Abstract
We developed a system combining visible-light photocatalysis with biological treatment for the continuous removal of phthalate esters (PAEs) from both synthetic and real aquaculture wastewater. We investigated the effects of different operating factors, including the coexistence of glucose or PAEs, on individual PAE removal by using a photobiological system (PBS). In wastewater containing a mixture of PAEs, that is, containing di-(2-ethylhexyl)phthalate (DEHP), dibutyl phthalate (DBP), and dimethyl phthalate (DMP), a coimmobilized bioreactor system comprising the bacterium Pseudomonas putida and the microalga Chlorella vulgaris demonstrated a higher removal efficiency than immobilized P. putida alone or a coculture of immobilized P. putida and suspended C. vulgaris did. The PBS employed for the continuous treatment of real aquaculture wastewater containing DEHP (0.62 ± 0.05 mg/L), DBP (8.7 ± 0.9 mg/L), and DMP (17.4 ± 1.5 mg/L) achieved at least 99.5% PAE removal and 99.2% mineralization efficiency under optimal operating conditions. After 42 days of treatment, inoculated Pseudomonas (98.12%) remained the predominant genus in the bioreactor. The results reveal that the symbiotic microalgal–bacterial system is a feasible alternative to a pure P. putida immobilized bioreactor for reducing CO2 emissions from mineralized PAEs through microalgal activity.
1. Introduction
Phthalate esters (PAEs) are crucial industrial chemicals and commonly used as plasticizers to improve the flexibility and processability of polymer materials [1]. Furthermore, PAEs are extensively used as plasticizers in polymer production. However, they can be released from plastic products into the environment [2]. PAEs can be categorized as short alkane chain and long alkane chain. Short-alkane-chain PAEs are commonly used in personal care products, cosmetics, and plastic bags, whereas long-alkane-chain PAEs are mainly used in plastic polymers, building materials, furniture, and food contact materials [3].
Numerous countries currently list PAEs as priority pollutants and endocrine-disrupting compounds for assessing and controlling phthalate pollution because of their association with various human and animal diseases [4]. PAEs can bind to cellular targets in the human body and interfere with hormone reactions, leading to various disorders. Moreover, PAEs are suspected to interfere with biological processes in wildlife, causing teratogenicity, mutagenicity, and carcinogenicity [5]. PAEs comprise diverse compounds with different structures and properties. Among them, di-(2-ethylhexyl)phthalate (DEHP), dibutyl phthalate (DBP), and dimethyl phthalate (DMP) have been discovered to be the most prevalent in the environment, wastewater, and sludge [6,7].
Advanced oxidation processes (AOPs), such as photocatalysis, have been suggested for the removal of PAEs from the environment [1]. Photocatalysis is highly effective in eliminating PAEs from water because it generates potent, nonselective oxidants through light-driven reactions [8]. However, achieving complete mineralization of PAEs is energy-intensive, rendering photocatalysis inefficient if used alone [9]. Biodegradation facilitated by microbes was also reported to be a key method of initiating PAE degradation. Although biological degradation of PAEs is both cost-effective and environmentally friendly [10], it is time-consuming, requiring an extended period before the amount of PAE is reduced to harmless levels [11].
Simultaneous coupling of photocatalytic and microbial processes can serve as an exceptionally efficient system, combining the advantages of both processes for the effective removal of PAEs [12]. In our previous studies, we developed a system involving TiO2 photocatalysis under visible-light irradiation [4]. This system uses the solar spectrum, incorporating the visible-light region into the TiO2 matrix to broaden its photoresponsive capability [4]. We analyzed the optimal operating conditions for PAE removal by using a visible-light photoreactor. When combined with a packed-bed reactor (PBR), our visible-light photo-pretreatment method achieved removal efficiencies of over 95% for individual PAEs in synthetic wastewater [4].
The concept of algae–bacteria consortia was initially proposed in 1981 [13]. Since then, these consortia have been used for the treatment of wastewater and detoxification of environmental pollutants. During photosynthesis, algae produce O2, which is used by bacteria. Subsequently, bacteria degrade organic pollutants, releasing CO2 that is beneficial for algal growth [14]. However, few studies have successfully developed a symbiotic algae–bacteria system for wastewater treatment [15]. We modified our previously designed packed-bed reactor, using a microalgae–bacteria consortia bioreactor rather than a PAE-degrading bacterial strain [4].
In a real wastewater environment, PAEs often coexist with other organic pollutants. We analyzed ten aquaculture ponds and found that the concentrations of DEHP ranged from 0.18~0.89 mg/L. We selected one aquaculture pond as our research model. The concentrations of DEHP, DBP, and DMP were 0.62 ± 0.05 mg/L, 8.7 ± 0.9 mg/L, and 17.4 ± 1.5 mg/L, respectively. In the present study, we investigated the efficiency of PBS in removing PAEs from synthetic and real wastewater. In addition, we investigated the effects of the coexistence of organic pollutants or PAEs on the removal efficiencies of individual PAEs. Furthermore, we evaluated changes in the bacterial community in the immobilized bioreactor before and after PBS treatment. The results of this study can provide insight into strategies that can be used for PAE removal in a real wastewater environment and CO2 reduction across various environments toward an environmentally friendly and sustainable future.
2. Materials and Methods
2.1. Materials
Analytical-grade DEHP, DBP, DMP, and titanium tetraisopropoxide (TTIP) were purchased from AccuStandard Chem. Co. (New Haven, CT, USA). To ensure a neutral pH in the biological system, we automatically adjusted the pH of the wastewater we used with 0.1 M HCl or 0.1 M NaOH in the storage tank. A PAE (DEHP, DBP, and DMP) stock solution (100 mg/L) was prepared as described in our previous studies [4]. We additionally used a mineral medium (g/L) containing 1.09 KH2PO4, 2.10 K2HPO4, 0.40 (NH4)2SO4, 0.29 CaCl2, 0.21 MgSO4, 0.21 MnSO4, and 0.02 FeSO4. All culture media, biochemical reagents, and chemicals were purchased from Sigma–Aldrich (Saint Louis, MO, USA) unless stated otherwise.
2.2. Synthesis of I-Doped TiO2 and Preparation of I-Doped-TiO2-Coated Beads
We synthesized I-doped TiO2 following the method reported by Štengl and Grygar [4] and Hung et al. [11] with some modifications. First, 0.89 mL TTIP was mixed with 10 mL isopropanol solution (0.3 M). The mixture was gently stirred for 30 min, and distilled water and alcohol were gradually added until the hydrogel solution appeared white and cloudy. Next, 35% HCl was added, and the pH of the solution was adjusted to 3–4 until the white turbidity in the solution changed to that of a white transparent gel. The resulting 100 mL transparent gel was mixed with 100 mL of 30% H2O2 solution, which caused it to form into a yellow gelatinous mass. This mass was subsequently mixed with 0.7 g of KI and heated at 80 °C until a yellowish-white precipitate formed. After standing for 30 h, the color of the precipitate changed to white, resulting in the formation of I-doped TiO2 powder.
I-doped-TiO2-coated beads were prepared using the same procedure as described in our previous studies [4]. First, 0.5 g of I-doped TiO2 was mixed with 1 mL of solution containing 0.1 mL acetylacetone. The mixture was gently stirred until a viscous paste was formed. Subsequently, the paste was combined with 1.7 mL distilled water and 0.05 mL Triton X-100 and then mixed with 15 g of silica glass beads (id = 2 cm). The beads were coated with I-doped TiO2 and were then removed from the liquid, heated for 10 min at 80 °C, and further heated for 30 min at 450 °C in an oven to immobilize the I-doped TiO2 on the beads. The concentration, specific surface area, and crystal size of the I-doped TiO2 on the surface of the glass beads were 0.38%, 205 m2/g, and 29.8 nm, respectively.
2.3. Microalgae and Bacterial Culture
Chlorella, a microalgae species, was selected because of its widespread use in wastewater treatment and its prevalence in municipal wastewater treatment systems [16]. In our preliminary investigations, we analyzed some Chlorella species and determined that Chlorella vulgaris exhibited favorable mutualistic growth when cocultivated with the PAE-degrading bacterial strain Pseudomonas putida [4]. C. vulgaris was isolated from an aquaculture pond in Tainan City. It was cultivated in 1 L Erlenmeyer flasks containing BG-11 medium under sterile conditions, was maintained at 25 °C, was exposed to white fluorescent light illumination at 60 μmole/m2/s, and was subjected to a 12:12 h light/dark cycle. The culture medium was then removed through centrifugation for 10 min at 5000 rpm, and the C. vulgaris biomass was diluted before it was introduced into the bioreactor. P. putida was routinely cultured in Luria–Bertani (LB) broth at 30 °C prior to being used in PAE treatment. As with C. vulgaris, P. putida biomass was collected through centrifugation for 10 min at 5000 rpm.
The C. vulgaris and P. putida biomasses were washed three times with 0.8% sterilized NaCl solution. The concentrations of C. vulgaris and P. putida were 1.5 × 106 cells/mL and 2 × 108 CFU/mL in the prepared inflow solution, respectively. The inoculum ratio of immobilized microalgae to bacteria was determined by considering the findings of the preliminary study.
2.4. Coupled PBS design
The coupled PBS mainly consisted of a photoreactor, a storage tank, and a bioreactor, as described in our previous studies [4]. The cylindrical concentric glass photoreactor (length 50 cm) was composed of an inner cylinder (id = 5 cm) and an outer cylinder (id = 7 cm). A xenon lamp served as the light source and was placed on the inner cylinder. I-doped-TiO2-coated beads were packed in the outer cylinder of the photoreactor (with a packing height of 30 cm and a packing volume of 565 mL). Synthetic wastewater (with a pH of 5.0) or real aquaculture wastewater containing different PAE concentrations was continuously introduced into the photoreactor using an upward flow mode through a peristaltic pump. The photolytic conditions were 30 °C, a liquid retention time (RT) of 5.5 min, and a light intensity of 300 W, and wavelength was 420–440 nm unless stated otherwise. The effluent’s pH value was automatically adjusted to 7 in the storage tank, and the effluent was continuously piped to the PBR with a peristaltic pump.
The bioreactor was a cylindrical packed-bed reactor (length = 150 cm, id = 20 cm) constructed from acrylic material. It had two ports at the top for measuring pH and dissolved oxygen (DO). Wastewater from the photoreactor outlet was directed into the storage tank and then continuously introduced into the PBR in an upward flow mode with a retention time of 4 h, unless stated otherwise.
Three types of microbiota consortia were used in the PBR: immobilized P. putida, a coimmobilized culture of C. vulgaris and P. putida, and a coculture of immobilized P. putida and suspended C. vulgaris. The inoculation methods were as follows: In the first consortium, 2.5 L packed volumes of plastic Raschig rings (rosette type; id = 2 cm) were placed in the PBR as packing material. LB broth containing P. putida (2 × 108 CFU/mL) was continuously introduced into the PBR at a retention time of 24 h for 2 weeks to achieve immobilization. In the second consortium, LB broth containing P. putida (2 × 108 CFU/mL) and C. vulgaris (1.5 × 106 cells/mL) was continuously introduced into the PBR at a retention time of 24 h for 2 weeks to achieve microalgae–bacteria coimmobilization. In the third consortium, P. putida was immobilized for 2 weeks, and precultured C. vulgaris (1.5 × 106 cells/mL) was then suspended in the PBR. In the preliminary test, we analyzed the survival rate of the test organisms (microalgae and bacteria) by I-doped TiO2, and the survival rate was not significantly decreased before or after the operation process (p > 0.05).
2.5. Effect of Operation Parameters on PAE Removal Efficiency
To evaluate the effect of the coexistence of PAEs on the efficiency of their removal, synthetic wastewater containing individual PAEs (20 mg/L) or a mixture (20 mg/L each of DEHP, DBP, and DMP) was continuously introduced into the coupled photoimmobilized P. putida biological system. To evaluate the matrix effects of real aquaculture wastewater on PAE removal efficiency, synthetic wastewater containing a mixture of PAEs (20 mg/L each of DEHP, DBP, and DMP) was mixed with easily degraded organic compounds (glucose) at 0–20 mg/L and introduced continuously into the coupled photoimmobilized P. putida biological system. To evaluate the effect of the microbiota consortium (immobilized P. putida, a coimmobilized culture of C. vulgaris and P. putida, and a coculture of immobilized P. putida and suspended C. vulgaris) in the PBR on PAE removal efficiency, synthetic wastewater containing a mixture of PAEs (20 mg/L each of DEHP, DBP, and DMP) was continuously introduced into the coupled PBS. To investigate the effect of shock loading on PAE removal efficiency, real PAE-containing wastewater was obtained from an aquaculture pond located in Tainan City (Taiwan), filtered through two layers of gauze, and introduced continuously into the coupled photo-coimmobilized C. vulgaris–P. putida biological system. Real PAE-containing wastewater was introduced into the photoreactor at a retention time of 5.5 min for 14 days, 4.5 min for the subsequent 14 days, and 5.5 min for the subsequent 14 days. The bioreactor was constantly operated for 42 days at a retention time of 4 h. The concentrations of PAEs were determined once every 30 min, and the results (that is, the removal efficiency) are expressed as an average value over 4 h. Furthermore, we analyzed the bacterial communities in the real PAE-containing wastewater, the bioreactor before system startup, and the bioreactor after 42 days of operation.
2.6. Identification of Bacterial Communities
Bacterial communities in both the influent (real PAE-containing wastewater) and the biofilm of the bioreactor were analyzed using next-generation sequencing (NGS). Cell lysis, DNA extraction, polymerase chain reaction amplification, and 454 pyrosequencing were conducted following processes described by Wu et al. [17]. DNA was extracted using a Fast DNA SPIN Kit (MP Biomedicals). The PCR primers GAGTTTGATCNTGGCTCAG (forward) and GTNTTACNGCGGCKGCTG (reverse) were used to amplify the eubacterial 16S rRNA fragment. The PCR protocol involved an initial denaturation step at 95 °C for 10 min followed by 35 cycles of denaturation at 94 °C for 45 s, with annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. All of the partial 16S rRNA gene sequences were preprocessed following methods described by Wu et al. [17]. Sequence analysis was performed using the QIIME software package (2020.2.0.). These analyzed sequences were clustered into operational taxonomic units (OTUs) with consideration of a 0.97 sequence similarity threshold by using the UCLUST algorithm. Representative OTUs were selected with consideration of the most abundant sequences, and taxonomic assignment was performed using the ribosomal database project classifier.
2.7. Chemical Analysis
The concentrations of PAEs were analyzed using a high-performance liquid chromatography system (Hitachi, Japan) equipped with two Hitachi L-6000 pumps and one L-5000 LC controller. These concentrations were detected at 280 nm by using a Hitachi L-4200 UV/VIS detector. The HPLC analysis was performed with a C18 column (4.6 mm ID × 250 mm, 5 μm). The mobile phase was isocratic CHCl3:C6H14 (3:7) and the flow rate was at 1 mL/min. The intermediate products of the PAEs generated through photocatalysis were obtained through solid-phase extraction. Subsequently, the extracted samples were evaporated into vials and injected into a gas chromatography (GC)–mass spectrometer (MS). The GC–MS system included an HP 5890 series Ⅱ GC and a HP 5971 MS detector (Agilent, Santa Clara, CA, USA). GC separation was performed using an Ultra-2 fused silica capillary column (0.2 mm ID × 25 m), with temperature programming ranging from 100 °C to 280 °C. Helium was used as the carrier gas and the gas flow rate was 31 cm/s. The injector and detector temperatures were set at 250 and 280 °C, respectively. The analysis of intermediate products was carried out in EI mode, 70 eV, and full scan.
3. Results and Discussion
3.1. Effect of Coexisting PAEs on PAE Removal in PBS
In a real wastewater environment, PAEs typically coexist as a mixture of more than one pollutant [18]. To investigate the effect of these coexisting PAEs on individual PAE removal, we analyzed the removal efficiencies of DEHP, DBP, and DMP in a PAE mixture by using PBS. The removal efficiencies of DEHP, DBP, and DMP were slightly affected by the coexistence of PAEs (Figure 1). Furthermore, the removal efficiencies of DEHP (98.1% ± 0.15%), DBP (99.0% ± 0.08%), and DMP (99.8% ± 0.03%) in the mixture were slightly lower than those in the samples of individual PAEs (DEHP: 99.6% ± 0.02%; DBP: 99.9% ± 0.005%; DMP: 100.0% ± 0.004%) at the same concentration. When only the photocatalytic efficiency was evaluated, whether PAEs were alone or in a mixture had a minimal effect on the removal efficiency. Coexisting PAEs mainly had an effect on the removal efficiency in the biotreatment stage. The removal of recalcitrant PAEs was affected when the PAE mixture was present in the wastewater, with DEHP removal being the most significantly affected. Xie et al. [19] indicated that the coexistence of PAEs in the environment affects microbial degradation. Even when bacteria exhibit a preference for a certain PAE as substrate, the addition of other PAEs might lead to an inhibitory effect, which does not occur in the presence of only a single PAE.
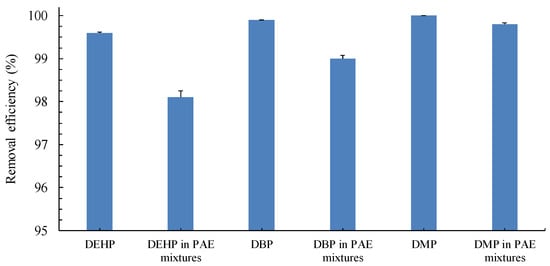
Figure 1.
Effect of the coexistence of PAEs on continuous PAE removal in the coupled PBS. Individual PAEs and synthetic wastewater containing a PAE mixture (20 mg/L each of DEHP, DBP, and DMP) were introduced. The photolytic conditions were as follows: light intensity, 300 W; temperature, 30 °C; pH, 5.0; and RT, 5.5 min. The photoreactor effluent was continuously introduced into the immobilized P. putida PBS at an RT of 4 h.
Unlike in our previous studies, in the current study, the PBS achieved higher PAE removal efficiencies than photoreactor treatment alone did (86.5% for DEHP at 20 mg/L) [4]. Furthermore, our system achieved a higher DEHP removal efficiency than the oxidative Fenton reaction (H2O2/Fe2+) system (85.6% for DEHP at 20 mg/L) [20] and the photo-Fenton–biological system did (60% for DEHP at 20 mg/L) [21]. In addition, our coupled PBS system achieved a similar DEHP removal efficiency (99.6%), even when the DEHP concentration was as high as 50 mg/L in the sample of DEHP alone [4]. This is the first study to report on the removal efficiency of individual PAEs from a coexisting PAE mixture in synthetic wastewater by using PBS.
3.2. Effects of Glucose on the Removal Efficiency of PAEs
Glucose is an easily degrading carbon source and is extensively used to induce bacterial growth [22]. In the present study, the removal efficiency of PAEs was evaluated under different glucose concentrations, which simulated the presence of coexisting organic compounds in real wastewater. When only photocatalysis was conducted, the coexistence of glucose did not exert any significant effect on PAE removal. However, the PAE removal efficiencies increased when the glucose concentration ranged from 0 to 10 mg/L and significantly decreased when the glucose concentration ranged from 10 to 20 mg/L in the immobilized P. putida PBR (Figure 2a). The coexistence of low glucose concentrations promoted P. putida growth (increase from 4.2 × 105 CFU/mL at 0 mg/L of glucose to 1.8 × 106 CFU/mL at 10 mg/L of glucose in the bioreactor), thus enhancing PAE removal. When the glucose concentration was >10 mg/L, P. putida preferentially degraded glucose instead of PAEs, resulting in a notable decrease in PAE removal. Correspondingly, the PAE removal efficiencies of the overall photobiological system were also decreased (Figure 2b). Short-chain DMP was more easily degraded than DEHP and DBP were (Figure 2a). Thus, their removal was not affected by the presence of coexisting glucose. Microorganisms can use glucose to metabolize or cometabolize refractory organic matter [23]. Hu and Wan [24] reported that when a mixture of PAEs and glucose serve as the carbon source, bacteria consume glucose first and then metabolize PAEs. However, the overall PAE removal efficiencies were maintained within the range of 99.6–100% in our PBS.
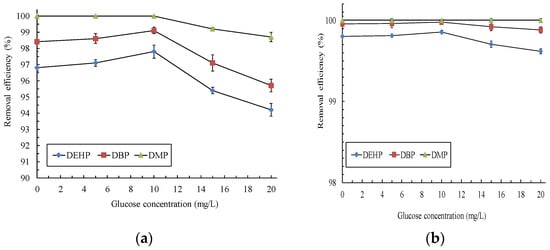
Figure 2.
Effect of glucose on continuous PAE removal in (a) the immobilized P. putida PBR and (b) the coupled PBS. Synthetic wastewater containing a PAE mixture (20 mg/L each of DEHP, DBP, and DMP) and different glucose concentrations were introduced. The photolytic conditions were as follows: light intensity, 300 W; temperature, 30 °C; pH, 5.0; and RT, 5.5 min. The photoreactor effluent was continuously introduced into the immobilized P. putida PBR at a RT of 4 h.
3.3. Comparison of PAE Removal Efficiencies with Different Microbiota Consortia
The efficiencies of removing DEHP, DBP, and DMP from a coexisting PAE mixture (20 mg/L each of DEHP, DBP, and DMP) in PBS were analyzed. In the experiment, three types of microbiota consortia were used in the PBR. A coculture of immobilized P. putida and C. vulgaris exhibited a higher DEHP removal efficiency (99.5% ± 0.08%) than did a pure culture of immobilized P. putida (98.0% ± 0.06%) and a coculture of immobilized P. putida and suspended C. vulgaris (96.8% ± 0.35%; Figure 3). Similar trends were observed for the removal of DBP and DMP (Figure 3). The results demonstrated that the PAEs were eliminated from wastewater more efficiently if microalgae coexisted in the system [16]. The findings further revealed that C. vulgaris preferred to remain immobilized in a matrix instead of being in free suspension during the PAE removal process. Moreover, the results indicated that P. putida might have a positive symbiotic relationship with C. vulgaris in a coimmobilized state. The adequate oxygen that is produced in the C. vulgaris–P. putida coimmobilized system is used as an electron acceptor for P. putida, which significantly enhances PAE degradation. Bahr et al. [25] demonstrated that a coimmobilized system completely degraded glucose. Furthermore, photosynthesis by C. vulgaris in the coimmobilized system can increase or balance the pH in the microenvironment, facilitating PAE biodegradation. In coimmobilized bacteria/microalgae systems, algal growth increases DO levels and pH values, leading to improved synergistic performance in pollutant removal [26].
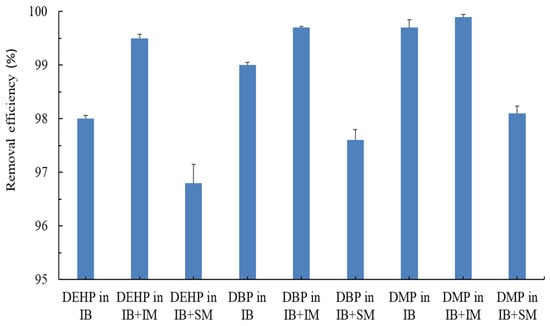
Figure 3.
Removal efficiency of PAEs in the coupled PBS. Synthetic wastewater containing a PAE mixture (20 mg/L each of DEHP, DBP, and DMP) was introduced. The bioreactor included immobilized P. putida (IB), a coimmobilized culture of P. putida and C. vulgaris (IB + IM), and a coculture of immobilized P. putida and suspended C. vulgaris (IB + SM).
Our results revealed that the coculture of immobilized P. putida and C. vulgaris exhibited a higher PAE removal efficiency than a pure culture of immobilized P. putida did. CO2 generated from the mineralization of PAEs or other organic pollutants by P. putida can be absorbed by C. vulgaris for photosynthesis. Thus, the coimmobilized bioreactor system of P. putida and C. vulgaris is a feasible and environmentally friendly alternative to a pure P. putida immobilized bioreactor for carbon reduction.
3.4. Effect of Shock Loading on the PBS
The water quality of the real aquaculture wastewater was as follows: total coliform, 3.2 × 102 CFU/mL; aerobic plate count, 6.5 × 105 CFU/mL; Cd2+, 8 × 10−4 mg/L; Pb2+, 2 × 10−2 mg/L; Cr6+, 2 × 10−3 mg/L; Cu2+, 6 × 10−4 mg/L; Zn2+, 3 × 10−3 mg/L; As5+, 7 × 10−4 mg/L; and Hg2+, 2 × 10−4 mg/L. The concentrations of DEHP, DBP, and DMP were 0.62 ± 0.05 mg/L, 8.7 ± 0.9 mg/L, and 17.4 ± 1.5 mg/L, respectively. The total organic carbon content (TOC) from organic compounds was 22.3 mg/L and that from non-PAE organic compounds was 5.04 mg/L.
Real wastewater is prone to shock loading. Therefore, the continuous and shock-loading operation of the PBS for treating wastewater containing PAEs must be tested. Figure 4 presents the results regarding the effect of shock loading on the PAE removal efficiencies in the coupled photo-coimmobilized C. vulgaris–P. putida biological system. For an initial 14 days, the photoreactor was operated at a RT of 5.5 min, and the bioreactor was operated at a RT of 4 h. On day 7, a decline in the PAE removal efficiency and mineralization was noted. An increase was noted in the amount of suspended solids in the photoreactor, with this change being observable with the naked eye, and this increase may have caused scattering and reduced the photocatalytic efficiency. Thus, the system was temporarily shut down, and a filtration system was introduced at the inlet to remove suspended solids. Subsequently, the PAE removal efficiency increased suddenly on day 8. For instance, the DEHP removal efficiency increased from 96.9% ± 0.15% to 99.6% ± 0.05%. From day 15 to 28, the photoreactor was operated at a RT of 4.5 min (shock loading), and the bioreactor was operated at a RT of 4 h, which had a minor effect on the PAE removal efficiencies but had a significant effect on mineralization (decrease from 99.9% ± 0.02% to 96.2% ± 0.28%) (Figure 4). This finding indicates that some organic pollutants in the wastewater other than PAEs could not easily be completely degraded at a low RT. From day 29 to 42, the photoreactor’s RT was restored to 5.5 min. During this period, the PAE removal efficiencies and mineralization were similar to those observed on days 8 to 14, indicating excellent PAE removal efficiency and mineralization (>99.2%). Throughout this period, the pH and ORP in the coimmobilized C. vulgaris–P. putida biological system were maintained at 6.3–7.5 and 248–302 mV, respectively. These results indicate that our PBS can adapt to shock loading and that the effect of coexisting substances on PAE removal is limited. Pajoumshariati et al. [27] reported that an effective bioreactor process should be insensitive to shock loading or only transiently affected by it.
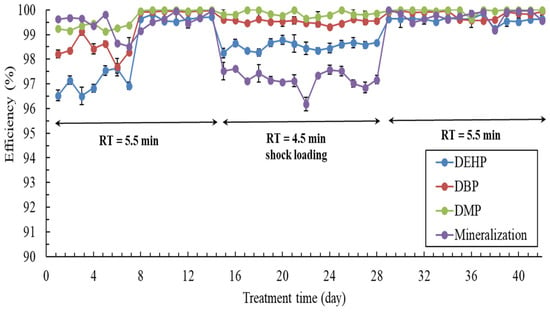
Figure 4.
Effect of shock loading on PAE removal efficiencies in the coupled photo-coimmobilized C. vulgaris–P. putida biological system. Real aquaculture wastewater containing a PAE mixture was introduced. On days 1 to 14, the photoreactor was operated at a RT of 5.5 min. On days 15 to 28, the photoreactor was operated at a RT of 4.5 min (shock loading). On days 29 to 42, the photoreactor was operated at a RT of 5.5 min. The bioreactor was constantly operated at an RT of 4 h.
Regarding the presence of intermediate PAE products, we observed more complex aromatic intermediate compounds in the aquaculture wastewater in the photocatalysis system than those previously reported in synthetic wastewater [4]. This finding indicates that some aromatic intermediate compounds might originate from other PAE chemical structures that are present in real aquaculture wastewater. Yang et al. [28] observed the presence of additional PAEs in the sediments of the Dianbao River (Kaohsiung City, Taiwan), which is generally used for aquaculture, environmental conservation, and irrigation. Furthermore, Chang et al. [29] detected trace amounts of DEHP in Taiwanese aquafarm shrimps. Although no immediate health risks were discovered to be associated with consumption of these shrimps, the finding indicates that PAEs must be removed from real aquaculture wastewater. In future research, we will collect more data and think of using Spearman and Pearson correlation matrices [30] to analyze the correlation between parameters and phthalate esters.
3.5. Changes in Bacterial Communities during the PAE Treatment Process
To investigate changes in the bacterial community during the PAE treatment process, we analyzed bacterial communities through NGS. Figure 5 presents the relative abundances of bacterial 16S rRNA gene sequences at the genus level in different situations. At least 16 bacterial genera were identified in the real aquaculture wastewater, with the most abundant being Flavorbacterium (20.64%), followed by Edwardsiella (12.27%), Exiguobacterium (9.04%), Roseomonas (8.12%), and Mycobacterium (7.38%). Exiguobacterium and Roseomonas are bacterial genera that are commonly found in freshwater culture ponds [31].
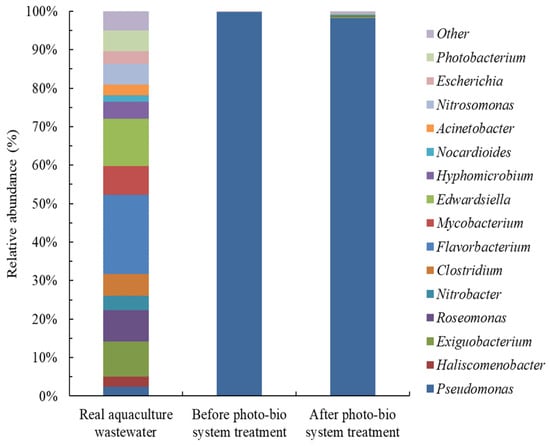
Figure 5.
Bacterial communities in real aquaculture wastewater analyzed in the biofilm of the bioreactor before system startup and after 42 days of operation. The system was a coupled photocatalysis and coimmobilized C. vulgaris–P. putida biological system.
In the present study, before the PBS was used for wastewater treatment, bacterial community analysis conducted using the bioreactor revealed the presence of only three genera, with the dominant bacterial genus being Pseudomonas (99.68%, inoculated bacteria), followed by Flavorbacterium (0.14%) and Escherichia (0.18%). Any miscellaneous bacteria beyond Pseudomonas that may have been detected may have resulted from contamination. Escherichia is commonly found in aquaculture ponds [32]. After we used the PBS to treat the real aquaculture wastewater, at least five bacteria genera were identified in the bioreactor, with Pseudomonas (98.12%) being the dominant genus, followed by Exiguobacterium (0.53%), Roseomonas (0.13%), Flavobacterium (0.26%), Edwardsiella (0.29%), and other genera (0.67%). These results indicate that most bacteria were killed during the photocatalytic process, which is consistent with the notion that photocatalysis inactivates bacteria [33]. Furthermore, the relative abundance of Roseomonas significantly decreased in the wetland in the presence of DBP [34]. Flavobacterium exhibited the ability to degrade PAEs [35], and Exiguobacterium displayed the ability to degrade polyaromatic hydrocarbons [36]. Edwardsiella is a typical pathogen abundant in aquaculture environments and is known to infect some fish species, with such infection being associated with high mortality rates [37]. The findings of the present study indicate that the coupled system not only effectively removed pathogens from real wastewater but also maintained the dominance of inoculated bacteria in the bioreactor. Thus, the coupled system represents a feasible means of removing PAEs from aquaculture wastewater.
4. Conclusions
This is the first study to use a coupled visible-light PBS for the treatment of real aquaculture wastewater containing a PAE mixture. The findings reveal that the DEHP removal efficiency was the most influenced by the coexistence of multiple PAEs (DEHP, DBP, DMP) in the wastewater. The coimmobilization of the microalga C. vulgaris with the bacterium P. putida improved the PAE removal efficiencies relative to those achieved with only immobilization of P. putida. Analysis of the bacterial communities in the aquaculture wastewater in the bioreactor before and after PBS treatment indicated that most bacterial genera were removed through the photocatalysis process, and the inoculated bacteria remained dominant. These results indicate that application of the PBS for continuous treatment of PAE-containing real wastewater is feasible. However, additional research is required to identify the optimal parameters for the coupled visible-light PBS and to determine its cost-effectiveness to enable direct utilization of sunlight for PAE photocatalysis.
Author Contributions
Conceptualization, C.-Y.C. and Y.-C.C.; writing—original draft preparation, C.-Y.C. and Y.-C.C.; writing—review and editing, C.-Y.C. and Y.-C.C.; supervision, Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Ministry of Science and Technology (Taiwan), grant number MOST 110-2637-B-266-001-.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pang, X.; Skillen, N.; Gunaratne, N.; Rooney, D.W.; Robertson, P.K. Removal of phthalates from aqueous solution by semiconductor photocatalysis: A review. J. Hazard. Mater. 2021, 402, 123461. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.P.; Kumar, M.A. Remediation of phthalate acid esters from contaminated environment—Insights on the bioremedial approaches and future perspectives. Heliyon 2023, 9, e14945. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Ju, Y.R.; Lim, Y.C.; Wang, M.H.; Patel, A.K.; Singhania, R.R.; Chen, C.W.; Dong, C.D. The effect of heavy rainfall on the exposure risks of sedimentary phthalate esters to aquatic organisms. Chemosphere 2022, 290, 133204. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wang, G.H.; Chang, Y.J.; Chen, Y.H.; Cheng, C.Y.; Chung, Y.C. Combination of a Highly Efficient Biological System and Visible-Light Photocatalysis Pretreatment System for the Removal of Phthalate Esters from Wastewater. Water 2022, 14, 3139. [Google Scholar] [CrossRef]
- Chang, W.H.; Herianto, S.; Lee, C.C.; Hung, H.; Chen, H.L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, L.; Fang, C.; Chen, L. Effects of di-n-butyl phthalate and di-2-ethylhexyl phthalate on pollutant removal and microbial community during wastewater treatment. Ecotoxicol. Environ. Saf. 2020, 198, 110665. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, J.; Li, B.; Geng, J.; Ma, L.; Qi, H.; Zhang, A.; Zhao, Z. The spatiotemporal distribution and potential risk assessment of 19 phthalate acid esters in wastewater treatment plants in China. Environ. Sci. Pollut. Res. 2021, 28, 67280–67291. [Google Scholar] [CrossRef]
- Zhou, X.; Xiong, W.; Li, Y.; Zhang, C.; Xiong, X. A novel simultaneous coupling of memory photocatalysts and microbial communities for alternate removal of dimethyl phthalate and nitrate in water under light/dark cycles. J. Hazard. Mater. 2022, 430, 128395. [Google Scholar] [CrossRef]
- Anandan, S.; Pugazhenthiran, N.; Lana-Villarreal, T.; Lee, G.J.; Wu, J.J. Catalytic degradation of a plasticizer, di-ethylhexyl phthalate, using Nx–TiO2−x nanoparticles synthesized via co-precipitation. Chem. Eng. J. 2013, 231, 182–189. [Google Scholar] [CrossRef]
- Khadka, S.; Nshimiyimana, J.B.; Zou, P.; Koirala, N.; Xiong, L. Biodegradation kinetics of diethyl phthalate by three newly isolated strains of Pseudomonas. Sci. Afr. 2020, 8, e00380. [Google Scholar] [CrossRef]
- Hung, C.H.; Yuan, C.; Li, H.W. Photodegradation of diethyl phthalate with PANi/CNT/TiO2 immobilized on glass plate irradiated with visible light and simulated sunlight—Effect of synthesized method and pH. J. Hazard. Mater. 2017, 322, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Štengl, V.; Grygar, T.M. The simplest way to iodine-doped anatase for photocatalysts activated by visible light. Int. J. Photoenergy 2011, 11, 13. [Google Scholar] [CrossRef]
- Nambiar, K.; Bokil, S. Luxury uptake of nitrogen in flocculating algal-bacterial system. Water Res. 1981, 15, 667–669. [Google Scholar] [CrossRef]
- Del Rio-Chanona, E.A.; Cong, X.; Bradford, E.; Zhang, D.; Jing, K. Review of advanced physical and data-driven models for dynamic bioprocess simulation: Case study of algae–bacteria consortium wastewater treatment. Biotechnol. Bioeng. 2019, 116, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Zhang, C.; Li, F.; Ren, N.Q.; Ho, S.H. Recent advances of algae-bacteria consortia in aquatic remediation. Crit. Rev. Environ. Sci. Technol. 2023, 53, 315–339. [Google Scholar] [CrossRef]
- Mujtaba, G.; Rizwan, M.; Lee, K. Removal of nutrients and COD from wastewater using symbiotic co-culture of bacterium Pseudomonas putida and immobilized microalga Chlorella vulgaris. J. Indust. Eng. Chem. 2017, 49, 145–151. [Google Scholar] [CrossRef]
- Wu, L.C.; Tsai, T.H.; Liu, M.H.; Kuo, J.L.; Chang, Y.C.; Chung, Y.C. A green microbial fuel cell-based biosensor for in situ chromium (VI) measurement in electroplating wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef]
- Kanaujiya, D.K.; Sivashanmugam, S.; Pakshirajan, K. Biodegradation and toxicity removal of phthalate mixture by Gordonia sp. in a continuous stirred tank bioreactor system. Environ. Technol. Innov. 2022, 26, 102324. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, X.; Shim, H. Degradation of dibutyl phthalate and diethyl phthalate by indigenous isolate Bacillus sp. MY156. IOP Conf. Ser. Earth Environ. Sci. 2023, 1171, 012057. [Google Scholar] [CrossRef]
- Esmaeli, R.; Hassani, A.; Eslami, A.; Ahmadimoghadam, M.; Safari, A. Di-(2-Ethylhexyl) Phthalate oxidative degradation by Fenton process in synthetic and real petrochemical wastewater. J. Environ. Health Sci. Eng. 2011, 8, 201–206. [Google Scholar]
- Chen, C.Y.; Wu, P.S.; Chung, Y.C. Coupled biological and photo-Fenton pretreatment system for the removal of di-(2-ethylhexyl) phthalate (DEHP) from water. Bioresour. Technol. 2009, 100, 4531–4534. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic acid bacteria: Physiology and carbon sources oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, Y.; He, X.; Li, W.; Yuan, L.; Wu, X.; Qin, Y.; Yuan, R.; Gong, X. Glucose addition enhanced the advanced treatment of coking wastewater. Water 2021, 13, 3365. [Google Scholar] [CrossRef]
- Hu, X.; Wan, J. Study of biodegradation properties of phthalate esters in aqueous culture conditions. J. Synth. Lubr. 2006, 23, 71–80. [Google Scholar] [CrossRef]
- Bahr, M.; Stams, A.J.; De la Rosa, F.; García-Encina, P.A.; Muñoz, R. Assessing the influence of the carbon oxidation-reduction state on organic pollutant biodegradation in algal–bacterial photobioreactors. Appl. Microbiol. Biotechnol. 2011, 90, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Ma, H.; Hursthouse, A.S. Removal of manganese (II) from acid mine wastewater: A review of the challenges and opportunities with special emphasis on Mn-oxidizing bacteria and microalgae. Water 2019, 11, 2493. [Google Scholar] [CrossRef]
- Pajoumshariati, S.; Zare, N.; Bonakdarpour, B. Considering membrane sequencing batch reactors for the biological treatment of petroleum refinery wastewaters. J. Membr. Sci. 2017, 523, 542–550. [Google Scholar] [CrossRef]
- Yang, G.C.; Wang, C.L.; Chiu, Y.H. Occurrence and distribution of phthalate esters and pharmaceuticals in Taiwan river sediments. J. Soils Sediments 2015, 15, 198–210. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yang, W.C.; Xue, Y.J.; Tsai, M.Y.; Wang, J.H.; Chang, G.R. Phthalates and organophosphorus insecticide residues in shrimp determined by liquid/gas chromatography–Tandem mass spectrometry and a health risk assessment. Mar. Pollut. Bull. 2019, 144, 140–145. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Pedrazzani, R.; Bellazzi, S.; Carnevale Miino, M.; Caccamo, F.M.; Baldi, M.; Abba, A.; Bertanza, G. Numerical Analysis of a Full-Scale Thermophilic Biological System and Investigation of Nitrate and Ammonia Fates. Appl. Sci. 2022, 12, 6952. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Liang, X.F.; Guo, W.; Lv, L.; Li, L. Influence of environmental factors and bacterial community diversity in pond water on health of Chinese perch through Gut Microbiota change. Aquac. Rep. 2021, 20, 100629. [Google Scholar] [CrossRef]
- Alagarsamy, S.; Thampuran, N.; Joseph, T.C. Virulence genes, serobiotypes and antibiotic resistance profile of Escherichia coli strains isolated from aquaculture and other sources. Aquac. Res. 2010, 41, 1003–1014. [Google Scholar]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Du, S.; Yuan, Y.; Zhu, M.; Li, Y.; Zhu, X. Removal of dibutyl phthalate and its effects on bacterial communities in lab-scale constructed wetlands. J. Environ. Account. Manag. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Kido, Y.; Tanaka, T.; Yamada, K.; Hachiyanagi, H.; Baba, H.; Iriguchi, T.; Uyeda, M. Complete degradation of the endocrine-disrupting chemical dimethyl phthalate ester by Flavobacterium sp. J. Health Sci. 2007, 53, 740–744. [Google Scholar] [CrossRef]
- Chikere, C.B.; Fenibo, E.O. Distribution of PAH-ring hydroxylating dioxygenase genes in bacteria isolated from two illegal oil refining sites in the Niger Delta, Nigeria. Sci. Afr. 2018, 1, e00003. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Pan, L.; Guan, W.; Lou, Y. Environmentally relevant concentrations of triclosan exposure promote the horizontal transfer of antibiotic resistance genes mediated by Edwardsiella piscicida. Environ. Sci. Pollut. Res. 2022, 29, 64622–64632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).