, 52, 937–947. [Google Scholar] [CrossRef]
- Wang-Li, L.; Li, Q.F.; Chai, L.; Cortus, E.L.; Wang, K.; Kilic, I.; Bogan, B.W.; Ni, J.Q.; Heber, A.J. The national air emissions monitoring study’s southeast layer site: Part Ⅲ. Ammonia concentrations and emissions. Trans. ASABE. 2013, 56, 1185–1197. [Google Scholar] [CrossRef]
- Ni, J.Q.; Chai, L.L.; Chen, L.; Bogan, B.W.; Wang, K.; Cortus, E.L.; Heber, A.J.; Lim, T.T.; Diehl, C.A. Characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter concentrations in high-rise and manure-belt layer hen houses. Atmos. Environ. 2012, 57, 165–174. [Google Scholar] [CrossRef]
- Huang, D.D.; Guo, H.Q. Toxic gas and respirable dust concentrations and emissions from broiler and cage-layer barns in the Canadian Prairies. Environ. Sci. Pollut. R. 2020, 27, 21680–21691. [Google Scholar] [CrossRef]
- EEA. Manure Management Regarding Nitrogen Compounds. In EMEP/CORINAIR Emission Inventory Guidebook; European Environment Agency: Copenhagen, Denmark, 2007; Available online: http://www.eea.europa.eu/publications/EMEPCORINAIR5 (accessed on 6 December 2007).
- EEA. Cultures with Fertilizers. In EMEP/CORINAIR Emission Inventory Guidebook; European Environment Agency: Copenhagen, Denmark, 2007; Available online: http://www.eea.europa.eu/publications/EMEPCORINAIR5 (accessed on 6 December 2007).
- Zhang, Y.; Dore, A.J.; Ma, L.; Liu, X.J.; Ma, W.Q.; Cape, J.N.; Zhang, F.S. Agricultural ammonia emissions inventory and spatial distribution in the North China Plain. Environ. Pollut. 2010, 158, 490–501. [Google Scholar] [CrossRef]
- Keener, H.M.; Elwell, D.L.; Grande, D. NH3 emissions and N-balances for a 1.6 million caged layer facility: Manure belt/composting vs. deep pit operation. Trans. ASAE 2002, 45, 1977–1984. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.F.; Xue, W.T.; Sun, Q.P.; Zhu, Z.P.; Tian, Z.; Li, X.R.; Zou, G.Y. Study of ammonia and particulate matter emission characteristics from large-scale cage layer house in winter. Trans. CSAE 2018, 34, 170–178. [Google Scholar] [CrossRef]
- Huang, X.; Song, Y.; Li, M.; Li, J.; Huo, Q.; Cai, X.; Zhu, T.; Hu, M.; Zhang, H. A high-resolution ammonia emission inventory in China. Global. Biogeochem. Cycles 2012, 26, GB1030. [Google Scholar] [CrossRef]
- Pedersen, S.; Sällvik, K. Fourth Report of Working Group on Climatisation of Animal Houses: Heat and Moisture Production at Animal and House Levels; Commission Internationale du Genie Rural: Horsens, Denmark, 2002. [Google Scholar]
- Wang-Li, L.; Li, Q.F.; Wang, K.; Bogan, B.W.; Ni, J.Q.; Cortus, E.L.; Heber, A.J. The National Air Emissions Monitoring Study’s Southeast Layer Site: Part I. Site characteristics and monitoring methodology. Trans. ASABE 2013, 56, 1157–1171. [Google Scholar] [CrossRef]
- Shepherd, T.A.; Zhao, Y.; Li, H.; Stinn, J.P.; Hayes, M.D.; Xin, H. Environmental assessment of three egg production systems—Part II. Ammonia, greenhouse gas, and particulate matter emissions. Poult. Sci. 2015, 94, 534–543. [Google Scholar] [CrossRef]
- Zhao, Y.; Shepherd, T.A.; Li, H.; Xin, H. Environmental assessment of three egg production systems–Part I: Monitoring system and indoor air quality. Poult. Sci. 2015, 94, 518–533. [Google Scholar] [CrossRef]
- Yang, P.; Lorimor, L.C.; Xin, H. Nitrogen losses from laying hen manure in commercial high-rise layer facilities. Trans. ASAE. 2000, 43, 1771–1780. [Google Scholar] [CrossRef]
- Alberdi, O.; Arriaga, H.; Calvet, S.; Estellés, F.; Merino, P. Ammonia and GHG emissions from an enriched laying hen facility. Biosyst. Eng. 2016, 144, 1–12. [Google Scholar] [CrossRef]
- Fournel, S.; Pelletier, F.; Godbout, S.; Lagacé, R.; Feddes, J.J.R. Odour emissions, hedonic tones and ammonia emissions from three cage layer housing systems. Biosyst. Eng. 2012, 112, 181–191. [Google Scholar] [CrossRef]
- Mendes, L.B.; Xin, H.; Li, H. Ammonia emissions of pullets and laying hens as affected by stocking density and manure accumulation time. Trans. ASABE 2011, 55, 1067–1075. [Google Scholar] [CrossRef]
- Ning, X. Feeding, Defecation, and Gaseous Emission Dynamics of W-36 Laying Hens. Mater’s Thesis, Iowa State University, Ames, IA, USA, 2008. [Google Scholar]
- Hayes, E.T.; Curran, T.P.; Dodd, V.A. Odour and ammonia emissions from intensive poultry units in Ireland. Bioresour. Technol. 2006, 97, 933–939. [Google Scholar] [CrossRef]
- Ministry of agriculture and rural affairs of the people’s republic of China. In Environmental Quality Standard for the Livestock and Poultry Farm; China Agricultural Press: Beijing, China, 1999; p. 81, NY/T 388-1999.
- Nicholson, F.A.; Chambers, B.J.; Walker, A.W. Ammonia emissions from broiler litter and laying hen manure management systems. Biosyst. Eng. 2004, 89, 175–185. [Google Scholar] [CrossRef]
- Meda, B.; Hassouna, M.; Aubert, C.; Robin, P.; Dourmad, J.Y. Influence of rearing conditions and manure management practices on ammonia and greenhouse gas emissions from poultry houses. Worlds Poult. Sci. J. 2011, 67, 441–456. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).