Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus brasiliensis Spores
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Fungal Growth and Spore Preparation
2.2. Soft Plasma Jet Device, Plasma-Activated Water Treatments, and Spore Viability
2.3. Morphology and Cell Wall Structure Analysis of A. brasiliensis Spores Treated with Plasma-Activated Water
2.4. Optical Spectroscopic Analyses of the PAW and A. brasiliensis Spores
2.5. Electrophoretic Analysis of Genomic DNA of the A. brasiliensis Spores Treated with the Plasma-Activated Water
3. Results and Discussion
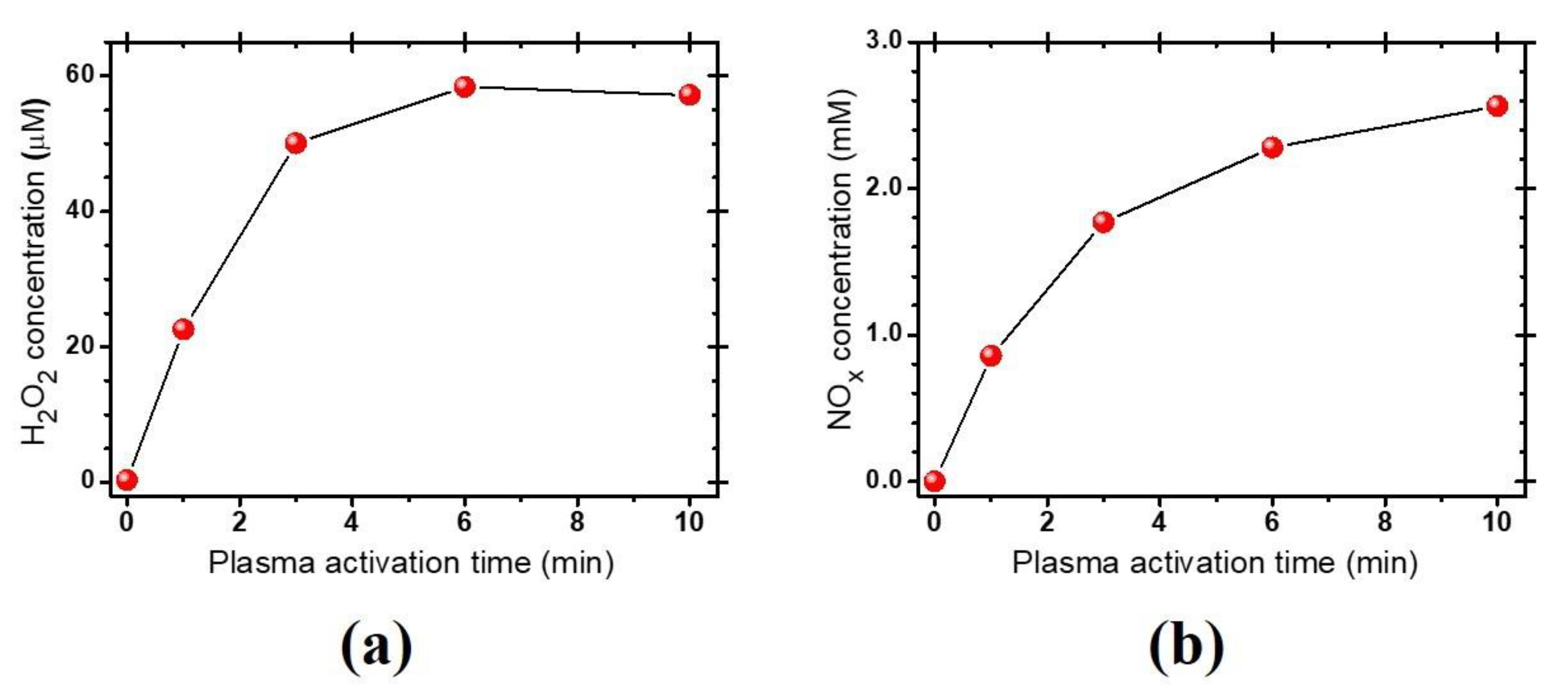
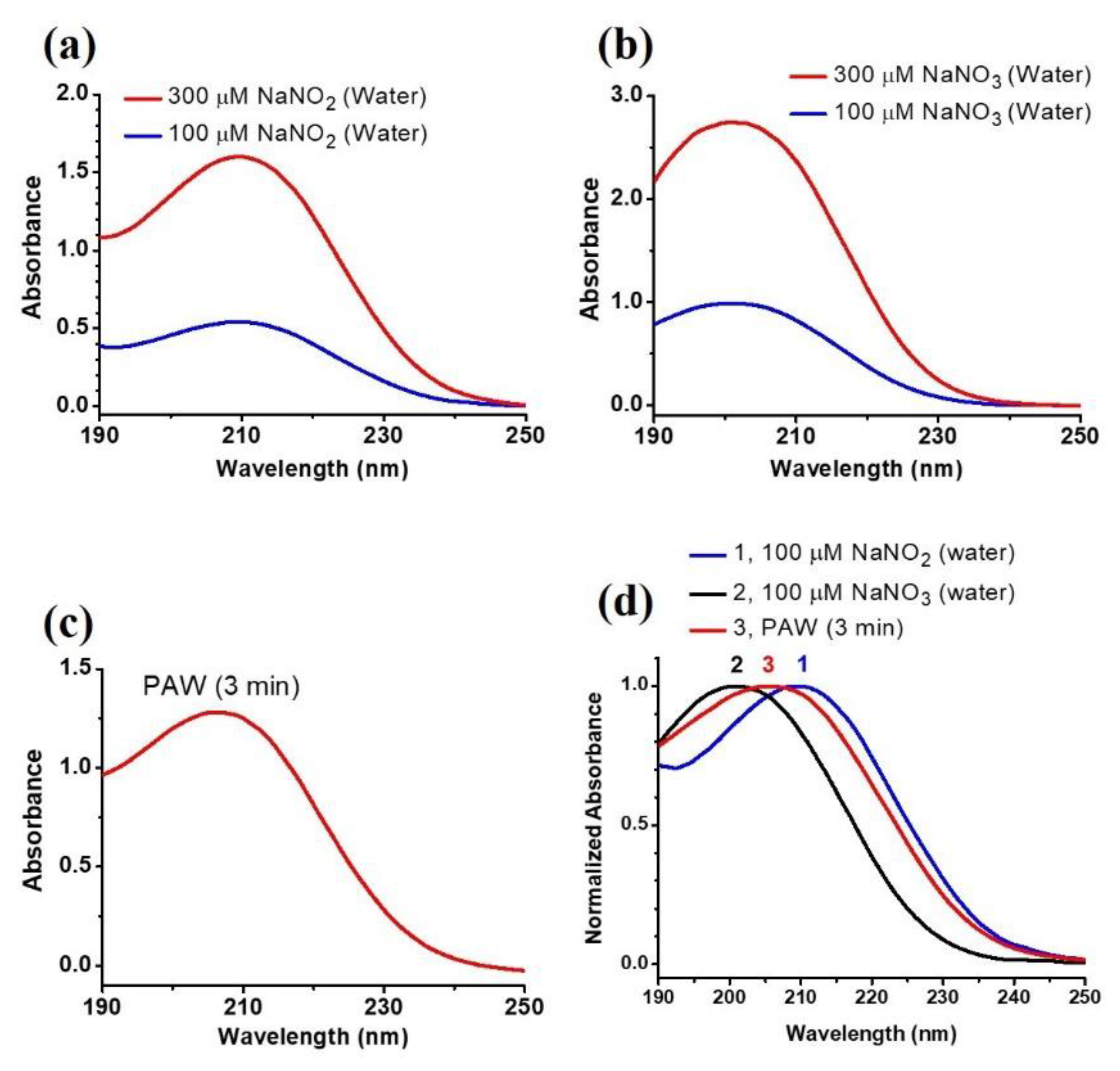
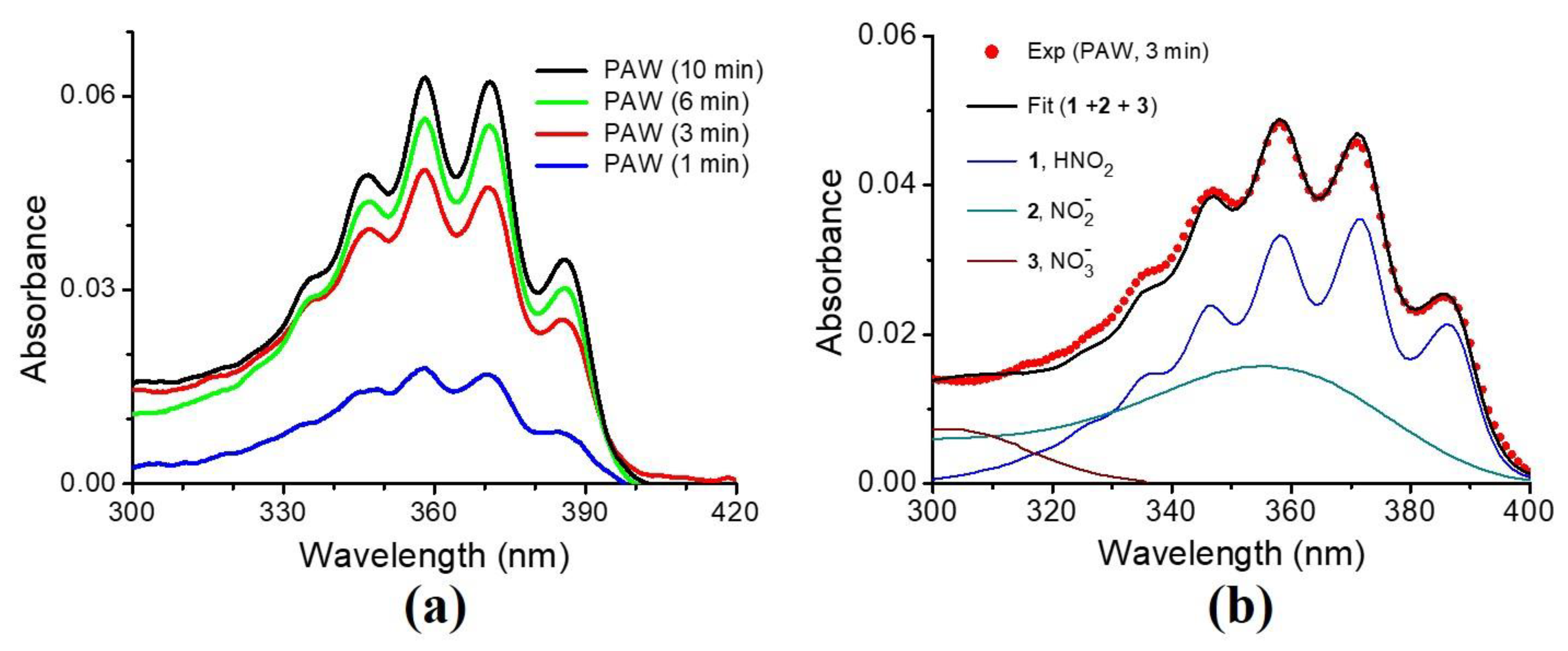
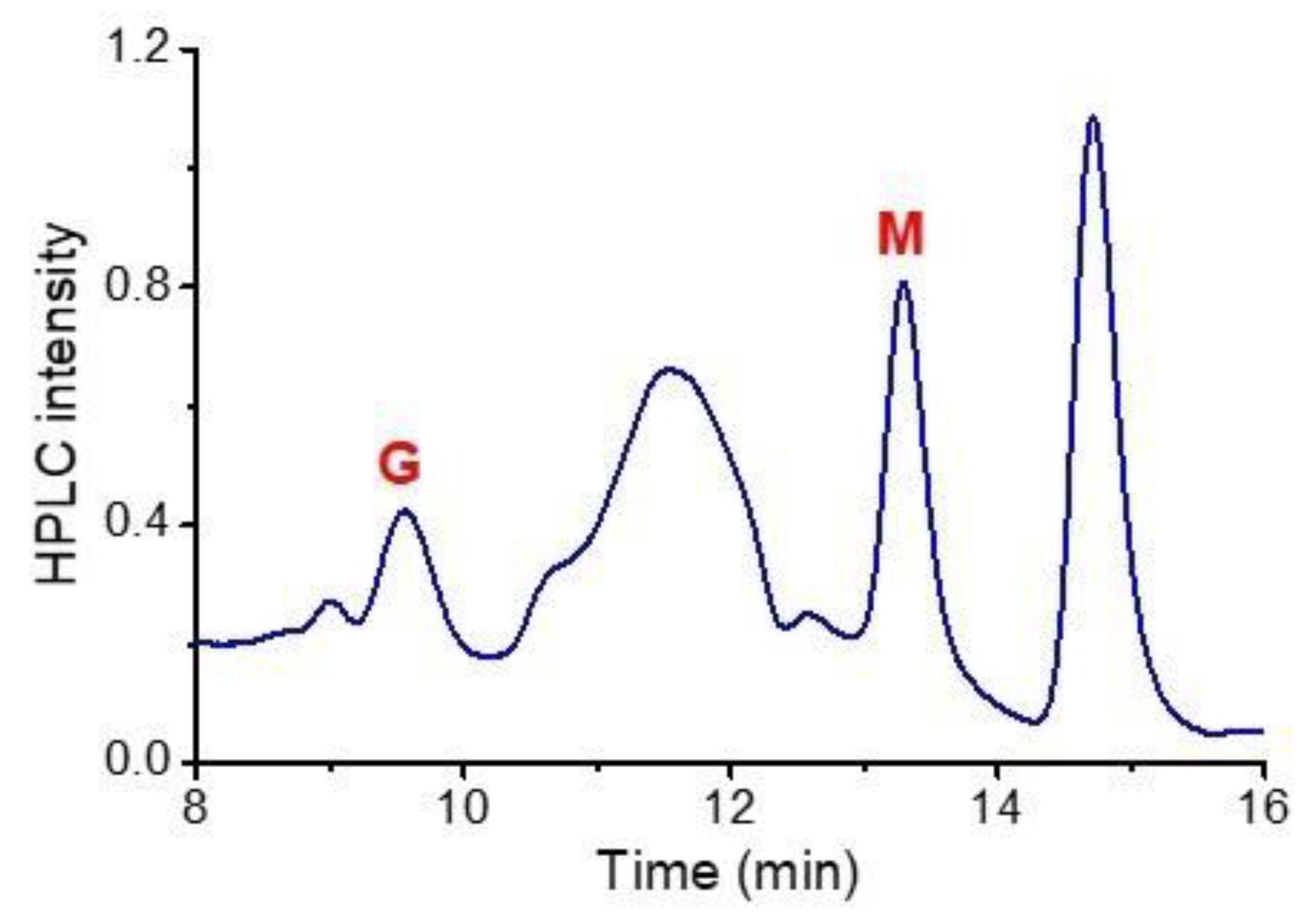
3.1. Characteristics of the Plasma-Activated Water Produced by the Soft Plasma Jet
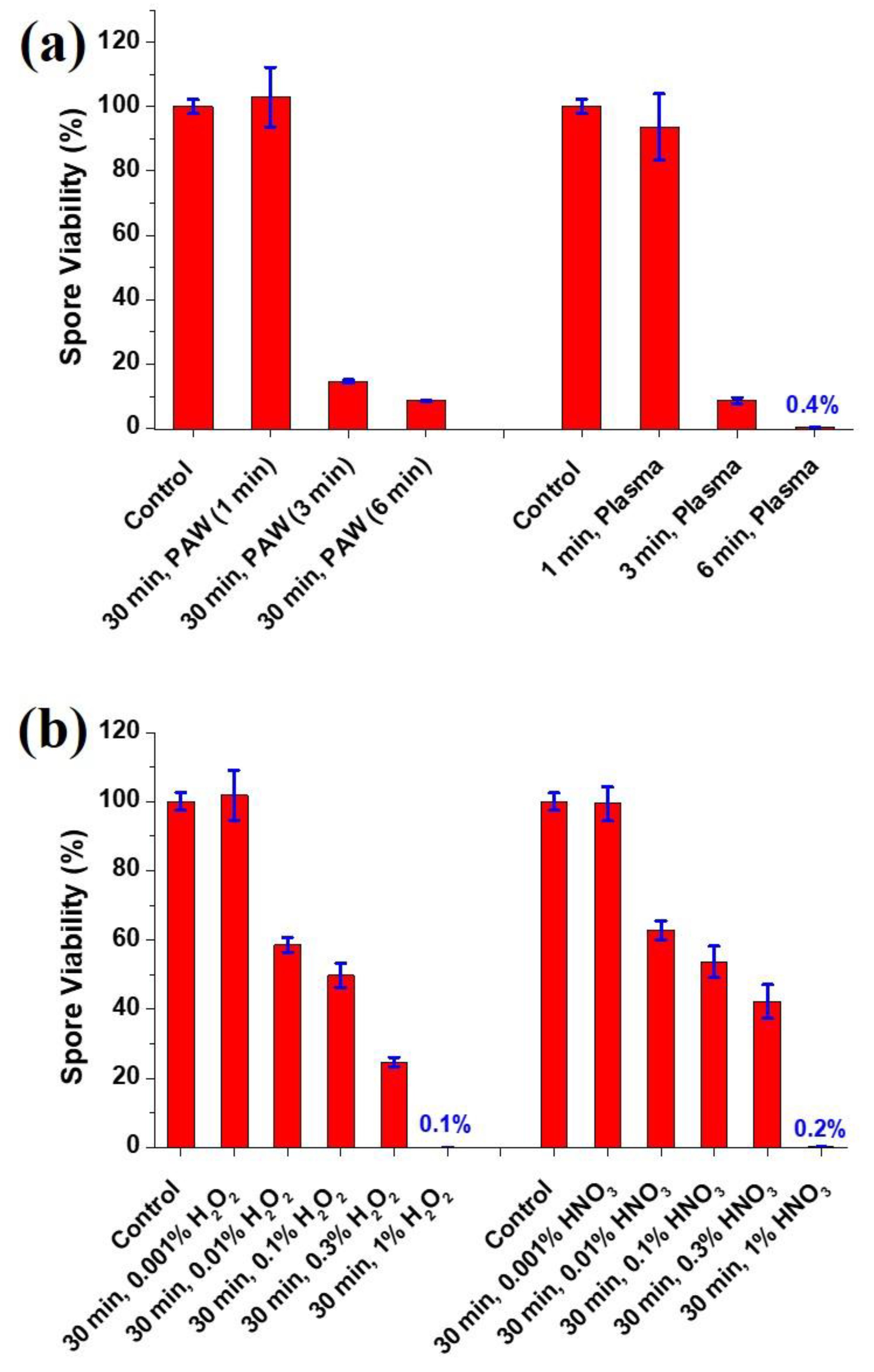
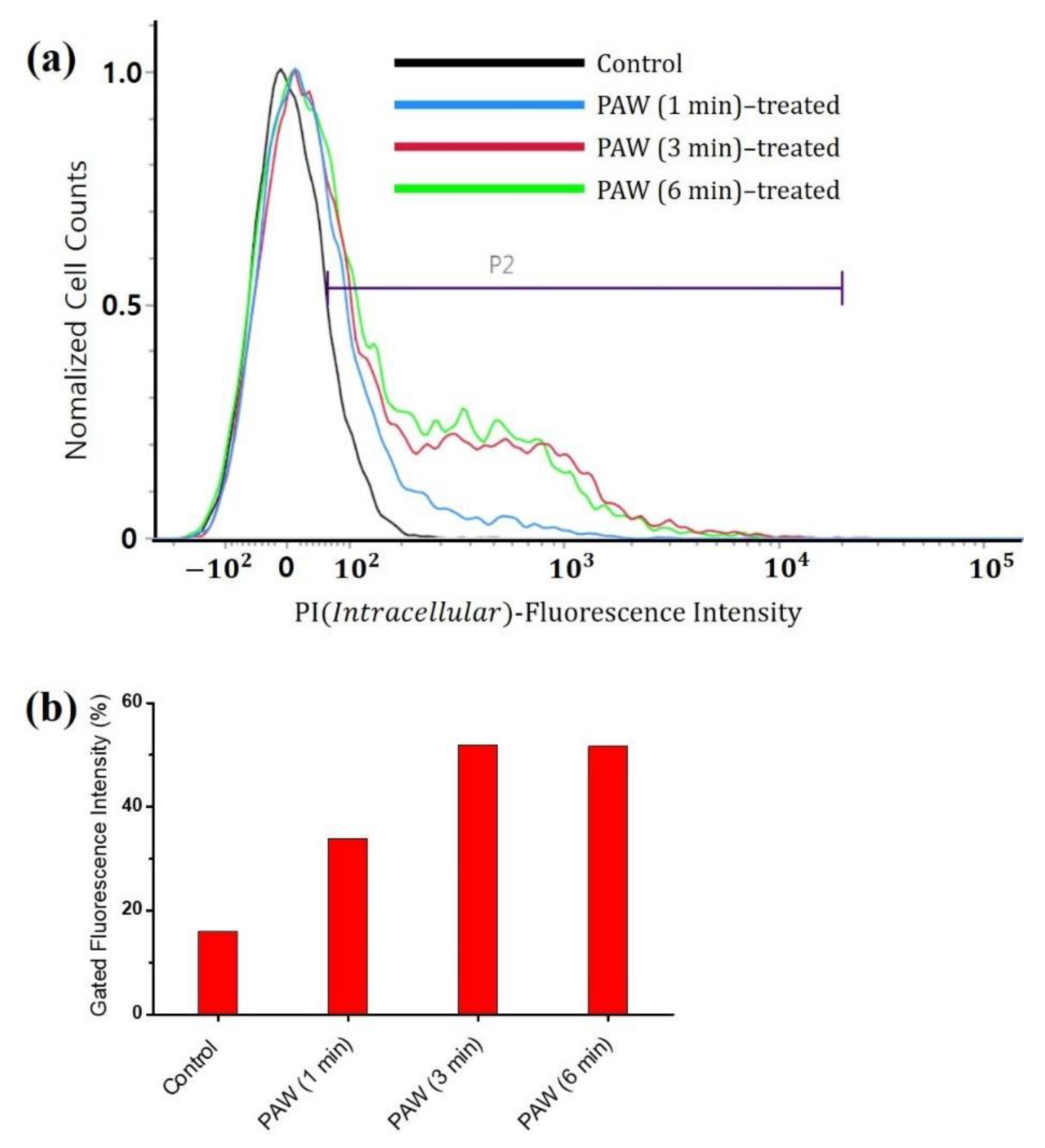
3.2. Viability of the A. brasiliensis Spores Treated with Plasma-Activated Water and Chemically Induced RONS Solutions
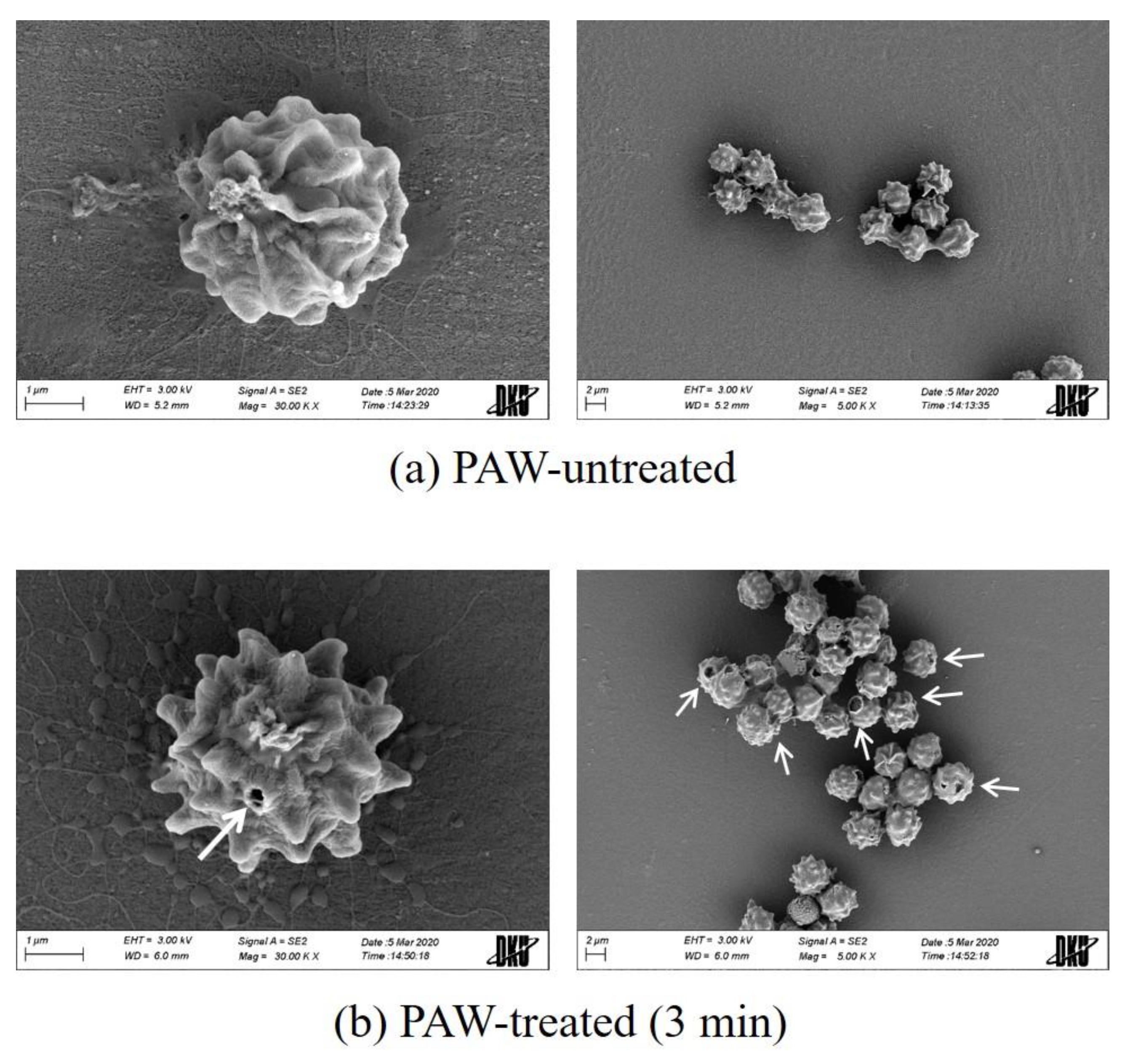
3.3. Morphology and Cell Wall Structure of the A. brasiliensis Spores Treated with Plasma-Activated Water
3.4. Electrophoretic Analysis of Genomic DNA Extracted from the A. brasiliensis Spores Treated with Plasma-Activated Water
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.M.; Vilgalys, R. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef]
- Schmit, J.P.; Mueller, G.M. An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 2007, 16, 99–111. [Google Scholar] [CrossRef]
- Hyde, K.D.; Al-Hatmi, A.M.S.; Andersen, B.; Boekhout, T.; Buzina, W.; Dawson, T.L., Jr.; Eastwood, D.C.; Jones, G.; de Hoog, S.; Kang, Y.; et al. The world’s ten most feared fungi. Fungal Divers. 2018, 93, 161–194. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Davis, S.; Alexiou, H.; Handke, R.; Bartley, R. Descriptions of Medical Fungi; University of Adelaide: Adelaide, Australia, 2007. [Google Scholar]
- Douglas, A.P.; Chen, S.C.A.; Slavin, M.A. Emerging infections caused by non-Aspergillus filamentous fungi. Clin. Microbiol. Infect. 2016, 22, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Worobo, R.W. Fungal spoilage in food processing. J. Food Protect. 2018, 81, 1035–1040. [Google Scholar] [CrossRef]
- Curtis, L.; Lieberman, A.; Stark, M.; Rea, W.; Vetter, M. Adverse health effects of indoor molds. J. Nutr. Environ. Med. 2004, 14, 261–274. [Google Scholar] [CrossRef]
- Boudaiffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science 2000, 287, 1658–1660. [Google Scholar]
- Madugundu, G.S.; Park, Y.; Sanche, L.; Wagner, J.R. Radiation-induced formation of 2′,3′-dideoxyribonucleosides in DNA: A potential signature of low-energy electrons. J. Am. Chem. Soc. 2012, 134, 17366–17368. [Google Scholar] [CrossRef][Green Version]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Li, Y.; Kang, M.H.; Uhm, H.S.; Lee, G.J.; Choi, E.H.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781. [Google Scholar] [CrossRef] [PubMed]
- Iza, F.; Kim, G.J.; Lee, S.M.; Lee, J.K.; Walsh, J.L.; Zhang, Y.T.; Kong, M.G. Microplasmas: Sources, particle kinetics, and biomedical applications. Plasma Process. Polym. 2008, 5, 322–344. [Google Scholar] [CrossRef]
- Lee, G.J.; Kwon, Y.W.; Kim, Y.H.; Choi, E.H. Raman spectroscopic study of plasma-treated salmon DNA. Appl. Phys. Lett. 2013, 102, 021911. [Google Scholar]
- Lackmann, J.W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Liu, F.; Sun, P.; Bai, N.; Tian, Y.; Zhou, H.; Wei, S.; Zhou, Y.; Zhang, J.; Zhu, W.; Becker, K.; et al. Inactivation of bacteria in an aqueous environment by a direct-current, cold-atmospheric-pressure air plasma microjet. Plasma Process. Polym. 2010, 7, 231–236. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.; Bai, N.; Zhou, H.; Wang, R.; Feng, H.; Zhu, W.; Zhang, J.; Fang, J. Inactivation of Bacillus subtilis spores in water by a direct-current, cold atmospheric-pressure air plasma microjet. Plasma Process. Polym. 2012, 9, 157–164. [Google Scholar] [CrossRef]
- Lee, G.J.; Choi, M.A.; Kim, D.; Kim, J.Y.; Ghimire, B.; Choi, E.H.; Kim, S.H. Influence of plasma-generated reactive species on the plasmid DNA structure and plasmid-mediated transformation of Escherichia coli cells. J. Appl. Phys. 2017, 122, 103303. [Google Scholar] [CrossRef]
- Lee, G.J.; Sim, G.B.; Choi, E.H.; Kwon, Y.W.; Kim, J.Y.; Jang, S.; Kim, S.H. Optical and structural properties of plasma-treated Cordyceps bassiana spores as studied by circular dichroism, absorption, and fluorescence spectroscopy. J. Appl. Phys. 2015, 117, 023303. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, I.H.; Kim, D.; Kim, S.H.; Kwon, Y.W.; Han, G.H.; Cho, G.; Choi, E.H.; Lee, G.J. Effects of reactive oxygen species on the biological, structural, and optical properties of Cordyceps pruinosa spores. RSC Adv. 2016, 6, 30699–30709. [Google Scholar] [CrossRef]
- Noh, H.; Kim, J.E.; Kim, J.Y.; Kim, S.H.; Han, I.; Lim, J.; Ki, S.H.; Choi, E.H.; Lee, G.J. Spore viability and cell wall integrity of Cordyceps pruinosa treated with an electric shock-free, atmospheric-pressure air plasma jet. Appl. Sci. 2019, 9, 3921. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Inactivation efficacies and mechanisms of gas plasma and plasma-activated water against Aspergillus flavus spores and biofilms: A comparative study. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Kocsube, S.; Toth, B.; Frisvad, J.C.; Perrone, G.; Susca, A.; Meijer, M.; Samson, R.A. Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution. Int. J. Syst. Evol. Microbiol. 2007, 57, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.C.; van Dijck, P.W.M. On the safety of Aspergillus niger—A review. Appl. Microbiol. Biotech. 2002, 59, 426–435. [Google Scholar]
- Yun, Y.H.; Hyun, M.W.; Suh, D.Y.; Kim, Y.M.; Kim, S.H. Identification and characterization of Eurotium rubrum isolated from Meju in Korea. Mycobiology 2009, 37, 251–257. [Google Scholar] [CrossRef]
- Aamir, S.; Sutar, S.; Singh, S.K.; Baghela, A. A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. Plant Pathol. Quar. 2015, 5, 74–81. [Google Scholar] [CrossRef]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Mol. Biol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef]
- Uhm, H.S. Generation of various radicals in nitrogen plasma and their behavior in media. Phys. Plasmas 2015, 22, 123506. [Google Scholar] [CrossRef]
- Uhm, H.S.; Na, Y.H.; Lee, C.B.; Choi, E.H.; Cho, G. Dissociation and excitation coefficients of nitrogen molecules and radical generation in nitrogen plasma. Curr. Appl. Phys. 2014, 14, S162–S166. [Google Scholar] [CrossRef]
- Bibinov, N.; Knake, N.; Bahre, H.; Awakowicz, P.; Schulz-von der Gathen, V. Spectroscopic characterization of an atmospheric pressure μ-jet plasma source. J. Phys. D Appl. Phys. 2011, 44, 345204. [Google Scholar] [CrossRef]
- Deng, X.L.; Nikiforov, A.Y.; Vanraes, P.; Leys, C. Direct current plasma jet at atmospheric pressure operating in nitrogen and air. J. Appl. Phys. 2013, 113, 023305. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- van Gils, C.A.J.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Fukuto, J.M.; Griscavage, J.M.; Rogers, N.E.; Byrns, R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: Comparison with enzymatically formed nitric oxide from l-arginine. Proc. Natl. Acad. Sci. USA 1993, 90, 8103–8107. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.; Rossi, M.J.; Ross, D.S. Kinetic and mechanistic aspects of the NO oxidation by O2 in aqueous phase. Int. J. Chem. Kinet. 1994, 26, 1207–1227. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/ radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- de Grey, A.D.N.J. : The forgotten radical. DNA Cell Biol. 2002, 21, 251–257. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ohshima, H. DNA damage induced by peroxynitrite: Subsequent biological effects. Nitric Oxide 1997, 1, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, T.; Miyake, T.; Hirakawa, T.; Sakugawa, H. pH-dependent photoformation of hydroxyl radical and absorbance of aqueous-phase N (III) (HNO2 and ). Environ. Sci. Technol. 1999, 33, 2561–2565. [Google Scholar] [CrossRef]
- Riordan, E.; Minogue, N.; Healy, D.; O’Driscoll, P.; Sodeau, J.R. Spectroscopic and optimization modeling study of nitrous acid in aqueous solution. J. Phys. Chem. A 2005, 109, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, C.; Chu, L. Photochemistry of nitrous acid (HONO) and nitrous acidium ion (H2ONO+) in aqueous solution and ice. Environ. Sci. Technol. 2009, 43, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, J.S.; Marley, N.A.; Cunningham, M.M. Measurement of the absorption constants for nitrate in water between 270 and 335 nm. Environ. Sci. Technol. 1992, 26, 207–209. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–281. [Google Scholar] [CrossRef]
- Gessler, N.N.; Averyanov, A.A.; Belozerskaya, T.A. Reactive oxygen species in regulation of fungal development. Biochemistry 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a major antioxidant: When, what for and why it fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef]
- Ghimire, B.; Lee, G.J.; Mumtaz, S.; Choi, E.H. Scavenging effects of ascorbic acid and mannitol on hydroxyl radicals generated inside water by an atmospheric pressure plasma jet. AIP Adv. 2018, 8, 075021. [Google Scholar] [CrossRef]
- Johnston, I.R. The composition of the cell wall of Aspergillus niger. Biochem. J. 1965, 96, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, C.F.B.; Visser, J. Polyol pools in Aspergillus niger. FEMS Microbiol. Lett. 1995, 134, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, G.J.G.; Bax, M.; Patel, H.; Flitter, S.J.; van de Vondervoort, P.J.I.; de Vries, R.P.; van Kuyk, P.A.; Visser, J. Mannitol is required for stress tolerance in Aspergillus niger conidiospores. Eukaryot. Cell 2003, 2, 690–698. [Google Scholar] [CrossRef]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The mechanistic targets of antifungal agents: An overview. Mini Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Macheleidt, J.; Foge, M.; Brakhage, A.A. The Aspergillus fumigatus cell wall integrity signaling pathway: Drug target, compensatory pathways, and virulence. Front. Microbiol. 2015, 6, 325. [Google Scholar] [CrossRef]
- Qin, G.; Liu, J.; Cao, B.; Li, B.; Tian, S. Hydrogen peroxide acts on sensitive mitochondrial proteins to induce death of a fungal pathogen revealed by proteomic analysis. PLoS ONE 2011, 6, e21945. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.G.; Mascio, P.D.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA damage as a source of genomic instability in cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef]
- Burrows, C.J.; Muller, J.G. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1152. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ki, S.H.; Noh, H.; Ahn, G.R.; Kim, S.H.; Kaushik, N.K.; Choi, E.H.; Lee, G.J. Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus brasiliensis Spores. Appl. Sci. 2020, 10, 6378. https://doi.org/10.3390/app10186378
Ki SH, Noh H, Ahn GR, Kim SH, Kaushik NK, Choi EH, Lee GJ. Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus brasiliensis Spores. Applied Sciences. 2020; 10(18):6378. https://doi.org/10.3390/app10186378
Chicago/Turabian StyleKi, Se Hoon, Hyeongjin Noh, Geum Ran Ahn, Seong Hwan Kim, Nagendra K. Kaushik, Eun Ha Choi, and Geon Joon Lee. 2020. "Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus brasiliensis Spores" Applied Sciences 10, no. 18: 6378. https://doi.org/10.3390/app10186378
APA StyleKi, S. H., Noh, H., Ahn, G. R., Kim, S. H., Kaushik, N. K., Choi, E. H., & Lee, G. J. (2020). Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus brasiliensis Spores. Applied Sciences, 10(18), 6378. https://doi.org/10.3390/app10186378







