Abstract
Freshwater inflows are linked to the abundance and catch rates of fish in estuaries. The role of terrestrial carbon resources brought into estuaries after inflows may be important, but this is currently not well understood. Therefore, we performed a study examining the effect of terrestrial dissolved organic matter (tDOM) dietary additions on the growth of food-limited juvenile Australian bass (Macquaria novemculeata). Crustaceous zooplankton Artemia franciscana (Artemia) were reared for two days under control conditions (no addition) or with additions of tDOM leachate at dissolved organic carbon (DOC) concentrations of 5 mg/L or 10 mg/L. Artemia were fed to juvenile bass in their treatment tanks over 42 days at feeding rates reduced by 65–75% of ad libitum. Juvenile fish from the 5 mg/L treatment exhibited no statistical difference in weight or standard, fork and total lengths compared to the control treatment. In contrast, the fish in the 10 mg/L tDOM treatment had significant increases (p < 0.05) in all length parameters after 42 days compared to the other treatments. The greater lengths of fish where tDOM is available indicate that tDOM can contribute to improved growth and development in juvenile Australian bass. While stable isotope analysis of fish tissue showed only minor changes toward terrestrial carbon signatures, increased terrestrial resource availability in the juvenile fish diets may have subsidised energetic needs, facilitating the greater utilisation of endogenous resources. Overall, the results indicate that freshwater inflows that deliver terrestrial resources may be important for the growth and development of estuarine fish.
1. Introduction
Freshwater inflows have been linked to estuarine and nearshore fishery abundance and catch rates for several decades [1,2,3]. The links between inflows and estuarine fish abundances are thought to be driven by a combination of facilitating longitudinal movement for fish, terrestrial nutrient delivery stimulating algal growth and increased habitat access [4,5,6]. In recent decades, researchers have also suggested that terrestrial organic carbon delivered during freshwater inflows may provide a direct energetic subsidy, supporting increased fish productivity [7,8,9,10].
Terrestrial organic carbon in aquatic food webs has been considered a poor energy source for the production of zooplankton and fish [11,12,13]. Firstly, it has a poor nutritional quality compared to algae, with low concentrations of the essential highly unsaturated fatty acids (HUFAs) necessary for the growth and development of zooplankton and fish [11,14,15]. Secondly, most terrestrial organic carbon is available in a dissolved form [16], requiring a higher number of trophic level transfers before the energy reaches the fish. This results in the majority of its energy and nutritional value being respired by microbes and other microplankton [17]. Despite these energetic limitations, when the flux of terrestrial carbon substantially outweighs the bulk of algal carbon, such as after flow events when terrestrial inputs are large and comparatively more bioavailable [18], terrestrial resources can become a dominant energy source, supporting the growth of bacterial, microzooplankton, mesozooplankton and benthic invertebrate communities in estuaries [19,20,21,22].
Increased prey densities stimulated by freshwater inflows and subsidised by terrestrial carbon have been suggested to increase the recruitment success of juvenile fish following higher inflow periods [8,9,23] despite the fact that the specific role of terrestrial carbon as an energetic subsidy remains unexplored. It is well established that increased prey density can result in increased larval and juvenile fish growth and body size, which in turn increases the success rate of juvenile recruitment [24,25]. Research has suggested that terrestrial subsidies, provided to juvenile fish after inflows through trophic transfer, may be an important resource for the early-life-history stages of fish and sub-adults as they feed on lower trophic levels within the food web [26]. Developing a better understanding of the role terrestrial carbon plays as a basal food web resource in supporting the growth and survival of fish during early life stages is important given global increases in river regulation [27] and the associated shift towards heterotrophic food web structures that can occur in regulated systems after inflows [28].
Manipulative studies on the impact and role of terrestrial resources on the somatic growth of juvenile fish are limited. Degerman et al. [29] found that additions of dissolved organic carbon (DOC) as glucose to an existing algal food web decreased the growth of planktivorous juvenile fish (Gasterosteus aculeatus) due to the channelling of energy through inefficient bacterial pathways. Meanwhile, Growns et al. [30] found that additions of terrestrial dissolved organic matter (tDOM) as leachate had no positive influence on the growth of carp gudgeons (Hypseleotris spp.), although they acknowledged that an abundance of algal resources may have overshadowed any potential influence of tDOM on growth. On this basis, they proposed that future investigations should use a diet where terrestrial resources were abundant and algal resources were excluded [30]. Such a terrestrially dominant diet may be more representative of the food web conditions that can occur in estuaries after larger inflows where terrestrially derived resources and heterotrophic processes dominate the food web and algal resources are limited [28,31].
Australian bass (Macquaria novemculeata) is an endemic catadromous species that has been widely impacted by river regulation and reductions in riverine-estuary connectivity [32,33]. Multiple links between Australian bass and inflows have been established, with the importance of inflows during spawning seasons well documented [32,33,34,35]. This study aimed to understand if increased terrestrial resource availability, such as after inflow events, could positively influence somatic growth of juvenile Australian bass. We conducted an experiment where juvenile Australian bass were fed zooplankton that had been exposed to varying levels of tDOM and limited algal food sources. Fish weight, length and stable isotope ratios were monitored to assess the incorporation of tDOM and the influence on growth and development. We hypothesised that increased carbon availability from the leachate would result in increased somatic growth of juvenile fish in the higher tDOM treatments.
2. Materials and Methods
2.1. Experimental Setup and Acclimation
Prior to the experiment, leachates were prepared from a mix of senesced Eucalyptus sp. and Casuarina glauca leaves (approximately 2 kg wet weight) added to 40 L bins of reverse osmosis water and left in a refrigerator overnight (3.8 °C) [36]. After 24 h, the leachate was filtered through pre-combusted glass fibre filter papers (GF/F grade, 47 mm, Whatman, Kent, UK), refrigerated and stored in batches. The batches were independently tested for dissolved organic carbon and nutrient levels to allow the treatment additions to be regulated. The average ratio of dissolved C:N:P in the leachate used was 156.3 (±41.2): 6 (±0.0): 1 (±0.0), highlighting how the added leachate would not be a significant source of nitrate and phosphorous to the system. This leachate is stoichiometrically representative of terrestrial organic matter in aquatic systems after freshwater inflows [37].
Australian bass juveniles were acquired 1 h post-feeding from Port Stephens Fisheries Institute (PSFI) hatchery at 40 days after hatching (DAH). Prior to collection, the fish were reared by PSFI Hatchery following the methods of Fielder and Heasman [38]. The fish were housed in 27 separate 20 L plastic tanks (length = 45 cm; width = 30 cm; depth = 30 cm) in a filtered flow-through system (5 L/h water exchange) with aeration systems in a temperature- and light-insulated room located at Port Stephens Fisheries Institute, NSW, Australia. The 27 tanks were established to accommodate for the treatments (n = 3, control, 5 mg/L and 10 mg/L) and sampling time points within the experiment (n = 3) and randomly allocated to 4 large water baths and set up as part of a flow-through system maintained at 20 °C (Table 1). Replicate tanks for different sampling time points were used to minimise the effects of manual handling on fish, repeated measures and growth depensation. An additional tank was used to house fish to sample for the “Initial” measurements prior to the application of the experimental treatments. A total of 200 fish of unknown sex (mean ± S.E. standard length = 12.8 mm ± 0.2 mm, weight = 0.064 g ± 0.002) were randomly allocated to each tank to provide a stocking density of 10 fish/L; a density previously used in experimental studies on the growth rates of the juveniles of the species [38]. All of the fish were acclimated to a salinity of 20 ppt (Table 1) over the next 24 h using gradual additions of pre-mixed 20 PSU water until it had filled the system. This salinity level was selected to reduce the mortality rates of the juvenile Australian bass [38]. Full-spectrum artificial light (Ledzeal, Malibu S series) was provided on a 12 h light:12 h dark cycle to replicate diurnal cycles, and the water was filtered (10 µm) prior to re-entering the system to reduce particulates entering the tanks. The tanks and filters were cleaned daily prior to feeding. Water temperature, salinity, pH and oxygen saturation were monitored daily with a multiprobe (WTW, Multi3430, Weilheim, Germany) to ensure that the conditions were stable (Table 1), and ammonia was measured with a test kit (Aqua One® Quick Drop Ammonia Test Kit, Sydney, Australia). Fish behaviour was observed in the half hour prior to and post feeding, with any deceased fish counted and removed prior to feeding.

Table 1.
Average water quality conditions in system over the 42-day experimental period.
2.2. Artemia and Feeding
Artemia were used to transfer the leachate across trophic levels, a process similar to the enrichment of Artemia through the bioencapsulation of algal supplements by the stocking program of the PSFI hatchery [38]. Artemia nauplii were chosen for two reasons; firstly, they are a commonly used food source for rearing juvenile fish [39], and secondly, they maintain a similar food web position to the planktonic crustacea that are the dominant food source of juvenile Australian Bass in estuaries [40]. Artemia were hatched in 100 L of seawater at 30 °C over 20 h using 30 g of Artemia cysts (Sep-Art® GSL Magnetic Artemia Cysts, Ogden, Utah, USA), yielding approximately 10 L of Artemia at a concentration of 500–600 Artemia/mL at the end of the hatching cycle. Artemia were then transferred to 20 L tanks of filtered (10 µm) brackish water (20 ppt, 20 °C) at a density of 40 Artemia/L and exposed to the DOC amendment treatments (0 mg/L, 5 mg/L and 10 mg/L) for 40 h in order to consume the provisioned terrestrial carbon material. DOC concentrations and the length of the experiment were analogous to the range and duration of DOC concentrations (between 5 and 12 mg/L) found in a post-inflow monitoring study of the nearby Williams River estuary, a known Australian bass spawning area, with elevated DOC concentrations present for up to a month and a half following the inflow, approximately the length of this experiment (Johnson et al., unpublished data). Carbon additions were administered to the on-growing tanks as they were filled with brackish water to homogenise the addition. The availability of in situ food resources for Artemia in the control treatment and for all treatments during the on-growing period was limited by the physical filtration of seawater but may have included smaller algal cells and POM (<10 µm).
Treatment batches of Artemia were prepared daily to ensure 40 h enriched Artemia were available for daily feeding with on-growing tanks cleaned with freshwater before being restocked. Prior to feeding, Artemia were enumerated daily to determine their concentration after on-growing. Triplicate 5 mL samples of Artemia were enumerated for each tank by light microscopy (Leica, MZ6, Wetzler, Germany). Artemia were fed to their corresponding treatment fish at a concentration of 2/mL/day which equated to approximately 200 Artemia/fish/day. The fish were fed daily between 8 and 10 am. During feeding, tank flow-through was turned off for half an hour to prevent the loss of Artemia from the tank and to maximise access to prey for fish. To optimise growth in the PSFI, production process juvenile bass are generally fed ad libitum 300 Artemia/fish/day [38]. By reducing this feeding rate, we were able to underfeed the fish, aiming to better reveal the effects on the growth of terrestrial carbon, which have been previously masked by feeding ad libitum [30], while still ensuring high survival rates. This feeding rate was selected with the advice of PSFC hatchery staff and was aligned with post-inflow densities of copepod nauplii in the nearby Williams River estuary, being between 2 and 5 individuals/mL for up to a month after the inflow (Johnson et al., unpublished data).
2.3. Bacteria and Fish Sampling
Technical replicates of bacterial samples were taken in triplicate directly from the Artemia on-growing tanks immediately after they were prepared (0 h) on the three fish sampling days to assess the differences in microbial activity due to the addition of leachate. Bacterial samples were also taken from the on-growing tanks used for feeding on the day of fish sampling (40 h), after the homogenising of the tank for Artemia enumeration, to characterise microbial activity across the 2-day on-growing period. The 3 analytical replicates for each treatment (control; 5 mg/L; 10 mg/L) from the 3 fish sampling days resulted in 9 replicates for each bacterial sampling time level (0 h; 40 h). Samples were taken with a 1 mL pipette and were stored in 1.6 mL cryovials (Sarstedt, CryoPure, Numbrecht, Germany) with 0.1 mL of glutaraldehyde for preservation. Samples were refrigerated (3 °C) before they were snap-frozen in liquid nitrogen within 2 days of sampling. The samples were then stored at −80 °C until they were processed [41].
When sampling the fish for analysis, all of the individuals were removed from the tanks using dipnets and then euthanised in a 150 mg/L benzocaine bath. After the cessation of opercular movement, the fish were removed and rinsed with RO water. Thirty fish from each tank were randomly selected and placed on glass microscope slides, weighed, and measured with calipers for standard, fork and total length. This suite of length measurements was used to reflect the variety of measurements used in historical and modern studies of juvenile Australian bass and allow conversions between them where necessary [38,42,43,44]. The remaining fish from each replicate tank were placed into plastic bags and frozen (−14 °C) for tissue analysis. The fish from the “Initial” measurement tank were sampled at the end of the acclimation period (T0), providing baseline measurements to be considered representative of the entire cohort. The fish were then sampled at 2, 4 and 6 weeks after the commencement of their new diet (referred to in figures as T1 = 2 weeks, T2 = 4 weeks, and T3 = 6 weeks). Replicate tanks for different time points were used to eliminate the impacts of density dependent growth, manual handling and repeated measures.
2.4. POM and Tissue analyses
Triplicate samples of on-grown Artemia from each on-growing treatment were taken on fish sampling days by filtering 500 mL of water from the on-growing tanks through a small 40 µm tow net, separating the Artemia from POM. The water that passed through the tow net was then filtered through GF/F filter papers to allow POM to be isotopically analysed. Artemia retained in the tow net were also placed on pre-combusted GF/F filter papers for stable isotope analysis. All filter papers were placed into plastic bags and then frozen (−14 °C). The frozen fish were dried at 50 °C for 96 h, crushed to homogenise the tissue and stored at -20 °C. POM, Artemia and homogenised fish material were analysed for stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) using a continuous flow-isotope mass spectrometer (GV IsoprimeEurovector EA 3000, Manchester, UK) and a Sercon Hydra [20,21,22] stable isotope ratio mass spectrometer, respectively. The results were determined against laboratory standard reference material IAEA-CH-6 and atmospheric nitrogen.
2.5. Statistical Analyses
We conducted a two-way ANOVA to determine if the mean bacterial cell counts (n = 9) differed among treatments (control; 5 mg/L; 10 mg/L) and bacterial sampling times (0 h; 40 h). Similarly, we conducted a two-way ANOVA to test for statistical differences in survival rates of the juvenile fish as well as the weight and length measurements between the tDOM treatments ((control; 5 mg/L; 10 mg/L) and experimental time points (Initial, T1, T2 and T3). Parametric assumptions were checked for all ANOVAs, and where they were not met, transformations were applied to satisfy the assumptions. This was only necessary for the survival rates of juvenile fish (%) and the weight (g) of juvenile fish, where square root transformations were applied. Significant differences between treatments and time points for survival rates, fish length, fish weight and bacterial concentrations were determined using Tukey’s post hoc test. All of the statistical analyses were undertaken in R [45]. Figures were also constructed in R using “ggplot2” and “ggpubr” [46,47].
3. Results
There were significant differences in bacterial concentrations with the interaction between treatments and bacterial sampling points in the Artemia on-growing tanks (ANOVA, F2,6 = 5.891, p = 0.0384). Post hoc testing indicated that bacteria concentrations were significantly higher in the 10 mg/L treatment at 0 h after the DOC additions compared to the other two treatments (Figure 1, Tukey’s HSD, p < 0.05). After 40 h, bacterial cell concentrations in the control treatment (0 mg/L) on-growing tanks were less than in the 5 and 10 mg/L treatment on-growing tanks (Figure 1) though non-significantly (Tukey’s HSD, p > 0.05).
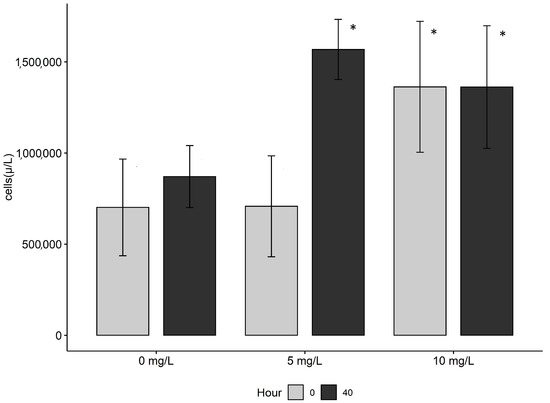
Figure 1.
Bacterial cell concentrations (cells/µL) for the 3 DOC addition treatments 0 mg/L, 5 mg/L, and 10 mg/L in the Artemia on-growing chambers during the bio-encapsulation period. 0 h measurements were taken minutes after the addition of leachate and Artemia to freshly prepared on-growing tanks. 40 h measurements were taken from pre-prepared on-growing tanks to be used for feeding that day, approximately 40 h after the addition of leachate and Artemia. Error bars represent standard error of the mean (n = 9), and asterisks (*) denote statistical differences.
The survival rates of juvenile fish throughout the study varied between treatments and time series (Table 2). The survival rates of juvenile fish decreased significantly across the study (Table 2, ANOVA, F2,18 = 6.945, p = 0.0058) with significant differences between the T1 and T3 time periods (Tukey’s HSD, p = 0.006). There was no significant difference in survival rates between treatments, although the survival rates of fish at the final time point (T3) were greatest in the 10 mg/L treatment (Table 2).

Table 2.
Survival rates of each treatment. Larger standard deviation in the T2 0 mg/L samples are due to swim bladder infections in 1 tank. This T2 control tank was impacted two days before sampling so growth depensation effects were disregarded.
Analysis of the average wet weight of fish through ANOVA showed there was a significant difference in weight between the treatments (0 mg/L, 5 mg/L and 10 mg/L) and time points (T0, T1, T2, and T3) (ANOVA, F4,839 = 5.99, p < 0.001). There were notable decreases in weight for all treatments during the first fortnight of the experiment (Tukey’s HSD, p < 0.001), with decreases from 0.064 g (±0.002) at T0 to 0.044 g (±0.001) for the control treatment, 0.042 g (±0.001) for the 5 mg/L treatment and 0.044 g (±0.001) for the 10 mg/L treatment by time point T1, 2 weeks into the study (Figure 2a). In the control treatment, the wet weight continued to fall across the experimental period to 0.037 g (±0.001) T3 at the end of the experiment (Figure 2a). Similarly, the wet weights in the 5 mg/L treatment also decreased between T1 and T2 to 0.035 (±0.001) but then increased to 0.040 g (±0.001) by T3 at the end of the experiment (Figure 2a). Similar to the 5 mg/L treatment, the fish from the 10 mg/L treatment decreased in average wet weight between T1 and T2 to 0.042 g (±0.001) and increased to 0.045 g (±0.001) by T3 at the conclusion of the study (Figure 2a). By the end of the experiment, the fish from the 10 mg/L treatment were significantly heavier than those from the control (Tukey’s HSD, p < 0.001) and the 5 mg/L (Tukey’s HSD, p = 0.024) treatments.
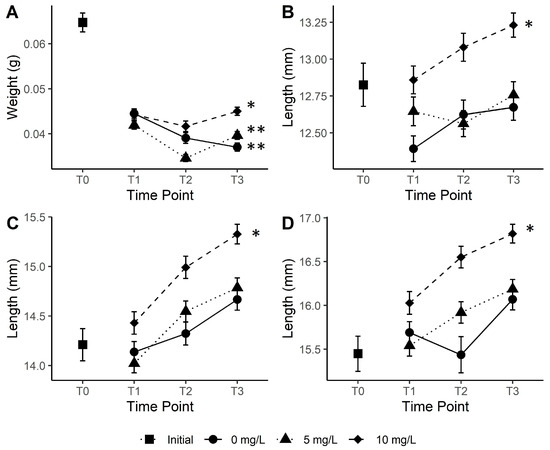
Figure 2.
Weight (A), standard length (B), fork length (C) and total length (D) of juvenile Australian bass for experimental carbon treatments of 0 mg/L, 5 mg/L and 10 mg/L across the study period. Measurements of fish when acquired are presented as “Initial” measurements. Error bars represent standard error of the mean (n = 30), and asterisks (* and **) denote statistical differences in measurements between treatments at the end of the study. T0 = time zero–start of experiment, T1 = sampling time point 1 (2 weeks from T0), T2 = sampling time point 2 (4 weeks from T0) and T3 = sampling time point 3 (6 weeks from T0).
There was no interaction factor between the treatment and time factors for any fish length measurements (standard, fork and total lengths). However, there were significant differences between time points and treatments for all length measurements (all p < 0.001). Fish length was significantly greater in the 10 mg/L treatment for standard and total length compared to other treatments by the conclusion of the study (Figure 2b,d, Tukey’s HSD, p < 0.001). Fork length was also greatest in the 10 mg/L treatment by T3 and significantly different to the control group and the 5 mg/L treatment by the end of the study (Figure 2c, Tukey’s HSD, p = 0.034). Over the course of the experiment, all length metrics increased continually regardless of the treatment, except for the 5 mg/L treatment for standard length and the control treatment for the total length, which both decreased between T1 and T2 before increasing again by T3 at the end of the experiment.
The leachate used in the experiment had an average δ13C and δ15N ratio of −29.4‰ (±0.529) and 3.2‰ (±0.834), respectively. Particulate organic matter in the on-growing tanks, which was a mixture of waste from biological processes occurring in the tanks, was more δ13C and δ15N depleted in the 10 mg/L treatment than in the control and 5 mg/L treatments (Figure 3). Artemia had similar δ13C ratios between treatments, although δ15N ratios for Artemia in the 5 and 10 mg/L treatments were higher than the control, being highest in the 10 mg/L treatment. Initial fish tissue measurements of δ13C and δ15N were −18.2‰ (±0.018) and 13.0‰ (±0.119), respectively (Figure 3). At the conclusion of the experiment, the fish in all treatments and control largely resembled their initial signatures, with signatures reflecting neither those of the leachate or Artemia. The fish from the 10 mg/L treatment were slightly more depleted in δ13C and more enriched in δ15N than the other treatments, which had similar δ13C and δ15N ratios (−17.7‰ and 12.5‰, respectively).
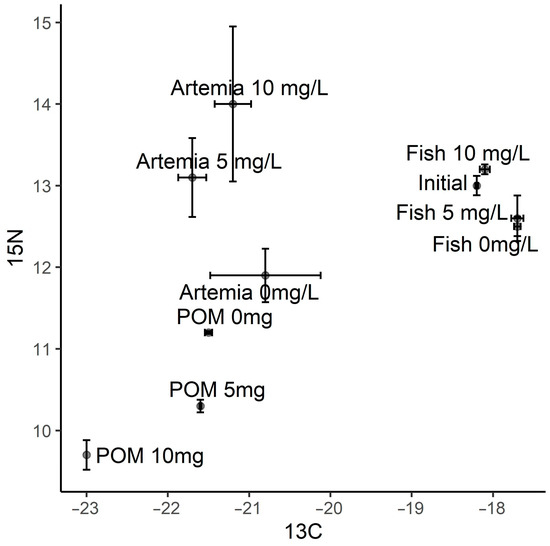
Figure 3.
δ13C (13C) and δ15N (15N) stable isotope ratios for fish tissues at the beginning (Initial) and conclusion of the experiment for all 3 treatments (Fish 0 mg/L, Fish 5 mg/L and Fish 10 mg/L). Isotopic ratios are also displayed for particulate organic matter samples from the Artemia on-growing tanks (POM 0 mg/L, 5 mg/L and 10 mg/L) and gut purged Artemia (Artemia 0 mg/L, 5 mg/L and 10 mg/L) from the 3 treatments. Error bars represent standard error of the mean for each sample.
4. Discussion
In this study, we investigated the influence of terrestrial dissolved organic matter (tDOM) on the somatic growth of juvenile Australian bass. Our results indicate that terrestrial carbon positively influenced the growth of juvenile Australian bass (Figure 2) in terms of body length when additions of DOC to Artemia were at higher concentrations (10 mg/L).
4.1. tDOM Influence on Growth
The juvenile fish in the 10 mg/L treatment were significantly larger and heavier than those of the other treatments, indicating that increased tDOM availability to Artemia had a positive influence on somatic growth relative to the control treatment. Although increases in size were small for all treatments across the study period due to the slow-growing nature of Australian bass [48], by the end of the study, the juveniles were roughly the same size (15–18 mm) as similarly aged individuals (12 weeks) found in the Sydney Basin [48]. However, the average lengths were smaller than optimally fed PSFI hatchery juveniles of the same age (20–25 mm, pers comms. Cheviot, L., PSFI, 2021). Although increases in the total length of the fish in all treatments continued throughout the experiment, there were significant reductions in weight (Figure 2a). The most significant reductions in weight occurred between T0 and T1 in all treatments. While osmotic water loss due to a movement between fresh and brackish water may have contributed to wet weight loss, these effects are generally short-lived, approximately 96 h [49,50]. It is more likely that the significantly reduced wet weights in all treatments are an indication of energetic limitation, with fish potentially utilising endogenous lipids and proteins to support themselves [38,51,52]. As the experimental design purposefully limited food availability by between 66 and 75% relative to PSFI hatchery diets [38], this process likely explains the weight loss of fish in all treatments. Interestingly, this feeding regime limitation did not impact the survival of juveniles, with survival rates remaining high across all treatments and time points of the study (Table 2), with T3 observations being similar to the survival rates found in the PSFI hatchery under optimal feeding conditions [38,39]. However, there was a consistent decline in fish survival across the experiment (Table 2) which may have been influenced by the feeding regime.
The minimal enrichment of the δ13C signatures of the juvenile fish relative to initial signatures across all treatments indicates that the added terrestrial carbon was not greatly assimilated into new tissue by juveniles over the duration of the experiment. Despite no evidence of direct consumption of tDOM [53,54], the enrichment of δ15N of fish tissue in the 10 mg/L treatment, relative to other treatments, may indicate that increased biological activity supported by increased tDOM led to the retention of enriched nitrogen in tissues. This is supported by the increased bacterial concentrations in the Artemia on-growing tanks (Figure 1), with leachate resulting in increased bacterial concentrations after 40 h compared to the controls (Figure 1). These increased bacterial concentrations in the on-growing tanks may have played a role in transferring additional resources in the 5 mg/L and 10 mg/L treatments to Artemia, thereby increasing the δ15N of Artemia by between 1 and 2‰ [54,55].
Our results contrast findings from field studies that have shown the assimilation of terrestrial carbon into tissue by juvenile fish [9,10]. This contrast may be due to the slow growth rate of the Australian bass [48] and the short time frame of the experiment, with lag effects in isotopic turnover in fish tissues limiting the degree to which tDOM dietary contributions could be seen in this study. Further, food limitation conditions used in this study may have masked changes in δ13C of fish tissues. Reduced prey densities were used to remove the potential masking of growth effects by ad libitum feeding in a similar experiment [30]. However, metabolic processes in fish tissues under energetic limitation can limit the equilibration of δ13C signatures between the food source and tissue, particularly in fish muscle tissue [56].
While the δ13C signatures indicate the minimal assimilation of terrestrial carbon into fish tissue (Figure 3), tDOM may have contributed to somatic growth by subsidising the energetic demands of juvenile fish. This phenomenon has been observed previously in zooplankton, where terrestrial resources low in HUFA were selectively catabolised to meet energetic demands while HUFA from algal resources were retained for growth and reproductive development [11]. In the absence of dietary HUFA, the fish in the 10 mg/L treatment may have been able to utilise tDOM for immediate energy demands while utilising endogenous lipid reserves to facilitate increased fish length (Figure 2). However, in the control and 5 mg/L treatments, endogenous resources may not have been sufficient to increase fish length without a substantial tDOM subsidy. This process may also account for the consumption of terrestrial carbon, yielding a minimal response in terms of assimilation into fish tissue, as tDOM resources were prioritised for meeting energetic demands rather than contributing to growth [11].
The 5 mg/L treatment elicited no significant effect on juvenile fish growth relative to the control, indicating that energy transfer losses through the trophic levels render this concentration of tDOM (as DOC) insufficient to provide a notable dietary subsidy. Therefore, biomass transfer and somatic growth was not observed at this concentration. Limitations of energetic transfer due to tDOM quantity were also found by Hitchcock et al. [36], where a 3 mg/L addition of DOC had a reduced influence on copepod productivity compared to an addition of 16 mg/L. Similarly, Karlsson [57] observed an increasing gradient of glucose additions between 0.11 and 3.4 mg/L produced increasingly positive responses in zooplankton growth rates. However, the transfer of terrestrial energy through the food web may have been enhanced due to the relatively short food web pathways used to transfer energy to juvenile fish and the use of tDOM with a high bioavailability [36]. Future studies would benefit from examining a wider range of terrestrial carbon sources and concentrations to better understand differences in carbon bioavailability and to identify optimum concentrations to support juvenile fish growth.
4.2. tDOM, Inflows and Juvenile Fish
Our experiment used a terrestrially dominant diet to mimic heterotrophic conditions following inflow periods where algal production is suppressed, and terrestrial carbon becomes the dominant energy resource [28,58,59]. This provides a notable point of difference to other experiments where terrestrial carbon additions have been given to juvenile fish diets and found contrasting responses in juvenile fish growth. For example, Growns et al. [30] conducted experiments on freshwater gudgeons (Hypseleotris spp.) and found no significant increase in fish size with increased terrestrial DOC availability as part of a zooplankton diet based on mixed algal/tDOM sources. However, food web efficiency experiments with juvenile three-spined stickleback (Gasterosteus aculeatus) as an apex predator and increased DOC availability (in the form of glucose) in the presence of an existing phytoplankton energy pathway resulted in reduced lengths of juvenile fish [29]. Our results, where fish growth increased with exposure to greater tDOM availability, albeit slightly, may contrast with these experiments due to (1) the use of a “terrestrially dominant” diet in our experiment, where the influence of algal resources was reduced and (2) a sufficient concentration of tDOC to overcome energy respiration losses by microbial and zooplankton trophic levels [57].
Numerous studies have demonstrated a positive relationship between inflows, terrestrial carbon assimilation and the growth of juvenile fish in estuaries, attributing this to increased prey resources following inflows [8,9,26,60]. Our results provide potential support to the notion that terrestrial carbon uptake by zooplankton after inflows [19,20,22] can positively influence the somatic growth of juvenile fish, providing increased energetic resources. However, there were relatively small changes in juvenile size within our experiment, both between treatments and across the experimental timeframe, and only minor changes in isotopic ratios of fish tissue. Considering that river regulation is on the rise globally [27] and has been associated with changes in food web structures [61,62], with heterotrophic energy pathways dominating after inflow periods in regulated estuaries [28], investigations into the importance of terrestrial carbon transfer and its potential as a food resource remain crucial to understanding the wider role of freshwater inflow events.
5. Conclusions
Our results indicate that terrestrial carbon can positively influence somatic growth in terms of the length of juvenile Australian bass (Macquaria novemculeata) after the addition of tDOM to the food web. The addition of tDOM at concentrations of 10 mg/L of DOC to Artemia nauplii on-growing tanks led to the increased body length of the food-limited juvenile Australian bass fed these zooplankton. However, tDOM was not assimilated into new tissue by the juvenile fish, as revealed by stable isotope analysis, though these results may have been influenced by food limitation. Our results suggest that tDOM may be catabolised for energetic demands and contribute indirectly to somatic growth by allowing more high-quality dietary resources to be used for growth when resources are scarce.
Author Contributions
Conceptualisation: E.B.J. and S.M.M.; Methodology: E.B.J., S.M.M., C.B., S.F. and W.H.; Formal analysis and investigation: E.B.J., J.H. and W.H.; Writing—original draft preparation: E.B.J. and S.M.M.; Writing—review and editing: C.B., J.H., W.H., S.F., J.A.F. and S.M.M.; Funding acquisition: C.B. and S.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this project was received from a UTS (University of Technology of Sydney) Research Excellence Scholarship and NSW DPI Water project “Carbon and nutrient transport, food webs and the effectiveness of high flow protection and end of system flows”.
Institutional Review Board Statement
The research was conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (8th Ed. 2013) with ethical approval from the University of Technology Sydney’s Animal Ethics Committee (No. ETH19–3895, Sydney, Australia).
Data Availability Statement
The datasets generated during and analysed during this study are available from the corresponding author upon reasonable request.
Acknowledgments
This project was also conducted with the support of NSW DPIE Fisheries staff Luke Cheviott and Graham Housefield.
Conflicts of Interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
References
- Loneragan, N.R.; Bunn, S.E. River Flows and Estuarine Ecosystems: Implications for Coastal Fisheries from a Review and a Case Study of the Logan River, Southeast Queensland. Aust. J. Ecol. 1999, 24, 431–440. [Google Scholar] [CrossRef]
- Gillanders, R.N.; Bronwyn, M.; Kingsford, M. Impact of Changes in Flow of Freshwater on Estuarine and Open Coastal Habitats and the Associated Organisms. In Oceanography and Marine Biology; Gibson, R.N., Barnes, M., Atkinson, R.J.A., Eds.; Taylor & Francis Ltd.: London, UK, 2002; Volume 40, pp. 233–309. [Google Scholar]
- Connolly, R.M.; Schlacher, T.A.; Gaston, T.F. Stable Isotope Evidence for Trophic Subsidy of Coastal Benthic Fisheries by River Discharge Plumes off Small Estuaries. Mar. Biol. Res. 2009, 5, 164–171. [Google Scholar] [CrossRef]
- ter Morshuizen, L.D.; Whitfield, A.K.; Paterson, A.W. Influence of Freshwater Flow Regime on Fish Assemblages in the Great Fish River and Estuary. S. Afr. J. Aquat. Sci. 1996, 22, 52–61. [Google Scholar] [CrossRef]
- Robins, J.B.B.; Halliday, A.I.; Staunton-Smith, J.; Mayer, G.D.; Sellin, J.M.; Halliday, I.A.; Staunton-Smith, J.; Mayer, D.G.; Sellin, M.J. Freshwater Flow Requirements of Estuarine Fisheries in Tropical Australia: A Review of the State of Knowledge and Application of a Suggested Approach. Mar. Freshw. Res. 2005, 56, 343–360. [Google Scholar] [CrossRef]
- Gillson, J. Freshwater Flow and Fisheries Production in Estuarine and Coastal Systems: Where a Drop of Rain Is Not Lost. Rev. Fish. Sci. 2011, 19, 169–186. [Google Scholar] [CrossRef]
- Salen-Picard, C.; Darnaude, A.M.; Arlhac, D.; Harmelin-Vivien, M.L. Fluctuations of Macrobenthic Populations: A Link between Climate-Driven River Run-off and Sole Fishery Yields in the Gulf of Lions. Oecologia 2002, 133, 380–388. [Google Scholar] [CrossRef]
- Darnaude, A.M.; Salen-Picard, C.; Polunin, N.V.C.; Harmelin-Vivien, M.L. Trophodynamic Linkage between River Runoff and Coastal Fishery Yield Elucidated by Stable Isotope Data in the Gulf of Lions (NW Mediterranean). Oecologia 2004, 138, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.C.; Bronk, D.A.; Olney, J.E. Contribution of Allochthonous Carbon to American Shad Production in the Mattaponi River, Virginia, Using Stable Isotopes. Estuaries Coasts 2007, 30, 1034–1048. [Google Scholar] [CrossRef]
- Abrantes, K.G.; Sheaves, M. Importance of Freshwater Flow in Terrestrial-Aquatic Energetic Connectivity in Intermittently Connected Estuaries of Tropical Australia. Mar. Biol. 2010, 157, 2071–2086. [Google Scholar] [CrossRef]
- Brett, M.T.; Kainz, M.J.; Taipale, S.J.; Seshan, H. Phytoplankton, Not Allochthonous Carbon, Sustains Herbivorous Zooplankton Production. Proc. Natl. Acad. Sci. USA 2009, 106, 21197–21201. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; Solomon, C.T.; Weidel, B.C.; Jones, S.E. Terrestrial Carbon Is a Resource, but Not a Subsidy, for Lake Zooplankton. Ecology 2014, 95, 1236–1242. [Google Scholar] [CrossRef]
- Taipale, S.J.; Brett, M.T.; Hahn, M.W.; Martin-Creuzburg, D.; Yeung, S.; Hiltunen, M.; Strandberg, U.; Kankaala, P. Differing Daphnia Magna Assimilation Efficiencies for Terrestrial, Bacterial, and Algal Carbon and Fatty Acids. Ecology 2014, 95, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estevez, A. Recent Developments in the Essential Fatty Acid Nutrition of Fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. Lipid Nutrition of Marine Fish during Early Development: Current Status and Future Directions. Aquaculture 1999, 179, 217–229. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.A.; McDowell, W.H.; Neff, J.C. Sources, Production, and Regulation of Allochthonous Dissolved Organic Matter Inputs to Surface Waters. In Aquatic Ecosystems: Interactivity of Dissolved Organic Matter; Findlay, S., Sinsabaugh, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 25–70. [Google Scholar]
- Ducklow, H.W.; Purdie, D.A.; Williams, P.J.L.B.; Davies, J.M. Bacterioplankton: A Sink for Carbon in a Coastal Marine Plankton Community. Science 1986, 232, 871–873. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M. Highs and Lows: The Effect of Differently Sized Freshwater Inflows on Estuarine Carbon, Nitrogen, Phosphorus, Bacteria and Chlorophyll a Dynamics. Estuar. Coast. Shelf Sci. 2015, 156, 71–82. [Google Scholar] [CrossRef]
- Asmala, E.; Autio, R.; Kaartokallio, H.; Stedmon, C.A.; Thomas, D.N. Processing of Humic-Rich Riverine Dissolved Organic Matter by Estuarine Bacteria: Effects of Predegradation and Inorganic Nutrients. Aquat. Sci. 2014, 76, 451–463. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M.; Hadwen, W.L.; Growns, I.O.; Rohlfs, A.M. Zooplankton Responses to Freshwater Inflows and Organic-Matter Pulses in a Wave-Dominated Estuary. Mar. Freshw. Res. 2016, 67, 1374–1386. [Google Scholar] [CrossRef]
- Bartels, P.; Ask, J.; Andersson, A.; Karlsson, J.; Giesler, R. Allochthonous Organic Matter Supports Benthic but Not Pelagic Food Webs in Shallow Coastal Ecosystems. Ecosystems 2018, 21, 1459–1470. [Google Scholar] [CrossRef]
- Berggren, M.; Bengtson, P.; Soares, A.R.A.; Karlsson, J. Terrestrial Support of Zooplankton Biomass in Northern Rivers. Limnol. Ocean 2018, 63, 2479–2492. [Google Scholar] [CrossRef]
- Kostecki, C.; le Loc’h, F.; Roussel, J.M.; Desroy, N.; Huteau, D.; Riera, P.; le Bris, H.; le Pape, O. Dynamics of an Estuarine Nursery Ground: The Spatio-Temporal Relationship between the River Flow and the Food Web of the Juvenile Common Sole (Solea solea, L.) as Revealed by Stable Isotopes Analysis. J. Sea Res. 2010, 64, 54–60. [Google Scholar] [CrossRef]
- Houde, E.D. Patterns and Consequences of Selective Processes in Teleost Early Life Histories. In Early Life History and Recruitment in Fish Populations; Springer Netherlands: Dordrecht, The Netherlands, 1997; pp. 173–196. [Google Scholar]
- Sogard, S.M. Size-Selective Mortality in the Juvenile Stage of Teleost Fishes: A Review. Bull. Mar. Sci. 1997, 60, 1129–1157. [Google Scholar]
- von Biela, V.R.; Zimmerman, C.E.; Cohn, B.R.; Welker, J.M. Terrestrial and Marine Trophic Pathways Support Young-of-Year Growth in a Nearshore Arctic Fish. Polar Biol. 2013, 36, 137–146. [Google Scholar] [CrossRef]
- Arthington, A.H.; Kennen, J.G.; Stein, E.D.; Webb, J.A. Recent Advances in Environmental Flows Science and Water Management—Innovation in the Anthropocene. In Freshwater Biology; Blackwell Publishing Ltd.: Oxford, UK, 2018; Volume 63, pp. 1022–1034. [Google Scholar]
- Hemraj, D.A.; Hossain, A.; Ye, Q.; Qin, J.G.; Leterme, S.C. Anthropogenic Shift of Planktonic Food Web Structure in a Coastal Lagoon by Freshwater Flow Regulation. Sci. Rep. 2017, 7, 44441. [Google Scholar] [CrossRef]
- Degerman, R.; Lefébure, R.; Byström, P.; Båmstedt, U.; Larsson, S.; Andersson, A. Food Web Interactions Determine Energy Transfer Efficiency and Top Consumer Responses to Inputs of Dissolved Organic Carbon. Hydrobiologia 2018, 805, 131–146. [Google Scholar] [CrossRef]
- Growns, I.; Ryder, D.; McInerney, P.; Bond, N.; Holt, G.; Lester, R.; Thompson, R. The Use of Fatty Acids to Identify Food Sources of Secondary Consumers in Wetland Mesocosms. J. Freshw. Ecol. 2020, 35, 173–189. [Google Scholar] [CrossRef]
- Bruesewitz, D.A.; Gardner, W.S.; Mooney, R.F.; Pollard, L.; Buskey, E.J. Estuarine Ecosystem Function Response to Flood and Drought in a Shallow, Semiarid Estuary: Nitrogen Cycling and Ecosystem Metabolism. Limnol. Ocean 2013, 58, 2293–2309. [Google Scholar] [CrossRef]
- Growns, I.; James, M. Relationships between River Flows and Recreational Catches of Australian Bass. J. Fish. Biol. 2005, 66, 404–416. [Google Scholar] [CrossRef]
- Harding, D.J.; Dwyer, R.G.; Mullins, T.M.; Kennard, M.J.; Pillans, R.D.; Roberts, D.T. Migration Patterns and Estuarine Aggregations of a Catadromous Fish, Australian Bass (Percalates Novemaculeata) in a Regulated River System. Mar. Freshw. Res. 2017, 68, 1544–1553. [Google Scholar] [CrossRef]
- Morrongiello, J.R.; Walsh, C.T.; Gray, C.A.; Stocks, J.R.; Crook, D.A. Environmental Change Drives Long-Term Recruitment and Growth Variation in an Estuarine Fish. Glob. Chang. Biol. 2014, 20, 1844–1860. [Google Scholar] [CrossRef]
- Stoessel, D.J.; Morrongiello, J.R.; Raadik, T.A.; Lyon, J.P.; Nicol, M.D. Determinants of Year Class Strength and Growth of Estuary Perch Macquaria Colonorum in a Highly Regulated System. Mar. Freshw. Res. 2018, 69, 1663–1673. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M.; Hadwen, W.L.; Roelke, D.L.; Growns, I.O.; Rohlfs, A.M. Terrestrial Dissolved Organic Carbon Subsidizes Estuarine Zooplankton: An in Situ Mesocosm Study. Limnol. Ocean 2016, 61, 254–267. [Google Scholar] [CrossRef]
- Lennon, J.T.; Pfaff, L.E. Source and Supply of Terrestrial Organic Matter Affects Aquatic Microbial Metabolism. Aquat. Microb. Ecol. 2005, 39, 107–119. [Google Scholar] [CrossRef]
- Fielder, S.; Heasman, M. Hatchery Manual for the Production of Australian Bass, Mulloway and Yellowtail Kingfish; Industry & Investment NSW: Orange, CA, USA, 2011; p. 176. ISBN 978 1 74256 058 8. [Google Scholar]
- van der Wal, E.J.; Nell, J.A. Effect of Food Concentration on the Survival and Growth of Australian Bass (Macquaria Novemaculeata) Larvae. Progress. Fish-Cult. 1986, 48, 202–204. [Google Scholar] [CrossRef]
- Sorgeloos, P.; Dhert, P.; Candreva, P. Use of the Brine Shrimp, Artemia spp., in Marine Fish Larviculture. Aquaculture 2001, 200, 147–159. [Google Scholar] [CrossRef]
- Carney, R.L.; Mitrovic, S.M.; Jeffries, T.; Westhorpe, D.; Curlevski, N.; Seymour, J.R. River bacterioplankton community responses to a high inflow event. Aquat. Microb. Ecol. 2015, 75, 187–205. [Google Scholar] [CrossRef]
- Harris, J.H. Diet of the Australian Bass, Macquaria Novemaculeata (Perciformes: Percichthyidae), in the Sydney Basin. Mar. Freshw. Res. 1985, 36, 219–234. [Google Scholar] [CrossRef]
- Mallen-Cooper, M. Swimming ability of juvenile Australian bass, Macquaria novemaculeata (Steindachner), and juvenile barramundi, Lates calcarifer (Bloch), in an experimental vertical-slot fishway. Mar. Freshw. Res. 1992, 43, 823–833. [Google Scholar] [CrossRef]
- Suthers, I.M.; Cleary, J.J.; Battaglene, S.C.; Evans, R. Relative RNA content as a measure of condition in larval and juvenile fish. Mar. Freshw. Res. 1996, 47, 301–307. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Australia, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. Package ‘Ggpubr’: “ggplot2” Based Publication Ready Plots; R Package Version 0.4.0; 2020. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 January 2023).
- Harris, J.H. Growth of Australian Bass Macquaria Novemaculeata (Perciformes:Percichthyidae) in the Sydney Basin. Mar. Freshw. Res. 1987, 38, 351–361. [Google Scholar] [CrossRef]
- Maceina, M.J.; Shireman, J.V. Grass Carp: Effects of Salinity on Survival, Weight Loss, and Muscle Tissue Water Content. Progress. Fish-Cult. 1979, 41, 69–73. [Google Scholar] [CrossRef]
- Altinok, I.; Galli, S.M.; Chapman, F.A. Ionic and Osmotic Regulation Capabilities of Juvenile Gulf of Mexico Sturgeon, Acipenser Oxyrinchus de Sotoi. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 120, 609–616. [Google Scholar] [CrossRef]
- Güroy, D.; Güroy, B.; Merrifield, D.L.; Ergün, S.; Tekinay, A.A.; Yiǧit, M. Effect of Dietary Ulva and Spirulina on Weight Loss and Body Composition of Rainbow Trout, Oncorhynchus Mykiss (Walbaum), during a Starvation Period. J. Anim. Physiol. Anim. Nutr. 2011, 95, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Falahatkar, B.; Akhavan, S.R.; Efatpanah, I.; Meknatkhah, B. Effect of Winter Feeding and Starvation on the Growth Performance of Young-of-Year (YOY) Great Sturgeon, Huso Huso. J. Appl. Ichthyol. 2013, 29, 26–30. [Google Scholar] [CrossRef]
- Deniro, M.J.; Epstein, S. Influence of Diet on the Distribution of Nitrogen Isotopes in Animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in Trophic Shift for Stable Isotope Ratios of Carbon, Nitrogen, and Sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Karlsson, J.; Jonsson, A.; Meili, M.; Jansson, M. Δ15N of Zooplankton Species in Subarctic Lakes in Northern Sweden: Effects of Diet and Trophic Fractionation. Freshw. Biol. 2004, 49, 526–534. [Google Scholar] [CrossRef]
- Colborne, S.F.; Robinson, B.W. Effect of Nutritional Condition on Variation in Δ13C and Δ15N Stable Isotope Values in Pumpkinseed Sunfish (Lepomis Gibbosus) Fed Different Diets. Environ. Biol. Fishes 2013, 96, 543–554. [Google Scholar] [CrossRef]
- Karlsson, J. Different Carbon Support for Respiration and Secondary Production in Unproductive Lakes. Oikos 2007, 116, 1691–1696. [Google Scholar] [CrossRef]
- Meunier, C.L.; Liess, A.; Andersson, A.; Brugel, S.; Paczkowska, J.; Rahman, H.; Skoglund, B.; Rowe, O.F. Allochthonous Carbon Is a Major Driver of the Microbial Food Web—A Mesocosm Study Simulating Elevated Terrestrial Matter Runoff. Mar. Environ. Res. 2017, 129, 236–244. [Google Scholar] [CrossRef]
- Andersson, A.; Brugel, S.; Paczkowska, J.; Rowe, O.F.; Figueroa, D.; Kratzer, S.; Legrand, C. Influence of Allochthonous Dissolved Organic Matter on Pelagic Basal Production in a Northerly Estuary. Estuar. Coast. Shelf Sci. 2018, 204, 225–235. [Google Scholar] [CrossRef]
- Wai, T.-C.; Yeung, J.W.Y.; Lam, V.Y.Y.; Leung, K.M.Y.; Dudgeon, D.; Williams, G.A. Monsoons and Habitat Influence Trophic Pathways and the Importance of Terrestrial-Marine Linkages for Estuary Sharks. Ecosphere 2012, 3, art8. [Google Scholar] [CrossRef]
- Harfmann, J.; Kurobe, T.; Bergamaschi, B.; Teh, S.; Hernes, P. Plant Detritus Is Selectively Consumed by Estuarine Copepods and Can Augment Their Survival. Sci. Rep. 2019, 9, 9076. [Google Scholar] [CrossRef] [PubMed]
- Ru, H.; Li, Y.; Sheng, Q.; Zhong, L.; Ni, Z. River Damming Affects Energy Flow and Food Web Structure: A Case Study from a Subtropical Large River. Hydrobiologia 2020, 847, 679–695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).