Abstract
We investigated the dispersal ability of Euphydryas aurinia provincialis in a local-scale analysis within a single habitat patch of the Colfiorito highlands metapopulation. Our findings indicate that inside a single node, the organization of nesting patches can be conceptualized as a metapopulation itself, where reproductive sites, despite their spatial proximity, can act as either source or sink habitats depending on environmental conditions. We conducted fieldwork in six nesting patches inside a single node, capturing, marking, and recapturing individuals to assess their spatial distribution and movement tendencies at a large landscape scale. We found a high degree of site fidelity among individuals, with many recaptures occurring within the original marking site, but also a sex-based difference in movement patterns; females dispersed farther than males, likely driven by reproductive strategies, while males remained more localized, prioritizing mate-searching. Our findings suggest a complex dynamic in habitat connectivity: pastures and abandoned fields, despite being open, seem to act like sink areas, while breeding sites with shrub and tree cover act as source habitats, offering optimal conditions for reproduction. Individuals, especially females, from these source areas were later compelled to disperse into open habitats, highlighting a nuanced interaction between landscape structure and population dynamics. These results highlight the importance of maintaining habitat corridors to support metapopulation dynamics and prevent genetic isolation; the abandonment of traditional grazing practices is leading to the rapid closure of these source habitats, posing a severe risk of local extinction. Conservation efforts should prioritize the preservation of these source habitats to ensure the long-term viability of E. a. provincialis populations in fragmented landscapes.
1. Introduction
In Italy, Euphydryas aurinia is considered a species complex comprising three taxa, (i) aurinia (Rottemburg, 1775), (ii) glaciegenita (Verity, 1928), and (iii) provincialis (Boisduval, 1828), although these have alternatively been considered as separate species or subspecies by different authors [1,2,3,4,5,6,7]. Euphydryas aurinia provincialis is limited to the Maritime Alps and the Apennine Mountains [8]. It is widely distributed on the Central Apennines, mostly on Monti Reatini [9], and it has been categorized as “Least Concern” in the recent Red List of Italian Butterflies [10,11,12]. Studies on this species have focused on several aspects of biology, on larval host plant preference [13], predators [14] and parasitoids [15], adult population dynamics, courtship, and mating behavior [9].
E. aurinia provincialis is a univoltine species, with adults emerging from late April to mid-June; females lay up to 300 eggs annually on the underside of the leaves of the larval host plant, particularly Knautia purpurea, Succisa pratensis, and Scabiosa columbaria [13,16,17]. These plants are critical for larval development, providing essential nutrition during the early growth phases. From late July, the first-instar larvae form communal silken nests that provide stability and protection, with larvae from different oviposition events often aggregating [13]. This gregarious feeding behavior continues until September–October across four developmental instars. As conditions worsen, larvae individually enter diapause in underground hibernacula (~1 cm3, 25 cm deep) [13]. In early spring, they regroup to resume feeding through the fifth and sixth instars, shifting to solitary dispersal for optimal food access before pupating under dead leaves or grass stems [13].
It is a species particularly linked to pasture environments; a remarkably high grazing pressure characterized the pastoral landscape for centuries, but recent decades have seen a marked trend of grazing cessation and mountain farming abandonment, and thus several sectors are currently abandoned and undergoing vegetation recovery and dynamic processes. Bonelli et al. (2011) [12] documented the extinction of 12 populations of E. aurinia due to habitat destruction, and this species suffered a severe decline in most European countries during the twentieth century [17,18]. Its presence is therefore essentially linked to a type of extensive agriculture and good management of pastures and grasslands, especially in mountainous areas. The conservation status of the Mediterranean and Alpine populations is “favourable” [5,6], but the risk of a severe decline remains very high, especially for E. a. provincialis if the current management trend of the Apennine Mountain landscape were to be maintained.
Dispersal strategies in the E. aurinia complex vary depending on habitat structure and resource distribution [5]; during the early instars, movement is limited, with larvae remaining within their natal patches. However, in later instars, particularly the fifth and sixth, larvae exhibit greater mobility, actively dispersing in search of more abundant or higher-quality food sources. The availability of host plants, such as Knautia purpurea, is a key factor in determining larval survival. Together with patch connectivity, it plays a crucial role in metapopulation dynamics. Fragmented landscapes may increase dispersal distances in adult butterflies, exposing individuals to higher predation risk and environmental stressors. However, well-connected patches facilitate successful movement between resource-rich areas, ensuring higher survival rates and genetic exchange between populations [6]. The source–sink dynamics observed in E. aurinia populations suggest that some patches function primarily as sources, where high larval survival and dispersal rates contribute to the maintenance of adjacent subpopulations. Conversely, sink patches rely on immigration to sustain population numbers, highlighting the importance of habitat connectivity in conservation planning [6].
In this study, we investigate on a local scale the dispersal ability of an E. a. provincialis population; the analysis focused within a single patch of the more complex ecological network of the Colfiorito highlands (Umbria, central Italy) to understand in detail how it is ecologically structured and if a single patch behaves like a small metapopulation. Warren (1994) [16] suggests that although it is thought to be a sedentary butterfly [19,20,21,22], E. aurinia is probably much more mobile, with a suggested colonization range of 15–20 km [23]. We believe that knowledge of the movements of a species on a fine scale can help to understand how a population patch is structured and to gain more information on its large-scale dispersal abilities, its extinction risk, recolonization, or the degree of geographical isolation.
2. Material and Methods
2.1. Study Area
Fieldwork was carried out in the highlands of Colfiorito (central Italy) on Mount Orve (950 m a.s.l Lat 43.024951° N; Long 12.883909° E; UTMWGS84) (Figure 1). The Colfiorito plateau system, located in the eastern part of the Umbria region near the Marche region border, comprises seven plains known as Annifo, Arvello, Colfiorito, Colfiorito marsh, Popoli and Cesi, Colle Croce, and Ricciano. These plateaus lie at altitudes between 700 and 800 m above sea level and are bordered to the east and west by two limestone mountain ridges reaching elevations of 1571 m (Mt. Pennino) and 1405 m (Mt. Tolagna), respectively. The inner landscape is characterized by rolling hills [24]. The catchment basin spans 9152 hectares and encompasses portions of the Umbria-Marche Apennine. It is the result of ancient lake systems that were gradually drained through both anthropogenic interventions and natural geomorphological processes [25]. A key ecological feature of the area is the Colfiorito marsh, whose value has been recognized at the international level. In 1977, the marsh was designated as a Wetland of International Importance under the Ramsar Convention. Furthermore, it has been included in the EU Natura 2000 as both a Special Protection Area (SPA) under the “Birds Directive” 79/409/EEC (then amended by the 2009/147/EC Directive) and a Special Area of Conservation (SAC) under the “Habitats Directive” 92/43/EEC [24].
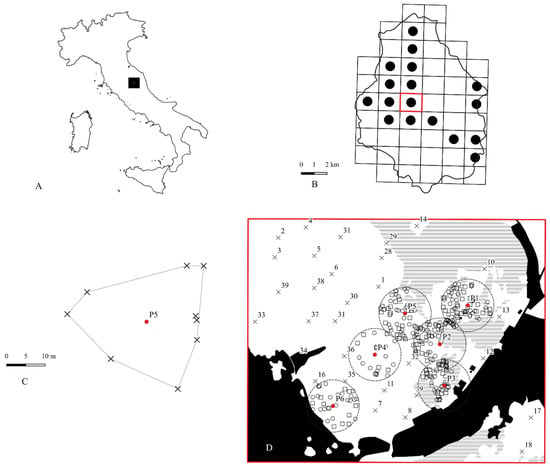
Figure 1.
(A) Black square: location of Colfiorito plateaus in the Apennines; (B) distribution of Euphydryas a. provincialis in the Colfiorito plateaus [26]; red square = study area; (C) example of nesting patch obtained using the MCP algorithm; black crosses = nests; red dot = nesting patch centroid; (D) study area in detail; dotted circles = MRRAs, mark release recapture areas (buffer of 150 m from nesting patch centroid); black regions = geographic barriers for E. a. provincialis; horizontal lines = forests, wood, pre-wooded communities; white regions = open land (pastures, crops, abandoned crops); squares = marked individuals; circles = recaptured individuals; crosses = random monitoring stations of marked individuals.
Information on the distribution of E. a. provincialis in the Colfiorito highlands was provided by Salomone et al. (2010) [26]. A UTM WGS84 rectangular grid was superimposed over the entire plateau system (Figure 1A), and for each grid cell, the presence/absence of the species was recorded. The species was detected in 17 out of 50 grid cells (about 34%). For this study, we selected the grid cell located closest to the centroid of the species’ dispersal nodes (Figure 1B) and conducted a complete census of E. a. provincialis larval nests. The selected grid cell covers an area of 235.5 hectares and includes a mosaic of different land uses: close-turf grassland, uncultivated fields, forest patches, and cultivated fields (Table 1). Changes in traditional land management practices have led to the expansion of shrublands, significantly altering habitat structure and contributing to the decline of several open grassland species. The study area lies within the temperate bioclimatic region, and it is characterized by the alternation of winter cold stress and summer drought stress, with different intensities depending on the elevation gradient and landform factors [27].

Table 1.
Summary of study area land cover.
2.2. Experimental Design and Data Collection
Field research on larval distribution was conducted from September to October 2008, resulting in the detection of 46 nests organized into six nesting patches within the study area. For each nest, the geographical coordinates (UTM, WGS84), host plant species, and number of observed larvae were recorded. Each nesting patch was obtained using the minimum convex polygon algorithm [28], starting from the position of each nest. The centroid of each minimum convex polygon was then obtained (Figure 1C). The centroid of the minimum convex polygon (nesting patch) represents the origin of a 150 m radius buffer (Figure 1D), i.e., the mark release recapture area (MMRA). Each MRRA (7.1 hectares) was picketed and divided into four quadrants (NE, SE, SW, and NW). Adult capture sessions were carried out from May 4th to June 10th 2009, with two visits per week for a total of 10 surveys. In each session, all six MRRAs were surveyed simultaneously, with a sampling effort of 45 min per quadrant. During these surveys, the GPS positions of marked or recaptured individuals were recorded, along with their food preferences and sex. The marking procedure followed the method described by Ehrlich and Davidson (1960) [29], as modified by Brussard (1971) [30] and outlined in Southwood (1978) [31]. Individuals were marked on the ventral surface of the wings using waterproof pens, with a different colour assigned to each patch (Figure 2).
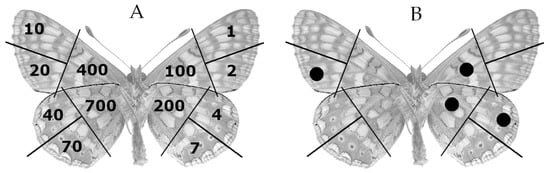
Figure 2.
(A) Insect marking system according to Brussard’s (1971) modification of Earlich and Davidson’s (1960) “1-2-4-7” system described by Southwood (1978). (B) Marking example of the 324th specimen (200 + 100 + 20 + 4 = 324).
2.3. Statistical Analysis
To assess the uniformity of capture, marking, and recapture efforts among patches, a Kruskal–Wallis test [32] was employed. This non-parametric method is suitable for comparing independent groups when the assumption of normality is not met.
Geographic distances were calculated using QGIS software (version 3.28.15) as Euclidean distances between the centroids of nesting patches or between the coordinates of individual marking and recapture events. Differences in dispersal distances between males and females were tested using the Wilcoxon rank-sum test [33], a non-parametric alternative to the t-test for independent samples.
Differences in dispersal among patches were analyzed using the Kruskal–Wallis test, which allows comparisons among multiple groups without assuming normality. To test the relationship between dispersal distance and time since marking, Hoeffding’s D test [34] was used, a non-parametric statistic capable of detecting both linear and non-linear dependencies. To assess monotonic relationships, Kendall’s tau correlation coefficient [35] was used, offering a rank-based approach that is more robust to non-normal data distributions and outliers.
Site fidelity was assessed using chi-square (χ2) tests to test for independence between site fidelity and sex and between site fidelity and patch location.
Patch connectivity was quantified using graph theory metrics [36,37,38,39]. Degree centrality measures the number of direct connections to each patch and indicates whether patches are dispersal hubs or isolated. Betweenness centrality highlights patches that act as key dispersal corridors and are crucial for maintaining network connectivity. Closeness centrality evaluates the overall efficiency with which a patch can reach the rest of the network, reflecting its integration within the dispersal system. Together, these metrics provide a comprehensive understanding of the function of the patches within the dispersal network, supporting conservation efforts that target both well-connected and peripheral patches. To assess the spatial structure of patch connectivity, Moran’s I test [40] was used to evaluate whether the degree centrality of patches exhibited spatial autocorrelation. The test was conducted under a randomization framework, with a spatial weight matrix constructed based on the nearest neighbours. To investigate the influence of geographic distance on patch connectivity further, a Mantel test [41] was performed to assess the correlation between the geographic distance matrix and the connectivity matrix (degree centrality).
Nine parameters were collected for the environmental analysis using QGIS 3.28.15 geoprocessing tools. For each nesting patch buffer (MRRA), the vegetation and geomorphological parameters (slope, exposure and distance from the hydrographic network) were measured. Vegetation parameters were collected entirely through field surveys and expressed as a proportion (p) of the available habitat in the study area. Vegetation types were first identified and categorized in the field based on their physiognomy (pre-wooded community, shrubland, grassland, meadow, marsh and hydrophytic vegetation, cropland). Then, some of these categories were described in more detail based on the vegetation structure and dominant species. Principal Component Analysis (PCA) [42] was applied to investigate changes in environmental parameters in relation to the ecological role of each nesting patch (source or sink). Component Analysis was performed on the correlation matrix, and the results are presented as biplots.
All statistical analyses were performed in RStudio 1.4.1717 [43] (R Core Team, 2024 [44]), using various packages: the stats for Wilcoxon rank-sum, Kruskal–Wallis, Kendall’s tau correlation, and chi-square tests; the Hmisc package for Hoeffding’s D test; the igraph package for calculating graph-theory connectivity metrics; the ape package for the Moran’s I index; and the vegan package for the Mantel test. The significance level was set at α = 0.05 for all tests.
3. Results
3.1. Mark Release Recapture Results
Field surveys conducted from September to October 2008 identified a total of 46 larval nests distributed across six distinct nesting patches (Figure 1D). The number of nests per patch varied, ranging from 5 to 9, with an average of 7.7 nests per patch (SD = 1.5) (Table 2). Geographical coordinates (UTM, WGS84), host plant species, and the number of larvae observed were recorded for each nest. The concentration of nests in certain patches suggests that some areas within the landscape provide more suitable microhabitats for larval survival.

Table 2.
Summary of the capture effort, marking and recapture. LAT = latitude (UTM/WGS84); LONG = longitude (UTM/WGS84); F% = recapture frequency, calculated as (Recaptured/Marked) × 100.
During the mark–recapture field surveys, a total of 3253 adult individuals were marked (Table 2), with 151 recaptures (91 males; 60 females), resulting in an overall recapture rate of 4.6%. Recapture rates varied among the patches, ranging from 2.5% in patch 4 to 6.0% in patches 1 and 2. The highest number of marked individuals was recorded in patch 5 (731 butterflies), whereas patch 6 had the lowest marking effort (436 butterflies). On average, 542.2 individuals were marked per patch, with a mean recapture count of 25.2 butterflies per patch. The Kruskal–Wallis test evaluating differences in capture rates across patches was not statistically significant (χ2 = 3.0745, df = 5, p = 0.688), and the same was true for recapture effort (χ2 = 3.304, df = 5, p = 0.653). Although the number of marked individuals varied among patches, this does not reflect differences in sampling effort; these variations may be due to differences in local adult abundance or patch-specific habitat conditions.
Most of the adults were captured on Thymus longicaulis longicaulis, which accounted for 44.02% of all the recorded observations made (n = 1432), followed by Hippocrepis comosa (22.65%, n = 737). Several other plant species were recorded at lower frequencies, including Sanguisorba minor (2.56%, n = 83) and Taraxacum officinale (1.78%, n = 58). Other species such as Acinos alpinus (8.55%, n = 278) and Crepis sancta (6.41%, n = 208) also contribute significantly to the presence of adults (Figure 3). Knautia calycina (C.Presl) Guss. (=K. purpurea auct. Fl. Ital. p.p.), known as a key larval host plant, in bloom during the capture sessions, was only marginally represented in adult captures (0.48%, n = 16), suggesting that adults do not frequently use it as a nectar source.
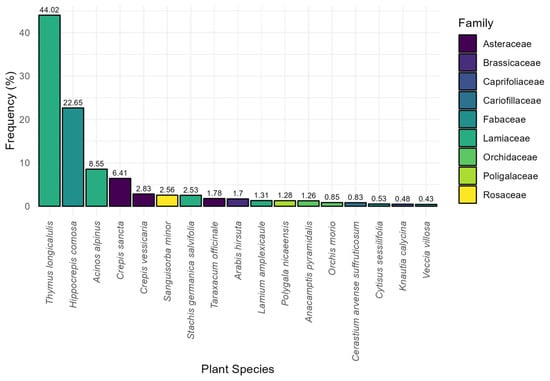
Figure 3.
Bar chart illustrating the percentage composition of plant species used by adults.
3.2. Descriptive Statistics of Dispersal Distances
The dispersal distances of recaptured E. a. provincialis individuals ranged from 5.4 to 740.0 m, with a mean distance of 149.3 m (SD = 132.3 m; CV= 73.2–171.7 m) and a median value of 120.0 m. The coefficient of variation (CV) is related to the interval between the first and third quartiles. Females exhibited greater dispersal distances (mean = 204.7 ± 176.6 m; CV= 89.1–256.7 m) compared to males (mean = 107.5 ± 57.1 m; CV= 68.6–138.9 m) (Figure 4), and the Wilcoxon test confirmed that these differences were statistically significant (W = 1858.6, p < 0.000). Conversely, the Kruskal–Wallis test showed no significant variation in dispersal distances among patches (χ2 = 5.51, df = 5, p = 0.360), indicating spatial consistency in movement patterns across the study area.
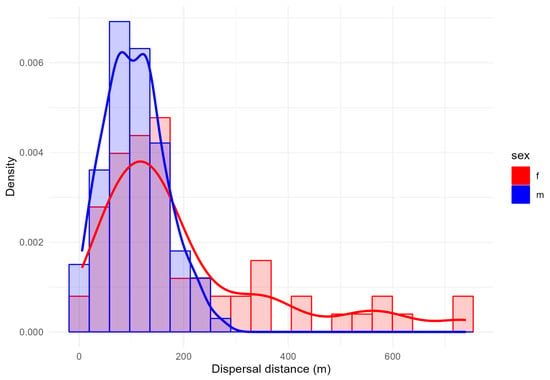
Figure 4.
Distribution frequencies of dispersal distances between sexes.
Site fidelity was generally high, with 89.4% of individuals recaptured in their original patch. Males showed significantly higher fidelity (98.8%) than females (76.9%) (χ2 = 16.525, df = 1, p < 0.000), while fidelity did not differ significantly among patches (χ2 = 6.74, df = 5, p = 0.240).
A weak but statistically significant correlation between days since marking and dispersal distance was detected in the overall dataset (Hoeffding’s D = 0.01, p = 0.035) (Figure 5). When analyzed by sex, males showed no correlation (D = 0.00, p = 0.5699), while females exhibited a slightly stronger and significant association (D = 0.02, p = 0.026), suggesting a limited temporal component in their dispersal behavior. Although the sex-based difference (ΔD = −0.023) was minor, it may indicate a tendency for females to extend their movement over time, potentially enhancing their ability to reach and colonize new habitat patches.
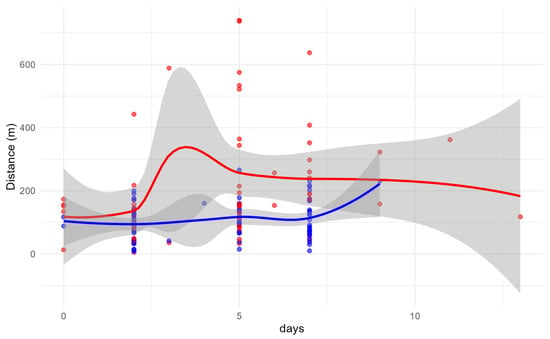
Figure 5.
Relationship between dispersal distance and days since marking for males (blue) and females (red). Points represent individual observations, with LOESS regression lines and confidence intervals. The annotations show Kendall’s p-value (0.965) and the difference in Hoeffding’s D (ΔD = −0.023), indicating a weak but significant time–distance relationship in females, which is absent in males.
When analyzed by sex and patch (Figure 6), no significant correlation between days since marking and dispersal distance was observed in males across all patches. In females, a significant positive association emerged only in patch 5 (Hoeffding’s D = 0.1, p = 0.022), suggesting that in this source patch, and to a lesser extent in patch 3, dispersal distance increased over time. These results indicate that temporal dynamics in female movement may be context-dependent.
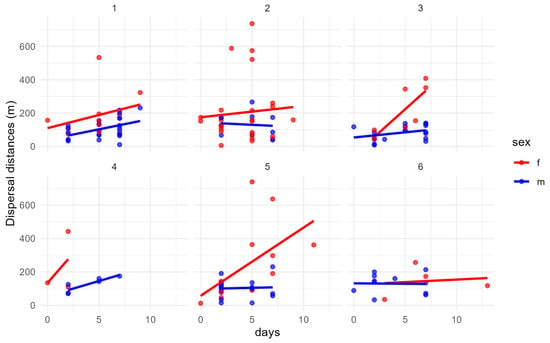
Figure 6.
Relationship between dispersal distance per patch and days since marking for males (blue) and females (red). In females, for patch 5, we have a significative dependence between days and dispersal distance over time.
3.3. Patch Connectivity Network
The dispersal dynamics of E. a. provincialis were analyzed to determine whether specific patches predominantly acted as sources (emitting more individuals than they receive) or sinks (receiving more individuals than they emit). The dispersal balance for each patch was calculated as the difference between emigrants and immigrants, where positive values indicate source patches and negative values indicate sink patches (Table 3). The results reveal that patches do not function equally in terms of dispersal. Patches differ in their dispersal roles, with some acting as key sources contributing to connectivity, while others primarily function as sinks receiving individuals from neighbouring areas.

Table 3.
Dispersion balance for each patch. Emigrants: individuals leaving the patch; Immigrants: individuals arriving. Balance = Emigrants − Immigrants. DP% = Emigrants/(Emigrants + Immigrants) × 100.
The patches do not have the same ecological role in the dispersal network (Table 4). Patch 2 emerges as the most connected and central node, exhibiting the highest degree of centrality and betweenness, indicating its function as a key dispersal hub or even a “super-source.”

Table 4.
Patch connectivity degree with the three centrality measures.
It interacts with multiple patches and maintains good proximity to others (closeness = 0.125), thereby reinforcing its role in facilitating movement across the network (Figure 7). In contrast, patch 6 exhibits the least influence, showing localized movements without broader connectivity, and does not act as a link between other patches, emphasizing its peripheral role in the network (Figure 7). Patch 5, on the other hand, exhibits the highest proximity and closeness centrality (0.143), suggesting its well-distributed connectivity within the network and a moderate bridging role (betweenness = 4). This patch likely represents a central hub with balanced dispersal flows (Figure 7). Patch 1 appears to be the most peripheral, functioning as a probable sink patch with limited interactions and low connectivity. It is the most spatially isolated from other patches, displaying the lowest closeness centrality (0.071) and connectivity function (betweenness = 3).
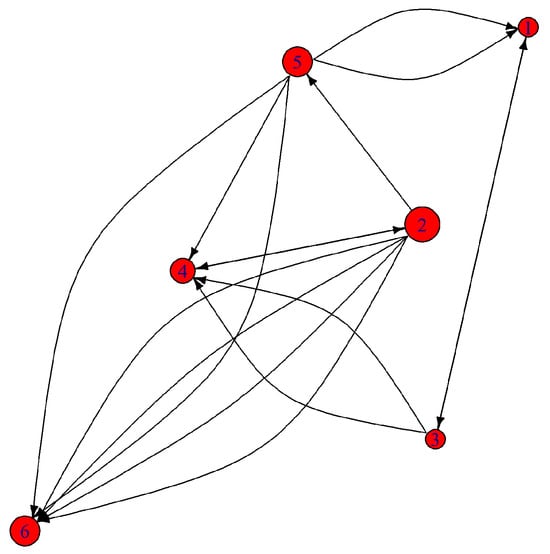
Figure 7.
Patch connectivity network; the size of the node is related to the number of connections the node has with other patches (degree values). The arrows indicate the direction of movement; if there are many arrows starting from a patch, it means that it is a source of dispersion (source).
A moderate positive correlation was found between patch connectivity and days since marking (Kendall’s tau τ = 0.4348, p = 0.045), indicating that more connected patches are involved in dispersal events over longer periods. This suggests that certain patches consistently function as stable sources within the dispersal network, supporting the role of connectivity in shaping temporal dispersal dynamics.
Although the spatial arrangement of dispersal pathways might suggest a possible relationship between the geographic position of patches and their functional role within the network (Figure 1D), the results do not support this assumption. Moran’s I test [40] did not exhibit significant spatial autocorrelation (Moran’s I = −0.068, p = 0.275); although the observed Moran’s I (Table 5) was slightly higher than the expected value under spatial randomness (−0.200), the difference was not statistically meaningful. This suggests that connectivity is not determined by geographic proximity alone and highly connected patches are not spatially clustered. The absence of a spatial pattern implies that the role of each patch within the dispersal network is shaped by other ecological drivers, such as habitat structure, resource availability, or species-specific behaviors, which may override simple spatial arrangement in defining dispersal dynamics.

Table 5.
Spatial autocorrelation and connectivity analysis results.
To further assess the relationship between geographic proximity and connectivity, a Mantel test [41] was performed to compare the geographical distance matrix with the connectivity matrix (degree centrality). The test yielded a negative Mantel statistic (−0.015), indicating a weak inverse correlation: patches that are further apart tend to have different connectivity levels (Table 5). However, this relationship was not statistically significant (p = 0.417), meaning there is no strong evidence that connectivity decreases with geographic distance. The null model, based on 719 permutations, showed that the observed correlation falls well within the expected range under random conditions. This further supports the conclusion that spatial distance alone does not determine patch connectivity.
Principal Component Analysis was conducted to determine the key environmental factors influencing the classification of patches as either source or sink. The first two principal components (PC1 and PC2) explain 78.4% of the total variance, with PC1 accounting for 44.2% and PC2 for 34.2% (Figure 8); PC1 is highly correlated with open pastures (0.91), closed pastures (0.93), and abandoned cultivated lands (0.44), representing a gradient from open to closed landscapes. Meanwhile, PC2 is mainly influenced by ruderal vegetation (0.81), urban areas (0.92), and abandoned fields (−0.82), indicating a gradient of anthropogenic influence (Table 6). These findings align with Moran’s I test and Mantel test (Table 5): ecological and environmental factors, such as land use or vegetation, play a significant role in shaping patch connectivity.
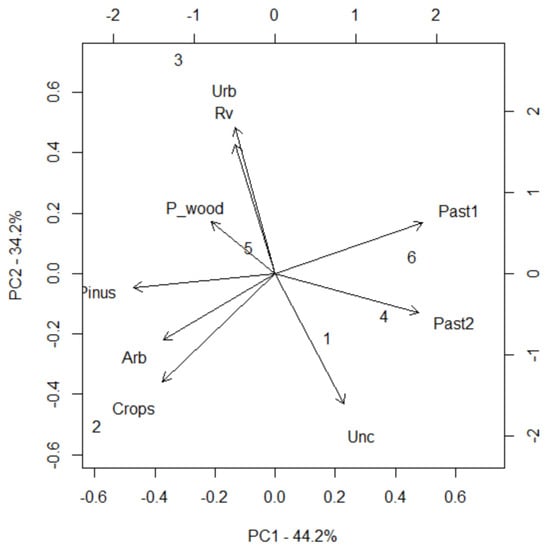
Figure 8.
PCA biplot illustrating the distribution of patches along PC1 and PC2, highlighting the ecological distinction between source (2, 3, 5) and sink patches (1, 4, 6).

Table 6.
Results of the Principal Component Analysis. PC I, II, III = Principal component I, II, and III. PC I is the gradient from open to closed landscapes, while the second indicates a gradient of anthropogenic influence. The factors most correlated with components are indicated in bold.
The distribution of PCA scores reveals a clear distinction between source and sink patches (Figure 8), with source patches (2, 3, 5) tending to have negative PC1 scores, indicating an association with ruderal vegetation and cultivated areas, and sink patches (1, 4, 6) displaying a positive PC1 values, suggesting a preference for open pastures and abandoned fields (Table 6). A Welch two-sample t-test confirmed that PC1 scores differ significantly between source and sink patches (p = 0.025, Table 7), indicating that this axis effectively separates the two groups. Conversely, PC2 does not show a statistically significant difference (p = 0.636), supporting the idea that anthropogenic factors are not the main drivers of patch function.

Table 7.
Results of the Welch two-sample t-test comparing PCA scores (PC1 and PC2) between source and sink patches. Mean scores values are presented with standard deviation (Mean ± SD).
4. Discussion
Our study on Euphydryas aurinia provincialis provides important insights into its movement ecology and the mechanisms underlying dispersal within a structured habitat network. This species is highly dependent on semi-natural grasslands, whose extent and quality are increasingly reduced by the abandonment of traditional management practices.
One of the most significant findings of our study is the limited dispersal ability of the species, with individuals generally moving over relatively short distances. Females dispersed farther than males and this pattern aligns with findings in other butterfly species where females tend to disperse further, likely driven by reproductive strategies requiring exploration of new oviposition sites [23,45,46,47]. This is consistent with prior findings in E. aurinia, where sex-based differences in movement are frequently reported as part of a reproductive strategy that optimizes fitness through dispersal [48,49]. The broader variability in female dispersal distances may reflect plasticity in movement behavior influenced by environmental heterogeneity and local resource distribution.
Site fidelity was high overall, but significantly greater in males, reinforcing the view that male behaviour is largely shaped by mate-searching within restricted areas [47]. Female behaviour, in contrast, appears more exploratory and spatially flexible, supporting their ecological role in the colonization of new habitat patches, a critical function in fragmented metapopulations. Interestingly, recent findings suggest that symbiotic infections, such as those caused by Wolbachia, may influence host behavior, reproduction, and dispersal patterns in Lepidoptera. Given that Wolbachia is maternally inherited and can affect the reproductive biology of host species, including inducing sex-ratio distortions and modifying dispersal-related behaviors, its potential role in shaping the observed sex-biased dispersal patterns in E. aurinia cannot be excluded [50].
While the influence of time on dispersal was weak overall, subtle sex-based differences emerged: female dispersal distances tended to increase slightly over time, potentially reflecting an incremental search process for oviposition sites. This trend was particularly evident in one patch, suggesting that local conditions may modulate this temporal effect. Although the overall magnitude of this difference was small, it may still contribute to colonization dynamics, particularly in fragmented landscapes where females play a pivotal role in connecting subpopulations.
The relationship between dispersal distances and time since marking in insects, particularly in Lepidoptera, has been a subject of interest in the scientific literature. In this regard, previous studies have shown that some butterfly species exhibit a correlation between the distance travelled and the number of days since marking. For example, research on Parnassius apollo has demonstrated that individuals tend to move over longer distances as time increases, suggesting an exploratory behavior linked to resource or mate searching [51]. However, this relationship is not universal among all butterfly species. In some populations, dispersal distance may be influenced by environmental variables, habitat availability, and species-specific ecological traits [48,51,52,53]. Therefore, while some species exhibit increasing distances over time, this relationship may be less evident or even absent in others.
This study also reveals that individual patches vary in their contribution to the dispersal network, with some functioning as sources and others as sinks. This heterogeneity suggests that, even on a very small scale, patches are not ecologically equivalent, and some may play a more critical role in maintaining metapopulation connectivity.
Patch connectivity metrics further reinforced these findings and highlight the existence of a structured dispersal network. These results indicate that dispersal is not spatially uniform but follows defined pathways shaped by habitat configuration and patch roles.
Despite the observed differences in connectivity, the spatial distribution of connected patches did not follow a clear pattern. Moran’s I test indicated a lack of spatial autocorrelation in patch connectivity, suggesting that highly connected patches are not necessarily clustered together. Similarly, the Mantel test found no significant correlation between geographic distance and connectivity, indicating that spatial proximity alone does not determine how well-connected a patch is within the dispersal network. This suggests that physical proximity alone does not explain movement patterns, as there are other factors, such as habitat quality, patch size, or species-specific movement preferences, that play a more important role in shaping connectivity. This interpretation aligns with Konvička et al. [48], who demonstrated that local vegetation structure within occupied habitats significantly affects adult behavior in E. aurinia, including basking, feeding, and movement choices.
Interestingly, while dispersal distances did not vary significantly among patches, connectivity differences suggest that some patches serve as critical nodes in maintaining movement pathways. Patches that function as key dispersal hubs may do so because of their habitat quality, availability of resources, or interactions with other landscape features that facilitate movement. These patterns confirm the results obtained on other populations of E. aurinia, in which the ecological role of habitat areas is irregular and strictly linked to microhabitat conditions [48,51]
These findings are further supported by the Principal Component Analysis (PCA), which identified key environmental factors influencing patch classification as either source or sink. These factors are represented by a gradient from open to closed landscapes and anthropogenic influence. Notably, patches classified as sources were associated with ruderal vegetation and cultivated areas, whereas sink patches corresponded to open pastures and abandoned fields. The Welch two-sample t-test confirmed the PCA results reinforcing the idea that within a metapopulation node, local landscape configuration governs source–sink dynamics, an idea in line with metapopulation ecology principles but also with emerging evidence on fine-scale patch functioning in butterflies. The internal structure of the landscape creates asymmetric movement and dispersal opportunities for specialist species; in this context, the insights from Konvička et al. [48], who analyzed the role of microhabitat structure on adult behavior, provide an important interpretative framework, supporting the view that both landscape-level configuration and local vegetation features jointly shape butterfly population dynamics and persistence.
5. Conclusions
These studies show that the dispersal of the butterfly E. a. provincialis is influenced by a combination of reproductive strategies, mate-seeking behaviors, and specific habitat characteristics. Landscape composition is very important in determining dispersal connectivity. While spatial arrangement alone does not dictate connectivity patterns, habitat type and structural heterogeneity appear to be key factors influencing movement. This suggests that conservation strategies should focus not only on maintaining patch proximity but also on preserving habitat diversity to facilitate dispersal. Understanding these connectivity metrics allows for targeted conservation strategies. The Colfiorito highlands have undergone significant landscape and economic transformations over time. Originally covered by forest and wetlands, human settlements gradually shaped the terrain through deforestation and drainage effort, promoting agriculture and pastoralism. During the Roman era, infrastructure development boosted trade and crop cultivation. The Middle Ages saw monastic land reclamation and a thriving wool industry. Rural depopulation during the 20th century led to a decline in traditional farming, and the strong reduction in grazing has caused a recovery in vegetation dynamism with a consequent closure of prairies, reforestation, and an increase in uncultivated areas. These changes pose a significant threat to species strictly dependent on pastures, such as E. a. provincialis, whose habitat is becoming increasingly fragmented into interconnected patches, with a high risk of local extinction. Future research should explore how additional ecological variables, such as resource availability and intra-patch vegetation complexity, influence movement decisions in E. a. provincialis. Genetic analyses could provide deeper insights into the factors shaping the dispersal networks
Author Contributions
A.B.: conceptualization, methodology, investigation, data curation, formal analysis, writing—original draft preparation, writing—review and editing. S.M.: conceptualization, methodology, investigation, data curation, visualization, writing—review and editing. F.M.T.: investigation, data curation, formal analysis. E.I.: conceptualization, methodology, investigation, data curation, resources, writing—review and editing, supervision, project administration. A.L.T.: conceptualization, resources, writing—review and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any funding.
Data Availability Statement
The data that support the findings of this study are available from the authors upon request.
Acknowledgments
The authors of the article would like to thank the Colfiorito Regional Park staff Biancarita Eleuteri and Alessandra Picchiarelli. The group would like to thank Pietro Salomone for his collaboration during the field surveys.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verity, R. Famiglie Apaturidae e Nymphalidae. In Le Farfalle Diurne d’Italia; Marzocco: Firenze, Italy, 1950; Volume IV, 380p. [Google Scholar]
- Hartig, F. Einige neue Lepidopterenrassen und -formen und eine wiederentdeckte Noctuide aus Süditalien. Reichenbachia 1968, 12, 1–13. [Google Scholar]
- Prola, C.; Provera, P.; Racheli, T.; Sbordoni, V. I Macrolepidotteri dell’Appennino Centrale. Parte I. Diurna, Bombyces e Sphinges. Fragm. Entomol. 1978, 14, 1–217. [Google Scholar]
- Parenzan, P.; Porcelli, F. I Macrolepidotteri Italiani Fauna Lepidopterorum Italiae (Macrolepidoptera). Phytophaga 2006, 40, 1–1051. [Google Scholar]
- Balletto, E.; Cassulo, L.A.; Bonelli, S. An annotated checklist of the Italian butterflies and skippers (Papilionoidea, Hesperiioidea). Zootaxa 2014, 3853, 1–114. [Google Scholar] [CrossRef] [PubMed]
- Casacci, L.P.; Cerrato, C.; Barbero, F.; Bosso, L.; Ghidotti, S.; Paveto, M.; Pesce, M.; Plazio, E.; Panizza, G.; Balletto, E.; et al. Dispersal and connectivity effects at different altitudes in the Euphydryas aurinia complex. J. Insect Conserv. 2015, 19, 265–277. [Google Scholar] [CrossRef]
- Korb, S.K.; Bolshakov, L.V.; Fric, Z.F.; Bartonova, A. Cluster biodiversity as a multidimensional structure evolution strategy: Checkerspot butterflies of the group Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera: Nymphalidae). Syst. Entomol. 2016, 41, 441–457. [Google Scholar] [CrossRef]
- Balletto, E.; Bonelli, S.; Zilli, A. Lepidotteri. In Specie e Habitat di Interesse Comunitario in Italia: Distribuzione, Stato di Conservazione e Trend; Angelini, P., Bianchi, E., Duprè, E., Ercole, S., Giacanelli, V., Ronchi, F., Stoch, F., Eds.; Rapporti ISPRA: Roma, Italy, 2014; pp. 118–130. [Google Scholar]
- Pinzari, M.; D’Alessandro, P.; Scalercio, S.; Biondi, M. Make it simple: Mating behaviour of Euphydryas aurinia provincialis (Lepidoptera: Nymphalidae). J. Nat. Hist. 2019, 53, 1811–1823. [Google Scholar] [CrossRef]
- Balletto, E.; Bonelli, S.; Barbero, F.; Casacci, L.P.; Sbordoni, V.; Dapporto, L.; Scalercio, S.; Zilli, A.; Battistoni, A.; Teofili, C.; et al. Lista Rossa IUCN Delle Farfalle Italiane—Ropaloceri; Comitato Italiano IUCN e Ministero Dell’Ambiente e Della Tutela del Territorio e del Mare: Roma, Italy, 2015; p. 47. [Google Scholar]
- Bonelli, S.; Cerrato, C.; Balletto, E. The Red List of Italian Butterflies. In Italian Red List of Threatened Species; Rondinini, C., Battistoni, A., Peronace, V., Teofili, C., Eds.; Comitato Italiano IUCN e Ministero Dell’Ambiente e Della Tutela del Territorio e del Mare: Rome, Italy, 2018; pp. 1–10. [Google Scholar]
- Bonelli, S.; Cerrato, C.; Loglisci, N.; Balletto, E. Population extinctions in the Italian diurnal lepidoptera: An analysis of possible causes. J. Insect Conserv. 2011, 15, 879–890. [Google Scholar] [CrossRef]
- Pinzari, M.; Pinzari, M.; Sbordoni, V. Egg laying behaviour, host plants and larval survival of Euphydryas aurinia provincialis (Lepidoptera, Nimphalidae) in a Mediterranean population (central Italy). Boll. Soc. Entomol. Ital. 2016, 148, 12–140. [Google Scholar]
- Pinzari, M. Predation by nymphs of Picromerus bidens (Heteroptera: Pentatomidae) on larvae of Euphydryas aurinia provincialis (Lepidoptera: Nymphalidae). Fragm. Entomol. 2016, 48, 131–134. [Google Scholar]
- Pinzari, M.; Scalercio, S.; Brandmayr, P. Associations between the larval-pupal parasitoids Erycia furibunda (Diptera: Tachinidae) and Cotesia bignellii (Hymenoptera: Braconidae) with the butterfly Euphydryas aurinia provincialis (Lepidoptera: Nymphalidae). J. Entomol. Acarol. Res. 2017, 49, 8582. [Google Scholar]
- Warren, M. The UK status and suspected metapopulation structure of a threatened European butterfly, the marsh fritillary Eurodryas aurinia. Biol. Conserv. 1994, 67, 239–249. [Google Scholar] [CrossRef]
- Van Swaay, C.; Collins, S.; Cuttelod, A.; Maes, D. European Red List of Butterflies; Publications Office of the European Union: Luxembourg, 2010; 46p. [Google Scholar] [CrossRef]
- Van Swaay, C.; Warren, M.; Lois, G. Biotope Use and Trends of European Butterflies. J. Insect Conserv. 2006, 10, 189–209. [Google Scholar] [CrossRef]
- Hula, V.; Konvicka, M.; Pavlicko, A.; Fric, Z. Marsh Fritillary (Euphydryas aurinia) in the Czech Republic: Monitoring, metapopulation structure, and conservation of an endangered butterfly. Entomol. Fenn. 2004, 15, 231–241. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Xu, R. Habitat utilization by ovipositing females and larvae of Marsh fritillary (Euphydryas aurinia) in a mosaic of meadows and croplands. J. Insect Conserv. 2006, 10, 351–360. [Google Scholar] [CrossRef]
- Schtickzelle, N.; Choutt, J.; Goffart, P.; Fichefet, V.; Baguette, M. Metapopulation dynamics and conservation of the marsh fritillary butterfly: Population viability analysis and management options for a critically endangered species in Western Europe. Biol. Conserv. 2005, 126, 569–581. [Google Scholar] [CrossRef]
- Fowles, A.P.; Smith, R.G. Mapping the habitat quality of patch networks for the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae) in Wales. J. Insect Conserv. 2006, 10, 161–177. [Google Scholar] [CrossRef]
- Zimmermann, K.; Fric, Z.; Jiskra, P.; Kopeckova, M.; Vlasanek, P.; Zapletal, M.; Konvicka, M. Marl Mark-recapture on large spatial scale reveals long distance dispersal in the Marsh Fritillary, Euphydryas aurinia. Ecol. Entomol. 2011, 36, 499–510. [Google Scholar] [CrossRef]
- Orsomando, E.; Catorci, A. Aspetti fitogeografici dei piani. In Gli Altipiani di Colfiorito, Appennino Umbro-Marchigiano; Orsomando, E., Ed.; Storia e Ambiente; Tipografia S. Giuseppe: Pollenza, Italy, 1998; 70p. (In Italian) [Google Scholar]
- Orsomando, E.; Pambianchi, G. Carta del Paesaggio Vegetale del Bacino Imbrifero dell’Altopiano di Colfiorito; Università di Camerino. S.EL.CA.: Firenze, Italy, 2002. (In Italian) [Google Scholar]
- Salomone, P.; Insom, E.; Brusaferro, A.; Marinsalti, S. Preliminary study on butterflies of the Colfiorito plateaus: Annifo plain and Colfiorito marsh (Lepidoptera). Boll. Soc. Entomol. Ital. 2010, 142, 3–20. [Google Scholar]
- Rivas-Martínez, S.; Rivas-Sáenz, S.; Penas, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1–634. [Google Scholar]
- Burt, W.H. Territoriality and home range concepts as applied to mammals. J. Mammal. 1943, 24, 346–352. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Davidson, S.E. Techniques for capture-recapture studies of Lepidoptera populations. J. Lepid. Soc. 1960, 14, 227–229. [Google Scholar]
- Brussard, P.F. Field techniques for investigation of population structure in a “ubiquitous” butterfly. J. Lepid. Soc. 1971, 25, 922. [Google Scholar]
- Southwood, T.R.E. Ecological Methods, with Particular Reference to the Study of Insect Populations; Methuen & Co., Ltd.: London, UK, 1978; p. XVIII + 391. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Hoeffding, W. A Non-Parametric Test of Independence. Ann. Math. Stat. 1948, 19, 546–557. [Google Scholar] [CrossRef]
- Kendall, M.G. A new measure of rank correlation. Biometrika 1938, 30, 81–93. [Google Scholar] [CrossRef]
- Freeman, L.C. A set of measures of centrality based on betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Freeman, L.C. Centrality in social networks: Conceptual clarification. Soc. Netw. 1978, 1, 215–239. [Google Scholar] [CrossRef]
- Freeman, L.C. Centrality in valued graphs: A measure of betweenness based on network flow. Soc. Netw. 1979, 2, 119–141. [Google Scholar] [CrossRef]
- Newman, M.E.J. Networks; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Moran, P.A.P. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Pearson, K. On lines and planes of closest fit to a system of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R, computer software v.0.98.1074; RStudio Inc.: Boston, MA, USA, 2015.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 24 July 2025).
- Hanski, I.; Ovaskainen, O. Metapopulation theory for fragmented landscapes. Theor. Popul. Biol. 2003, 64, 119–127. [Google Scholar] [CrossRef]
- Bergerot, B.; Merckx, T.; Van Dyck, H.; Baguette, M. Habitat fragmentation impacts mobility in a common and widespread woodland butterfly: Do sexes respond differently? BMC Ecol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Clobert, J.; Danchin, E.; Dhondt, A.A.; Nichols, J.D. Dispersal; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Konvička, M.; Hula, V.; Vlasanek, P.; Zimmermann, K.; Faltynek Fric, Z. Within-habitat vegetation structure and adult activity patterns of the declining butterfly Euphydryas aurinia. J. Insect Conserv. 2023, 27, 335–346. [Google Scholar] [CrossRef]
- Hanski, I.; Alho, J.; Moilanen, A. Estimating the parameters of survival and dispersal in a metapopulation: Comparison of Mark–Recapture and Habitat Occupancy data. Ann. Zool. Fenn. 2004, 41, 421–435. [Google Scholar]
- Smee, M.R. Population Ecology and Genetics of the Marsh Fritillary Butterfly Euphydryas aurinia. Ph.D. Thesis, University of Exeter, Exeter, UK, 2011. Available online: https://ore.exeter.ac.uk/repository/bitstream/handle/10036/3223/SmeeM.pdf?sequence=2 (accessed on 24 July 2025).
- Matter, S.F.; Roland, J.; Moilanen, A.; Hanski, I. Migration and survival of Parnassius apollo: Effects of landscape structure and population size. Ecology 2004, 85, 139–150. [Google Scholar]
- Franzén, M.; Nilsson, S.G. Both population size and patch quality affect local extinctions and colonizations. Proc. R. Soc. B Biol. Sci. 2010, 277, 79–85. [Google Scholar] [CrossRef]
- Schneider, C. The influence of landscape structure on butterfly movement in a mixed agricultural landscape: A mark–release–recapture study. J. Insect Conserv. 2003, 7, 233–242. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).