Abstract
Occurrence of micropollutants in water and their potential impact on the environment and human health are arising concerns. The micropollutants are not removed efficiently by current wastewater treatment and a small amount of them get released into receiving waters accompanying the discharging of the treated wastewater effluents. Therefore, it is useful to investigate an advanced or alternative technology to remove traces of micropollutants in Lake Constance water during drinking water treatment. Among various oxidation processes, ferrate(VI) has received extensive attentions due to its superior dual properties of oxidation and coagulation. The work in this communication is the first trial using ferrate(VI) in comparison with FeCl3/ozonation to treat lake water and to remove micropollutants in the region. The results of pilot-scale trials showed that 10% of metformin, benzotriazole and acesulfam can be removed by ferrate(VI) at a dose of 0.1 mg L−1 from raw water, but FeCl3 with or without pre-ozonation cannot achieve the same performance. The degradability of three additional micropollutants by ferrate(VI) oxidation followed the sequences of bisphenol-S (BS) > azithromycin (AZM) > imidacloprid (IMP) was evaluated, and 100% concentration reduction of BS was achieved. The work suggests that ferrate(VI) is a potential alternative to the existing treatment processes for drinking water treatment.
1. Introduction
Lake Constance is the third largest lake in Europe and located at the northwest edge of the Alps. Lake Constance has a surface of 535 km2, a maximum depth of 253 m and occupies 4.84 × 1010 m3. Its catchment area is 11,500 km2, locating it in Germany, Austria and Switzerland. The mean annual inflow is about 1.2 × 1010 m3, of which more than 80% are from glacial origin and comes from the Alps by Alpine Rhine, Bregenzerach and Dornbirnerach [1]. In total, 17 drinking water treatment plants from the three countries withdraw about 1.70 × 108 m3 of surface water annually from Lake Constance to supply more than 5 million people with drinking water [1,2].
Although over 95% of the wastewater in the catchment area of Lake Constance is treated before entering the lake [1], micropollutants are not removed efficiently by wastewater purification and are discharged into receiving waters of Lake Constance. Therefore, it is useful to investigate the occurrence and removal efficiency of micropollutants in the water of Lake Constance by an oxidative drinking water treatment process.
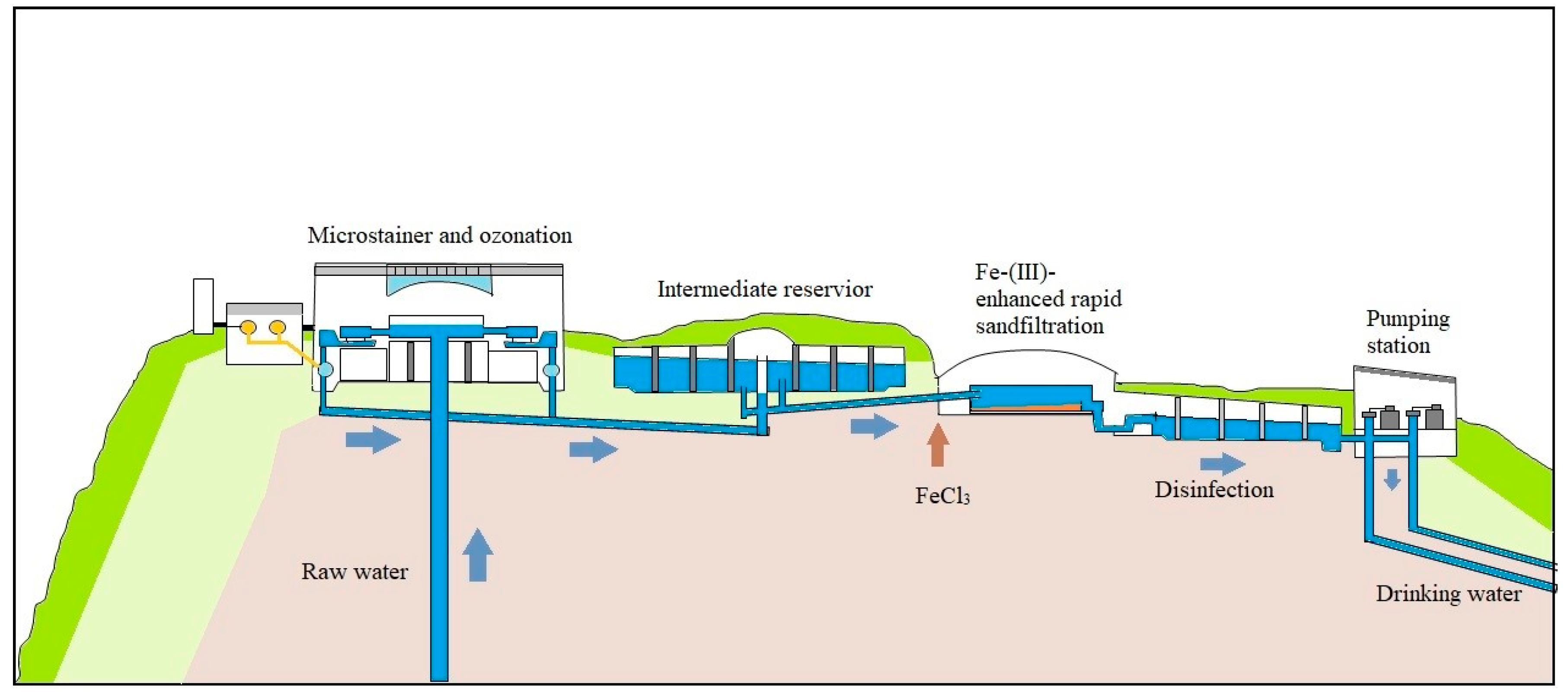
Figure 1 shows the Lake Constance Water Supply treatment processes. The raw water is drawn from a depth of 60 m of the Lake Constance. Micro-strainers with a mesh size of 15 µm remove phytoplankton, zooplankton and suspended matter. Thereafter, microalgae, bacteria and viruses are inactivated by ozonation with an initial ozone dose of 1.0 to 1.2 mg L−1. The mean ozone contact time is about 1.5 to 2.5 h depending on the overall water flow through the treatment plant. The last step of the treatment is a ferric-enhanced rapid sand filtration with a FeCl3 dosage of 100 µg L−1 to remove all particles with a size ≥ 1 µm. After the treatment process, chlorine is added to the drinking water so that the mean free chlorine concentration is 0.20 to 0.25 mg L−1.
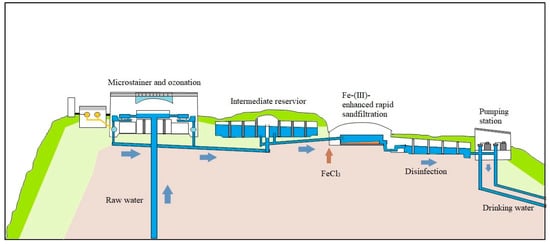
Figure 1.
Schematic diagram of processes at the Lake Constance Water Supply Treatment Plant.
Considerable emerging micropollutants’ (EMPs) residuals have been found in the water courses, which can cause adverse effects on the environment and living species health. Endocrine disrupting chemicals (EDCs) are one type of EMPs, which can cause disordered hormones and lead to depress the reproduction and growth of some species (e.g., fishes and birds) [3]. Antibiotics were also categorized as a main EMP; the misuse of antibiotics leads to its residuals being present in water, which can inhibit non-target organisms in the environment. Additionally, antibiotic-resistant bacteria make it difficult to cure infections of humans and livestock and also cause adverse public health issues [4].
Although high water quality of drinking water is achievable using the current treatment processes at Lake Constance Water Supply Treatment Plant (Figure 1), we are seeking more efficient and alternative oxidation processes in order to tackle the issue of micropollutants and to meet potential legislations that will regulate the concentrations of the given micropollutants in the near future [5,6].
Among various oxidation processes, ferrate(VI) has received extensive attentions due to its superior dual properties of oxidation and coagulation [7]. Ferrate(VI) has redox potentials varying from +2.2 V at pH 1 to +0.7 V at pH 14, respectively [8], and can perform oxidation, coagulation, adsorption, sedimentation, disinfection, decolorization and deodorization, which are unmatched by other water treatment reagents. After dosing ferrate(VI) into the water to be treated, more active species, such as Fe(V) and Fe(VI), are suggested to be generated [9], and even free radicals, such as ·O2 and ·OH-, could also be produced during the ferrate self-decomposition [10]; this can contribute to superior oxidation performance in some cases. However, due to its unstable property, ferrate(VI) cannot be stored for more than a couple of months, which means that it has limited practical application. Further, an in situ synthesis and application of ferrate(VI) has been considered in a pilot-scale wastewater treatment trial [11], which demonstrated that a potential use of ferrate(VI) for wastewater treatment could be possible.
Therefore, this research aims to demonstrate that ferrate(VI) could be a potential alternative to the existing treatment processes for drinking water treatment by studying the performance of ferrate(VI) in comparison with that of ferric chloride and degrading the selected three micropollutants by ferrate(VI) and ozonation.
2. Materials and Methods
2.1. Materials
Water samples of this study were raw lake water and treated drinking water. Characteristic properties of lake water can be seen in Table 1. Gabapentin, metformin, 10, 11-dihydroxycarbamazepin, 4-AAA, AMPA, 1, 4-dioxan, benzotriazole, 4-methyl-benzotriazole, 5-methyl-benzotriazole, acesulfam, cyclamat, sucralose, imidacloprid, bisphenol-S and azithromycin in analytical grade were purchased from Sigma-Aldrich (Glasgow, Scotland, UK). Dehydrated Biofix-Lumi luminescent bacteria (Vibro fisheri) and bacteria reactivated reagent were purchased from Envitech Ltd (Cardiff, Wales, UK), which were stored in the freezer at temperature of −15℃, and the bacteria reagent was stored in the fridge at temperature of 4 °C.

Table 1.
Occurrence of selected micropollutants in raw water and drinking water, Lake Constance Water Supply Treatment Plant in 2017–2019.
2.2. Pilot Plant Setup
The pilot plant followed the main plant’s processes: water flows through a micro sieve filter (15 µm), and then flows into the customized ozone mixer followed by seven contact tanks. Then, K2FeO4 (ferrate(VI)) and FeCl3 were dosed into two flowing waters separately by peristaltic pumps with the required volume dosage (equivalent to 0.1 mg L−1 as Fe). Water/coagulant mixtures were directed into two separated chambers where suitable flocculation occurred before the flow entered two parallel filter columns (Figure 2) with similar flow conditions. The flow rate for each stream was 1.5 m3 h−1, and the daily test period was 5 h. The two parallel filtration columns have the same configurations (Everzit N and quartz sand) and can be run with either raw water or ozonated water. When using raw water, no ozonation was operated, and then the performance of ferrate(VI) can be compared with that of FeCl3. Both filtrates were collected for the analysis of water qualities. A detailed pilot-plant setup can be seen in [12].

Figure 2.
Pilot plant facilities.
2.3. Additional Jar Tests
Additional jar tests were performed to compare the removal efficiency of three extra micropollutants, namely, imidacloprid (IMP), bisphenol-S (BS) and azithromycin (AZM). They represent pesticides (IMP), endocrine disrupting chemicals (BS) and pharmaceuticals (AZM), respectively. The three compounds are typical EMPs, which have been received interest by the Lake Constance Water Supply Treatment Plant since the increasing use of those three compounds in the area poses potential risks on the quality of Lake Constance water in the future. They were treated by either ferrate(VI) or ozonation. The equivalent dose (0.036 mM) of either ferrate(VI) or ozone was chosen, which was based on the pre-determined tests of ferrate(VI) to achieve the best removal efficiency, and dosed into the pre-prepared test solutions (1000 µg L−1 and pH 7 of each micropollutant, and pH 7 represents neutral acidic level and fits to the drinking water standards). The jar test was performed using Stuart™ SW6 flocculator (Cole-Parmer UK, Cambridgeshire, UK). The fast and slow mixing strength and duration were 250 rpm for 2 min and 40 rpm for 20 min, respectively. The subsequent sedimentation duration was 30 min. The treated samples were filtered through 0.45 µm cellulose filter. The filtered samples were analyzed for the concentrations of micropollutants studied and dissolved organic carbon (DOC).
2.4. Analysis
Micropollutants were analyzed by liquid chromatography with high-resolution mass spectrometry (LC-HRMS, Q-Exactive, Thermo-Fisher Scientific GmbH, Dreieich, Germany) after filtration and direct injection (DI) or after solid-phase extraction according to DIN 38407-47:2017-07 [13] and DIN ISO 16308:2017-09 [14], respectively. One of pollutants, 1, 4-dioxan, was analyzed according to EPA 522 [15] by solid-phase extraction (400 mL sample) and gas chromatography–mass spectrometry but with tandem mass spectrometry (GC-MS/MS, TSQ 8000evo, Thermo-Fisher Scientific GmbH, Dreieich, Germany). The limit of detection of all micropollutants for LC-HRMS and GC-MS/MS methods was between 10 and 20 ng L−1.
2.5. Toxicity Assessment
After treatment with ferrate(VI) and ozonation, the resultant toxicity of the chosen micropollutants, imidacloprid (IMP), bisphenol-S (BS) and azithromycin (AZM), was assessed by the laboratory’s test using Vibro fisheri, which are nonpathogenic, marine, luminance bacteria, as well as by International Standard ISO 11348-3:2007 (E) [16] and the Ecological Structure–Activity Relationships (ECOSAR) modelling tool (ECOSAR V2.0), which is a useful reference for the experimental results of toxicity [17].
3. Results and Discussion
3.1. Quality of Lake Constance Water
Raw water of Lake Constance consists of various microcontaminants (see Table 1), including residuals of pharmaceuticals like gabapentin, metformin, 4-acetylaminoantipyrine (4-AAA) and 4-formylaminoantipyrine (4-FAA); pesticides; and industry chemical residuals, such as benzotriazole and artificial sweeteners like cyclamat, which were detectable in the concentrations between 10 and 270 ng L−1. In treated drinking water, many investigated micropollutants were below the limit of detection (i.e., <10 ng L−1), but metformin, 1, 4-dioxan, benzotriazole, 4-methyl-benzotriazole and acesulfam were detected with concentrations between <10 and 64 ng L−1 (Table 1). The overall water quality of drinking water in Lake Constance is shown in Table 2.

Table 2.
Quality characteristics of Lake Constance drinking water.
3.2. Comparative Treatment Performance
The results of pilot-scale trials showed that 10% of metformin, benzotriazole and acesulfam can be removed by ferrate(VI) at a dose of 0.1 mg L−1 from both raw water and ozonated water, but FeCl3 with or without pre-ozonation cannot achieve the same performance (Table 3). The lower ferrate(VI) dose used in the pilot plant trials followed the Fe dose of the main plant, aiming at equally comparing the performance of removing micropollutants. Neither ferrate(VI) nor ozonation with FeCl3 reduced DOC concentration due to low original DOC in the lake water and low Fe dose (0.1 mg L−1), which simulated the main treatment plant conditions. Additionally, due to low EMPs concentrations (Table 1), no oxidation compounds after ferrate treatment or ozonation could be detected [12].

Table 3.
Comparative performance of ferrate(VI) and FeCl3 (+ozonation).
3.3. Additional Jar Test Results
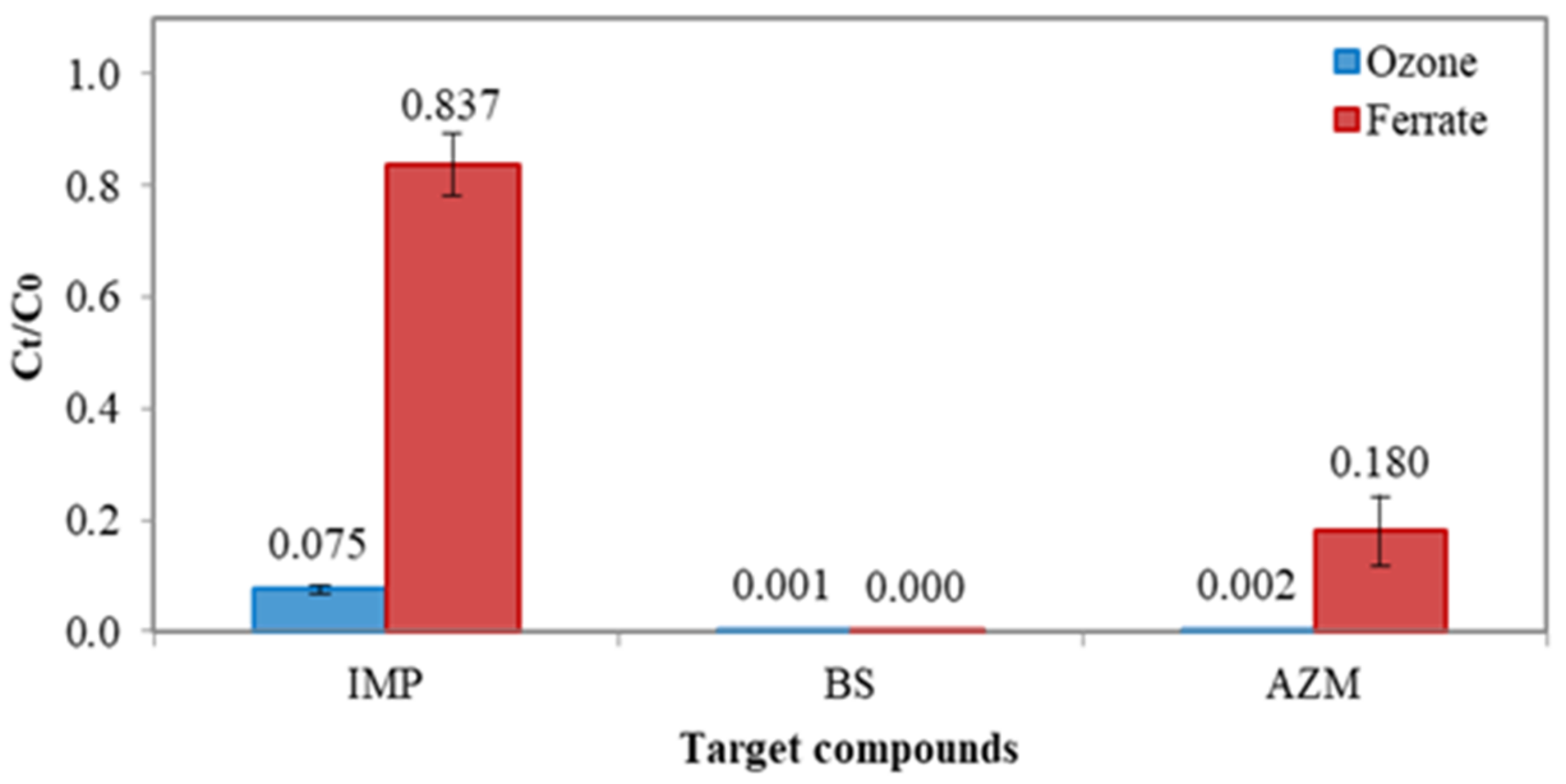
Due to extremely low concentrations of micropollutants in Lake Constance water and in the treated drinking water, additional jar tests were conducted in order to assess the treatment efficiency of either ferrate(VI) or ozonation. Figure 3 shows the results of concentration reduction of imidacloprid (IMP), bisphenol-S (BS) and azithromycin (AZM), where each solution with a concentration of 1000 μg L−1 and pH 7 was treated by ferrate(VI) or ozone, at the same dose of 0.036 mM. For IMP, the removal efficiency of ozonation and ferrate(VI) oxidation was greatly different; it was >95% removal by ozonation, whereas it was 14% removal by ferrate(VI). For BS, almost 100% concentration reductions were achieved by both ozonation and ferrate(VI). For AZM, there was 99.8% removal by ozonation and 82% by ferrate(VI). Overall, the degradability of three micropollutants by ozonation and ferrate(VI) oxidation followed the sequences of BS > AZM > IMP, which could be attributed to the characteristics of functional groups existing in three pollutants. Ferrate(VI) is a selective oxidant, which is more effective in reacting the compounds with electron-rich moieties (ERMs) than electron withdrawal groups (EWGs). BS consists of two phenyl groups where aromatic rings and hydroxylates are ERMs [18,19]. In contrast, IMP consists of EWGs such as halogen pyridine and imidazole. Thus, the ferrate(VI) is more effective in degrading BS than IMP. For AZM, the two heterocyclic groups in its molecule are more electron-enriched; ferrate(VI) can thus effectively treat more AZM than IMP. Comparing the performance of ozonation with that of ferrate(VI), the higher reactivity of ozone could be attributed to its relative greater redox potential than that of ferrate(VI) under the reaction’s conditions.
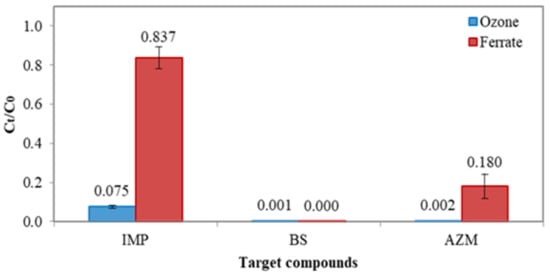
Figure 3.
Mean concentration reduction of IMP, BS and AZM with ferrate (VI) and ozonation, respectively. The dosage of ferrate(VI) and ozone was 0.036 mM. Test solution concentration = 1000 µg L−1 and pH = 7.
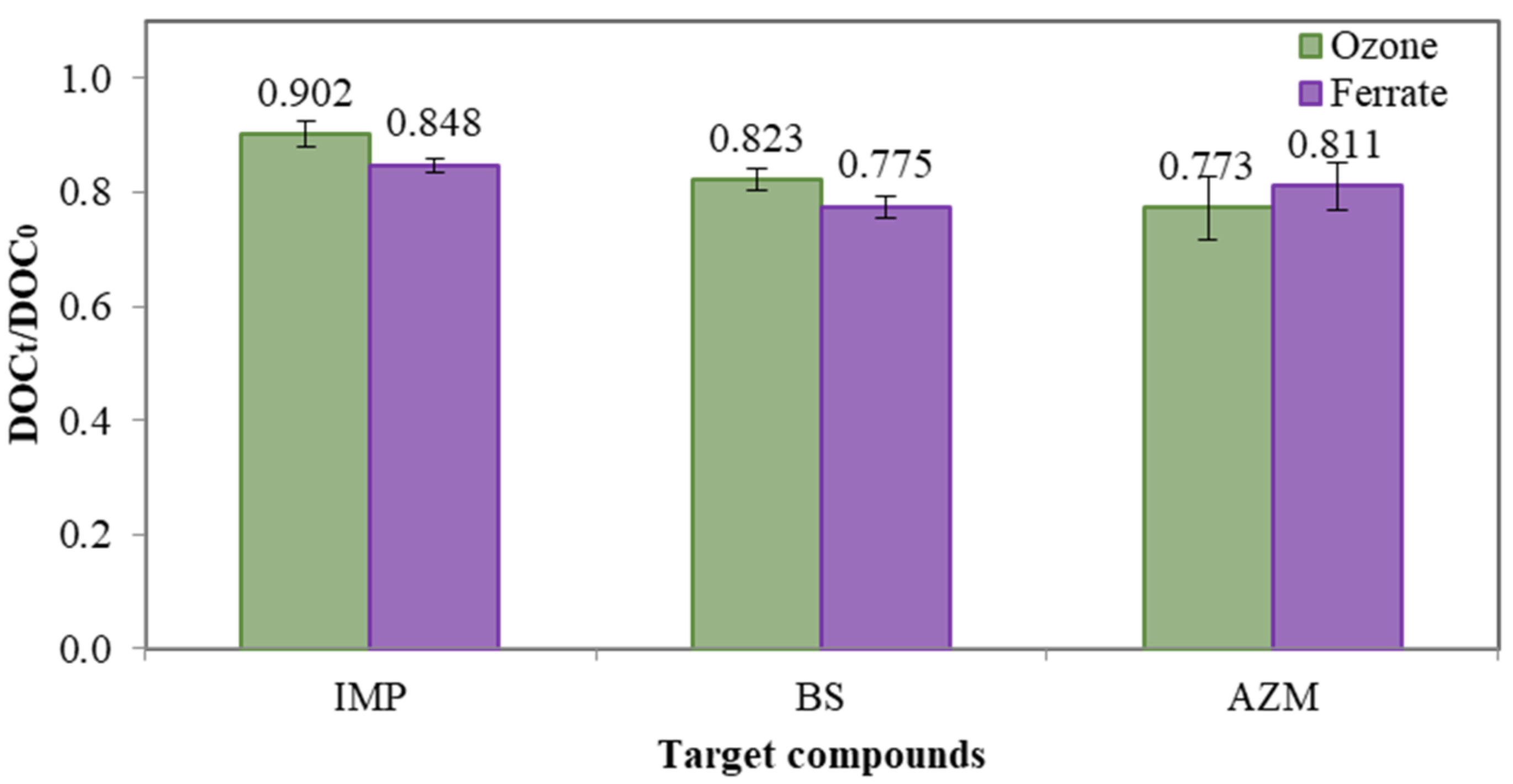
Figure 4 shows that approximately 10–20% of DOC removal in the degradation of IMP, BS and AZM by ozonation and ferrate(VI) treatment, which was relatively lower than the reduction of the compounds’ concentration. Moreover, there was no significant difference between DOC reductions by ferrate(VI) treatment and ozonation. DOC reduction indicates the mineralization of organic compounds, i.e., original organic compounds are converted to form more bio-assimilable molecules such as methane and carbon dioxide [20]. Limited mineralization by ferrate(VI) suggests that ferrate(VI)’s reactivity is mainly based on the functional groups and chemical bonds in the compounds to be treated. On the other hand, limited DOC reduction by ozonation in this study is attributed to the reactions of ozone molecules with the pollutants, which go through the selective reactions as well.
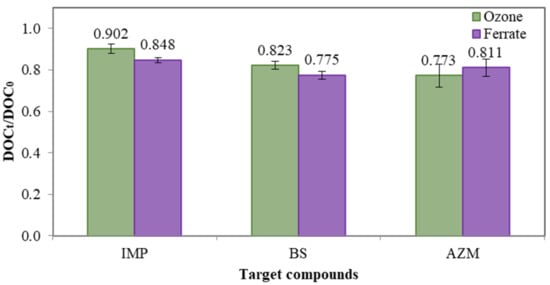
Figure 4.
Mean DOC reduction of IMP, BS and AZM with ferrate(VI) and ozonation, respectively. The dosage of ferrate(VI) and ozone was 0.036 mM. Test solution. concentration = 1000 µg L−1 and pH = 7.
3.4. Toxicity Assessment Results
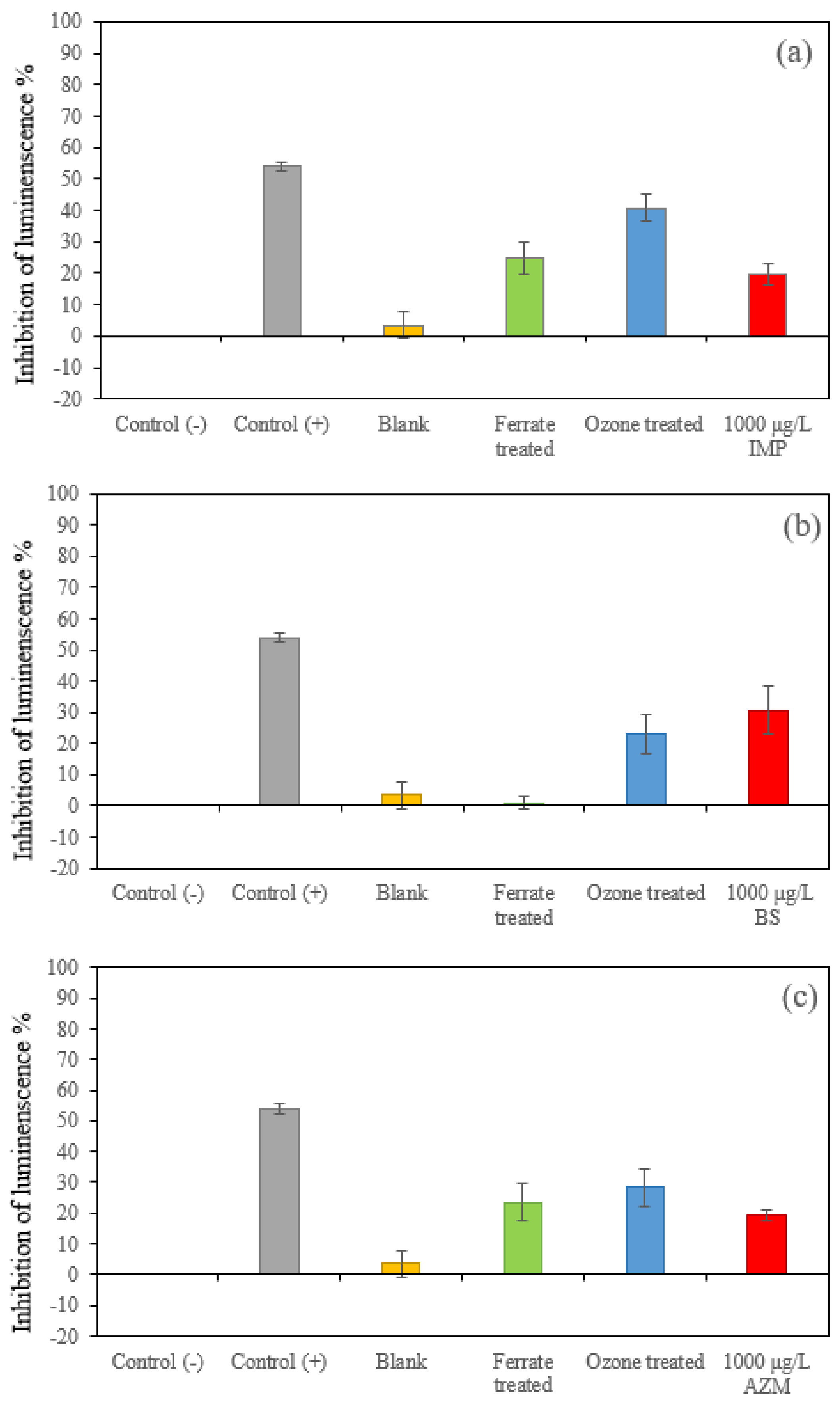
The positive control sample was a 5.29 g L−1 K2Cr2O7 solution, made by 2% NaCl. The results of 20–80% inhibition validated the toxicity test. The negative control sample was made of 2% NaCl solution, which was applied to correct the effect of attenuation of luminescence. It can be observed from Figure 5 that those untreated solutions containing IMP, BS and AZM at a concentration of 1000 μg L−1 had inhibiting effects on the growth of luminescent bacteria (or had toxicity). In all cases, the blank samples (treated drinking water) had slightly positive values, suggesting these samples had a minor inhibiting effect on the growth of luminescent bacteria, though such effects can be neglected in reference to the positive control sample. For BS samples (Figure 5b), the toxicity of the ferrate(VI)-treated samples significantly decreased, but the toxicity by ozonation was reduced less. In contrast, both IMP (Figure 5a) and AZM (Figure 5c) exhibited elevated toxicity after ferrate(VI) treatment and ozonation. The increase in the toxicity is attributed to the formation of oxidation products; they possessed higher toxicity than that of the original pollutants. The results are consistent to those of other studies (e.g., [21]). Moreover, variations of the toxicity of three target compounds reflect the nature of the properties and characteristics of raw compounds and their oxidation products.
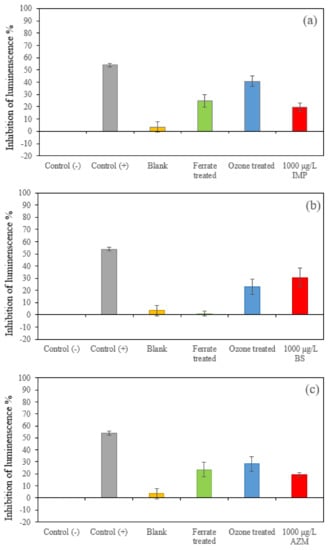
Figure 5.
Toxicity of the blank (treated drinking water) and the target three compounds. (1000 μg L−1), with and without being treated by 0.036 mM ferrate(VI) and ozone at pH 7, respectively. (a) IMP samples, (b) BS samples and (c) AZM samples.
Additionally, the toxicity of both untreated compounds and the oxidation products after ferrate(VI) treatment was assessed using the ecological structure–activity relationships modelling (ECOSAR Program V2.0) and the underlying methodology [22]. Based on the data shown in Table 4, the final oxidation products of IMP and AZM exhibit higher acute and chronic toxicity in three aquatic species than those of raw IMP and AZM. However, for BS, the toxicity of oxidation product reduced significantly in comparison with that of raw BS. In summary, the toxicity assessment by ECOSAR shows consistent results with that obtained from the laboratory bio-luminescence tests.

Table 4.
Aquatic toxicities resulting from IMP, BS and AZM and the relevant final oxidation product, calculated by ECOSAR program.
3.5. Overall Remarks on Future Work Consideration
Conventional wastewater treatment plants receive a large spectrum of micropollutants from domestic, industrial and hospital wastewater, which might not be completely eliminated during treatment processes. These contaminants need to be considered once drinking water is produced from downstream surface and lake waters. An integrated water protection management should include a better source water protection with effective wastewater treatment and have an advanced drinking water treatment process to be considered in the place. As shown in this study, the results of ferrate(VI) are encouraging, and the technology is a potential new drinking water treatment process, but further trials are needed to validate the cost effectiveness of using ferrate(VI) in full-scale drinking water treatment.
4. Conclusions
In the raw water of Lake Constance, 13 micropollutants were detectable. The concentrations of gabapentin, metformin acid, 4-AAA, 4-FAA and other monitored pollutants ranged between 10 and 270 ng L−1. In finished drinking water, many investigated micropollutants were below the limit of detection (i.e., <10 ng L−1), but metformin, 1, 4-dioxan, benzotriazole, 4-methyl-benzotriazole and acesulfam were detected with concentrations between <10 and 64 ng L−1.
Moreover, ferrate(VI) technology has been tested in a pilot-scale trial that demonstrated that ferrate(VI), at a dose of 0.1 mg L−1 as Fe, can remove 10% metformin, benzotriazole and acesulfam from raw water, but FeCl3 with or without pre-ozonation cannot. Additional jar tests demonstrated that the degradability of three chosen micropollutants by ozonation and ferrate(VI) oxidation followed the sequences of bisphenol-S (BS) > azithromycin (AZM) > imidacloprid (IMP), which could attribute to the characteristics of functional groups existing in three pollutants. Additionally, limited mineralization or DOC reduction by ferrate(VI) and ozonation were observed in this study, suggesting that both the ferrate(VI) and ozone selectively reacted with IMP, BS and AZM, and their reactivity is mainly based on the characteristics of functional groups of the pollutant compounds.
Finally, the toxicity assessment by both bio-luminescence tests and the ECOSAR program showed consistent results that the toxicity of BS samples after treatment by ferrate(VI) and ozonation decreased significantly; in contrast, both IMP and AZM samples exhibited elevated toxicity after ferrate(VI) treatment and ozonation.
Author Contributions
Conceptualization, J.-Q.J.; methodology, J.-Q.J., M.P. and S.Z.; validation, J.-Q.J.; formal analysis, all authors; investigation, all authors; writing—original draft preparation, J.-Q.J., M.P. and S.Z.; writing—review and editing, J.-Q.J.; supervision, J.-Q.J.; project administration, J.-Q.J.; funding acquisition, J.-Q.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Lake Constance Water Supply and Glasgow Caledonian University, grant number RIE-18-212.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to Glasgow Caledonian University and German Lake Constance Water Supply for offering a studentship to the PhD study of S.Z. Special thanks go to staff in the laboratory at German Lake Constance Water Supply for their support when running the field research. The views expressed in this paper are not necessary representing those from the water company.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mührle, U.; Ortlepp, J.; Rey, P. Der Bodensee Zustand–Fakten–Perspektiven; IGKB: Bregenz, Austria, 2004. [Google Scholar]
- Petri, M. Water Quality of Lake Constance. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; Volume 5, Part L; pp. 127–138. [Google Scholar]
- Patil, A.L.; Patil, P.N.; Gogate, P.R. Degradation of imidacloprid containing wastewaters using ultrasound based treatment strategies. Ultrason. Sonochem. 2014, 21, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Mesdaghinia, A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci. Total Environ. 2018, 619, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/105/EC; European Union Directive on Environmental Quality Standards. European Union: Brussels, Belgium, 2008.
- COM (2011) 876; EC-Proposal for a Directive Amending the WFD and EQSD. European Union: Brussels, Belgium, 2011.
- Jiang, J.-Q. Advances in the development and application of ferrate(VI) for water and wastewater treatment. J. Chem. Technol. Biotech. 2014, 89, 165–177. [Google Scholar] [CrossRef]
- Tiwari, D.; Kim, H.U.; Lee, S.M.; Yang, J.K.; Kim, H.O. Ferrate (VI) for wastewater treatment: Oxidation of cyanide in aqueous medium. Environ. Eng. Res. 2006, 11, 318–324. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mishra, S.K.; Nesnas, N. Oxidation of sulfonamide antimicrobials by ferrate (VI). Environ. Sci. Technol. 2006, 40, 7222–7227. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Dong, W.; Wang, H.; Ma, H.; Gu, Y.; Tian, Y. Degradation of tetrabromobisphenol A by a ferrate (vi)–ozone combination process: Advantages, optimization, and mechanistic analysis. RSC Adv. 2019, 9, 41783–41793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Q.; Stanford, C.; Alsheyab, M. The online generation and application of ferrate (VI) for sewage treatment—A pilot scale trial. Sep. Purif. Technol. 2009, 68, 227–231. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Stanford, C.; Petri, M. Practical application of ferrate(VI) for water and wastewater treatment—Site study’s approach. Water Energy Nexus 2018, 1, 42–46. [Google Scholar] [CrossRef]
- DIN 38407-47; Bestimmung Ausgewählter Arzneimittelwirkstoffe und Weitere Organischer Stoffe—Verfahren Mittels HPLC-MS/MS Oder—HRMS Nach Direktinjektion. Beuth Verlag: Berlin, Germany, 2017.
- DIN ISO 16308:2017-09; Wasserbeschaffenheit—Bestimmung von Glyphosat und AMPA—Verfahren Mittels Hochleistungs-Flüssigkeitschromatographie (HPLC) Mit Tandem-Massenspektrometrischer Detektion. Beuth Verlag: Berlin, Germany, 2018.
- Munch, J.W.; Grimmett, P. Method 522—Determination of 1,4-Dioxane in Drinking Water by Solid Phase Extraction (SPE) and Gas Chromatography Mass Spectrometry (GC/MS) with Selected Ion Monitoring (SIM); United States Environmental Protection Agency: Washington, DC, WA, USA, 2008. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality: Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test). Method Suring Liquid-Dried Bacteria; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Mayo-Bean, K.; Moran-Bruce, K.; Meylan, W.; Ranslow, P.; Lock, M.; Nabholz, V.; Von Runnen, J.; Cassidy, L.; Tunkel, J. Methodology Document for the Ecological Structure–Activity Relationship Model (ECOSAR) Class Program, Version 2; Office of Pollution Prevention and Toxics, US Environmental Protection Agency: Washington, DC, USA, 2017. [Google Scholar]
- Jiang, J.-Q.; Zhou, Z. Removal of pharmaceutical residues by ferrate (VI). PLoS ONE 2013, 8, e55729. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate(VI) and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Jaeger, M. On the photodegradation of azithromycin, erythromycin and tylosin and their transformation products–A kinetic study. Sustain. Chem. Pharm. 2017, 5, 131–140. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Lyu, B.; Tang, Y.; Zhang, Y.; Chen, F.; Korshin, G. Degradation of typical macrolide antibiotic roxithromycin by hydroxyl radical: Kinetics, products, and toxicity assessment. Environ. Sci. Poll. Res. 2019, 26, 14570–14582. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shu, J.; Sharma, V.K.; Liu, C.; Xu, X.; Nesnas, N.; Wang, H. Unveiling the mechanism of imidacloprid removal by ferrate (VI): Kinetics, role of oxidation and adsorption, reaction pathway and toxicity assessment. Sci. Total Environ. 2022, 805, 150383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).