Abstract
In recent decades, the pollution of water with micropollutants has become an increasing environmental concern. Since 2019, increased stormwater pollution from chlorine-based disinfectants has been recorded due to the COVID-19 pandemic. Runoff from disinfected areas and the residual chlorine present in stormwater are transported to surface water bodies, posing a risk to aquatic flora and fauna. The objectives of this study were (1) to evaluate the efficiency of different low-cost and recyclable filter materials in removing residual chlorine, and (2) to test plants’ ability to reduce residual chlorine concentrations through phytoremediation. Experiments were conducted in the laboratory (column and batch) and in the field (raised garden bed) to assess the efficiency of various filter materials (peat, wood chips, sawdust and the lightweight aggregates) in retaining residual chlorine to be implemented in green infrastructure. The best retainers of chlorine were sawdust (96%) and the LWA Leca (76%). No harmful effects of residual chlorine (changes in growth, color, leaf size, etc.) on plants (Tagetes patula or Pisum savitum) were observed and the residual chlorine in the leachate samples was below the equipment’s detection limit. Our research results will contribute to future studies aiming to remove various micropollutants from stormwater using remediation technologies.
1. Introduction
Untreated stormwater contains various pollutants and is one of the main sources of urban water bodies’ contamination. At present, a growing environmental challenge is the pollution of stormwater by micropollutants, which are, through runoff, transferred to rivers and lakes. The European Commission is concerned that the yearly loads of micropollutants are getting worse and more complicated, in addition to the long-term risk they pose to aquatic ecosystems even at low concentrations [1]. Therefore, stormwater treatments must meet the highest requirements to avoid a negative impact on ecosystems and the deterioration of the water quality of rivers and lakes. Proper stormwater treatment requires permanent monitoring and quality analyses [2,3]; it also contributes to the sustainable management of water resources and the implementation of circular solutions, provides water security and resiliency, saves water resources, and improves the quality of surface waters [4,5]. The European Union’s water policy aims to encourage and facilitate water reuse [6]. Following the recommendations of the Baltic Sea Environmental Protection Commission, since June 2021, stormwater must be managed in a way that reduces the amount of pollutants entering surface water bodies [7].
The European Commission highlights the potential use of green infrastructure (GI), also referred to as nature-based solutions, to remove micropollutants from stormwater in order to protect the water bodies [1]. GI complements grey infrastructure (pipes, ditches, swales, culverts and retention ponds) and also contributes to the cost reduction and economic development of existing infrastructure [8,9]. However, despite urbanization, the frequent natural disasters caused by climate change make grey infrastructure less effective and call for cities to implement innovative green stormwater management solutions [10]. Cities are encouraged to apply nature-based solutions to mitigate the negative effects of climate change. The integration of green infrastructure into stormwater management systems is an effective tool to retain and absorb pollutants [11,12] using the natural sorbents, as well the abilities of vegetation and soil’s sorption to remove various harmful substances [13]. The major advantages of green infrastructure are not only increased environment protection, but also the creation of aesthetically attractive landscapes or additional recreational spaces, thus improving residents’ quality of life [14,15].
This article focuses on a discussion of possible filter materials and remediation technologies that could reduce the concentrations of residual chlorine in stormwater. The need to analyze chlorine and chlorine compounds’ impact on surface water increased during the pandemic, when countries intensively disinfected public spaces. The number of studies presenting the environmental pollution of chlorine-based substances due to public outdoor disinfection increased several-fold. Outdoor surfaces (spa centers, nursing homes, etc.) needed to use permanent disinfection to avoid the spread of infections and viruses [16]. The resulting disinfected surfaces were washed, and their chlorine-containing runoff and stormwater were stored in reservoirs. Increased amounts of residual chlorine and disinfection by-products have been found in rivers and lakes as well [17,18]; studies demonstrate that more than 0.4 mg/l of residual chlorine and about 8.8 µg/L of disinfection by-products have been obtained from these water bodies [19,20]. Other studies show that some countries use swimming pool water for the irrigation of green areas [21], which contributes to the release of residual chlorine into the environment.
Chlorine can be present in water as free residual chlorine or as combined chlorine. Residual chlorine is the small amount of chlorine remaining in water for a certain period as part of its contact time after its initial application. Studies show that the formation of free residual chlorine depends on the initial dose of sodium hypochlorite [22,23]. Residual and combined chlorine exist in the same water and together determine the total chlorine in that water (Figure 1). Free residual chlorine is present as hypochlorous acid or a hypochlorite ion. Combined chlorine exists as monochloramine, dichloramine, nitrogen trichloride, etc.

Figure 1.
Forms of chlorine in water.
Chlorine and chlorine compounds influence the formation of harmful secondary products (trihalomethanes, haloacetic acids, trihalophenols, etc.), which pose risks to aquatic environments [24,25]. Studies have demonstrated that the impact of residual chlorine on various water microorganisms lasts for up to 14 days and that even a low, continuous concentration of chlorine can affect water ecosystems. An impact on aquatic fauna was detected at low chlorine concentrations (0.1 mg/L) [26]. Outdoor disinfection using sodium hypochlorite also causes surface corrosion due to the strong oxidizing features of chlorine [27,28] and its reaction with metals [29]. These findings raise a concern about the adverse effects of chlorine and its compounds on the environment due to the intensive disinfection of public spaces and surfaces [30]. The increased use of chlorine-based disinfectants and residual chlorine toxicity in water bodies have increased our need to analyze residual chlorine’s impact on water environments and provide possible methods and materials for its reduction. Previous studies have revealed that stormwater contaminated by chlorine and chlorine compounds can be treated using natural sorbents [31]. The novelty of this research is that it analyzes the efficiency of waste materials and phytoremediation in the removal of residual chlorine from water. There is a lack of investigation on chlorine’s impact on water environments and its retention processes from stormwater [16,32]. The removal of residual chlorine depends on the following characteristics: the structure of filter material and the material’s particle size, pore dimensions, pore volume and specific surface area [31]. The key hypothesis of the present research is that low-cost adsorbents and selected plants can be efficient in retaining residual chlorine and reducing its concentration in stormwater. This research was conducted with the following specific objectives: (1) to evaluate the efficiency of different low-cost and recyclable filter materials in removing residual chlorine; (2) to test plants’ efficiency in reducing residual chlorine concentrations through phytoremediation; and (3) to share our findings on the materials that could be used in green infrastructure in order to reuse stormwater.
2. Materials and Methods
Experiments were conducted in the laboratory (column and batch) and in the field (raised garden bed) to assess the efficiency of various natural, low-cost and recyclable filter materials in retaining residual chlorine and preventing its release into the environment. The column and batch tests were used to analyze different materials’ capacities to reduce chlorine concentrations. The raised garden bed was used to assess plants’ phytoremediation capacities, as well as the combined effect of plants and their substrate on chlorine pollution. The research materials were chosen by considering their ability to remove pollutants from stormwater, as well as by following the main principles of sustainability (cheap waste materials that are accessible in the European Union market). The experiments were carried out using the following materials (Figure 2 and Figure 3):
- -
- Peat (0.1–5 mm) is an inexpensive and effective sorbent suitable for removing various environmental pollutants [33]. Peat has good adsorption properties and is often used as an effective filter material for suspended and dissolved solid particles. Decomposed peat has a relatively high porosity of about 95% [34].
- -
- Wood chips (20–50 mm) and sawdust (0.1–2 mm) are wood by-products, waste materials and low-cost sorbents applied mainly to the removal of organic compounds from wastewater [35,36]. They are also used in green infrastructure to remove the pollutants in stormwater before they enter the environment via runoff.

Figure 2.
Filter materials used in the experiments: (a) peat; (b) wood chips; and (c) sawdust.
- -
- Lightweight aggregates (LWAs) are light expanded clay aggregates made of bloated particles of burnt clay. LWAs have good physical properties (high porosity, low water absorption) which enable them to be used as filter media [37]. Pollytag (fraction size: 8–11 mm) is a cheap adsorbent material and a type of LWA produced by granulating and sintering fly ash at a temperature of 1000–1350 °C. Batch tests were carried out on four lightweight aggregates: Polski, Leca, Pollytag and Ceski (Figure 3).

Figure 3.
Researched LWA materials: (a) Polski; (b) Leca; (c). Pollytag; and (d) Ceski.
Previous studies have shown that LWAs can retain pollutants in green infrastructure [38]. Pollytag is a commercial product manufactured from the fly ashes of a thermal electric power station which, due to its efficient absorption characteristics, is used in green infrastructure as a water retention layer. Its main compounds are SiO2 (58%), Al2O3 (22%), CaO (2.2%) and MgO (1.4%). Leca is a light expanded clay aggregate containing small particles of burnt clay. It is used as a construction material for flooring and roofing, as well as bio-filtration (wastewater treatment) and agriculture. Its main compounds are SiO2 (54%), Fe2O3 (14%), Al2O3 (12%), MgO (2%) and CaO (0.6%). Polski is a natural processed mineral, with good absorption properties, that is used for the removal of pollutants. Ceski is a light expanded clay aggregate widely used in gardening, building and the construction industry.
Sodium hypochlorite (NaOCl) is a clear, yellow-colored solution with a pungent smell. NaOCl is an effective disinfectant widely used for the decontamination of surfaces, public spaces and pools. NaOCl is characterized by its strong high-energy-consumption corrosive effect and is toxic to the aquatic environment. Its molar mass is 74.44 g/mol, density is 1.11 g/cm3, melting point is 18 °C, and boiling point is 101 °C. The WHO recommends using a 1000 ppm concentration for disinfection [39]. For the batch tests, a sodium hypochlorite solution was prepared using distilled water. The stormwater samples that fed the column experiment were collected at stormwater outlets in a territory permanently disinfected using sodium hypochlorite. The stormwater quality indicators were found to be within the following limits: pH 7.3–7.5, conductivity 254–273 µs/cm, and turbidity 0.067–0.098 NTU. In the field-scale experiments, plants were watered with a solution of stormwater and sodium hypochlorite (at a concentration of 1000 ppm, according to WHO recommendations).
Test water and collected leachates (column and field experiments) were analyzed for their residual chlorine using a Chlorine meter CL200 ExStik, which has a measuring range from 0.01 ppm to 10 ppm, an accuracy (±10%) of ±0.01 ppm, a temperature range −5 to +90 °C, automatic self-calibration and complies with ISO-9001. After a contact time of 30 min (the optimal detention time for the main reactions investigated) in the batch experiments, the concentration of total (residual) chlorine was measured using a Hach DR/2400 Portable Spectrophotometer. This device is used for testing the residual and total chlorine and chloramines in water, wastewater, storm water, estuary water, seawater, etc. When using the Hach DR/2400, samples must be analyzed immediately and cannot be preserved for later analysis. After adding the reagent (DPD total chlorine reagent powder pillows, 10 mL), a pink color will develop if chlorine is present.
Batch test. Batch experiments were conducted to determine the capacities of different types of drainage construction materials (LWAs) to absorb residual chlorine. For the batch sorption test, glass jars (with a diameter of 7 cm) were filled with 5 cm of the tested material—different types of LWAs—and this was tested using 450 mL of a solution prepared by mixing distilled water with sodium hypochlorite following WHO recommendations (a concentration of 1000 ppm). At the first stage, three glass jars (J1, J2, J3) were filled with Polski (J1—159.93 g; J2—178.36 g; J3—155.34 g) and three glass jars (J4, J5, J6) were filled with Leca (J4—65.16 g; J5—71.70 g; J6—72.43 g) (Figure 4). After a contact time of 30 min, the concentration of residual chlorine in the solution was measured in mg/l.
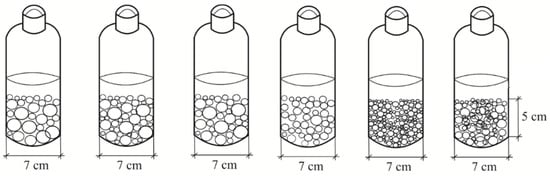
Figure 4.
Batch test.
At the second stage, the batch experiments were repeated with different types of LWA; three glass jars (J7, J8, J9) were filled with Pollytag (J7—172.51 g; J8—185.02 g; J9—159.75 g) and three glass jars (J10, J11, J12) were filled with Ceski (J10—181.97 g.; J11—187.53 g; J6—190.73 g). After a contact time of 30 min, the concentration of total (and residual) chlorine in the solution was measured.
Column experiment. The column test involves glass columns (of 5 cm diameter) filled with LWAs (Pollytag, fraction size: 8–11 mm) as a drainage layer (20 cm), as well as different filter materials (peat, wood chips, sawdust, each layer 20 cm) (Figure 5) and 2000 mL of a solution made from a stormwater sample polluted with sodium hypochlorite (following World Health Organization recommendations: 1000 ppm). Test samples were collected at stormwater outlets in a territory permanently disinfected using sodium hypochlorite. The samples were placed into hermetically sealed containers (10 L) and transported to the laboratory. The first experiment was conducted using peat (0.1–5 mm) as the filter material, the second experiment using wood chips (20–50 mm) and the third using sawdust (0.1–2 mm). After a contact time of 30 min, the sample was measured for its pH, conductivity, turbidity, color intensity and residual chlorine. Each experiment was repeated three times. Test stormwater samples were measured for their pH, conductivity and turbidity on site using portable devices. Their minimum, medium and maximum values were determined. The samples were measured for microelements, with an acceptance criterion of ±10% of the known value and a deviation of less than 3%. Their statistical values (maximum, minimum, median, standard deviation and coefficient of determination) were calculated. The assessment of the stormwater’s initial indicators is important for the further evaluation of residual chlorine’s impact on stormwater. The data were analyzed using MathCad statistical software, with a type I error (a) of 0.05. Appropriate normal and nonparametric statistics were also applied.
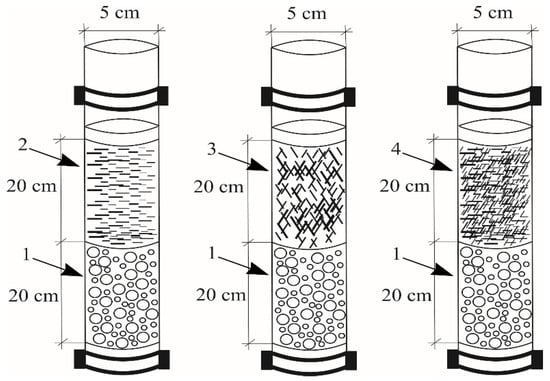
Figure 5.
Column test: 1—LWA (Pollytag); 2—peat (0.1–5 mm); 3—wood chips (20–50 mm); 4—sawdust (0.1–2 mm).
Raised garden bed. The field test was carried out to analyze plants’ capacities to filter stormwater and retain the residual chlorine that pollutes stormwater after the disinfection of surfaces. A raised garden bed cross-section is presented in Figure 6.
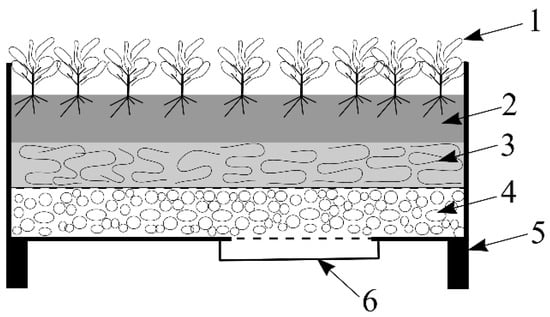
Figure 6.
Raised garden bed: 1. Plants; 2. Peat layer (15 cm); 3. Water-filtering layer (10 cm); 4. Drainage layer (LWA, 5 cm); 5. Wooden bed frame; and 6. Water tank.
The raised garden bed includes a wooden bed frame (1 m long and 1 m wide), a peat layer (15 cm), a water-filtering layer (Rockwool, 10 cm), a drainage layer (LWA Leca 5 cm) and a reservoir to collect stormwater runoff. The materials used for the test bed’s construction were selected considering the construction layers used in green infrastructure; studies demonstrate that the efficiency of the removal of pollutants depends on these construction materials. The purpose of the water-filtering layer is stormwater filtration as well as the protection of the drainage layer from the fine particles present in the soil substrate. Rockwool was used as the water-filtering layer because it is a proper medium for plant roots (provides conditions suitable for the uptake of oxygen) and has a good absorption capacity. The drainage layer must be resistant to cold and mechanical impacts, chemically neutral, harmless to plants and have the ability to drain excess water. LWAs are resistant to chlorine, have a low density and do not affect the structure of soil. The LWA Leca (with a fraction size of 0.25–4 mm evaluated by ISO standard) was used as the drainage layer because its characteristics improve roots’ breathing, eliminate weeds and provide porosity and rot-resistance.
For the first field test, Tagetes patula and Pisum sativum—annual plants that do not require extensive care, with excellent flowering and foliage characteristics—were selected. In order to achieve a large removal of pollutants, it is very important to choose the right plants. Mainly, it is recommended to use native plants in green infrastructure [40]. The pollutant removal efficiency depends on their oxygen and nutrient concentration, temperature, pH and other abiotic factors [41,42]. Studies have demonstrated that the organic pollutants’ removal efficiency by phytoremediation is about 56% [43]. Tagetes patula and Pisum sativum have been selected due to their excellent adsorption properties and their phytoremediation capacities [44,45].
During the experiment, plants were planted at the beginning of June and continuously watered with a solution of stormwater and sodium hypochlorite to determine how they reacted to residual chlorine. In the middle of November, the plants were harvested and transported to the laboratory for analysis. These samples were dried and analyzed using an XRF analyzer to measure the presence of chlorine and other compounds.
3. Results and Discussion
This research was carried out to investigate how different filter materials with a low environmental impact (e.g., recycled materials) remove the residual chlorine present in stormwater after the disinfection of outdoor spaces. Previous studies have confirmed the efficiency of natural filter materials (sorbents) in removing pollutants from stormwater [46]. Our experiments revealed that all the materials used in the laboratory tests removed the residual chlorine from stormwater, affecting its conductivity, pH, turbidity and color.
3.1. Batch Test
The adsorption process was tested using a static method for determining the capacities of natural outdoor covers to retain chlorine. Different types of LWA were used for the batch test to evaluate the adsorption efficiency of these construction materials. Glass jars with a volume of 500 mL were filled with a 5 cm high layer of LWA and with 450 mL of a solution with a total chlorine concentration of 1000 ppm. Studies show that some countries used higher concentrations than is recommended by the WHO for outdoor disinfection [47]. After 30 min of contact time, the total chlorine concentration was measured. The first stage of the experiment tested the LWAs Polski (J1, J2, J3) and Leca (J4, J5, J6) (Table 1). The experimental runs are related to the periods during which stormwater with sodium hypochlorite was flowing to the experimental devices.

Table 1.
Batch test results (experimental runs I, II, III and IV according to the residual chlorine concentration).
Table 1 presents the capacities of Polski (J1, J2, J3) and Leca (J4, J5, J6) to retain total chlorine. The results indicate that Polski (151.34–178.36 g.) retained total chlorine in the range of 2.00–3.89 ppm. The total chlorine retention of Leca (65.16–72.43 g.) was 0.81–1.97 mg/L. The total chlorine concentration of the stormwater after contact with Polski decreased about 1.1–1.7 times, and Polski’s removal efficiency of chlorine reached approximately 43%, while that of Leca was about 76%. This can be explained by the LWAs’ size, porous structure and overall structure; a higher fraction size increases the porosity and water immersion of the LWA. The second stage of batch test experiments followed the same method but with following LWAs: Pollytag (J7, J8, J9) and Ceski (J10, J11, J12) (Table 2).

Table 2.
Batch test results (experimental runs I, II, III and IV according to the residual chlorine concentration).
Pollytag (159.75–185.02 g) retained total chlorine within the range of 1.58–3.78 ppm, and Ceski (181.97–190.74 g) did so within 1.64–3.86 ppm. These results indicate that the LWA Pollytag has a chlorine retention efficiency of about 16%, while Ceski’s retention efficiency is 18%. These results show that outdoor covers partially retain residual chlorine. The LWA Leca reached a 76% retention efficiency and could be recommended for use in green infrastructure as a drainage layer; fraction size was evaluated through the mesh diameter of the sieve in mm. The results are presented in Figure 7.
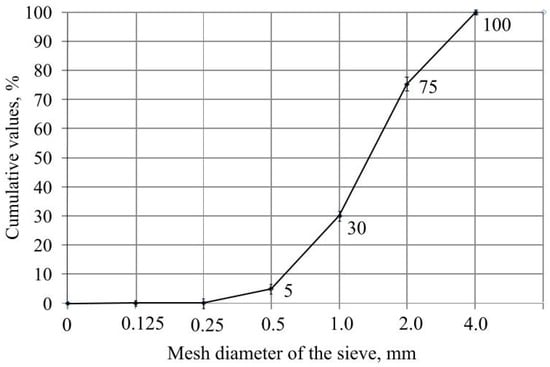
Figure 7.
Fraction size evaluation.
3.2. Column Experiment
A column experiment was conducted using peat with a fraction size of 0.1–5 mm, wood chips with a fraction size of 20–50 mm and sawdust with a fraction size of 0.1–2 mm, as well stormwater test samples contaminated with sodium hypochlorite with a concentration following the WHO recommendations (1000 ppm). First, the experiment was carried out using a 20 cm peat layer as the filter material. A control test was conducted using the initial stormwater test samples to evaluate the tested water’s indicators (pH, conductivity, turbidity).
Table 3 presents the indicator values of the initial stormwater before and after filtration. The experiments revealed that an amount of residual chlorine is washed away and enters the environment through runoff. The control test determined that the filtration of the stormwater samples using peat increased the values of the water’s indicators.

Table 3.
Stormwater indicators for control and after filtration.
Table 3 shows that the medium pH before filtration was 6.92; meanwhile, the medium pH obtained after filtration was 7.46. The medium conductivity of the water before filtration was 92.6 µs/cm; after filtration it was 189.4 µs/cm. The water medium turbidity before filtration was 0.15 NTU; after filtration it was 0.21 NTU. It is assumed that these changes in the stormwater indicators might be influenced by the contact between the tested water sample and the filter material as well as the type of filter material used.
The next stage of the column test was used to investigate peat’s capacity to retain residual chlorine and conducted using stormwater synthetically polluted with sodium hypochlorite (1000 ppm, as per WHO recommendations). The measured stormwater indicators (pH, conductivity, turbidity, color) and the concentration of residual chlorine are presented in Table 4.

Table 4.
Residual chlorine removed via peat filtration (experimental runs I, II, III, IV, V and VI).
This experiment obtained pHs that varied between 5.5 and 6.8; compared with the initial stormwater pH, this alkaline water became acidic. The conductivity varied between 492 and 501 µS/cm and the color varied between 0.003 and 0.169 AV after the peat filtration of the tested water. After filtration, the water’s turbidity varied between 0.003 and 0.180 NTU. The results demonstrate that turbidity-causing substances and colored substances were removed in a similar manner. The residual chlorine concentration was reduced from 0.6 ppm to below the detection limit after the stormwater sample was filtered through peat. The peat’s filtration efficiency depends on the properties of the peat and the test water. Later experiments were carried out by changing the filter material. Further column experiments were conducted using pine wood chips and pine sawdust instead of peat. Table 5 presents the results obtained when using wood chips with a fraction size of 20–50 mm to filter synthetic stormwater samples contaminated with sodium hypochlorite.

Table 5.
Residual chlorine removed via wood chip filtration (experimental runs I, II, III, IV, V and VI).
The results show that the pH value changed from 8.9 to 10.8; the stormwater test sample was made more alkaline. This can be explained by the mutual reactions between the disinfectant and the natural fiber present, as the sample’s conductivity ranged from 481 to 615 µS/cm. The presence of soluble substances in the filter medium impacted the sample’s conductivity. Functional groups on the surface of the filter medium participate in the reactions occurring between the solid surface and liquid. The water’s turbidity varied slightly, between 1.217 and 1.396 NDV, and its color improved approximately two-fold, from 0.119 to 0.224 AV. This shows that the substances that caused the turbidity were removed faster than the substances that caused intense color. After filtering with wood chips, the concentration of residual chlorine in the water sample was determined to be from 0.15 to 0.39 ppm. The wood chip’s chlorine removal efficiency was about 84–92%. Table 6 presents the measurements obtained when the test water was filtered through pine sawdust (with a fraction size of 0–2 mm). Studies have demonstrated that small-sized sawdust is an effective and low-cost waste material that can be used for the removal of various pollutants from stormwater [35,48].

Table 6.
Residual chlorine removal via sawdust filtration (experimental runs I, II, III, IV, V and VI).
The tested water’s pH varied between 7.6 and 9.8 (making it an acidic medium), its conductivity changed from 124 to 764 µS/cm, its turbidity from 1.131 to 1.249 NTU, and its color from 0.067 to 0.131 AV. Compared with the initial stormwater indicators, the water’s turbidity and color changed slightly after this filtration. The concentration of residual chlorine in the water after filtration was established to be in the range of 0.08–0.48 ppm. It is assumed that the intensity of the tested water’s turbidity is influenced by its contact with the filter material. Conductivity is an important property of water; a higher conductivity leads to higher concentrations of dissolved electrolyte ions in the water. An increase in conductivity indicates that the filter material effectively adsorbs disinfectants. This experiment determined that the efficiency of sawdust in removing residual chlorine varied from 80 to 96%.
3.3. Raised Garden Bed
The field experiments in the raised garden bed aimed to analyze different plants’ abilities to retain residual chlorine, and the results are presented in Table 7. The samples were analyzed using X-ray fluorescence spectrometry (XRF) with a detection limit of approximately 10 µg/g and a standard deviation of 0.00012–0.04.

Table 7.
Results of plants’ phytoremediation of residual chlorine.
Table 7 presents the results obtained from the testing of plants (Tagetes patula or Pisum savitum) watered with a sodium hypochlorite solution by analyzing the GI layers applied in the raised bed’s construction (filtering layer, drainage layer). The experiment revealed that the residual chlorine in the test samples was below the equipment’s detection limit. It is assumed that the experiment’s results were caused by the plants’ ability to transpirate chlorine through their vegetation system, results that are explained by the plants’ ability to survive in stressful conditions due to their ability to limit the entry of toxic ions into their cells [49]. During the experiment, no harmful effects of residual chlorine (changes in plant growth, plant color, leaf size, etc.) were detected in the selected plants. This has shown that residual chlorine has worked as a useful microelement for plant nutrition. Studies have highlighted that, in some cases, low concentrations of pollutants might have a positive impact on plants, but that higher doses have a harmful effect [50]. The raised bed test was a preliminary experiment used to analyze the phytoremediation capacities of plants towards residual chlorine. This field experiment needs to be continued in order to verify the obtained results and to evaluate the plants’ ability to reduce chlorine concentrations. The important implications and limitations of this study are the determination of the properties of waste materials that render them useful for chlorine removal and the additional involvement of phytoremediation for some commonly used and chlorine-resistant plants.
4. Conclusions
In order to achieve sustainable stormwater management, as well as the Green Deal and circular economy goals, the use of low-cost, recyclable materials and plants is recommended in the treatment of stormwater polluted with chlorine-based disinfectants. Our research revealed that the efficiency of a stormwater treatment depends on the type of filter material, the LWA and plants species used.
These experiments determined the impact of residual chlorine on stormwater’s quality indicators (pH, conductivity, turbidity, color). Its medium pH before filtration was 6.92; meanwhile, after filtration it was 7.46. Its medium conductivity before filtration was 92.6 µs/cm, while after filtration it was 189.4 µs/cm. Its medium turbidity before filtration was 0.15 NTU, while after filtration it was 0.21 NTU. These changes in the stormwater’s quality indicators might be influenced by the contact between the test water samples and the filter materials, as well as the type of filter material used.
Our experiments show that filtration efficiency depends on various factors: the type of filter material used, the concentration of chlorine-based disinfectants used, the solution’s acidity and the contact time between the filter material and the polluted stormwater. Our column and batch tests demonstrated the efficiency of wood chips, sawdust and LWAs at retaining chlorine; the sawdust’s efficiency reached approximately 96%, while the LWA Leca’s efficiency was approximately 76%.
The difference in these findings could be caused by climatic conditions, the contact time between the filter material and polluted stormwater, etc. Therefore, it is necessary to continue this experimental research under field conditions.
The use of phytoremediation systems and lightweight aggregates is well established in the removal of chlorine. These green solutions can be used to remove chlorine from stormwater and partially regenerate raised plant beds.
Author Contributions
Conceptualization, I.A. and M.V.; methodology, M.V. and A.K.; software, R.Z.; validation, I.A., M.V., A.K. and R.Z.; formal analysis, I.A.; investigation, I.A.; resources, A.K.; data curation, A.K.; writing—original draft preparation, I.A.; writing—review and editing, I.A., M.V., A.K. and R.Z.; visualization, R.Z.; supervision, M.V. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Commission Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Concerning Urban Wastewater Treatment (Recast) COM/2022/541 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022PC0541 (accessed on 26 November 2023).
- Zhang, W.; Li, J.; Sun, H.C.; Che, W. Pollutant first flush identification and its implications for urban runoff pollution control: A roof and road runoff case study in Beijing, China. Water Sci. Technol. 2021, 83, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, S. Innovative and Reliable Assessment of Polluted Stormwater Runoff for Effective Stormwater Management. Water 2024, 16, 16. [Google Scholar] [CrossRef]
- Feng, W.; Liu, Y.; Gao, L. Stormwater treatment for reuse: Current practice and future development—A review. J. Environ. Manag. 2022, 301, 113830. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz-Zabłocka, J.; Capodaglio, A.G. Analysis of Alternatives for Sustainable Stormwater Management in Small Developments of Polish Urban Catchments. Sustainability 2020, 12, 10189. [Google Scholar] [CrossRef]
- European Commision. 2020 REGULATION (EU) 2020/741 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on minimum requirements for water reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741&from=EN (accessed on 26 November 2023).
- HELCOM. 2021 Recommendation 23/5-Rev. Reduction of Discharges from Urban Areas Byt the Proper Management of Stormwater Systems. Available online: https://helcom.fi/wp-content/uploads/2021/06/Rec-23-5-Rev.1.pdf (accessed on 26 November 2023).
- Hopkins, K.G.; Grimm, N.B.; York, A.M. Influence of governance structure on green stormwater infrastructure investment. Environ. Sci. Policy 2018, 84, 124–133. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Singh, A. Assessment of Green Infrastructure for sustainable urban water management. Environ. Dev. Sustain. 2023. [Google Scholar] [CrossRef]
- Xu, C.; Hong, J.; Jia, H.; Liang, S.; Xu, T. Life cycle environmental and economic assessment of a LID-BMP treatment train system: A case study in China. J. Clean. Prod. 2017, 149, 227–237. [Google Scholar] [CrossRef]
- European Union. 2013 European Union: European Commission, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Green Infrastructure (GI)—Enhancing Europe’s Natural Capital, COM/2013/0249 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52013DC024 (accessed on 26 November 2023).
- Mobilia, M.; Longobardi, A.; Sartor, J.F. Including A priori Assessment of Actual Evapotranspiration for Green Roof Daily Scale Hydrological Modelling. Water 2017, 9, 72. [Google Scholar] [CrossRef]
- Stefanakis, A.I. The Role of Constructed Wetlands as Green Infrastructure for Sustainable Urban Water Management. Sustainability 2019, 11, 6981. [Google Scholar] [CrossRef]
- Grădinaru, S.R.; Hersperger, A.; Green, M. infrastructure in strategic spatial plans: Evidence from European urban regions. Urban For. Urban Green. 2019, 40, 17–28. [Google Scholar] [CrossRef]
- Orta-Ortiz, S.; Geneletti, D.M. What variables matter when designing nature-based solutions for stormwater management? A review of impacts on ecosystem services. Environ. Impact Assess. Rev. 2022, 95, 106802. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Wang, C.; Huang, J.; Zhou, M. Chlorination in the pandemic times: The current state of the art for monitoring chlorine residual in water and chlorine exposure in air. Sci. Total Environ. 2022, 838, 156193. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Fang, C.; Deng, Y.; Xu, Z. Intensified Disinfection Amid COVID-19 Pandemic Poses Potential Risks to Water Quality and Safety. Environ. Sci. Technol. 2021, 55, 4084–4086. [Google Scholar] [CrossRef] [PubMed]
- Sotirov, A. Increasing quantity of disinfectants at the environment. Acad. Lett. 2021, 2, 1290. [Google Scholar] [CrossRef]
- Yin, W.; Wang, C.; Zhang, H.; Lei, P. Impact of the use of disinfectants on water environment in Wuhan during COVID-19 pandemic. Yangtze River 2020, 51, 29–33. [Google Scholar]
- Li, Z.; Song, G.; Bi, Y.; Gao, W.; He, A.; Lu, Y.; Wang, Y.; Jiang, G. Occurrence and Distribution of Disinfection Byproducts in Domestic Wastewater Effluent, Tap Water, and Surface Water during the SARS-CoV-2 Pandemic in China. Environ. Sci. Technol. 2021, 55, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Poćwiardowski, W. The potential of swimming pool rinsing water for irrigation of green areas: A case study. Environ. Sci. Pollut. Res. 2023, 30, 57174–57177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ling, H.; Huang, X.; Li, J.; Li, W.; Yi, C.; Zhang, T.; Jiang, Y.; He, Y.; Deng, S.; et al. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020, 74, 40445. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Joshi, L.T. 2021 Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. 2022, 29, 85742–85760. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: Halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res. 2014, 65, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Lu, T.; Zhang, J.; Sun, L.; Hu, B.; Hu, J.; Peñuelas, J.; Zhu, L.; Qian, H. Residual chlorine disrupts the microbialcommunities and spreads antibiotic resistance in freshwater. J. Hazard Mater. 2022, 423, 127152. [Google Scholar] [CrossRef] [PubMed]
- Valentukeviciene, M.; Andriulaityte, I.; Chadysas, V. Assessment of Residual Chlorine Interaction with Different Microelements in Stormwater Sediments. Molecules 2023, 28, 5358. [Google Scholar] [CrossRef] [PubMed]
- Bonin, L.; Vitry, V.; Olivier, M.G.; Bertolucci-Coelho, L. Covid-19: Effect of disinfection on corrosion of surfaces. Corros. Eng. Sci. Technol. 2020, 55, 693–695. [Google Scholar] [CrossRef]
- Costa, R.D.F.S.; Barbosa, M.L.S.; Silva, F.J.G.; Sousa, S.R.; Sousa, V.F.C.; Ferreira, B.O. Study of the Chlorine Influence on the Corrosion of Three Steels to Be Used in Water Treatment Municipal Facilities. Materials 2023, 16, 2514. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sher, F.; Kumar, R.; Karahmet, E.; Haq, U.A.S.; Zafar, A.; Lima, E.C. Environmental and health impacts of spraying COVID-19 disinfectants with associated challenges. Environ. Sci. Pollut. Res. 2021, 29, 85648–85657. [Google Scholar] [CrossRef] [PubMed]
- Valentukeviciene, M.; Andriulaityte, I.; Zurauskiene, R. Experimental Research on the Treatment of Stormwater Contaminated by Disinfectants Using Recycled Materials—Hemp Fiber and Ceramzite. Int. J. Environ. Res. Public Health 2022, 19, 14486. [Google Scholar] [CrossRef] [PubMed]
- Nabi, G.; Wang, Y.; Hao, Y.; Khan, S.; Wu, Y.; Li, D. Massive use of disinfectants against COVID-19 poses potential risks to urban wildlife. Environ. Res. 2020, 188, 109916. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, S.A.; Hayrapetyan, S.S.; Hayrapetyan, M.S.; Khachatryan, H.G. Express evaluation of sorption mechanism on peat containing materials. Period. Tche Quim. 2020, 17, 469–477. [Google Scholar] [CrossRef]
- Arabia, T.; Basri, H.; Manfarizah; Zainabun; Mukhtaruddin. Physical and chemical characteristics in peat lands of Aceh Jaya District, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 499, 012004. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Laohaprapanon, S.; Marques, M.; Hogland, W. Removal of Organic Pollutants from Wastewater Using Wood Fly Ash as a Low-Cost Sorbent. Clean Soil Air Water 2010, 38, 1055–1061. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A.; Baryla, A. Wybór materiału reaktywnego do usuwania fosforu z wód i ścieków na przykładzie kruszywa popiołoporytowego Pollytag® (Choosing of reactive material for phosphorous removal from water and wastewater on the example of lightweight aggregate Pollytag®). Inżynieria Ekol. 2014, 39, 33–41. (In Polish) [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baryła, A.; Bus, A. Effect of P-Reactive Drainage Aggregates on Green Roof Runoff Quality. Water 2014, 6, 2575–2589. [Google Scholar] [CrossRef]
- World Health Organization. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/332096 (accessed on 26 November 2023).
- Malaviya, P.; Sharma, R.; Sharma, P.K. Rain Gardens as Stormwater Management Tool. In Sustainable Green Technologies for Environmental Management; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Khan, S.; Faiq, M.E.; Elahi, S.; Hashmi, S.I.; Akhtar, S.; Jamil, A.; Sharif, M.; Ullah, S.; Zakerullah; Mansoor, S.; et al. Phytoremediation of pollutants from wastewater using hydrophytes: A case study of Islamabad, Pakistan. J. Biodivers. Environ. Sci. 2021, 19, 36–49. Available online: https://www.innspub.net/wp-content/uploads/2022/05/JBES-V19-No5-p36-49.pdf (accessed on 27 November 2023).
- Obinna, I.B.; Ebere, E.C. A Review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Anal. Methods Environ. Chem. J. 2019, 2, 5–38. [Google Scholar] [CrossRef]
- Kulandaiswamy, N.D.M.; Nithyanandam, M. Feasibility Studies on Treatment of Household Greywater Using Phytoremediation Plants. Res. Sq. 2021, 1–15. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Phytoremediation efficacy assessment of polycyclic aromatic hydrocarbons contaminated soils using garden pea (Pisum sativum) and earthworms (Eisenia fetida). Chemosphere 2019, 229, 227–235. [Google Scholar] [CrossRef]
- Biswal, B.; Singh, S.K.; Patra, A.; Mohapatra, K.K. Evaluation of phytoremediation capability of French marigold (Tagetes patula) and African marigold (Tagetes erecta) under heavy metals contaminated soils. Int. J. Phytoremediation 2022, 24, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Fridrick, L.; Valentukevičienė, M. Hemp as a Sorbent for Disinfectant—Polluted Watertreatment. Conference: AQUA 2021, Plock, Poland. Available online: https://www.researchgate.net/publication/358125963_HEMP_AS_A_SORBENT_FOR_DISINFECTANT-POLLUTED_WATER_TREATMENT (accessed on 26 November 2023).
- Hu, L.; Mao, J.; Zhong, R.; Zhao, H. Assessment of heavy metals mobilization in road-deposited sediments induced by COVID-19 disinfection. Water Res. 2023, 243, 120393. [Google Scholar] [CrossRef]
- Cheng, Z.; Guan, H.; Meng, J.; Wang, X. Dual-Functional Porous Wood Filter for Simultaneous Oil/Water Separation and Organic Pollutant Removal. ASC Omega 2020, 5, 14096–14103. [Google Scholar] [CrossRef]
- Šimėnaitė, R. Fitoremediacija: Augalų Įvairovė ir Ekspozicijos Įrengimas. Available online: https://www.botanikos-sodas.vu.lt/files/fitor1.pdf (accessed on 27 November 2023).
- Ansari, A.A.; Naeem, M.; Gill, S.S.; Alzuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).