Plastics at an Offshore Fish Farm on the South Coast of Madeira Island (Portugal): A Preliminary Evaluation of Their Origin, Type, and Impact on Farmed Fish
Abstract
1. Introduction
2. Materials and Methods
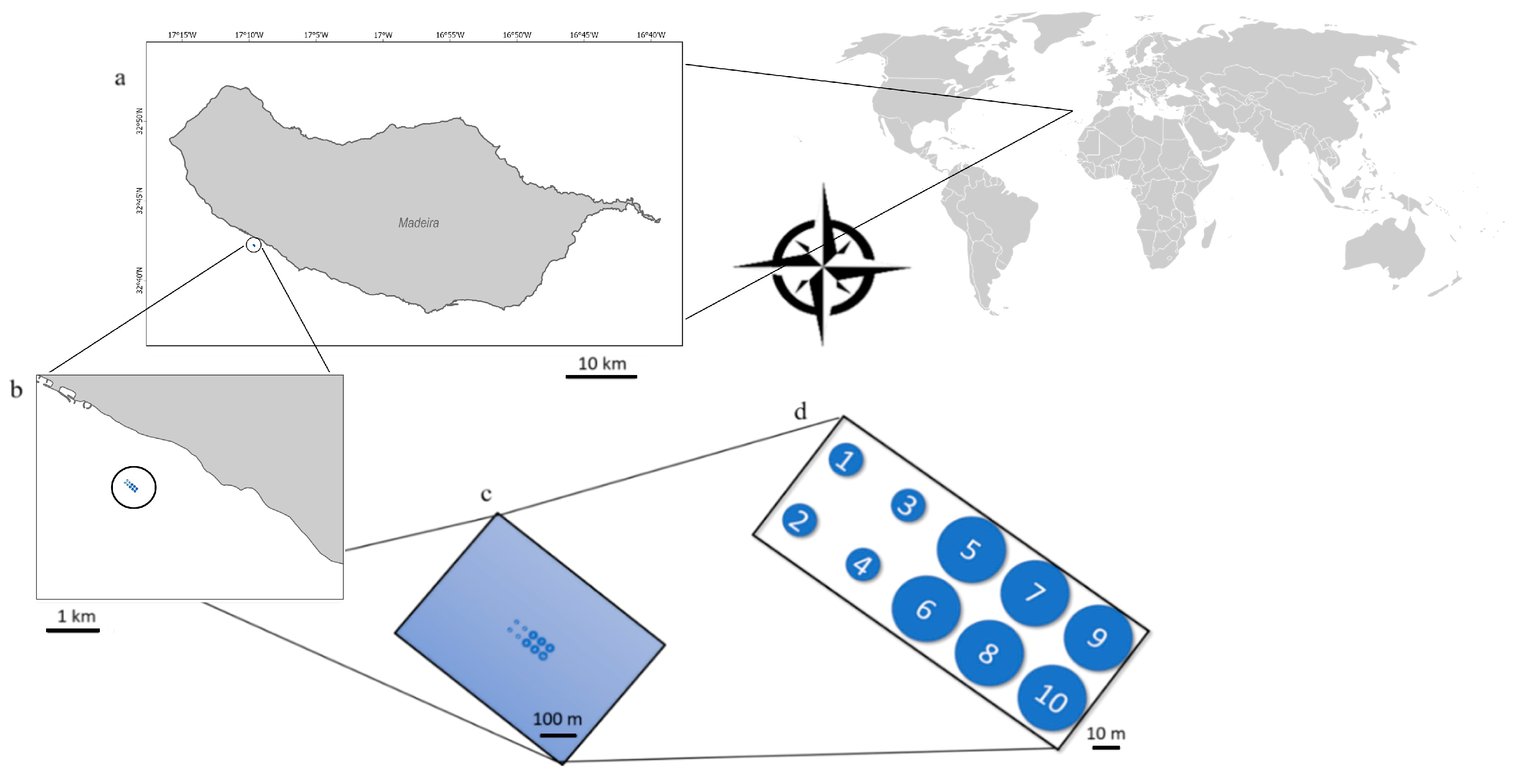
2.1. Sampling Site and Period
2.2. Monitoring Plastic in Fish Farm Cages: Videography
2.3. Climate and Oceanographic Parameters
2.4. Monitoring of Plastic in Fish Gut
2.5. Statistical Analysis
3. Results
3.1. Monitoring of Plastic in Fish-Farm Cages: Videography
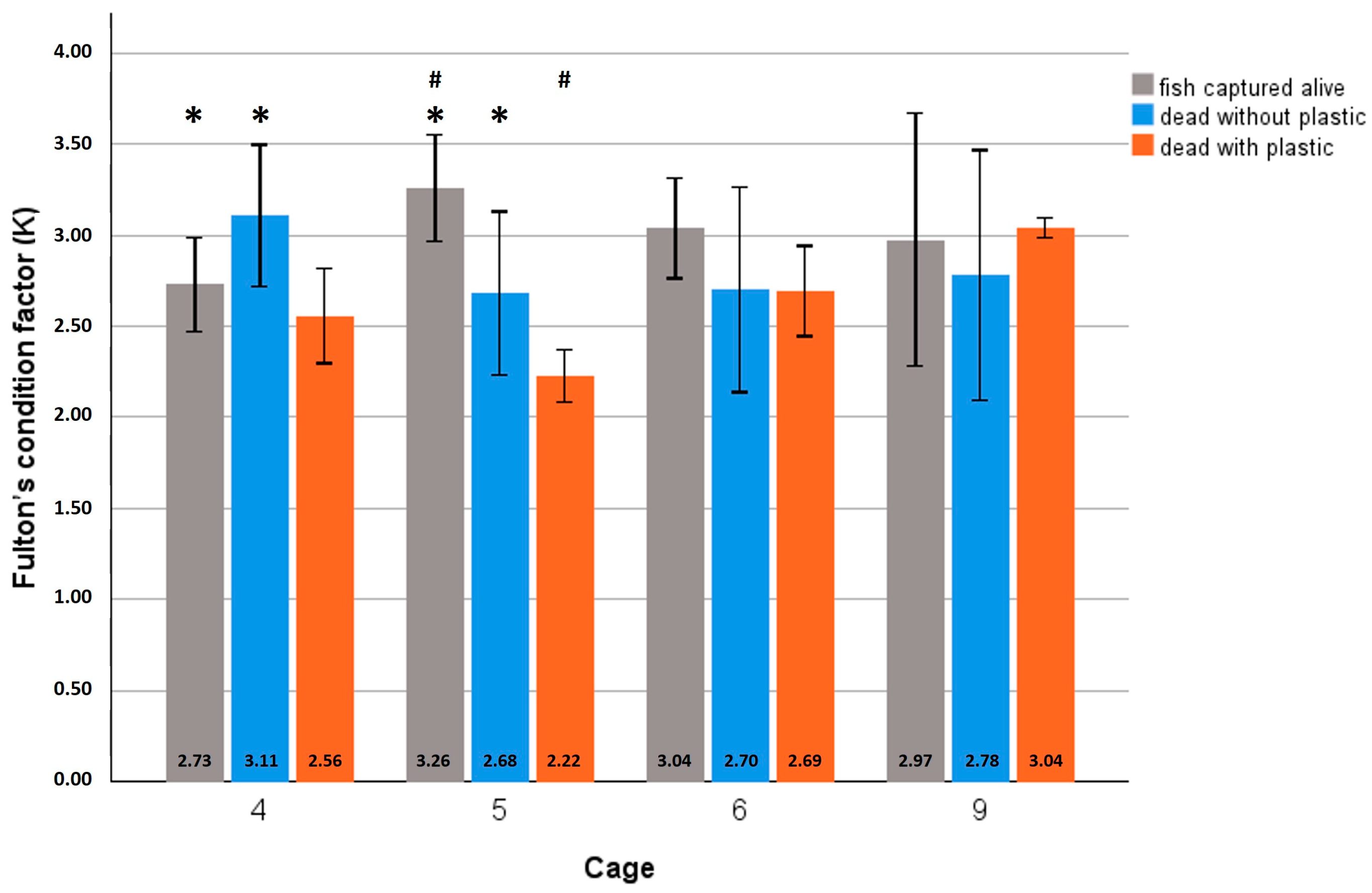
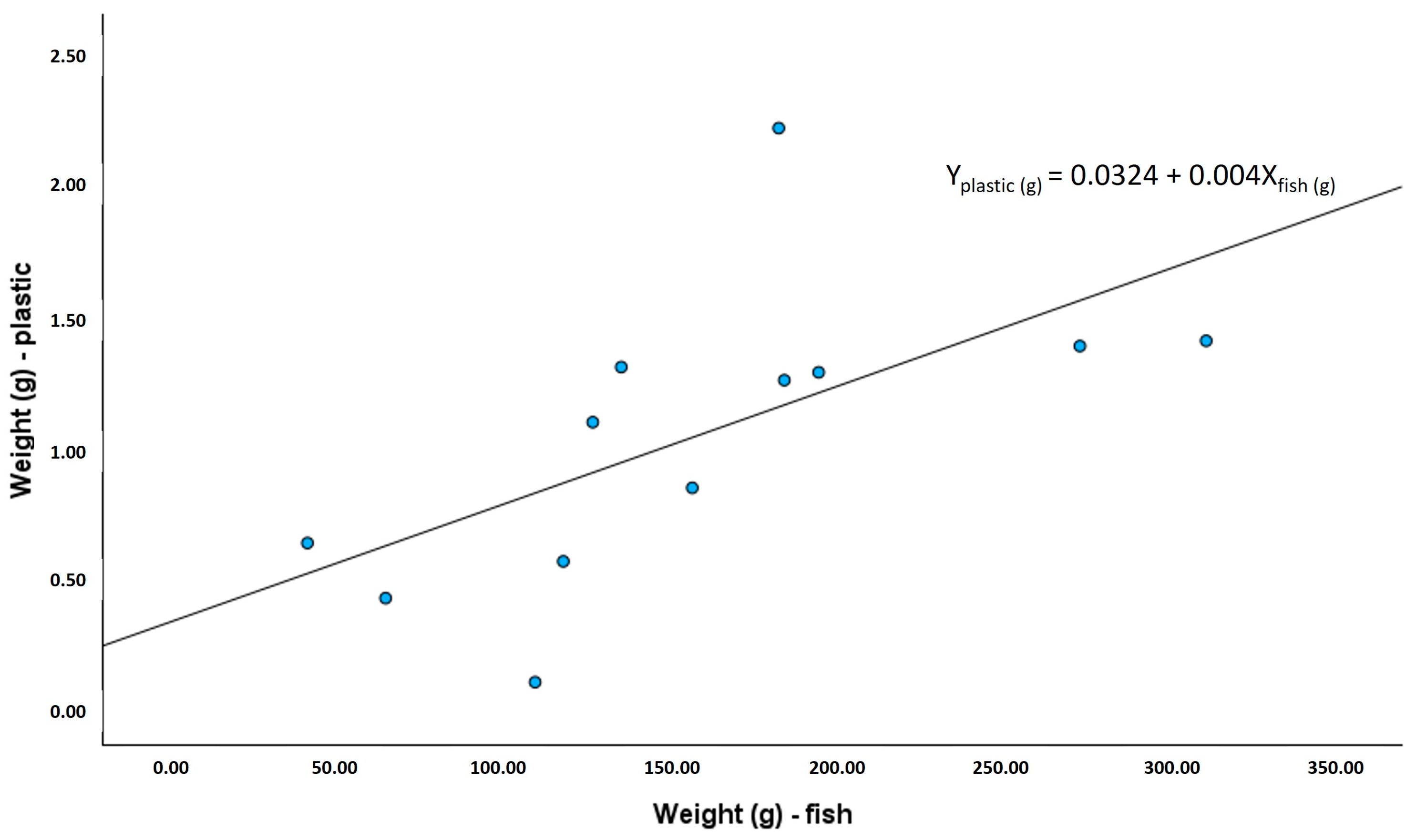
3.2. Monitoring of Plastic in Fish Gut
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz Orejon Sanchez Pastor, L.; Tornero Alvarez, M.V.; Boschetti, S.; Hanke, G. Marine Strategy Framework Directive—Review and analysis of EU Member States’ 2018 Reports—Descriptor 10, Marine Litter; EUR 30665 EN; Publications Office of the European Union: Luxembourg, 2021; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC124701 (accessed on 17 June 2024).
- Williams, A.T.; Rangel-Buitrago, N. The past, present, and future of plastic pollution. Mar. Poll. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: Review. Mar. Poll. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R.; Andrady, A.L. Plastics in the marine environment. Plast. Environ. 2003, 379, 389–390. [Google Scholar] [CrossRef]
- Viejo, J.; Martí, E.; Ignacio, J.; Montero, E.; Arroyo, G.M.; Hanke, G.; Salvo, V.S.; Basurko, O.C.; Mallos, N.; Lebreton, L.; et al. An inshore–offshore sorting system revealed from global classification of ocean litter. Nat. Sustain. 2021, 4, 484–493. [Google Scholar] [CrossRef]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Poll. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Bråte, I.L.N.; Huwer, B.; Thomas, K.V.; Eidsvoll, D.P.; Halsband, C.; Almroth, B.C.; Lusher, A. Micro-and Macro-Plastics in Marine Species from Nordic Waters; Nordic Council of Ministers: Copenhagen, Denmark, 2017; p. 549. Available online: https://norden.diva-portal.org/smash/record.jsf?pid=diva2%3A1141513&dswid=2343 (accessed on 17 June 2024).
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental implications of plastic debris in marine settings entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Marine Litter in the North-East Atlantic Region: Assessment and Priorities for Response; OSPAR: London, UK, 2009; 127p.
- Lusher, A. Microplastics in the Marine Environment: Distribution, Interactions and Effects. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 245–307. [Google Scholar]
- Bentivegna, F.; Travaglini, A.; Matiddi, M.; Baini, M.; Camedda, A.; De Lucia, A.; Fossi, M.C.; Giannetti, M.; Mancusi, C.; Marchiori, E.; et al. First data on ingestion of marine litter by loggerhead sea turtles, Caretta caretta in Italian waters (Mediterranean Sea). In Proceedings of the Biology and Ecotoxicology of Large Marine Vertebrates: Potential Sentinels of Good Environmental Status of Marine Environment, Implication on European Marine Strategy Framework Directive, Siena, Italy, 5–6 June 2013. [Google Scholar]
- Van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.L.; Heubeck, M.; Jensen, J.-K.; Le Guillou, G.; et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Poll. 2011, 159, 2609–2615. [Google Scholar] [CrossRef]
- Simmonds, M.P. Cetaceans and marine debris: The great unknown. J. Mar. Biol. 2012, 2012, 684279. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Poll. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.M.; Morrison, L.; Croot, P.L.; Allcock, A.L.; MacLoughlin, E.; Savard, O.; Brownlow, H.; Doyle, T.K. Frequency of Microplastics in Mesopelagic Fishes from the Northwest Atlantic. Front. Mar. Sci. 2018, 5, 339138. [Google Scholar] [CrossRef]
- Hanachi, P.; Karbalaei, S.; Walker, T.R.; Cole, M.; Hosseini, S.V. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ. Sci. Poll. Res. 2019, 26, 23777–23787. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hollman, P.C.H.; Mendoza-Hill, J.J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2017; 125p. [Google Scholar]
- Skirtun, M.; Sandra, M.; Strietman, W.J.; van den Burg, S.W.K.; De Raedemaecker, F.; Devriese, L.I. Plastic pollution pathways from marine aquaculture practices and potential solutions for the North-East Atlantic region. Mar. Poll. Bull. 2022, 174, 113178. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: Part Two of a Global Assessment; Kershaw, P.J., Rochman, C.M., Eds.; IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection; GESAMP: London, UK, 2016; Volume 93, 220p. [Google Scholar]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Iheanacho, S.; Ogbu, M.; Bhuyan, M.S.; Ogunji, J. Microplastic pollution: An emerging contaminant in aquaculture. Aqua. Fish. 2023, 8, 603–616. [Google Scholar] [CrossRef]
- Krüger, L.; Casado-Coy, N.; Valle, C.; Ramos, M.; Sánchez-Jerez, P.; Gago, J.; Carretero, O.; Beltran-Sanahuja, A.; Sanz-Lazaro, C. Plastic debris accumulation in the seabed derived from coastal fish farming. Environ. Poll. 2020, 257, 113336. [Google Scholar] [CrossRef]
- Sanz-Lazaro, C.; Casado-Coy, N.; Calderero, E.M.; Villamar, U.A. The environmental effect on the seabed of an offshore marine fish farm in the tropical Pacific. J. Environ. Manag. 2021, 300, 113712. [Google Scholar] [CrossRef]
- Chen, F.; Lao, Q.; Liu, M.; Huang, P.; Chen, B.; Zhou, X.; Chen, P.; Chen, K.; Song, Z.; Cai, M. Impact of intensive mariculture activities on microplastic pollution in a typical semi-enclosed bay: Zhanjiang Bay. Mar. Poll. Bull. 2022, 176, 113402. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Pham, C.K. Marine litter on the seafloor of the Faial-Pico Passage, Azores Archipelago. Mar. Poll. Bull. 2017, 116, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.J.; Walker, T.R.; Brown, C.J.; Wilson, B.R.; Gazzola, V.; Sameoto, J.A. Benthic marine debris in the Bay of Fundy, eastern Canada: Spatial distribution and categorization using seafloor video footage. Mar. Poll. Bull. 2020, 150, 110722. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J. Monitoring Marine Debris; Report of University of Gothenburg, Faculty of Sciences: Gothenburg, Sweeden, 2013; 22p. [Google Scholar]
- Fleet, D.; Vlachogianni, T.; Hanke, G. A Joint List of Litter Categories for Marine Macrolitter Monitoring; EUR 30348 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-21445-8. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can 1975, 191, 1–382. [Google Scholar]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Poll. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J. Floating plastic debris in the Mediterranean. Mar. Poll. Bull. 1980, 11, 125. [Google Scholar] [CrossRef]
- Aliani, S.; Griffa, A.; Molcard, A. Floating debris in the Ligurian Sea, Northwestern Mediterranean. Mar. Poll. Bull. 2003, 46, 1142–1149. [Google Scholar] [CrossRef]
- Hinojosa, I.A.; Thiel, M. Floating marine debris in fjords, gulfs and channels of southern Chile. Mar. Poll. Bull. 2009, 58, 341–350. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Guo, H.; Lin, H.; Zhang, Y.; Long, Z.; Huang, H. Study of marine debris around a tourist city in East China: Implication for waste management. Sci. Total Environ. 2019, 676, 278–289. [Google Scholar] [CrossRef]
- Sheavly, S.B.; Register, K.M. Marine Debris & Plastics: Environmental Concerns, Sources, Impacts and Solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar] [CrossRef]
- Cataldi, E.; Cataudella, S.; Monaco, G.; Rossi, A.; Tancioni, L. A study of the histology and morphology of the digestive tract of the sea-bream, Sparus aurata. J. Fish. Biol. 1987, 30, 135–145. [Google Scholar] [CrossRef]
- Andrew, J.E.; Holm, J.; Huntingford, F.A. The effect of pellet texture on the feeding behaviour of gilthead sea bream (Sparus aurata L.). Aquaculture 2004, 232, 471–479. [Google Scholar] [CrossRef]
- de Azevedo, A.M.; Fontanillas, R.; Owen, M.A.G.; Busti, S.; Parma, L.; Bonaldo, A.; Witten, P.E.; Huysseune, A. A quantitative analysis of gilthead seabream (Sparus aurata) juvenile dentition as a tool to assess the effect of diet. Can. J. Zool. 2021, 99, 548–556. [Google Scholar] [CrossRef]
- Veiga, J.; Fleet, D.; Kinsey, S.; Nilsson, P.; Vlachogianni, T.; Werner, S.; Galgani, F.; Thompson, R.; Dagevos, J.; Gago, J.; et al. Identifying Sources of Marine Litter; MSFD GES TG Marine Litter Thematic Report; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Bettencourt, S.; Lucas, C.; Costa, S.; Caeiro, S. Monitoring marine litter on Funchal beaches (Madeira Island): Insights for litter management. Reg. Stud. Mar. Sci. 2023, 63, 102991. [Google Scholar] [CrossRef]
- Parretti, P.; Almeida, S.; Monteiro, J.G.; Canning-Clode. Marine Litter and Offshore Aquaculture: A Survey-Based Case Study in Madeira. Project CleanAtlantic—Tackling Marine Litter in the Atlantic Area (Interreg Atlantic Area). Available online: https://www.cleanatlantic.eu/wp-content/uploads/2021/09/Report_AquacultureSurvey_CleanAtlantic_VersaoFinal.pdf (accessed on 19 February 2021).
- Knapp, G. The development of offshore aquaculture: An economic perspective. In Expanding Mariculture Farther Offshore: Technical, Environmental, Spatial and Governance Challenges; Lovatelli, A., Aguilar-Manjarrez, J., Soto, D., Eds.; FAO Technical Workshop; FAO Fisheries and Aquaculture Proceedings; FAO: Rome, Italy, 2013; pp. 201–244. [Google Scholar]
- Wu, H.; Hou, J.; Wang, X. A review of microplastic pollution in aquaculture: Sources, effects, removal strategies and prospects. Ecotoxicol. Environ. Saf. 2023, 252, 114567. [Google Scholar] [CrossRef]


| Video Samples, n | Plastics, n | |
|---|---|---|
| Month | ||
| January | 30 | 6 |
| February | 16 | 1 |
| March | 19 | 5 |
| April | 15 | 0 |
| May | 23 | 0 |
| Cage | ||
| 5 | 20 | 0 |
| 6 | 23 | 6 |
| 7 | 7 | 1 |
| 8 | 15 | 1 |
| 9 | 21 | 3 |
| 10 | 17 | 1 |
| Type-Code | J-Code | Name | n | Source |
|---|---|---|---|---|
| pl_nn_bag_smbg_ | J4 | small plastic bags | 1 | Domestic Use |
| pl_nn_bag_cabg_ | J3 | plastic shopping/carrier/grocery bags | 7 | Domestic Use |
| pl_fc_b&c_lids_drnk_ | J21 | plastic caps/lids drinks | 1 | Domestic Use |
| pl_nn_idp_idnf_ | J241 | other identifiable non-foamed plastic items | 1 | Fisheries/Aquaculture |
| pl_nn_rps_strg_nodr_ | J242 | plastic string and cord (diameter less than 1cm) not from dolly ropes or unidentified | 1 | Fisheries/Aquaculture |
| pl_nn_frg_nofp_smal_ | J79 | fragments of non-foamed plastic ≥ 2.5cm, ≤50cm | 1 | Fisheries/Aquaculture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, M.; Pombo, A.; Mendes, S.; Andrade, C.A.P. Plastics at an Offshore Fish Farm on the South Coast of Madeira Island (Portugal): A Preliminary Evaluation of Their Origin, Type, and Impact on Farmed Fish. Environments 2024, 11, 202. https://doi.org/10.3390/environments11090202
Martins M, Pombo A, Mendes S, Andrade CAP. Plastics at an Offshore Fish Farm on the South Coast of Madeira Island (Portugal): A Preliminary Evaluation of Their Origin, Type, and Impact on Farmed Fish. Environments. 2024; 11(9):202. https://doi.org/10.3390/environments11090202
Chicago/Turabian StyleMartins, Mariana, Ana Pombo, Susana Mendes, and Carlos A. P. Andrade. 2024. "Plastics at an Offshore Fish Farm on the South Coast of Madeira Island (Portugal): A Preliminary Evaluation of Their Origin, Type, and Impact on Farmed Fish" Environments 11, no. 9: 202. https://doi.org/10.3390/environments11090202
APA StyleMartins, M., Pombo, A., Mendes, S., & Andrade, C. A. P. (2024). Plastics at an Offshore Fish Farm on the South Coast of Madeira Island (Portugal): A Preliminary Evaluation of Their Origin, Type, and Impact on Farmed Fish. Environments, 11(9), 202. https://doi.org/10.3390/environments11090202







