Abstract
Mild cognitive impairment (MCI) represents a heterogeneous state between normal aging and dementia, with varied transition pathways. While factors influencing MCI progression are known, their role in cognitive reversal is unclear. This study analyzed 756 Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants, classified as progressive MCI (pMCI, N = 272, mean age = 75.10 ± 7.34 years), reversible MCI (rMCI, N = 52, mean age = 69.94 ± 7.98 years) and stable MCI (sMCI, N = 432, mean age = 73.34 ± 7.44 years) based on 36-month follow-up. We compared demographic, lifestyle, clinical, cognitive, neuroimaging, and biomarker data across groups and developed a prediction model. Patients in the rMCI group were significantly younger and had a higher level of education compared with those in the pMCI group. Memory, general cognition, daily functional activities, and hippocampal volume effectively distinguished all three groups. In contrast, Aβ, tau, and other brain regions were able to distinguish only between progressive and non-progressive cases. Informant-reported Everyday Cognition (Ecog) scales outperformed self-reported Ecog scales in differentiating subtypes and predicting progression. Multinomial regression revealed that higher education, larger hippocampal volume, and lower daily functional impairment were associated with reversion, whereas APOE ε4, poorer memory, and greater brain atrophy predicted progression (model accuracy: 78%). The results confirm the significant utility of hippocampal volume, education level, and daily functional activities for assessing baseline disparities and predicting reversion. This study highlights the differential contributions of cognitive abilities and brain regions on MCI reversal, advancing understanding of MCI heterogeneity and providing evidence for precise diagnosis and treatment in early MCI.
1. Introduction
Dementia is a prevalent neurodegenerative disorder among the elderly population. While several interventions and treatment approaches currently exist—including symptomatic therapies and emerging disease-modifying agents—they primarily slow disease progression rather than providing a cure. There remains a long path ahead before we can truly realize the goal of curing dementia (Fox et al., 2025; Zhi et al., 2025). The transitional state between normal cognitive aging and dementia is defined as mild cognitive impairment (MCI) (Albert et al., 2011; Luo et al., 2022; Petersen, 2004). However, MCI exhibits marked heterogeneity. While MCI represents a high-risk state for dementia development, not all individuals will progress (Lopez et al., 2012). Based on the future developmental trends of MCI, patients may progress to dementia (progressive MCI, pMCI), revert to normal cognition (NC) (reversible MCI, rMCI), or remain in a stable condition (stable MCI, sMCI) (Iraniparast et al., 2022; Petersen et al., 2001). According to the recent studies, the transition rate from MCI to NC has been reported to range from 4.5% to 30%, which may reflect differences in clinical versus community-based screening methods as well as variations in follow-up duration (Koepsell & Monsell, 2012; Malek-Ahmadi, 2016; Nordlund et al., 2010; Sha et al., 2022; Wood, 2016; Xue et al., 2019; Yu et al., 2025). These findings suggest that a substantial proportion of MCI patients have the potential to revert to NC. Therefore, targeting therapeutic interventions on rMCI may represents a promising strategy to optimize MCI management and to delay or even prevent the onset of dementia.
The progression of MCI is influenced by a multitude of factors. For example, older age, a higher number of risk alleles, lower educational levels, poorer baseline cognitive function and psychological factors (e.g., depression) are all associated with a greater likelihood of MCI progression (Li et al., 2016; Marquié et al., 2023; Nakahata et al., 2021; Pink et al., 2015; Serrano-Pozo et al., 2021; Tucker-Drob, 2019). Nevertheless, the roles of these factors in MCI reversal remain unclear. Previous studies have demonstrated significant differences in these factors between progressive and non-progressive groups (W. Wang et al., 2023). Few studies have investigated whether stable and reversible subtypes can be distinguished within the non-progressive group. Specifically, previous studies have reported that age—considered one of the most critical predictors of MCI progression—carries the greatest weight in progression-related risk models. However, its predictive utility in cognitive reversal remains uncertain (Hu et al., 2017). While some evidence suggests that younger individuals have a higher likelihood of reverting to normal cognitive status (Pandya et al., 2017), other studies indicate that the predictive influence of age on progression cannot be directly extrapolated to predict reversal (M. Wang et al., 2023). APOE (apolipoprotein E) is a polymorphic protein, and the APOE ε4 allele is a well-established genetic risk factor for late-onset Alzheimer’s disease (AD), significantly increases the likelihood of developing AD (Serrano-Pozo et al., 2021). Thus, carriers of the APOE ε4 allele exhibit a markedly higher risk of progression to AD compared to non-carriers (Scarabino et al., 2016). However, whether ε4 non-carriers are more likely to experience cognitive reversal remains an open question. Some studies have reported that fewer APOE ε4 alleles are associated with a higher probability of cognitive reversal (Caselli et al., 2009), whereas others suggest that having fewer APOE ε4 alleles may reduce progression risk without directly predicting reversal (Ward et al., 2020). Educational attainment is closely associated with cognitive reserve, which, in turn, may influences the progression trajectory of MCI (Iraniparast et al., 2022; Tucker-Drob, 2019). Higher cognitive reserve may delay the progression from MCI to AD, but its role in cognitive reversal is not yet well established (Mazzeo et al., 2019; H. Xu et al., 2020). Furthermore, baseline cognitive status is a well-established predictor: lower baseline scores strongly predict progression to dementia, while higher baseline cognitive performance increases the likelihood of reversal (Grassi et al., 2019; Jack et al., 2010). Neuroimaging markers (e.g., gray matter atrophy rates) have been extensively validated as bidirectional markers of cognitive change and demonstrate significant heterogeneity across distinct MCI subtype, showing moderate predictive utility for both disease progression and reversion (Lissek & Suchan, 2021; Luo et al., 2022; Pyun et al., 2021; Serrano-Pozo et al., 2021). Nevertheless, their predictive accuracy requires further elucidation. For instance, although hippocampal atrophy is a well-established neuroimaging biomarker of AD, it remains unclear whether preserved hippocampal volume can indicate better cognitive performance in individuals exhibiting cognitive reversal (Yang et al., 2022).
Additionally, non-neurodegenerative conditions can also influence MCI conversion. In terms of lifestyle factors, individuals with a history of smoking, alcohol use are at significantly higher risk of MCI progression (Durazzo et al., 2018; W. Xu et al., 2017). Similarly, dietary habits and activities are also influential factors that should not be overlooked. Consumption of fresh fruits is associated with an increased rate of reversion from MCI, while cognitively stimulating activities (e.g., reading) not only helps slow the decline of cognitive decline but also promote MCI reversion from MCI (Katayama et al., 2021; Sha et al., 2022). Marital status in later life reduces the risk of MCI and dementia, though no direct evidence currently links it to MCI reversion (Skirbekk et al., 2023). In terms of physiological and psychological factors, research has identified sleep disorders are a well-established risk factor for both MCI and dementia, and treating sleep disorders may lead to measurable improvements in cognitive function (Mayer et al., 2024). Depression, apathy, diabetes mellitus and hyperlipidemias also should not be overlooked when investigating the processes underlying MCI conversion mechanisms (Li et al., 2016). However, the absence of such lifestyle factors or medical conditions may only serve to slow MCI progression rather than actively promote reversion (Sha et al., 2022). In summary, while these non-neurodegenerative factors are strongly associated with MCI progression, the majority of their correlation with cognitive reversion remains unclear. Moreover, the presence of multiple risk factors—which may interact with each other—makes it difficult to determine the individual predictive power of each factor.
Therefore, in this study, we categorized MCI participants into three groups—pMCI, rMCI, and sMCI—based on data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. We aimed to systematically investigate the characteristic differences among these subpopulations within a unified analytical framework, delineate the distinct features of each MCI subgroup, and develop a prediction model of whether baseline MCI status is likely to transition.
2. Methods
2.1. Participant Characteristics
Participants in this study were drawn from ADNI (http://adni.loni.usc.edu/). ADNI is a longitudinal multicenter study that has recruited participants aged 55+ years, including individuals with NC, MCI, and AD. It collects comprehensive multimodal data covering neuroimaging, biomarkers, clinical assessments, and genetic profiles. Prior to data collection, ADNI obtained approval from the Institutional Review Board (IRB) of each participating site and written informed consent from all its participants or their legally authorized representatives.
All participants included in the present study had a baseline diagnosis of MCI. In the ADNI database, the diagnostic criteria for MCI include: (1) Subjective Memory Concern; (2) Objective Cognitive Impairment, determined via standardized cognitive assessments, with the following specifications: (i) a Clinical Dementia Rating (CDR) (Berg, 1988) score of 0.5 and Mini-Mental State Examination (MMSE) (Folstein et al., 1975), scores between 24–30; (ii) intact activities of daily living; and (iii) objective memory loss measured by education adjusted scores.
Participants fulfilling both criteria were diagnosed with MCI.
MCI participants were further subclassified based on their cognitive trajectories over a 36-month follow-up period, using the following criteria:
Progressive MCI (pMCI): Individuals who experienced cognitive decline during the follow-up, meeting the threshold for a diagnosis of dementia. These participants transitioned from MCI to a clinical diagnosis of AD or other kinds of dementia.
Reversible MCI (rMCI): Individuals whose cognitive function improved during the follow-up, returning to a normal cognitive range and no longer meeting the criteria for MCI.
Stable MCI (sMCI): Individuals who maintained relatively stable cognitive performance throughout the follow-up period, with no significant improvement or deterioration.
After initially selecting MCI participants who exhibited progression, reversion, or remained stable over 36-month follow-up, we specifically verified the completeness of their demographic data and key genetic variable, namely age, sex, education level, and number of APOE ε4 alleles. Only participants with complete data for all four variables were included in the final analysis. Finally, total of 756 participants from ADNI-1, ADNI-GO, ADNI-2, and ADNI-3 were included in this study.
2.2. Demographic Variables
Demographic variables included age, sex, years of education, and the number of APOE ε4 alleles (0/1/2). Marital status was categorized as either living with a partner (married) or living alone (not married/divorced/widowed).
2.3. Lifestyle and Clinical Characteristics
Lifestyle variables included living arrangement, obesity status, smoking history, alcohol using history. Body Mass Index (BMI) was calculated using height and weight; individuals with a BMI ≥ 28 were classified as obese, while those with lower BMI were considered non-obese. Clinical characteristics consisted of medical history variables (Neurological & Psychiatric Disorders/Cardiovascular Diseases/Hypertension/Stroke/Respiratory Diseases/Endocrine Disorders/Major Surgical History), and Geriatric Depression Scale (GDS) (Sheikh, 1986) was used for assessing depressive symptoms.
2.4. Behavioral Data
Cognitive assessments covered five domains. General Cognition: CDR Sum of Boxes (CDRSB), MMSE, Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) (Rosen et al., 1984); Memory: Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1958), Logical Memory Delayed Recall Total (LDELtotal) (Wechsler, 1987); Executive Function: Trail Making Test (TMT) (Reitan, 1958) Daily Function: Functional Activities Questionnaire (FAQ) (Pfeffer et al., 1982); and Language: Category Fluency (Animal Naming) Test (CFT) (Butters et al., 1987). For the TMT, Part B–Part A difference scores were calculated. The Everyday Cognition (Ecog) (Farias et al., 2008) was administered to assess patient and caregiver ratings across distinct cognitive domains, capturing self-reported (participant) and informant-reported (study partner) perspectives on functional abilities. In general, study partners are someone who knows the patient well, such as caregivers.
2.5. Neuroimaging Data and Biomarkers
Neuroimaging data from the ADNI database included volumetric measurements of the whole brain and five specific brain regions: ventricles, hippocampus, entorhinal cortex, fusiform gyrus, and middle temporal gyrus. Biomarkers data included cerebrospinal fluid (CSF) tau, p-tau and Aβ datas.
2.6. Statistical Analyses
In the analysis of demographic characteristics, categorical variables such as sex and APOE ε4 allele count were analyzed using the chi-square test, while continuous variables such as age and education were analyzed using One-Way Analysis of Variance (one-way ANOVA). Additionally, non-parametric tests were conducted to examine the distribution of age and education.
A general linear model (GLM) was applied to all behavioral data, brain region volumes, and biomarker measurements treated as continuous variables to assess group differences. Age, sex, years of education, and the number of APOE ε4 alleles were included as covariates. For brain region data, total intracranial volume (TIV) was additionally included as a covariate. Except for BMI and GDS, all variables related to medical history, lifestyle, and clinical characteristics were binary and analyzed using the chi-square test. BMI and GDS were analyzed using one-way ANOVA. Additionally, a mixed-design ANOVA was conducted on the Ecog data, with source (self vs. informant) as the within-subjects factor, group (pMCI, rMCI, sMCI) as the between-subjects factor, and the Ecog subscales as the dependent variables. We evaluated self-reported and informant-reported subscores within the Ecog scale to determine whether these two types of reports significantly differed, and to assess their utility in predicting MCI reversion. All post hoc test was corrected using the Bonferroni method.
To investigate the relationship between neuroimaging data and behavioral data, partial correlation analyses were conducted in each group between the volumes of specific brain regions (ventricles, hippocampus, entorhinal cortex, fusiform gyrus, middle temporal gyrus) and cognitive measure, with age, sex, years of education, number of APOE ε4 alleles, and TIV included as covariates. The results were corrected for multiple comparisons using the false discovery rate (FDR) method.
Predictors of MCI transformation were examined using multinomial logistic regression. The model was adjusted for age, sex, education, and APOE ε4 status. Significant predictors (p < 0.05 in univariable analyses) were then entered into the multivariable model, with final variable selection determined by likelihood ratio tests. In the regression model, the sMCI group was set as the reference category. All continuous variables underwent z-score, and regional brain volumes were normalized as percentages of total intracranial volume (TIV) prior to z-score transformation.
All analysis processes were conducted using SPSS 25.
3. Results
3.1. Demographic Characteristics
A total of 756 participants were included in the study (male: 449; female: 307), comprising 52 were rMCI (male: 28; female: 24), 432 were sMCI (male: 265; female: 167), and 272 were pMCI (male: 156; female: 116). Significant differences were observed in the mean and distribution of age (ps < 0.05) and years of education (ps < 0.05) across groups (Table 1). Participants in the rMCI (p = 0.03) and sMCI (p = 0.033) groups were significantly younger than the pMCI group. In addition, the rMCI group had significantly higher education levels compared to pMCI (p = 0.007) and sMCI (p = 0.034).

Table 1.
Demographic characteristics and behavior data.
Significant differences were observed in obesity levels (p = 0.001), with the sMCI group showing significantly higher values compared to the pMCI group. No significant differences were found in the total score of the GDS. Among the clinical characteristics, Only the presence of neurological conditions (p = 0.019) and stroke (p = 0.035) showed a significant difference.
Results from the GLM for behavioral data indicated significant between-group differences across all cognitive domains (ps < 0.05). Further analysis revealed that MMSE, RAVLT-immediate recall, RAVLT-delayed recall, and CFT followed a descending pattern across groups (rMCI > sMCI > pMCI). FAQ scores also significantly differentiated the three groups (rMCI < sMCI < pMCI). For CDRSB, LDELtotal and the TMT(B-A) difference, the pMCI group performed significantly worse than the rMCI and sMCI groups (Table 1).
3.2. Ecog Cognitive Test
A mixed-design ANOVA was conducted to compare ratings between two sources (participants and study partners, as the within-subjects factor) and across three MCI groups (pMCI, rMCI, sMCI, as the between-subjects factor) on the Ecog scales. Significant main effects were observed across all subscales (Table 2, Figure 1). Group-wise analyses indicated that Memory and Total scale scores could differentiate all three groups, whereas other scale scores only distinguished the pMCI group the rMCI and sMCI groups. No significant main effect was observed for source (self vs. informant). However, a significant interaction between group and Ecog source suggested that group influenced the discrepancies between self and informant-assessments. Self-reported Language, Executive, and Attention did not differ significantly across groups, while other self-reported subscales showed at least one significant pairwise difference. In contrast, all informant-reported subscales significantly distinguished rMCI and sMCI from pMCI, with Memory and Total scores further differentiating rMCI from sMCI. These results indicate that informant ratings in these domains effectively distinguish among the three MCI subtypes (p < 0.05, Bonferroni-corrected).

Table 2.
Everyday cognitive performance of three groups.
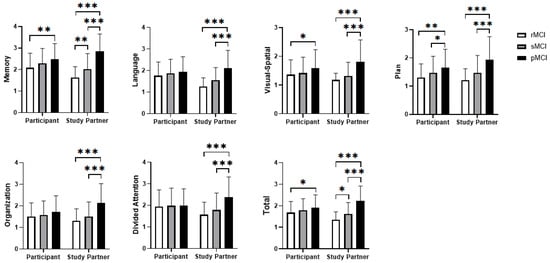
Figure 1.
Differences between self-reported and informant-reported scales. Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Neuroanatomical Characteristics
Significant group differences were observed in the ventricles, hippocampus, fusiform gyrus, middle temporal gyrus, and entorhinal cortex (Figure 2, Supplementary Table S1). Specifically, ventricle volume was largest in the pMCI group, whereas volumes of the other brain regions were smallest in this pMCI group. Notably, hippocampus effectively distinguished all three groups, while the remaining brain regions only differentiated the progressive (pMCI) group from the non-progressive (rMCI and sMCI).
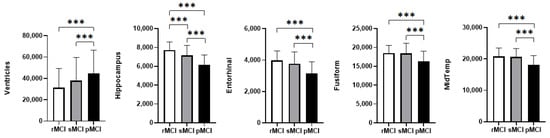
Figure 2.
Group differences in brain region volumes among the three MCI subtypes. Significance levels: *** p < 0.001. Abbreviations: Entorhinal: entorhinal cortex; Fusiform: fusiform gyrus; MidTemp: middle temporal gyrus.
3.4. Biomarker Characteristics of Participants
All biomarkers showed significant group differences (p < 0.001, Supplementary Table S2), indicating substantial variations in the underlying AD pathology levels among the three groups. Pairwise comparisons revealed that pMCI had the most pathological biomarker levels. Specifically, Aβ levels were lowest, while CSF tau and p-tau levels were highest in the pMCI group (ps < 0.001). In contrast, no significant differences were observed within the non-progressor cohort (rMCI vs. sMCI).
3.5. Partial Correlation Analysis
Partial correlation analyses were conducted for the entire sample, as well as separately within the rMCI, sMCI, and pMCI subgroups. Across the total sample, significant correlations were observed between cognitive performance and regional brain volumes after controlling for age, sex, education, APOE ε4 status, and total intracranial volume, with all results surviving FDR correction. Specifically, larger ventricular volumes were associated with poorer cognitive performance, whereas greater volumes of the hippocampus, entorhinal cortex, fusiform gyrus, and middle temporal gyrus were associated with better cognitive function.
Subgroup analyses revealed that significant brain–behavior associations were predominantly observed in the sMCI and pMCI groups (ps < 0.05, FDR correction). In the sMCI group: ventricular volume was positively correlated with TMTB-A scores; hippocampal and entorhinal cortex volumes were negatively correlated with ADAS13 and positively correlated with RAVLT_delay and LDELtotal scores; fusiform gyrus volume showed significant correlations with LDELtotal, TMTB-A, and FAQ scores; middle temporal gyrus volume correlated positively with LDELtotal and CFT scores. In the pMCI group: hippocampal and entorhinal cortex volumes were significantly correlated with memory measures (RAVLT_delay, LDELtotal); the middle temporal gyrus volume was negatively correlated with TMTB-A scores. In contrast, in the rMCI group, no correlations reached statistical significance after correction (Supplementary Table S3).
3.6. Multinomial Logistic Regression Analysis of Predictive Factors
The optimized multinomial logistic regression model included ADAS-cog, RAVLT_immediate, FAQ, hippocampus, and fusiform volumes, using a data-driven forward entry approach. The model achieved a classification accuracy of 78%, with the sMCI group serving as the reference category.
Significant predictors of reversion from sMCI to rMCI were education, FAQ scores and hippocampal volume. Higher education, lower FAQ scores, and larger hippocampal volumes associated with an increased probability of reversion (Table 3, Figure 3a).

Table 3.
Multivariate logistic regression analysis of groups.
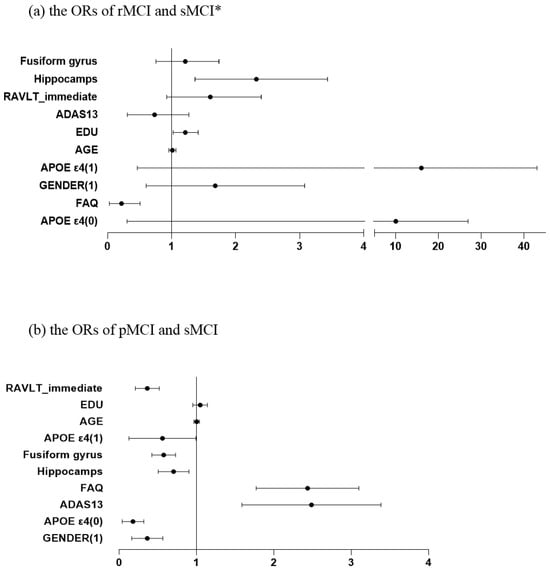
Figure 3.
The odds ratios (ORs) for different variables. * X-axis segmented into intervals: [0, 4] [5, 45]. Abbreviations: Fusiform: fusiform gyrus; EDU: Years of education; ADAS: Alzheimer’s Disease Assessment Scale; FAQ: Functional Activities Questionnaire; RAVLT_immediate: Rey Auditory Verbal Learning Test (Immediate Recall).
Significant predictors of progression from sMCI to pMCI were gender, RAVLT immediate recall scores, APOE ε4 allele, hippocampal volume, fusiform gyrus thickness, FAQ and ADAS-cog total scores. Among categorical predictors, male participants were less likely to progress to pMCI. APOE ε4 alleles of 0 or 1 was protective compared to 2 alleles. Among continuous predictors, higher ADAS-cog and FAQ scores increased the likelihood of progression, whereas Higher RAVLT-immediate recall scores reduced the risk of pMCI. Larger hippocampal and fusiform gyrus volumes were associated with a reduced risk of progression (Table 3, Figure 3b).
4. Discussion
This study demonstrates that subjects who converted within 3 years exhibited significant differences in baseline cognitive performance compared to non-converters. Among demographic variables, age distinguished the pMCI group, whereas educational attainment differentiated the rMCI group (Iraniparast et al., 2022)—suggesting that education plays a more critical role in the likelihood of MCI reversal.
Variables differentiating the three participant groups primarily involve general cognition, memory, daily functional ability, and language fluency. Specifically, performance on the ADAS-Cog and delayed recall measures effectively distinguished the three groups. Memory impairment represents a hallmark deficit in dementia. Research has consistently demonstrated that amnestic MCI (aMCI) has a higher progression rate to dementia than non-amnestic subtypes (Mitchell & Shiri-Feshki, 2009; Qin et al., 2023). However, despite the fact that this study’s population was predominantly aMCI (due to the enrollment criterion of objective memory impairment), we were able to observe significant differences in memory performance among participants. Our results lend additional support to the conclusion that individuals progressing to dementia within 36 months demonstrated markedly poorer baseline memory performance. Furthermore, participants who experienced cognitive reversal displayed superior baseline memory scores relative to other groups, further supporting the notion that memory plays a pivotal role in the transition of MCI. In addition, the FAQ differentiated the three groups, indicating that functional discrepancies are already present at baseline, despite MCI generally having minimal impact on daily functioning compared with dementia. Although these differences may not yet interfere with daily life, they are nonetheless detectable. Results from the CFT indicate that language functions are an influential factor in MCI progression. Although language impairment is not considered a core deficit in the early stages of AD, some studies have suggested that certain semantic tasks may serve as early cognitive markers during the prodromal phase of AD (Marra et al., 2021). Future research should further investigate the potentia role of language functions in MCI reversal.
Most other variables, including CDRSB, logical memory and TMT, distinguished pMCI from the general cohort but did not differentiate the two non-progressive groups. These findings suggest that pMCI and rMCI likely differ in their rates of cognitive decline, with pMCI typically exhibiting a more pronounced decrease in cognitive performance. Transitions from stability to progression are relatively common, whereas reversal is comparatively rare. Furthermore, although previous studies have identified risk factors including smoking history, alcohol consumption, obesity, hypertension, and a history of late-life depression—as influential factors in MCI progression (Durazzo et al., 2018; Marquié et al., 2023; Moody et al., 2021; W. Xu et al., 2017; Yu et al., 2024), our study did not observe significant differences. This discrepancy may be attributable to sample population, other unmeasured factors, the duration of follow-up, or the degree of variability (Johnson et al., 2021; Li et al., 2016), or associated with the degree of variability (Sundermann et al., 2017). Some risk factors may influence cognition differently depending on their intensity or duration, with effects emerging only beyond certain thresholds. Taking alcohol consumption as an example, studies have demonstrated that excessive drinking impairs cognitive function, whereas moderate intake is associated with better cognitive performance (W. Xu et al., 2017). Although obesity is recognized as a risk factor for dementia, elevated BMI has been suggested to confer metabolic reserve, potentially exerting a protective effect against MCI progression (Moody et al., 2021). Consequently, these factors may exhibit a non-linear relationship with MCI transition. Potential interactions among these factors may also exist. Previous studies have indicated that cognitive improvement requires the combined effects of multiple interventions, includes non-neurodegenerative factors (Ornish et al., 2024). Future investigations should adopt refined stratification approaches to disentangle the differential effects of distinct risk factor dimensions. Moreover, other potential factors, such as sleep disorders, were not considered in the analysis. Future research should therefore incorporate a broader set of factors to clarify their collective roles in MCI reversion.
Significant differences were observed across all brain regions; the pMCI group exhibited significantly larger ventricular volumes as well as reduced parenchymal volumes in the entorhinal cortex, medial temporal lobe, hippocampus, and fusiform gyrus compared to non-progressors, indicating distinct neurodegenerative patterns in pMCI. This finding is consistent with previous research demonstrating a close association between AD and brain atrophy (Li et al., 2016). However, neuroimaging data revealed that only hippocampal volume could differentiate all three groups, whereas other brain regions were effective solely in distinguishing progressive from non-progressive cases. Consistent with previous evidence demonstrating that preserved hippocampal volume predicts a higher likelihood of cognitive reversal (Dang et al., 2023), our findings confirmed this association. The hippocampus appears to be more vulnerable to cortical atrophy than other brain regions, and significant differences in cortical atrophy in non-hippocampal regions may only emerge during more advanced stages of the disease.
Our findings also suggest that caregivers are more perceptive of disease progression than patients themselves. Self-assessment tends to be biased, with both rMCI and pMCI individuals more likely to make conservative judgments about their condition, while caregivers are more sensitive to changes in assessment scores and better able to detect cognitive decline. These resluts align with prior research, which has consistently demonstrated an association between caregiver sensitivity and accurate detection of disease progression (Manjavong et al., 2024; Thomas et al., 2024). Studies have shown significant discrepancies between caregiver and patient assessments, particularly in evaluating social functioning, with caregivers often providing more accurate distinctions in severity levels (Rangel et al., 2025). Furthermore, the severity of patient symptoms is positively correlated with the burden experienced by caregivers (Rebolo et al., 2025). Future research and clinical practice should place greater emphasis on caregiver-reported assessments of patients’ functional and cognitive status. Allocating increased attention and resources to caregivers is crucial for enhancing patient quality of life, improving the predictability of disease progression, and safeguarding the psychological well-being of the caregivers themselves.
Nearly all participants exhibited significant associations between brain regions and cognitive measures in the partial correlation analyses, This finding is consistent with previous research highlighting the close relationship between brain structure and cognitive function (Zhang et al., 2021). Interestingly, in the subgroup analyses, a greater number of significant correlations were observed in the sMCI group. This may be partly explained by the larger sample size in this group. Alternatively, the relationship between brain volume changes and cognition may not follow a strictly linear trajectory, and potential inflection points could exist. Another possible explanation is that sMCI individuals possess more stable neuroanatomical features compared to the other two groups, whose brain regions may be undergoing more latent dynamic changes. This finding highlights a potentially overlooked aspect: sMCI may, in certain respects, more clearly reflect the underlying pathological characteristics of MCI. Future studies would benefit from employing segmented or stratified analytical approaches to further elucidate this phenomenon.
In the final model, factors significantly associated with cognitive improvement were educational attainment, daily functional ability, and hippocampal volume. In contrast, progression was influenced by gender, immediate recall ability, the number of APOE4 alleles, ADAS-cog score, and the volumes of the hippocampus and fusiform gyrus. These well-established risk factors for progression were confirmed in our study. The differences in predictive variables between the two models underscore the distinct mechanisms underlying progression and reversal.
Although gender did not differ significantly across groups, the model indicated that males are less likely to progress to pMCI, a finding partially supported by prior research (Altmann et al., 2014). It is noteworthy that previous studies have found the progression rates differ between males and females across various age ranges (Bun et al., 2023; Mian et al., 2024). This may be attributable to inherent biological factors, such as hormonal fluctuations, socio-cognitive differences between genders or interaction effects with other factors. The remaining variables were consistent with previous findings. Notably, age—typically regarded as a major risk factor for MCI progression (M. Wang et al., 2023)—did not emerge as a significant predictor in the model. This unexpected result may be explained by severa factors. First, age may exhibit collinearity with other variables (such as disease-related or protective factors). Second, the relatively short follow-up period and narrow age range of the cohort may have reduced the statistical power to detect age-related effects (Malek-Ahmadi, 2016).
This study also has several limitations that should be acknowledged. (1) The rMCI group had a small sample size, which may lead to some potential differences that are difficult to detect. Nevertheless, these cases were identified from a large-scale database, and their prevalence aligns with previously reported epidemiological estimates. Furthermore, we implemented stringent screening criteria for participants, ensuring that the reversed cognitive status remained stable throughout the follow-up period. Future studies should aim to increase the sample size of rMCI participants to improve statistical power. (2) The neuroimaging analysis was was restricted to volumetric comparisons of known brain region, without a comprehensive investigation of neuroimaging biomarkers. Future studies should place greater emphasis on exploring neuroimaging biomarkers, particularly structural changes in the hippocampal and peri-hippocampal. (3) Due to sample size limitations, while biomarkers can reveal the underlying pathological status of participants, no significant associations were found between the biomarkers and cognitive reversal status in this study. While our between-group analyses elucidated their role in disease progression, future studies are warranted to further investigate the reversion of it. This study utilized CSF biomarkers, future research could explore other types of biological markers. (4) Given the need to establish temporally precise predictors for targeted early intervention, this study adopted a 36-month follow-up period to optimally capture critical transition windows in disease progression. However, the follow-up remains relatively short, and longitudinal changes beyond this period were not assessed. Future studies should extend the follow-up duration to evaluate the long-term effects of various contributing factors. (5) Our study revealed that even among MCI participants with baseline memory impairment, memory continues to exhibit predictive value. However, due to the limitations of the ADNI dataset, we were unable to examine the role of other cognitive impairments in reversion. Future studies should prioritize targeted analyses based on aMCI and naMCI subtypes to further refine and validate these findings. (6) Cultural background is a meaningful variable that cannot be overlooked in studies of MCI progression, as it may influence symptom perception, diagnostic processes, or healthcare utilization. Due to limitations of the ADNI dataset—specifically the lack of direct cultural assessment indicators (e.g., cultural values, language-related factors)—we were unable to conduct in-depth analyses of cultural influences in our study. Future research should consider cross-cultural cohort studies that integrate culturally specific assessment tools to clarify the extent to which cultural factors modulate MCI progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bs15111552/s1, Table S1: Group differences in brain region volumes among the three MCI subtypes; Table S2: Group differences in biomarker characteristics among the three MCI subtypes; Table S3: Correlation between brain regions and cognitive measure.
Author Contributions
Conceptualization, J.G. and J.F.; methodology, formal analysis and writing—original draft preparation, J.G.; writing—review and editing, J.F., J.G., L.L. and Z.Y.; visualization, J.G.; supervision, J.F.; project administration, J.F.; study design and implementation of ADNI, data curation (data collection), ADNI. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Liaoning Province (2024-BS-160), the Education Department Project of Liaoning Province (LJ212410165004), the Ph.D. Start-up Project of Liaoning Normal University (2024BSL004) and the Scientific Research and Innovation Team of Liaoning Normal University.
Institutional Review Board Statement
All ADNI protocols were formally approved by the Institutional Review Boards (IRBs) at the participating institutions, ensuring adherence to ethical guidelines for data collection. We obtained permission from the ADNI before using the data and have complied with their requirements in using the data.
Informed Consent Statement
ADNI obtained informed consent from the subjects when they initially collected the data. Since our research is conducted in accordance with the protocols of ADNI, informed consent is not applicable to our study.
Data Availability Statement
All data are available upon reasonable request or can be obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).
Acknowledgments
Data collection and sharing for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) is funded by the National Institute on Aging (National Institutes of Health Grant U19AG024904). The grantee organization is the Northern California Institute for Research and Education. In the past, ADNI has also received funding from the National Institute of Biomedical Imaging and Bioengineering, the Canadian Institutes of Health Research, and private sector contributions through the Foundation for the National Institutes of Health (FNIH) including generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MCI | mild cognitive impairment |
| pMCI | progressive mild cognitive impairment |
| rMCI | reversible mild cognitive impairment |
| sMCI | stable mild cognitive impairment |
| APOE | apolipoprotein E |
| CDRSB | Clinical Dementia Rating Scale Sum of Boxes |
| ADAS | Alzheimer’s Disease Assessment Scale |
| MMSE | Mini-Mental State Examination |
| RAVLT_immediate | Rey Auditory Verbal Learning Test (Immediate Recall) |
| RAVLT_delay | Rey Auditory Verbal Learning Test Delayed Recall |
| LDELtotal | Logical Memory Delayed Recall Total |
| TMTB-A | Trail Making Test Part B Score- Trail Making Test Part A Score |
| FAQ | Functional Activities Questionnaire |
| CFT | Category Fluency (Animal Naming) Score. |
| EcogPt | Everyday Cognition Scale—Participant Version |
| EcogSP | Everyday Cognition Scale—Study Partner Version |
| EDU | Years of education |
| Entorhinal | entorhinal cortex |
| Fusiform | fusiform gyrus |
| MidTemp | middle temporal gyrus |
References
- Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. M., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. [Google Scholar] [CrossRef]
- Altmann, A., Tian, L., Henderson, V. W., Greicius, M. D., & Alzheimer’s Disease Neuroimaging Initiative Investigators. (2014). Sex modifies the APOE -related risk of developing Alzheimer disease. Annals of Neurology, 75(4), 563–573. [Google Scholar] [CrossRef]
- Berg, L. (1988). Clinical dementia rating (CDR). Psychopharmacology Bulletin, 24(4), 637–639. [Google Scholar] [PubMed]
- Bun, S., Suzuki, K., Niimura, H., Shikimoto, R., Kida, H., Shibata, M., Honda, T., Ohara, T., Hata, J., Nakaji, S., Maeda, T., Ono, K., Nakashima, K., Iga, J., Takebayashi, M., Ninomiya, T., Mimura, M., & the JPSC-AD Study Group. (2023). Gender and age influence the association between gait speed and mild cognitive impairment in community-dwelling Japanese older adults: From the Japan prospective studies collaboration for ageing and dementia (JPSC-AD). Psychogeriatrics, 23(6), 918–929. [Google Scholar] [CrossRef] [PubMed]
- Butters, N., Granholm, E., Salmon, D. P., Grant, I., & Wolfe, J. (1987). Episodic and semantic memory: A comparison of amnesic and demented patients. Journal of Clinical and Experimental Neuropsychology, 9(5), 479–497. [Google Scholar] [CrossRef]
- Caselli, R. J., Dueck, A. C., Osborne, D., Sabbagh, M. N., Connor, D. J., Ahern, G. L., Baxter, L. C., Rapcsak, S. Z., Shi, J., Woodruff, B. K., Locke, D. E. C., Snyder, C. H., Alexander, G. E., Rademakers, R., & Reiman, E. M. (2009). Longitudinal modeling of age-related memory decline and the APOE ε4 effect. New England Journal of Medicine, 361(3), 255–263. [Google Scholar] [CrossRef]
- Dang, M., Yang, C., Chen, K., Lu, P., Li, H., Zhang, Z., & for the Beijing Aging Brain Rejuvenation Initiative, for the Alzheimer’s Disease Neuroimaging Initiative. (2023). Hippocampus-centred grey matter covariance networks predict the development and reversion of mild cognitive impairment. Alzheimer’s Research & Therapy, 15(1), 27. [Google Scholar] [CrossRef]
- Durazzo, T. C., Meyerhoff, D. J., & Yoder, K. K. (2018). Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug and Alcohol Dependence, 192, 277–284. [Google Scholar] [CrossRef]
- Farias, S. T., Mungas, D., Reed, B. R., Cahn-Weiner, D., Jagust, W., Baynes, K., & Decarli, C. (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology, 22(4), 531–544. [Google Scholar] [CrossRef]
- Folstein, M. F., Folstein, S. E., & Mchugh, P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [Google Scholar] [CrossRef]
- Fox, N. C., Belder, C., Ballard, C., Kales, H. C., Mummery, C., Caramelli, P., Ciccarelli, O., Frederiksen, K. S., Gomez-Isla, T., Ismail, Z., Paquet, C., Petersen, R. C., Perneczky, R., Robinson, L., Sayin, O., & Frisoni, G. B. (2025). Treatment for Alzheimer’s disease. The Lancet, 406(10510), 1408–1423. [Google Scholar] [CrossRef]
- Grassi, M., Rouleaux, N., Caldirola, D., Loewenstein, D., Schruers, K., Perna, G., Dumontier, M., & Alzheimer’s Disease Neuroimaging Initiative. (2019). A novel ensemble-based machine learning algorithm to predict the conversion from mild cognitive impairment to Alzheimer’s disease using socio-demographic characteristics, clinical information, and neuropsychological measures. Frontiers in Neurology, 10, 756. [Google Scholar] [CrossRef]
- Hu, C., Yu, D., Sun, X., Zhang, M., Wang, L., & Qin, H. (2017). The prevalence and progression of mild cognitive impairment among clinic and community populations: A systematic review and meta-analysis. International Psychogeriatrics, 29(10), 1595–1608. [Google Scholar] [CrossRef]
- Iraniparast, M., Shi, Y., Wu, Y., Zeng, L., Maxwell, C. J., Kryscio, R. J., St John, P. D., SantaCruz, K. S., & Tyas, S. L. (2022). Cognitive reserve and mild cognitive impairment: Predictors and rates of reversion to intact cognition vs. progression to dementia. Neurology, 98(11), e1114–e1123. [Google Scholar] [CrossRef]
- Jack, C. R., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., Petersen, R. C., & Trojanowski, J. Q. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9(1), 119–128. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A. L., Nystrom, N. C., Piper, M. E., Cook, J., Norton, D. L., Zuelsdorff, M., Wyman, M. F., Flowers Benton, S., Lambrou, N. H., O’Hara, J., Chin, N. A., Asthana, S., Carlsson, C., & Gleason, C. E. (2021). Cigarette smoking status, cigarette exposure, and duration of abstinence predicting incident dementia and death: A multistate model approach. Journal of Alzheimer’s Disease, 80(3), 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Katayama, O., Lee, S., Bae, S., Makino, K., Shinkai, Y., Chiba, I., Harada, K., & Shimada, H. (2021). Lifestyle changes and outcomes of older adults with mild cognitive impairment: A 4-year longitudinal study. Archives of Gerontology and Geriatrics, 94, 104376. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, T. D., & Monsell, S. E. (2012). Reversion from mild cognitive impairment to normal or near-normal cognition: Risk factors and prognosis. Neurology, 79(15), 1591–1598. [Google Scholar] [CrossRef]
- Li, J.-Q., Tan, L., Wang, H.-F., Tan, M.-S., Tan, L., Xu, W., Zhao, Q.-F., Wang, J., Jiang, T., & Yu, J.-T. (2016). Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. Journal of Neurology, Neurosurgery & Psychiatry, 87(5), 476–484. [Google Scholar] [CrossRef]
- Lissek, V., & Suchan, B. (2021). Preventing dementia? Interventional approaches in mild cognitive impairment. Neuroscience & Biobehavioral Reviews, 122, 143–164. [Google Scholar] [CrossRef]
- Lopez, O. L., Becker, J. T., Chang, Y.-F., Sweet, R. A., DeKosky, S. T., Gach, M. H., Carmichael, O. T., McDade, E., & Kuller, L. H. (2012). Incidence of mild cognitive impairment in the Pittsburgh cardiovascular health study–cognition study. Neurology, 79(15), 1599–1606. [Google Scholar] [CrossRef]
- Luo, Y., Qiao, M., Liang, Y., Chen, C., Zeng, L., Wang, L., & Wu, W. (2022). Functional brain connectivity in mild cognitive impairment with sleep disorders: A study based on resting-state functional magnetic resonance imaging. Frontiers in Aging Neuroscience, 14, 812664. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M. (2016). Reversion from mild cognitive impairment to normal cognition: A meta-analysis. Alzheimer Disease & Associated Disorders, 30(4), 324–330. [Google Scholar] [CrossRef]
- Manjavong, M., Diaz, A., Ashford, M. T., Aaronson, A., Miller, M. J., Kang, J. M., Mackin, S., Tank, R., Landavazo, B., Truran, D., Farias, S. T., Weiner, M., & Nosheny, R. L. (2024). Performance of a short version of the everyday cognition scale (ecog-12) to detect cognitive impairment. The Journal of Prevention of Alzheimer’s Disease, 11(6), 1741–1750. [Google Scholar] [CrossRef]
- Marquié, M., García-Gutiérrez, F., Orellana, A., Montrreal, L., De Rojas, I., García-González, P., Puerta, R., Olivé, C., Cano, A., Hernández, I., Rosende-Roca, M., Vargas, L., Tartari, J. P., Esteban-De Antonio, E., Bojaryn, U., Ricciardi, M., Ariton, D. M., Pytel, V., Alegret, M., … Valero, S. (2023). The synergic effect of AT(N) profiles and depression on the risk of conversion to dementia in patients with mild cognitive impairment. International Journal of Molecular Sciences, 24(2), 1371. [Google Scholar] [CrossRef] [PubMed]
- Marra, C., Piccininni, C., Masone Iacobucci, G., Caprara, A., Gainotti, G., Costantini, E. M., Callea, A., Venneri, A., & Quaranta, D. (2021). Semantic memory as an early cognitive marker of Alzheimer’s disease: Role of category and phonological verbal fluency tasks. Journal of Alzheimer’s Disease, 81(2), 619–627. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G., Frohnhofen, H., Jokisch, M., Hermann, D. M., & Gronewold, J. (2024). Associations of sleep disorders with all-cause MCI/dementia and different types of dementia—Clinical evidence, potential pathomechanisms and treatment options: A narrative review. Frontiers in Neuroscience, 18, 1372326. [Google Scholar] [CrossRef]
- Mazzeo, S., Padiglioni, S., Bagnoli, S., Bracco, L., Nacmias, B., Sorbi, S., & Bessi, V. (2019). The dual role of cognitive reserve in subjective cognitive decline and mild cognitive impairment: A 7-year follow-up study. Journal of Neurology, 266(2), 487–497. [Google Scholar] [CrossRef]
- Mian, M., Tahiri, J., Eldin, R., Altabaa, M., Sehar, U., & Reddy, P. H. (2024). Overlooked cases of mild cognitive impairment: Implications to early Alzheimer’s disease. Ageing Research Reviews, 98, 102335. [Google Scholar] [CrossRef]
- Mitchell, A. J., & Shiri-Feshki, M. (2009). Rate of progression of mild cognitive impairment to dementia—Meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica, 119(4), 252–265. [Google Scholar] [CrossRef]
- Moody, J. N., Valerio, K. E., Hasselbach, A. N., Prieto, S., Logue, M. W., Hayes, S. M., Hayes, J. P., & Alzheimer’s Disease Neuroimaging Initiative (ADNI). (2021). Body mass index and polygenic risk for Alzheimer’s disease predict conversion to Alzheimer’s disease. The Journals of Gerontology: Series A, 76(8), 1415–1422. [Google Scholar] [CrossRef]
- Nakahata, N., Nakamura, T., Kawarabayashi, T., Seino, Y., Ichii, S., Ikeda, Y., Amari, M., Takatama, M., Murashita, K., Ihara, K., Itoh, K., Nakaji, S., & Shoji, M. (2021). Age-related cognitive decline and prevalence of mild cognitive impairment in the Iwaki health promotion project. Journal of Alzheimer’s Disease, 84(3), 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, A., Rolstad, S., Klang, O., Edman, A., Hansen, S., & Wallin, A. (2010). Two-year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. Journal of Neurology, Neurosurgery & Psychiatry, 81(5), 541–546. [Google Scholar] [CrossRef]
- Ornish, D., Madison, C., Kivipelto, M., Kemp, C., McCulloch, C. E., Galasko, D., Artz, J., Rentz, D., Lin, J., Norman, K., Ornish, A., Tranter, S., DeLamarter, N., Wingers, N., Richling, C., Kaddurah-Daouk, R., Knight, R., McDonald, D., Patel, L., … Arnold, S. E. (2024). Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: A randomized, controlled clinical trial. Alzheimer’s Research & Therapy, 16(1), 122. [Google Scholar] [CrossRef]
- Pandya, S. Y., Lacritz, L. H., Weiner, M. F., Deschner, M., & Woon, F. L. (2017). Predictors of reversion from mild cognitive impairment to normal cognition. Dementia and Geriatric Cognitive Disorders, 43(3–4), 204–214. [Google Scholar] [CrossRef]
- Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. [Google Scholar] [CrossRef]
- Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., Ritchie, K., Rossor, M., Thal, L., & Winblad, B. (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985. [Google Scholar] [CrossRef]
- Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H., Chance, J. M., & Filos, S. (1982). Measurement of functional activities in older adults in the community. Journal of Gerontology, 37(3), 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pink, A., Stokin, G. B., Bartley, M. M., Roberts, R. O., Sochor, O., Machulda, M. M., Krell-Roesch, J., Knopman, D. S., Acosta, J. I., Christianson, T. J., Pankratz, V. S., Mielke, M. M., Petersen, R. C., & Geda, Y. E. (2015). Neuropsychiatric symptoms, APOE ε4, and the risk of incident dementia: A population-based study. Neurology, 84(9), 935–943. [Google Scholar] [CrossRef]
- Pyun, J.-M., Park, Y. H., Lee, K.-J., Kim, S., Saykin, A. J., Nho, K., & for the Alzheimer’s Disease Neuroimaging Initiative. (2021). Predictability of polygenic risk score for progression to dementia and its interaction with APOE ε4 in mild cognitive impairment. Translational Neurodegeneration, 10(1), 32. [Google Scholar] [CrossRef]
- Qin, Y., Han, H., Li, Y., Cui, J., Jia, H., Ge, X., Ma, Y., Bai, W., Zhang, R., Chen, D., Yi, F., & Yu, H. (2023). Estimating bidirectional transitions and identifying predictors of mild cognitive impairment. Neurology, 100(3), e297–e307. [Google Scholar] [CrossRef]
- Rangel, R. C., Belfort, T. T., Brandt, M. M., Lima Nogueira, M., & Dourado, M. C. N. (2025). Emotional recognition: A comparative study of people with mild cognitive impairment and Alzheimer’s disease self-report with caregiver perspectives. Journal of Geriatric Psychiatry and Neurology, 38(6), 457–466. [Google Scholar] [CrossRef]
- Rebolo, M., Maroco, J., De Mendonça, A., & Melo, G. (2025). Caregiver burden in mild cognitive impairment due to Alzheimer’s disease—A longitudinal study. Psychogeriatrics, 25(3), e70033. [Google Scholar] [CrossRef]
- Reitan, R. M. (1958). Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills, 8(3), 271–276. [Google Scholar] [CrossRef]
- Rey, A. (1958). L’examen clinique en psychologie. Presses Universitaries De France. [Google Scholar]
- Rosen, W. G., Mohs, R. C., & Davis, K. L. (1984). A new rating scale for Alzheimer’s disease. The American Journal of Psychiatry, 141(11), 1356–1364. [Google Scholar]
- Scarabino, D., Broggio, E., Gambina, G., Maida, C., Gaudio, M. R., & Corbo, R. M. (2016). Apolipoprotein E genotypes and plasma levels in mild cognitive impairment conversion to Alzheimer’s disease: A follow-up study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(8), 1131–1138. [Google Scholar] [CrossRef]
- Serrano-Pozo, A., Das, S., & Hyman, B. T. (2021). APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. The Lancet Neurology, 20(1), 68–80. [Google Scholar] [CrossRef]
- Sha, F., Zhao, Z., Wei, C., & Li, B. (2022). Modifiable factors associated with reversion from mild cognitive impairment to cognitively normal status: A prospective cohort study. Journal of Alzheimer’s Disease, 86(4), 1897–1906. [Google Scholar] [CrossRef]
- Sheikh, J. (1986). Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. Clinical Gerontology A Guide to Assessment & Intervention, 5, 165–173. [Google Scholar] [CrossRef]
- Skirbekk, V., Bowen, C. E., Håberg, A., Jugessur, A., Engdahl, B., Bratsberg, B., Zotcheva, E., Selbæk, G., Kohler, H.-P., Weiss, J., Harris, J. R., Tom, S. E., Krokstad, S., Stern, Y., & Strand, B. H. (2023). Marital histories and associations with later-life dementia and mild cognitive impairment risk in the HUNT4 70+ study in Norway. Journal of Aging and Health, 35(7–8), 543–555. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, E. E., Katz, M. J., & Lipton, R. B. (2017). Sex differences in the relationship between depressive symptoms and risk of amnestic mild cognitive impairment. The American Journal of Geriatric Psychiatry, 25(1), 13–22. [Google Scholar] [CrossRef]
- Thomas, K. R., Bangen, K. J., Rotblatt, L. J., Weigand, A. J., Edwards, L., Tosun, D., Galasko, D., & for the Alzheimer’s Disease Neuroimaging Initiative. (2024). Self- and study partner–reported cognitive decline in older adults without dementia: The role of α-synuclein and amyloid biomarkers in the Alzheimer’s disease neuroimaging initiative. Alzheimer’s & Dementia, 20(11), 7777–7787. [Google Scholar] [CrossRef]
- Tucker-Drob, E. M. (2019). Cognitive aging and dementia: A life-span perspective. Annual Review of Developmental Psychology, 1(1), 177–196. [Google Scholar] [CrossRef]
- Wang, M., Sajobi, T. T., Hogan, D. B., Ganesh, A., Seitz, D. P., Chekouo, T., Forkert, N. D., Borrie, M. J., Camicioli, R., Hsiung, G. R., Masellis, M., Moorhouse, P., Tartaglia, M. C., Ismail, Z., & Smith, E. E. (2023). Expert elicitation of risk factors for progression to dementia in individuals with mild cognitive impairment. Alzheimer’s & Dementia, 19(10), 4542–4548. [Google Scholar] [CrossRef]
- Wang, W., Peng, J., Hou, J., Yuan, Z., Xie, W., Mao, G., Pan, Y., Shao, Y., & Shu, Z. (2023). Predicting mild cognitive impairment progression to Alzheimer’s disease based on machine learning analysis of cortical morphological features. Aging Clinical and Experimental Research, 35(8), 1721–1730. [Google Scholar] [CrossRef]
- Ward, D. D., Wallace, L. M. K., & Rockwood, K. (2020). Cumulative health deficits, APOE genotype, and risk for later-life mild cognitive impairment and dementia. Journal of Neurology, Neurosurgery & Psychiatry, 92, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. (1987). Wechsler memory scale-revised. Psychological Corporation. [Google Scholar]
- Wood, H. (2016). Meta-analysis finds high reversion rate from MCI to normal cognition. Nature Reviews Neurology, 12(4), 189. [Google Scholar] [CrossRef]
- Xu, H., Yang, R., Dintica, C., Qi, X., Song, R., Bennett, D. A., & Xu, W. (2020). Association of lifespan cognitive reserve indicator with the risk of mild cognitive impairment and its progression to dementia. Alzheimer’s & Dementia, 16(6), 873–882. [Google Scholar] [CrossRef]
- Xu, W., Wang, H., Wan, Y., Tan, C., Li, J., Tan, L., & Yu, J.-T. (2017). Alcohol consumption and dementia risk: A dose–response meta-analysis of prospective studies. European Journal of Epidemiology, 32(1), 31–42. [Google Scholar] [CrossRef]
- Xue, H., Hou, P., Li, Y., Mao, X., Wu, L., & Liu, Y. (2019). Factors for predicting reversion from mild cognitive impairment to normal cognition: A meta-analysis. International Journal of Geriatric Psychiatry, 34(10), 1361–1368. [Google Scholar] [CrossRef]
- Yang, Y., Chen, Y., Sang, F., Zhao, S., Wang, J., Li, X., Chen, C., Chen, K., & Zhang, Z. (2022). Successful or pathological cognitive aging? Converging into a “frontal preservation, temporal impairment (FPTI)” hypothesis. Science Bulletin, 67(22), 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H., Tan, C.-C., Huang, S.-J., Zhang, X.-H., Tan, L., & Xu, W. (2024). Predicting the reversion from mild cognitive impairment to normal cognition based on magnetic resonance imaging, clinical, and neuropsychological examinations. Journal of Affective Disorders, 353, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H., Tan, L., Jiao, M.-J., Lv, Y.-J., Zhang, X.-H., Tan, C.-C., Xu, W., & for the Alzheimer’s Disease Neuroimaging Initiative. (2025). Dissecting the clinical and pathological prognosis of MCI patients who reverted to normal cognition: A longitudinal study. BMC Medicine, 23(1), 260. [Google Scholar] [CrossRef]
- Zhang, J., Liu, Y., Lan, K., Huang, X., He, Y., Yang, F., Li, J., Hu, Q., Xu, J., & Yu, H. (2021). Gray matter atrophy in amnestic mild cognitive impairment: A voxel-based meta-analysis. Frontiers in Aging Neuroscience, 13, 627919. [Google Scholar] [CrossRef]
- Zhi, N., Ren, R., Qi, J., Liu, X., Yun, Z., Lin, S., Hu, Y., Li, H., Xie, X., Wang, J., Li, J., Zhu, Y., Gao, M., Yang, J., Wang, Y., Jing, Y., Geng, J., Cao, W., Xu, Q., … Wang, G. (2025). The China Alzheimer report 2025. General Psychiatry, 38(4), e102020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).