Abstract
The effect of ester gum, a widely used weighting agent, on Ostwald ripening in model beverage emulsions formulated using different food-grade surfactants was examined. A microfluidizer was used to prepare 5% orange oil-in-water emulsions stabilized by a series of ethylene glycol alkyl ether surfactants. Emulsions prepared using only orange oil exhibited an appreciable increase in droplet size during a 14-day storage, independent of surfactant type or concentration. Incorporation of ester gum into the oil phase of the emulsions effectively inhibited droplet growth at concentrations ≥20%. The inhibition of droplet growth by ester gum depended on the surfactant type (hydrophilic group size) and concentration. Overall, ester gum stabilized the emulsions by acting as an Ostwald ripening inhibitor, as well as a weighting agent.
1. Introduction
As flavoring agents, essential oils are generally incorporated into beverage products in an emulsified form because of their poor water-solubility [1]. Essential oil emulsions are more prone to gravitational separation (creaming) than vegetable oil emulsions because the density contrast between essential oils and water is greater than that between vegetable oils and water [2]. Emulsified essential oils are also highly prone to droplet growth caused by Ostwald ripening [3]. Ostwald ripening occurs via the diffusion of oil molecules from smaller droplets to larger droplets through the aqueous solution that separates them [4]. This phenomenon does not usually cause destabilization of emulsified vegetable oils because of the extremely low water-solubility of long-chain triglycerides. Conversely, it promotes destabilization of emulsified essential oils because of the much higher water-solubilities of these oils [5]. The increase in droplet size caused by Ostwald ripening also increases their susceptibility to creaming and coalescence. Consequently, to confer long-term stability to emulsified essential oils, it is important to inhibit or prevent both creaming and Ostwald ripening.
Creaming can be prevented by reducing the density gradient between the oil and aqueous phases, thereby extending the shelf life of the emulsion. In beverage applications, weighting agents are typically added to the oil phase of essential oil emulsions to increase its density and make it more similar to that of the surrounding aqueous phase, thereby reducing the driving force for gravitational separation [6]. According to previous studies, the rate of Ostwald ripening in emulsions is affected by the initial droplet size distribution, the water-solubility of the used oils, and the oil phase composition [1,7]. The tendency for emulsified essential oils to undergo Ostwald ripening can be reduced by incorporating strongly hydrophobic oils into the oil phase prior to homogenization [8]. These strongly hydrophobic oils are usually referred to as ripening inhibitors and have a very low water-solubility. Ripening inhibitors slow down droplet growth due to an entropy of mixing effect that counter-balances the interfacial curvature effect that drives Ostwald ripening. As the water-soluble essential oils move from smaller to large droplets, their concentration in the small droplets decreases whereas their concentration in the large droplets increases. As a result, there is a concentration gradient, which generates an osmotic pressure, which favors the movement of essential oil molecules from the larger to the smaller oil droplets—a process known as compositional ripening. After a certain time, the Ostwald ripening and compositional ripening processes balance each other and droplet growth is inhibited.
Weiss et al. [9] found that the molecular characteristics of the surfactants coating the oil droplets in emulsions affected the diffusion of oil molecules from the droplets. The surfactant type may impact molecular diffusion processes in emulsions by altering the interfacial tension, thickness, rigidity, or permeability. For instance, Meinders and van Vliet [10] reported that sufficiently rigid interfacial coatings could inhibit Ostwald ripening by resisting droplet shrinkage and expansion. Similarly, it has been reported that multilayer-coatings formed by electrostatic deposition can inhibit Ostwald ripening, which was attributed to their increased rigidity [11,12]. We therefore hypothesized that the surfactant type would impact the rate of Ostwald ripening in model beverage emulsions, and selection of an appropriate surfactant may be a useful tool for slowing down droplet growth through this process.
In this study, we examined the combined effects of ripening inhibitor concentration on the stability of model beverage emulsions (emulsified orange oil) stabilized using poly (ethylene glycol) alkyl ether surfactants having various hydrophilic head sizes. Ester gum, which is typically prepared by esterification of refined wood resin with food-grade glycerol, is widely used as a weighting agent in the beverage industry for stabilizing essential oil emulsions by increasing the specific gravity of the oil phase [6]. However, it has been shown that ester gum can also act as an effective ripening inhibitor for suppressing droplet growth in essential oil emulsions caused by Ostwald ripening [13]. This effect is attributed to the extremely low water-solubility of the ester gum. Previous studies have reported that the rate of Ostwald ripening in emulsified corn oil/orange oil mixtures was strongly dependent on interfacial characteristics [9,14]. In the current study, we focused on the impact of interfacial characteristics on the effectiveness of ester gum to inhibit Ostwald ripening in orange oil emulsions, because ester gum is one of the most widely used weighting agents in the beverage industry. Interfacial characteristics were varied by using different kinds of food-grade surfactants to formulate the model beverage emulsions.
2. Materials and Methods
2.1. Materials
Poly(ethylene glycol) alkyl ether surfactants (poly(oxyethylene glycol) (10) stearyl ether (P10SE), poly(oxyethylene glycol) (20) stearyl ether (P20SE), poly(oxyethylene glycol) (23) lauryl ether (P23LE), and poly(oxyethylene glycol) (100) stearyl ether (P100SE) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The molecular structures of the poly(ethylene glycol) alkyl ether surfactants used in this study are present in Figure 1. Orange oil was purchased from Ernesto Ventós (Barcelona, Spain) and food-grade ester gum (Starez3136GF) was supplied by Forestar Chemical Co., Ltd. (Guangzhou, China). All other chemicals were of a reagent grade.
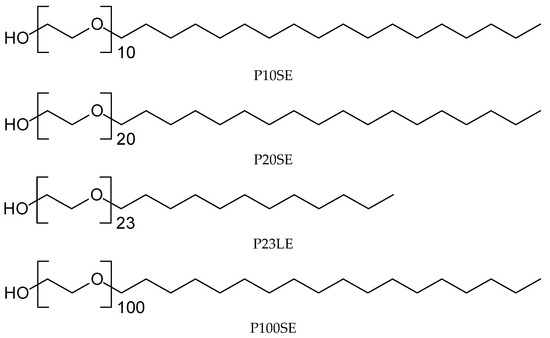
Figure 1.
Molecular structures of ethylene glycol alkyl ether emulsifiers.
2.2. Emulsion Preparation
Initially, aqueous phases were prepared by dispersing P10SE, P20SE, P23L, and P100SE in 10 mM phosphate buffer solutions (pH 7) to 1.00, 1.79, 2.93, and 3.17 mM, respectively, while oil phases were prepared by dissolving different concentrations of ester gum in orange oil (0, 2.5, 5, 10, and 20% w/w). Coarse emulsions were prepared by homogenizing the oil phase (5%, w/w) and aqueous phase (95%, w/w) for 2 min at 25 °C using a high-speed mixer (T18 Basic Ultra-Turrax, Ika, Staufen, Germany) at 11,000 rpm. Fine emulsions were then prepared by homogenizing the coarse emulsions five times using a microfluidizer (MN400BF, Micronox, Seongnam, Korea) at 100 MPa. The resulting fine emulsions were then stored at 25 °C in the dark.
2.3. Determination of Droplet Size
The change in the droplet size of the orange oil emulsions was monitored periodically. Emulsions were gently stirred for 2 min and then diluted to a droplet concentration of ≈0.005% (w/w) using a 10 mM phosphate buffer solution (pH 7) to prevent multiple scattering effects. The particle size distributions of diluted emulsions were measured using a laser diffraction particle size analyzer (BT-9300ST; Bettersize Instruments, Dandong, China) with continuous stirring to ensure homogeneity. The particle size data are reported as the volume-weighted mean diameter, , where is the number of particles with diameter .
2.4. Interfacial Tension Measurement
Interfacial tensions between orange oil and surfactant solutions were determined using a spinning-drop tensiometer (Krüss, Hamburg, Germany) at 30 °C. A drop of orange oil (2 µL) was injected into the rotating capillary containing the surfactant solution kept at a constant temperature of 30 °C. After allowing sufficient time to ensure the equilibrium at rotating velocities between 2500 and 5500 rpm at 30 °C, the average value obtained at 5 s intervals between 750 and 1000 s was used as the interfacial tension. For calibration of spinning drop tensiometer, an air bubble was injected using a microliter syringe to a rotating capillary tube containing the distilled/deionized water at 30 °C.
2.5. Viscosity Measurement
The viscosity of the oil phase was measured using a sine-wave vibro-viscometer (SV-10, A & D Company, Tokyo, Japan) at 25 °C. This viscometer measures viscosity by detecting the drive current necessary to resonate the two sensor plates at a constant frequency of 30 Hz.
2.6. Statistical Analysis
All the experiments were performed in triplicate and the data are expressed as the mean ± standard deviation. Analysis of variance (ANOVA) was performed and mean separations were performed using Duncan’s multiple-range test (p < 0.05). All statistical analyses described above were conducted using the SPSS software version 20.0 (IBM Corp., Armonk, NY, USA).
3. Results and Discussion
3.1. Impact of Surfactant Type and Concentration of Emulsion Stability
Previous studies have shown that the concentration of certain types of surfactant may impact the rate of Ostwald ripening in an emulsion [15]. This effect is mainly attributed to the ability of non-adsorbed surfactants to form micelles that can solubilize and transport non-polar molecules between the oil droplets. Excess ionic surfactants typically have little impact on the rate of Ostwald ripening [16,17], which may be because the strong electrostatic repulsion between the oil droplets and surfactant micelles keeps them apart. Conversely, excess nonionic surfactants may accelerate Ostwald ripening [18], possibly because these micelles can transfer oil molecules between droplets. Therefore, to investigate the impact of the type of surfactant used to create emulsions and ester gum on the Ostwald ripening of orange oil emulsions, it is important to minimize the effect of the surfactant micelles on the Ostwald ripening of orange oil emulsions. Based on our previous study, the concentrations of P100SE, P23LE, P20SE, and P10SE were determined to produce medium chain triglyceride emulsions where there was little non-adsorbed surfactant present and they were 1.00, 1.79, 2.93, and 3.17 mM for P10SE, P20SE, and P100SE, respectively [14].
The initial droplet size of all the emulsions was not significantly different (Table 1), suggesting that all the surfactants were effective at forming small oil droplets during homogenization. Droplet growth was observed immediately after homogenization in all of the emulsions (Figure 2). The observed droplet growth may be due to coalescence and/or Ostwald ripening. The size of the droplets in the P100SE-stabilized emulsions increased progressively throughout storage leading to a relatively large final mean particle diameter, whereas the size of the droplets in the other emulsions leveled off after 1-day storage leading to much smaller mean particle diameters. The droplet growth profile of the P100SE-stabilized orange oil emulsion was very similar to the finding in the previous studies, which highlights its susceptibility to Ostwald ripening [14]. However, the droplet growth profiles of P10SE-, P20SE-, and P23LE-stabilized emulsions differed from the previous findings [14]. The extent of droplet growth did depend on the nature of the surfactant used. The influence of surfactant type on droplet growth may have been due to their impact on surface properties, such as interfacial tension, thickness, rigidity, or permeability [1]. Ostwald ripening theory predicts that the rate of droplet growth should increase as the oil-water interfacial tension increases, because the free energy change associated with droplet curvature differences become greater [4]. For this reason, the interfacial tensions of oil-water interfaces saturated with the different surfactants were measured: 0.205d, 0.302c, 0.385b, and 0.589a mN/m for P10SE, P20SE, P23LE, and P100SE, respectively. Here, the values with different superscripts were found to be significantly different using Duncan’s multiple range test (p < 0.05). Although orange oil is quite polar, these interfacial tension values seemed smaller than the values reported in the previous study [19]. It was possibly due to the various compositions of commercial orange oils [20] and it may be because the interfacial tension measurement method was different from the previous studies [19]. Since increasing the hydrophilic size (or length) in surfactants decreases the effectiveness of reducing water surface tension, according to the previous studies [21,22], therefore, the difference in interfacial tension between P10SE- and P20SE-stabilized emulsions came from the difference in the number of oxyethylene groups in their hydrophilic groups because the surfactant concentration of the P10SE- and P20SE-stabilized emulsions was similar (surfactant concentration in the P10SE-stabilized emulsion was only 1.08 times greater than in the P20SE-stabilized one). The observed differences in the resistance of the emulsions to droplet growth could therefore be due to differences in the interfacial tension values associated with the different surfactants. In particle, the surfactant that gave the highest interfacial tension (P100SE) also gave the highest instability to Ostwald ripening, which is consistent with the predictions of the theory. When emulsions have the same interfacial membrane to a densely or loosely packed one, their long-term stability to Ostwald ripening might depend on the size of hydrophilic group of surfactants, attributed to the interfacial tension.

Table 1.
Effect of ester gum concentration in the oil phase on the apparent viscosity and the initial droplet diameters (d43, μm) of orange oil emulsions stabilized using various emulsifiers.
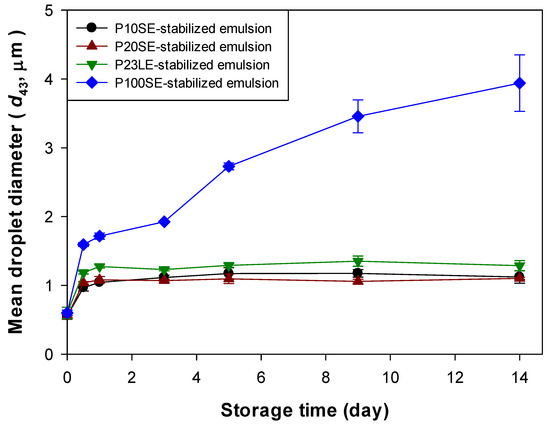
Figure 2.
Changes in the mean droplet diameter (volume-length mean diameter, d43) of orange oil-in-water emulsions stabilized with ethylene glycol alkyl ether emulsifiers.
3.2. Influence of Ester Gum Addition on Ostwald Ripening
Strongly hydrophobic compounds can be incorporated into the oil phase of essential oil emulsions to inhibit Ostwald ripening [23,24]. Ester gum is widely used in the beverage industry as a weighting agent [1], but it may play a dual role in stabilizing beverage emulsions by also acting as a ripening inhibitor. The main constituents in ester gum are strongly hydrophobic (Figure 3) [25], which means that they have a very low water-solubility. For this reason, we examined the impact of ester gum addition on the stability of model beverage emulsions formulated using different kinds of surfactant.
Figure 3.
Molecular structures of the main components in commercially available ester gum.
As shown in Figure 4, the addition of ester gum to the oil phase prior to homogenization retarded droplet growth in the emulsified orange oil during storage, which suggests that it was effective at inhibiting Ostwald ripening. The presence of the ester gum also impacted the droplet size of the initial emulsions, in a manner that depended on the surfactant type (Table 1). The initial droplet size of the P100SE-stabilized emulsions did not depend on the ester gum content. Conversely, there was a significant decrease in the initial droplet size of the orange oil emulsions stabilized using P10SE, P20SE, and P23LE at the highest ester gum level used (20% in oil phase). These results suggest that the presence of the ester gum either facilitated droplet disruption in the homogenizer during emulsion formation, or more likely, inhibited droplet growth promoted by Ostwald ripening during and after homogenization. Under fixed homogenization conditions, the efficiency of droplet disruption usually decreases with an increase in the viscosity of the oil phase or the oil-water interfacial tension [26]. In our study, the viscosity of the oil phase increased as the ester gum concentration increased (Table 1). Moreover, the interfacial tension increased as the amount of ester gum added to the orange oil increased. For instance, the interfacial tension was 0.205 (100%), 0.237 (116%), 0.231 (113%), 0.240 (117%), and 0.280 (139%) mN/m for P10SE-stabilized emulsions containing 0, 2.5, 5, 10, and 20% ester gum, respectively. Similarly, it was 0.589 (100%), 0.593 (101%), 0.620 (105%), 0.666 (113%), and 0.797 (135%) mN/m for the P10SE-stabilized emulsions containing 0, 2.5, 5, 10, and 20% ester gum, respectively. Consequently, one would expect that the initial droplet size should increase with the increasing ester gum level. Therefore, our results suggest that the reduction of droplet size with the increasing ester gum concentration was more likely due to its ability to inhibit droplet growth during homogenization, than its ability to facilitate droplet breakup.
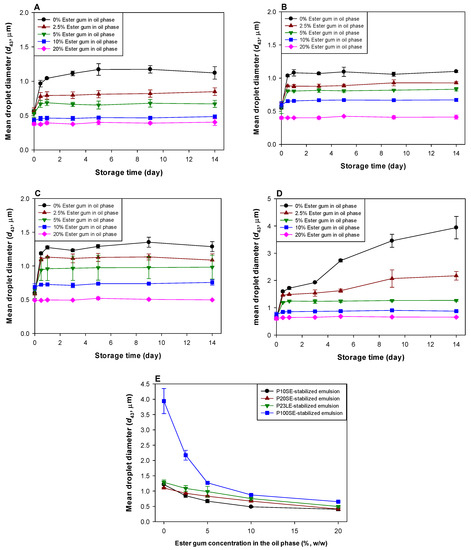
Figure 4.
Influence of the addition of ester gum into the oil phase on the mean droplet diameter (volume-length mean diameter, d43) of orange oil emulsions stabilized with ethylene glycol alkyl ether emulsifiers. (A): P10SE-stabilized emulsion, (B): P20SE-stabilized emulsion, (C): P23LE-stabilized emulsion, (D): P100SE-stabilized emulsion, (E): droplet size in emulsions after 14-day storage. For changes in the mean droplet diameter (area-volume mean diameter, d32) of orange oil emulsions, see Supplementary Figure S1.
Ostwald ripening was not observed in any of the emulsions when the ester gum concentration in the oil phase was 20% (Figure 4), which can be attributed to an “entropy of mixing” phenomenon [1]. When the oil phase of an emulsion consists of a mixture of a water-soluble oil (such as essential oil) and a water-insoluble oil (such as ester gum), the water-soluble oils diffuse from the small to the large droplets much faster than the water-insoluble ones. Eventually, the concentration of water-soluble oil is higher in the smaller droplets than in the larger ones (and vice versa), leading to a concentration gradient in the system. A concentration gradient between the oil droplets is thermodynamically unfavorable due to the entropy of mixing effects, which favors a net movement of the water-soluble oil from the larger to the smaller droplets. Eventually, the driving force for the entropy of mixing is the same as the driving force for Ostwald ripening, after which the droplet size and composition remain constant. Our results suggest that 20% of ester gum was sufficient to completely inhibit Ostwald ripening in the orange oil emulsions during or just after homogenization, independent of the surfactant type or concentration used.
At lower ester gum levels (e.g., 2.5, 5 or 10%), a steep increase in droplet size was observed in all the emulsions during the first day of storage, after which it remained relatively constant in most systems. This result suggests that these levels of ester gum were not sufficient to inhibit Ostwald ripening. Nevertheless, the final extent of droplet growth did decrease as the ester gum level was raised, which suggests that Ostwald ripening was arrested sooner. In general, it has been shown that the rate and extent of Ostwald ripening in an emulsion containing mixed oils depends on the volume fraction and water solubility of the oils [27].
Our results also showed that the ability of the ester gum to inhibit Ostwald ripening was influenced by the nature of the surfactant used to coat the oil droplets (Figure 4). These effects may have been due to differences in the packing and interactions of the surfactant molecules at the droplet surfaces, which could influence the swelling or shrinking of the oil droplets, as well as the transport of oil molecules across the surfactant monolayer. For instance, ester gum inhibited Ostwald ripening more effectively in the emulsions stabilized by surfactants with a small hydrophilic group (P10SE) than those stabilized with surfactants having a large hydrophilic group (P20SE). This may have been because the surfactants with a smaller hydrophilic group were packed together more tightly at the droplet surfaces, which made it more difficult for oil molecules to diffuse through.
According to the previous study [28], the type and concentration of emulsifiers determine whether coalescence or Ostwald ripening mainly contributes to the growth of the droplets in emulsions containing relatively polar oils. The ability of emulsifiers to solidify the interfacial layer adsorbing them is also one of important factors controlling coalescence and/or Ostwald ripening. Dimitrova, Boneva, Danov, Kralchevsky, Basheva, Marinova, Petkov, and Stoyanov [28] reported that Ostwald ripening in emulsions prepared from limonene was not observed. Therefore, it should be noted that droplet coalescence could also contribute to the droplet growth within the homogenizer and during storage in this study. However, we believe that our results are better explained by the Ostwald ripening mechanism. First, since limonene, the major component (94–96%, depending on biological origin) in orange oil, could be dissolved in water to 13.8 mg/L [29], orange oil is known to be relatively polar oil [13,24], which makes it highly susceptible to Ostwald ripening. Ostwald ripening tends to occur more rapidly when the droplet size is small, which is a possible reason why Ostwald ripening occurred rapidly in our study, unlike the previous finding [28] (droplets prepared in this study were approximately 10 times smaller than those reported in the previous study). Second, the ability of ester gum to inhibit droplet growth is also consistent with this mechanism due to the entropy of mixing effect mentioned earlier.
4. Conclusions
This research has shown that the molecular structures and concentration of surfactants affect the rate of Ostwald ripening in orange oil emulsions. It also showed that adding a sufficiently high concentration of ester gum to the oil phase inhibited Ostwald ripening. Ester gum is usually employed in the beverage industry as a weighting agent to stop creaming but this study shows that it can also act as a ripening inhibitor to retard droplet growth due to Ostwald ripening. However, the mechanism by which the molecular structures of the surfactants and their concentration affect the efficacy of ester gum as an Ostwald ripening inhibitor in emulsions must be further studied.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/16/5588/s1. Figure S1: Influence of the addition of ester gum into oil phase on (area-volume mean diameter, d32) of orange oil emulsions stabilized with ethylene glycol alkyl ether emulsifiers. A: P10SE-stabilized emulsion, B: P20SE-stabilized emulsion, C: P23LE-stabilized emulsion, D: P100SE-stabilized emulsions, E: droplet size in emulsions after 14-day storage.
Author Contributions
Conceptualization, S.J.C.; methodology, J.P. and J.L.; software, J.P. and J.L.; validation, J.P. and J.L.; formal analysis, J.P. and J.L.; investigation, J.P. and J.L.; writing—original draft preparation, S.J.C.; writing—review and editing, D.J.M. and S.J.C.; J.P. and J.L. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Research Program funded by the SeoulTech (Seoul National University of Science and Technology) (2019-0368).
Conflicts of Interest
The authors declare no conflict of interest.
References
- McClements, D.J. Food emulsions in practice. In Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; McClements, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 547–575. [Google Scholar]
- Noureddini, H.; Teoh, B.C.; Clements, L.D. Densities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1184–1188. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Kabalnov, A.S.; Shchukin, E.D. Ostwald ripening theory: Applications to fluorocarbon emulsion stability. Adv. Colloid Interface Sci. 1992, 38, 69–97. [Google Scholar] [CrossRef]
- Taisne, L.; Walstra, P.; Cabane, B. Transfer of oil between emulsion droplets. J. Colloid Interface Sci. 1996, 184, 378–390. [Google Scholar] [CrossRef]
- Piorkowski, D.T.; McClements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- De Smet, Y.; Danino, D.; Deriemaeker, L.; Talmon, Y.; Finsy, R. Ostwald ripening in the transient regime: A cryo-TEM study. Langmuir 2000, 16, 961–967. [Google Scholar] [CrossRef]
- McClements, D.J.; Henson, L.; Popplewell, L.M.; Decker, E.A.; Choi, S.J. Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J. Food Sci. 2012, 77, C33–C38. [Google Scholar] [CrossRef]
- Weiss, J.; Cancelliere, C.; McClements, D.J. Mass transport phenomena in oil-in-water emulsions containing surfactant micelles: Ostwald ripening. Langmuir 2000, 16, 6833–6838. [Google Scholar] [CrossRef]
- Meinders, M.B.J.; van Vliet, T. The role of interfacial rheological properties on Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 2004, 108-109, 119–126. [Google Scholar] [CrossRef]
- Mun, S.; McClements, D.J. Influence of interfacial characteristics on Ostwald ripening in hydrocarbon Oil-in-water emulsions. Langmuir 2006, 22, 1551–1554. [Google Scholar] [CrossRef]
- Zeeb, B.; Gibis, M.; Fischer, L.; Weiss, J. Influence of interfacial properties on Ostwald ripening in crosslinked multilayered oil-in-water emulsions. J. Colloid Interface Sci. 2012, 387, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Baik, M.Y.; Decker, E.A.; Henson, L.; Popplewell, L.M.; McClements, D.J.; Choi, S.J. Stabilization of orange oil-in-water emulsions: A new role for ester gum as an Ostwald ripening inhibitor. Food Chem. 2011, 128, 1023–1028. [Google Scholar] [CrossRef]
- Han, S.W.; Song, H.Y.; Moon, T.W.; Choi, S.J. Influence of emulsion interfacial membrane characteristics on Ostwald ripening in a model emulsion. Food Chem. 2018, 242, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ariyaprakai, S.; Dungan, S.R. Influence of surfactant structure on the contribution of micelles to Ostwald ripening in oil-in-water emulsions. J. Colloid Interface Sci. 2010, 343, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kabalnov, A.S. Can micelles mediate a mass transfer between oil droplets? Langmuir 1994, 10, 680–684. [Google Scholar] [CrossRef]
- Hoang, T.K.N.; La, V.B.; Deriemaeker, L.; Finsy, R. Ostwald ripening of alkane in water emulsions stabilized by sodium dodecyl benzene sulfonate. Langmuir 2002, 18, 10086–10090. [Google Scholar] [CrossRef]
- Weiss, J.; Herrmann, N.; McClements, D.J. Ostwald ripening of hydrocarbon emulsion droplets in surfactant solutions. Langmuir 1999, 15, 6652–6657. [Google Scholar] [CrossRef]
- Arneodo, C.; Baszkin, A.; Benoit, J.P.; Fellous, R.; Thies, C. Interfacial studies of essential oil-water systems. Colloid Surf. 1988–1989, 34, 159–169. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Impact of lemon oil composition on formation and stability of model food and beveage emulsions. Food Chem. 2012, 134, 749–757. [Google Scholar] [CrossRef]
- Smit, B.; Schlijper, A.G.; Rupert, L.A.M.; van Os, N.M. Effects of chain length of surfactants on the interfacial tension: Molecular dynamics simulations and experiments. J. Phys. Chem. 1990, 94, 6933–6935. [Google Scholar] [CrossRef]
- Zhang, T.; Marchant, R.E. Novel polysaccharide surfactants: The effect of hydrophobic and hydrophilic chain length on surface active properties. J. Colloid Interface Sci. 1996, 177, 419–426. [Google Scholar] [CrossRef]
- Ryu, V.; McClements, D.J.; Corradini, M.G.; McLandsborough, L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018, 245, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Park, J.; Song, H.Y.; Choi, S.J. Ostwald ripening rate of orange oil emulsions: Effects of molecular structure of emulsifiers and their oil composition. J. Food Sci. 2019, 84, 440–447. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of glycerol esters of wood rosin (E 445) as a food additive. EFSA J. 2018, 16, e05370. [Google Scholar] [CrossRef] [PubMed]
- Pandolfe, W.D. Effect of dispersed and continuous phase viscosity on droplet size of emulsions generated by homogenization. J. Dispers. Sci. Technol. 1981, 2, 459–474. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion stability. In Food Emulsions: Principles, Practice, and Techniques, 3rd ed.; McClements, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 289–382. [Google Scholar]
- Dimitrova, L.M.; Boneva, M.P.; Danov, K.D.; Kralchevsky, P.A.; Basheva, E.S.; Marinova, K.G.; Petkov, J.T.; Stoyanov, S.D. Limited coalescence and Ostwald ripening in emulsions stabilized by hydrophobin HFBII and milk proteins. Colloid Surf. A Physicochem. Eng. Asp. 2016, 509, 521–538. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Carà, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).