Abstract
Impregnated activated carbon (IAC) is an efficient adsorbent for the filtration of hazardous gases from the air. However, it tends to lose its efficiency after exposure to high humidity, where it adsorbs water molecules. Water adsorption causes changes in metal impregnation, resulting in a loss of adsorption efficiency for certain toxic gases, particularly gases that are adsorbed via chemisorption. Here, an innovative method was developed for the regeneration and reactivation of aged IAC. The method is based on dripping a regeneration solution composed of ammonium hydroxide and ammonium carbonate onto the aged IAC. The developed regeneration method was applied to ASZMT, a common commercially used IAC, that had undergone accelerated aging for six months. After the regeneration process, the protection capacity of the IAC against cyanogen chloride (CK) and toluene was almost fully restored to its initial value. Elemental analysis by energy dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS) showed that after the regeneration procedure, the concentrations of zinc and copper on the external surface of the IAC were decreased. This provides evidence that they were partially incorporated back into the pores. It is reasonable to believe that the developed method can be applied to IACs other than ASZMT impregnated with different kinds of materials. The implementation of this method has economic and environmental consequences. In the future, it may allow the reuse of aged IAC and even the restoration of filters.
Keywords:
adsorption; filtration; aging; regeneration; reactivation; impregnation; activated carbon; hazardous gases 1. Introduction
Activated carbon (AC) is an effective adsorbent for organic compounds that exhibit mild or low volatility (e.g., a boiling point greater than 65 °C). To enable adsorption of low-molecular weight gases and volatile organic compounds, such as chlorine, ammonia, sulfur dioxide, hydrogen cyanide (HCN), cyanogen chloride (CK), arsine, and formaldehyde, AC is impregnated with metal additives [1,2,3,4,5,6,7,8,9]. The impregnated metals settle inside the pores of the AC and chemically adsorb the target gases, which is more effective than the physical adsorption by the activated carbon itself. Initially, chromium, together with copper and silver, was found to be effective for impregnation. However, due to its carcinogenic properties, the use of chromium has been discontinued. Commonly used chromium-free impregnated activated carbon (IAC) can be impregnated with zinc, copper, molybdenum, silver, and triethylenediamine (TEDA). The most commonly used commercially available IAC in the United States in NBC filters containing such additive compositions is ASZM-TEDA® (referred to as ASZMT), manufactured by Calgon Carbon Corporation.
It is well known that IAC gradually loses its efficiency when exposed to humid air. The exact mechanism of the aging effect in chromium-free IAC, such as ASZMT, is not well known. However, it has been shown that adsorbed water leads to variations in the crystalline size of the metal compounds [10,11] and causes their migration to the external surface of the AC [12,13,14]. During aging, the impregnated metals gradually lose their efficiency against chemically adsorbed noxious gases, particularly the blood chemical warfare agent HCN and, most drastically, CK [3,13], for which the efficacy is well below the acceptable threshold. The addition of TEDA and molybdenum to ASZ carbon, which is composed of copper and zinc only, to obtain ASZMT IAC reduced the copper migration and improved the aged carbon performance against HCN [12]. However, the performance of ASZMT carbon against chemically adsorbed gases still decreases significantly during aging even though the material contains molybdenum.
In a previous publication [15], we showed that the addition of phosphate to AC impregnated with copper, zinc, molybdenum, and TEDA significantly decreased the aging rate; the decrease in the protection values after accelerated aging was remarkably lower than that of the aged IAC without the additive. In the present study, an additional new path to cope with the aging phenomenon is proposed, namely, the regeneration of aged IAC. The proposed regeneration method reactivates the impregnated metals in the aged carbon via the addition of a regeneration solution to the IAC, thereby restoring the protection value against CK to approximately that of new IAC. To evaluate the efficiency of the regeneration method, CK was utilized as the main testing agent since the protection capacity against CK is drastically reduced during aging [3,13].
2. Experimental
2.1. Chemicals
The coal-based impregnated activated carbon ASZM-TEDA®, with a mesh size of 12 × 30 (Calgon Carbon Corporation, Pittsburgh, PA, USA), referred to here as ASZMT, was used for the breakthrough experiment. This IAC contained zinc (5.2 wt.%), copper (5.2 wt.%), molybdenum (1.8 wt.%), silver (0.05 wt.%), and TEDA (3 wt.%). Ammonium carbonate (30 wt.% NH3, Alfa Aesar, Lancashire, UK) and a solution of ammonium hydroxide (25 wt.%, Bio Lab) were used to prepare the impregnation solution. TEDA (98 wt.%, Glentham, Corsham, UK) was used as the organoamine additive for the IAC. CK cylinders (>98.5%, Liquidgas Ltd., Pardesia, Israel) and toluene (99.5 wt.%, Merck, Darmstadt, Germany) were used as the testing agents in the breakthrough experiments.
2.2. Preparation of IAC Beds
Using the “snowfall” technique, an aluminum column was packed with 33.8 g of ASZMT IAC to prepare a 60 mm diameter bed [16]. A detailed description of the preparation of the IAC beds is given in reference [15]. The carbon layer depth was 1.85 cm and the bulk density was 0.63 g/cm3.
2.3. IAC Pretreatments
Prior to breakthrough measurements, the following treatments were used on most IAC beds: humidification, accelerated aging, and drying.
2.3.1. Humidification of the Carbon Beds
Prehumidification was performed for all the IAC beds before the CK breakthrough measurements and before the IAC was stored in the thermostatic chamber for aging. The IAC beds were prehumidified by passing an airflow of 20 L/min at a temperature of 18.0 ± 0.2 °C and a relative humidity (RH) of 85 ± 1%. The airflow relative humidity was monitored by a humidity sensor (HyCal Model 829, El Monte, CA, USA). Two consecutive weighings were performed at 2 h intervals and equilibrium was considered when the bed weight remained within 0.05 g. A constant water content (31.0 ± 1 wt.%, per dried IAC weight) was obtained in less than 48 h.
2.3.2. Accelerated Aging
After prehumidification, some of the IACs were sealed in a closed jar and stored at an elevated temperature of 50 ± 0.5 °C in a thermostatic chamber. The aging periods were 1.5, 3, and 6 months.
2.3.3. Drying of the IAC Beds
Some of the IAC beds were predried for 3 h at a temperature of 80 °C. No additional water loss (>0.02 g) was observed for a longer period. We chose to dry the carbons at a temperature of 80 °C to avoid the sublimation of TEDA that may occur at higher temperatures. After drying, the water content of the IAC was approximately 1 wt.%.
2.4. CK dynamic Adsorption
CK dynamic breakthrough experiments on IAC beds were carried out on new IACs, as well as on samples after aging. The IAC beds were exposed to 940 ppm of CK (2.4 mg/L) (generated from a CK cylinder), at an airflow rate of 10 L/min (linear flow velocity of 5.9 cm/s), a temperature of 18 °C, and an RH of 75%. This airflow rate corresponds to 30 L/min through a typical personal gas-mask filter with a carbon bed diameter of 105 mm.
The breakthrough measurements were carried out on prehumidified IAC. This means that new carbons were prehumidified before the breakthrough test, and aged carbons were tested without additional modification because they were already humidified before aging. In a previous study, it was shown that the effectiveness of IAC after aging is lower against CK when it is tested moist, i.e., without drying after aging. Breakthrough measurements of moist, aged IAC better reflect the effectiveness of the impregnated metals compared to those of dried, aged IAC [15].
When the effluent concentration of CK reached 0.59 ppm (C0/Cx~1500, 1.5 μg/L), the breakthrough time was defined. As described in the reference [15], a colorimetric method was used to determine this concentration.
2.5. Toluene Dynamic Adsorption
Toluene dynamic breakthrough experiments were performed at an airflow rate of 15 L/min (linear flow velocity of 8.8 cm/s), an inlet concentration of 1000 ppm (3.5 mg/L), an RH >20%, and a temperature of 40 °C. This airflow rate corresponds to 45 L/min through a typical personal gas-mask filter with a carbon bed diameter of 105 mm.
The toluene effluent concentration was monitored continuously using a photoionization detector (ppbRAE 3000, RAE Systems, San Jose, CA, USA). When the effluent concentration of toluene reached 0.35 μg/L (0.1ppm, C0/Cx~10,000), the breakthrough time was identified as that time. All the toluene breakthrough measurements were carried out after drying of the aged and new carbon beds. The IACs were dried to avoid the effect of adsorbed water on the physical adsorption of toluene.
2.6. TEDA Evaporation
During the study, we found that on some occasions, pre-evaporation of TEDA from carbon improved the efficiency of the regeneration procedure. TEDA evaporation was carried out prior to the regeneration procedure. The carbon was put into a tray in a thin layer and heated at 180 °C for 24 h. The absence of TEDA in the carbon after the evaporation process was verified by extracting TEDA from the carbon with methanol and analyzing the extract using GC-NPD.
2.7. Regeneration of the Aged IAC (Impregnation Reactivation)
2.7.1. Dry Thermal Regeneration
The aged IAC was put into a tray in a thin layer and heated at 180 °C for 8 h.
2.7.2. Wet Regeneration
For the wet regeneration procedure, the aged IAC was regenerated by the incipient wetness method. First, the aged carbons were dried; thereafter, while stirring, the regeneration solution was slowly added to the IAC until it started to adhere to the glass walls. An aqueous regeneration solution comprising ammonia in the form of ammonium hydroxide and ammonium carbonate was prepared. These two compounds were designed to allow the dissolution of the insoluble impregnation of zinc and copper. The regeneration efficiency was tested for 3 types of solutions, in which the ammonium hydroxide to ammonium carbonate ratio remained constant, but their concentration in the solution was changed by adding different concentrations of water to dilute the solution. The solution composition is presented in Section 3.3.
The regeneration solution was dripped onto the carbon at a volume of 0.52 mL per 1 g, which was slightly less than the wetness volume. The copper and zinc ions that might have migrated to the external surface of the carbon granule during aging were partially incorporated back into the pores of the activated carbon. The IAC was then dried at a temperature of 100 °C for 30 min, 130 °C for 30 min, 160 °C for 45 min, and finally at 180 °C for 45 min. During this drying process, the ammonium carbonate, ammonia, and water evaporated. As a result of the high temperature, these ions were probably partially converted to copper oxide (CuO) and zinc oxide (ZnO) [17,18]; both of these have a very low solubility in water.
2.8. TEDA Impregnation
During the drying stage of the regeneration procedure, the carbon was exposed to a high temperature (180 °C), causing sublimation of the TEDA from the carbon. Therefore, after the regeneration procedure, TEDA solution was added to the IAC while stirring at a volume of 0.43 mL/g using the incipient wetness impregnation method. The added TEDA quantity was 3 wt.% relative to the final weight of the IAC. The IAC was dried at 80 °C, which was a low enough temperature to ensure a negligible amount of TEDA evaporation from the IAC.
2.9. IAC Characterization
The changes in the textural properties and concentrations of the impregnated metals on the external surface of the IAC granules were characterized using the following methods (all carbons were dried before characterization):
- The Brunauer–Emmett–Teller (BET) surface area and micropore volume of the IACs were calculated from N2 adsorption–desorption isotherms at a temperature of 77 K using a NOVA 1200e (Quantachrome, Graz, Austria) system. Before the measurement, all the IAC samples were subjected to outgassing under vacuum at 80 °C. The surface area of the IAC micropores was determined using t-plot analysis of the sorption isotherm. Outgassing was carried out at a temperature of 80 °C, since significant TEDA sublimation may occur at higher temperatures. Moreover, the sublimation rate may vary for different types of carbons.
- Energy dispersive X-ray spectroscopy (EDS) was used to analyze changes in the surface element composition of the IACs with scanning electron microscopy (SEM) using a PhenomX instrument (Phenom-Word B.V., Eindhoven, The Netherlands), at a 15 keV electron acceleration voltage. Since the distribution of the impregnated metals on the external surface of the IAC is non-uniform even on a given carbon particle, the element concentrations were determined as the average of 10 different areas obtained from different carbon particles, and the area analyzed in each measurement was large, at 316 μm × 316 μm (magnification of X850).
- A second surface element composition analysis was performed ESCALAB X+ apparatus (Thermo Fisher Scientific, Waltham, MA USA) with a basic pressure of 5 × 10−10. For X-ray photoelectron spectroscopy (XPS) measurements, the carbon samples were irradiated with monochromatic Al Kα X-rays at 1486.6 eV. The X-ray beam size was 500 μm. Survey spectra were recorded with a pass energy (PE) of 150 eV, and high-energy resolution spectra were recorded with a PE of 20 eV. AVANTAGE, software (version 6.6.0) was used for data acquisition and analysis. The atomic concentrations were calculated using elemental sensitivity factors without applying any standardization procedure.
2.10. Determination of the Copper Oxide Dissolving Capacity of the Regeneration Solutions
The dissolution capacity of the regeneration solutions was determined by dissolving 14 g of copper oxide in 70 mL of regeneration solution. The solution was stirred for two hours until equilibrium was reached. The solution with the excess undissolved copper oxide was filtered under vacuum. Then, the precipitated copper oxide was rinsed with water and dried at 120 °C overnight. The dissolution capacity of the regeneration solutions (g/mL), i.e., the solubility of the copper oxide in the regeneration solution, was determined from the difference between the initial weight of copper oxide introduced to the solution and the weight of the precipitate after filtering and drying divided by the initial solution volume.
3. Results and Discussion
3.1. CK Protection Values of Aged IAC
Humidified ASZMT carbon beds were exposed to CK vapor until breakthrough occurred, before and after accelerated aging. Figure 1 shows the protection values (Ct, g min/m3), which were calculated as the product of the influent concentration (C0, g/m3) and the breakthrough time (tB, min).
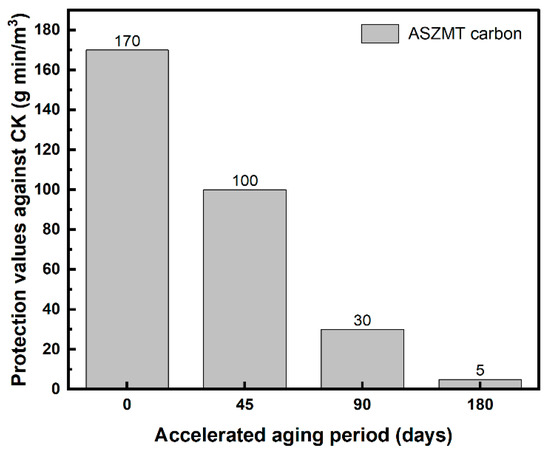
Figure 1.
Effect of aging on the protection values of IAC against CK (g min/m3). Data adapted from Ref. [15].
CK protection values were drastically decreased after 90 days of accelerated aging, rendering the IAC virtually ineffective. After 180 days of accelerated aging, the IAC lost 97% of its initial protection value. In addition, after the drying and rehumidification of IAC that had been aged for 90 days, the protection value was only 33 g min/m3 (not presented in Figure 1), very similar to that of carbon after aging that had not been subjected to drying and rehumidification.
3.2. Thermal Regeneration Efficiency
First, it is important to examine whether heating alone, without the addition of the regeneration solution to the carbon, can restore the protection capacity of aged IAC. Heating the IAC, followed by TEDA addition (to replace the TEDA that was sublimated during the drying period), was not expected to efficiently cause the impregnated metal, which might have migrated to the external surface of the IAC particles during aging, to move back to the pores in the granule’s interior. However, it is possible that during the heating process (180 °C, 8 h), the chemical configuration of the metals can return, completely or partially, to their initial metal oxide forms before aging [13,17,18]. This change may partially restore the protection values.
The effect of thermal processing (dry regeneration) on the protection values against CK is depicted in Figure 2. The IAC was humidified after heat treatment and TEDA addition but before CK exposure.
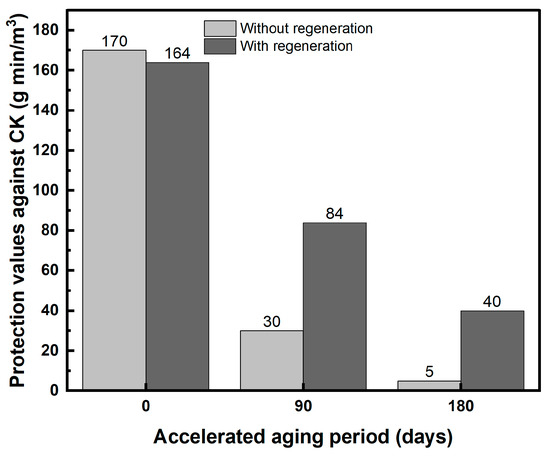
Figure 2.
Effect of dry thermal regeneration on the protection values against CK for new and aged IAC.
The regeneration procedure for new carbon was conducted to determine whether the regeneration process itself (heating of the carbon), regardless of aging and TEDA addition, affects the initial protection values of a new IAC. It can be seen in Figure 2 that the protection values remain high after the regeneration procedure (~164 g min/m3). However, for aged carbon, thermal dry regeneration had a low efficiency in restoring the protection value that had been lost during aging, especially after six months of accelerated aging. The protection values after regeneration were only 24% and 51% for IACs after three and six months of accelerated aging, respectively.
3.3. Wet Regeneration Efficiency
The wet regeneration method was based on dripping a solution with the capability of dissolving the impregnated metal species, including water-insoluble metal oxides, metal carbonates, or metal hydroxides, onto aged IAC. This solution redissolved the impregnated metals, enabling them to enter the pores of the carbon. In this way, metals that had migrated to the external surface of the IAC particles during aging partially returned into the pores, possibly in the places where the metals crystallized during the impregnation procedure of IAC production. Thereafter, the IAC was dried at an elevated temperature, similar to the drying temperatures applied during the impregnation procedures of AC [5,15]. Due to the high temperature (180 °C) and the presence of ammonium hydroxide and ammonium carbonate, the metals may have been converted, at least partially, to the desired metal substances, such as zinc oxide (ZnO) and copper oxide (CuO) [13,17,18], that formed during the activated carbon impregnation process. These two processes may partially restore the impregnated metals to their initial state (before aging), in terms of their dispersion within the granule and their chemical state. It is hoped that this process will restore the protection value to a level similar to that of new IAC.
The regeneration solution contained the same components as the solutions used for impregnation but without the metal salts themselves. That is, the solution contained ammonium hydroxide, ammonium carbonate, and distilled water for dilution [5,15]. During the drying process, all the solution ingredients evaporated: the ammonium ions as ammonia and the carbonate as CO2. The concentration of the regeneration solution affected the metal dissolving capacity. Therefore, we examined the regeneration efficiency for three solutions with different concentrations of dilution water so that the ratio between the components of the solution remained constant, but their concentration varied. The composition of the three regeneration solutions is presented in Table 1.

Table 1.
Composition (wt.%) of the three regeneration solutions.
Solution R0, which was the most dilute of the three solutions, was similar in composition to the impregnation solution used in our lab [15], with the exception of removing the metals from the solution. That is, the mass of ammonium hydroxide and ammonium carbonate in a given volume of solution was the same as that in the impregnation solution.
Since amines such as TEDA can form a complex with impregnated metals [19,20], their presence in the carbon during the dripping and subsequent drying of the regeneration solution may have interfered with the dissolution of the metals and affected the species that crystallize during drying. To improve the regeneration efficiency, we also examined the effect of early evaporation of TEDA from the carbon on this process.
The efficiency of wet regeneration on the protection values against CK is depicted in Figure 3. The regeneration process was applied on carbon after six months of accelerated aging.
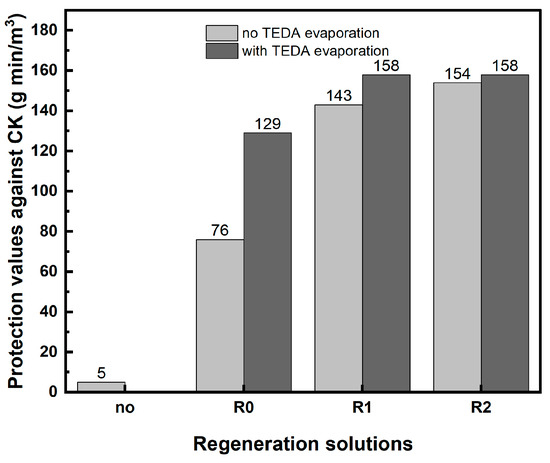
Figure 3.
The effect of wet regeneration with different solutions (R0, R1, and R2) on the protection values against CK for IAC after accelerated aging for 6 months.
Figure 3 shows that for solution R0, TEDA pre-evaporation significantly improved the efficiency of the regeneration solution to restore the protection value against CK. Nevertheless, even with TEDA pre-evaporation, the protection value after regeneration with this solution was not completely restored but remained at 75% of its original value.
The concentrated solutions R1 and R2 performed better than solution R0. In these two solutions, the protection values were higher (158 g min/m3) and approached the protection value of new carbon. Even without the pre-evaporation of TEDA, the protection values obtained after regeneration with solution R1 or R2 (143 g min/m3 and 154 g min/m3, respectively) were close to the protection value of new carbon, corresponding to 84% and 91% of the initial values. Using the R2 solution almost completely eliminated the need for the pre-evaporation of TEDA.
The higher efficiency of the concentrated solutions R1 and R2 compared to solution R0 may be explained by the higher solubility of the impregnated metals in solutions R1 and R2, because higher concentrations of ammonium hydroxide and ammonium carbonate could coordinate with the metal ions in the carbon to form a soluble metal–ammonium complex. Indeed, the CuO dissolving capacity of the R2 regeneration solution was found to be significantly higher than the dissolving capacity of the R0 solution: 0.193 g/mL and 0.132 g/mL, respectively. In addition, a higher concentration of ammonium carbonate and ammonium hydroxide in the regeneration solution may allow for a larger amount of impregnated metal in the carbon (copper or zinc) to be released from the complex with TEDA (forming a metal ammonium complex).
3.4. The Effect of the Regeneration Process on Toluene Adsorption
The fact that after a regeneration procedure, the protection value against CK approached the value of new IAC suggested that the regeneration efficiency for other gases that are chemically adsorbed may be satisfactory as well. However, it is important to verify that the regeneration process does not adversely affect the adsorption capability of the IAC against vapors that are adsorbed by the physical adsorption mechanism. Figure 4 shows the effect of accelerated aging and wet regeneration on the protection values against the organic compound toluene. Regeneration was achieved with solution R2 without the pre-evaporation of TEDA. All the carbon beds were dried before the toluene breakthrough measurements.
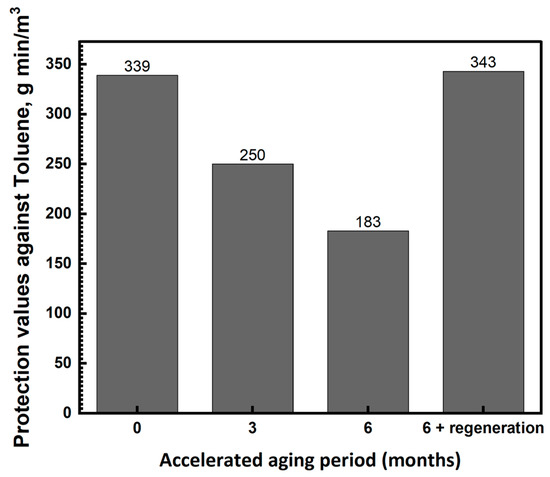
Figure 4.
The effect of accelerated aging and wet regeneration processes on the protection values against toluene.
Figure 4 shows that aging decreases the protection values of the IAC against toluene (physical adsorption). After six months, the protection value decreased by 46%. The regeneration process restored the protection value to its initial value. A decrease in the protection value of aged IAC against toluene may be related to the processes of dissolution and recrystallization of the metals during aging. These processes resulted in changes in the distribution and coverage of the impregnated metal on the carbon surface and consequently reduced its affinity to toluene. The regeneration process restored the state of the impregnated metal close to the original state, so that the protection value against toluene was the same as that of new IAC.
3.5. IAC Characterization
Effect of the Regeneration Process on the Textural Properties of IAC
The textural properties of the IACs were studied by plotting N2 adsorption–desorption isotherms. Figure 5 depicts the adsorption–desorption isotherms of N2 at 77 K, and Table 2 presents the textural properties.
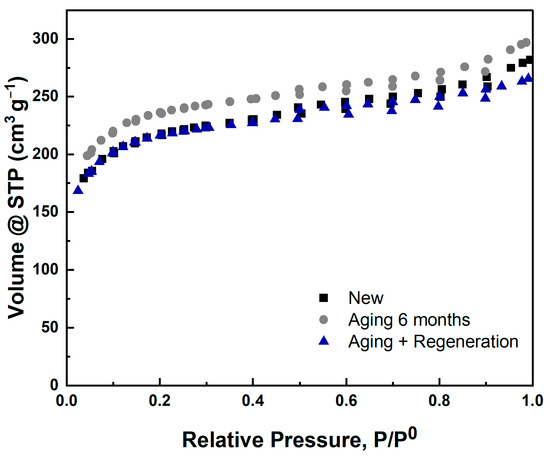
Figure 5.
N2 adsorption–desorption isotherms of IACs before and after regeneration.

Table 2.
Effect of aging and the regeneration process on the textural properties of IAC. Data for new IAC and IAC after 6 months of accelerated aging were adapted from [15].
The data for new IAC and IAC after 6 months of accelerated aging were adapted from Ref. [15].
The N2 adsorption–desorption isotherms presented in Figure 5 are typical of a microporous material (type I), such as microporous AC [21]. Neither aging nor the regeneration process changed the isotherm shape; i.e., no indication of micropore blockage was observed.
Table 2 shows that neither aging nor the regeneration process significantly affected the micropore volume or the surface area of the IACs. The slight increase in the micropore volume and the surface area after aging may have been due to the migration of the impregnated metal from the internal micropores to the external surface.
4. Elemental Analysis
The impregnated metal’s migration to the external surface of the IAC is one of the main characteristics of aging [12,13,14]. Thus, it is of interest to determine whether the metals migrate back into the pores during the regeneration procedure. For this reason, an EDS elemental analysis was performed on the external surface of the carbon. The results are presented in Figure 6.
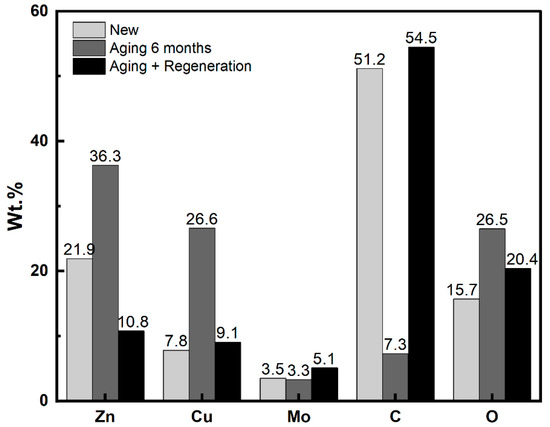
Figure 6.
Elemental analysis (EDS) of Zn, Cu, Mo, C, and O (wt.%) on the external surface of IAC granules.
As mentioned in previous publications [12,15,22], for AC impregnated with zinc and copper, the metal concentration, particularly the zinc concentration on the external surface of the IAC particles, was higher than the bulk concentration (5.2 wt.% Zn and 5.2 wt.% Cu). This implied that the metal distribution, especially of zinc, was not uniform. These results may be attributed to the migration of zinc from the interior to the external surface during drying after the addition of the metal impregnation solution to the IAC.
The zinc and copper migration to the external surface during aging was reflected by the significant increase in their concentration on the external surface, accompanied by a dramatic decrease in the carbon concentration. After accelerated aging for six months, the concentrations of zinc and copper increased from 7.8 and 21.9 wt.% to 26.6 and 36.3 wt.%, respectively.
Following the regeneration process, in which the metals partially migrated back into the pores, the zinc and copper concentrations decreased, while the concentration of carbon increased.
Table 3 presents the results of the elemental ratio analysis (w/w) of the impregnated metals using XPS and SEM-EDS. In both methods, a clear migration trend of zinc and copper to the external surface of the IAC particles during aging can be observed, as manifested by increases in the Zn/C and Cu/C ratios. After regeneration, a decrease in the value of both ratios was found, evidence of the partial migration of the metals back into the interior of the granules. As expected, the absolute values of the concentration ratios obtained by the two methods are not the same. This is mainly due to the fact that the XPS method provides an analysis to a depth of 30–50 angstroms only, while the SEM-EDS method, used for carbon samples with an energy of 15 keV, provides analysis to a greater depth, approximately 1–1.5 microns.

Table 3.
EDS and XPS elemental ratio analyses (w/w) of the external surface of the new and aged IAC particles.
5. Conclusions
A method for regenerating aged IAC was developed, with the aim of reactivating the impregnated metals and restoring the protective capacity to the level of new carbon. The method was based on dripping a regeneration solution, which was composed of ammonium hydroxide and ammonium carbonate, onto the IAC, drying it at a temperature of up to 180 °C, and replacing the TEDA that was evaporated during drying. After regeneration, the protection values against CK and toluene of the aged ASZMT carbon were almost fully restored to their initial protection values. It is reasonable to conclude that the presented regeneration method may also be efficient for aged IAC with impregnation compositions different from that of ASZMT. The implementation of the method has economic and environmental consequences. It may allow for the reuse of aged IAC and even a restoration of collective filters.
Author Contributions
Conceptualization, I.N., V.S. and H.R.; methodology, I.N. and V.S.; software, V.S. and L.R.; validation, I.N. and V.S.; formal analysis, I.N. and V.S.; investigation, I.N., V.S., L.R., A.L., L.A., T.A.-R. and H.R.; resources, I.N., V.S., L.R., A.L., L.A., T.A.-R. and H.R.; data curation, I.N., V.S., L.R., A.L., L.A.,T.A.-R. and H.R.; writing—original draft preparation, I.N. and H.R.; writing—review and editing, I.N., T.A.-R. and H.R.; visualization, I.N., T.A.-R. and H.R.; supervision, I.N., V.S., T.A.-R. and H.R.; project administration, I.N., V.S., T.A.-R. and H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This study was performed for the CBRN Defense Division, Israeli Ministry of Defense.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to have influenced the work reported in this paper. The contents of this manuscript have no conflict of interest with Life Science Research Israel Ltd.
References
- Wilson, R.E.; Whetzwl, J.C. Imtrgmated Carbon and Process of Making Same. U.S. Patent 1,519,470A, 16 December 1924. [Google Scholar]
- Noyes, W.A.J. Military Problems with Aerosols and Non-Persistent Gases; Summary Technical Report of the National Defence Research Committee (NDRC), Division 10; National Defense Research Committee: Washington, DC, USA, 1946.
- Lodewyckx, P.; Lodewyckx, P. Chapter 10 Adsorption of Chemical Warfare Agents. In Activated Carbon Surfaces in Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 475–528. ISBN 9780123705365. [Google Scholar]
- Kiani, S.S.; Farooq, A.; Ahmad, M.; Irfan, N.; Nawaz, M.; Irshad, M.A. Impregnation on Activated Carbon for Removal of Chemical Warfare Agents (CWAs) and Radioactive Content. Environ. Sci. Pollut. Res. 2021, 28, 60477–60494. [Google Scholar] [CrossRef] [PubMed]
- Doughty, D.T.; Knebel, W.J.; Cobes, J.W. Chromium-Free Impregnated Activated Universal Respirator Carbon for Adsorption of Toxic Gases and/or Vapors. EP. Patent 0 405 404 B1, 1993. [Google Scholar]
- Wang, S.; Nam, H.; Lee, D.; Nam, H. H2S Gas Adsorption Study Using Copper Impregnated on KOH Activated Carbon from Coffee Residue for Indoor Air Purification. J. Environ. Chem. Eng. 2022, 10, 108797. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Mathuray Veeran, L.S.; Noor Azam, A.M.I.; Masdar, M.S.; Wan Isahak, W.N.R. Effect of Bimetallic-Activated Carbon Impregnation on Adsorption–Desorption Performance for Hydrogen Sulfide (H2S) Capture. Materials 2022, 15, 5409. [Google Scholar] [CrossRef]
- Wang, X.; Jing, X.; Wang, F.; Ma, Y.; Cheng, J.; Wang, L.; Xu, K.; Cheng, C.; Ning, P. Coupling Catalytic Hydrolysis and Oxidation on Metal-Modified Activated Carbon for HCN Removal. RSC Adv. 2016, 6, 57108–57116. [Google Scholar] [CrossRef]
- Smith, J.W.H.; Romero, J.V.; Dahn, T.R.; Dunphy, K.; Croll, L.M.; Dahn, J.R. The Effect of Co-Impregnated Acids on the Performance of Zn-Based Broad Spectrum Respirator Carbons. J. Hazard. Mater. 2012, 235–236, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ehrburger, P. Aging of Cupric Oxide Supported on Activated Carbon. J. Catal. 1986, 100, 429–436. [Google Scholar] [CrossRef]
- Brown, P.N.; Jayson, G.G.; Thompson, G.; Wilkinson, M.C. Effect of Ageing and Moisture on the Retention of Hydrogen Cyanide by Impregnated Activated Charcoals. Carbon 1989, 27, 821–833. [Google Scholar] [CrossRef]
- Rossin, J.A.; Morrison, R.W. The Effects of Molybdenum on Stabilizing the Performance of an Experimental Copper/Zinc Impregnated, Activated Carbon. Carbon 1993, 31, 657–659. [Google Scholar] [CrossRef]
- Rossin, J.A.; Morrison, R.W. Spectroscopic Analysis and Performance of an Experimental Copper/Zinc Impregnated, Activated Carbon. Carbon 1991, 29, 887–892. [Google Scholar] [CrossRef]
- Rossin, J.; Petersen, E.; Tevault, D. Effects of Environnental Weathering on the Properties of ASC-Whetlerite. Carbon 1991, 29, 197–205. [Google Scholar] [CrossRef]
- Nir, I.; Shepelev, V.; Pevzner, A.; Marciano, D.; Rosh, L.; Amitay-Rosen, T.; Rotter, H. Phosphate Additives for Aging Inhibition of Impregnated Activated Carbon against Hazardous Gases. Int. J. Mol. Sci. 2023, 24, 13000. [Google Scholar] [CrossRef] [PubMed]
- Afandizadeh, S.; Foumeny, E.A. Design of Packed Bed Reactors: Guides to Catalyst Shape, Size, and Loading Selection. Appl. Therm. Eng. 2001, 21, 669–682. [Google Scholar] [CrossRef]
- Smith, J.W.H.; Westreich, P.; Croll, L.M.; Reynolds, J.H.; Dahn, J.R. Understanding the Role of Each Ingredient in a Basic Copper Carbonate Based Impregnation Recipe for Respirator Carbons. J. Colloid Interface Sci. 2009, 337, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.H.; Romero, J.V.; Dahn, T.R.; Dunphy, K.; Sullivan, B.; Mallay, M.; Croll, L.M.; Reynolds, J.H.; Andress, C.; Dahn, J.R. The Effect of Heating Temperature and Nitric Acid Treatments on the Performance of Cu- and Zn-Based Broad Spectrum Respirator Carbons. J. Colloid Interface Sci. 2011, 364, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Deitz, V.R.; Karwacki, C.J. Chemisorption of Cyano-Containing Vapors by Metal-Ligand Structures Adsorbed by Activated Carbon. Carbon 1994, 32, 703–707. [Google Scholar] [CrossRef]
- Morrow, J.R.; Trogler, W.C. Hydrolysis of Phosphate Triesters with Copper(II) Catalysts. Inorg. Chem. 1989, 28, 2330–2333. [Google Scholar] [CrossRef]
- Choma, J.; Jaroniec, M.; Jaroniec, M. Chapter 3 Characterization of Nanoporous Carbons by Using Gas Adsorption Isotherms. In Activated Carbon Surfaces in Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 107–158. ISBN 9780123705365. [Google Scholar]
- Park, S.H.; McClain, S.; Tian, Z.R.; Suib, S.L.; Karwacki, C. Surface and Bulk Measurements of Metals Deposited on Activated Carbon. Chem. Mater. 1997, 9, 176–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).