How Characterization of Particle Size Distribution Pre- and Post-Reaction Provides Mechanistic Insights into Mineral Carbonation †
Abstract
1. Introduction
2. Common, Complementary, and Alternate Particle Size Distribution Methodologies
2.1. Sieving Analysis
2.2. Laser Diffraction Analysis
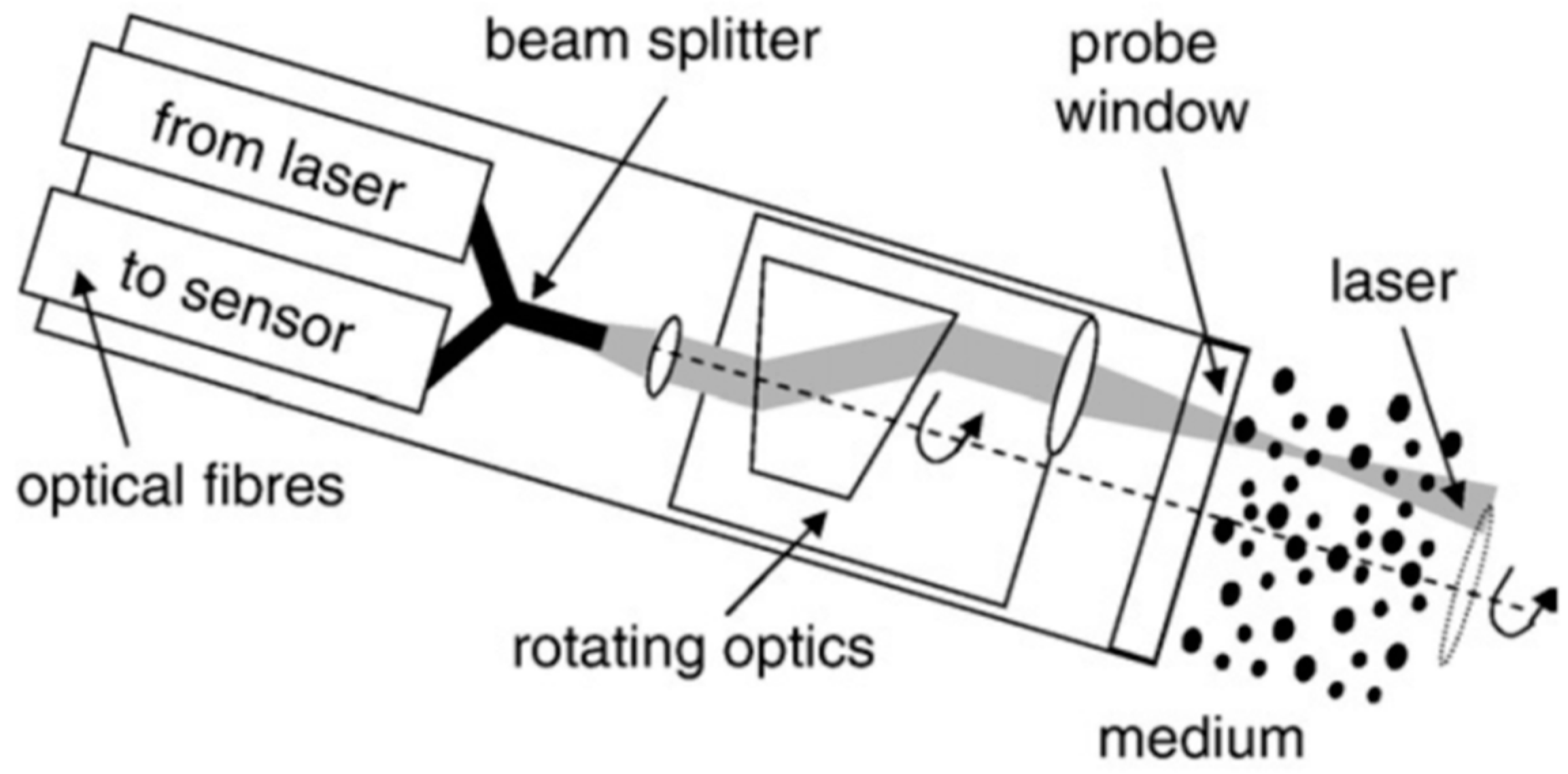
2.3. Complementary and Alternate Techniques (Laser Reflectance, Ultrasonic Extinction, Acoustic Spectrometry)
2.3.1. Laser Reflectance
2.3.2. Ultrasonic Extinction/Acoustic Spectrometry
3. Discussion of PSD Utilization in Mineral Carbonation Research
3.1. Use of Particle Size Distribution In Carbonation Research of Santos et al.
3.1.1. Ultrasound-Intensified Mineral Carbonation
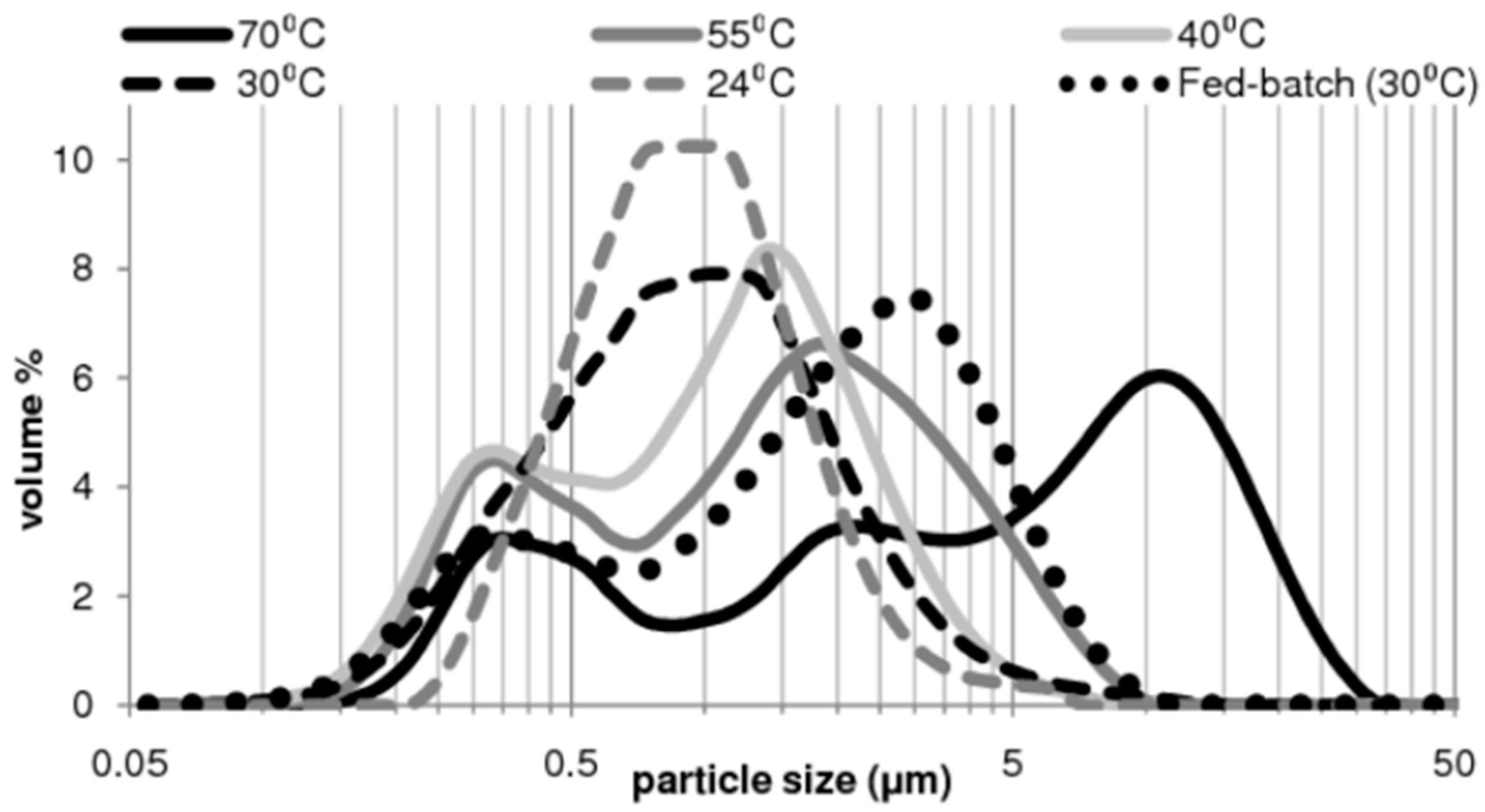
3.1.2. Synthesis of Pure Aragonite by Sonochemical Mineral Carbonation
3.1.3. Self-Regenerative Additive for the Mineral Carbonation of Calcium-Rich Materials
3.2. Use of Particle Size Distribution by Various Other Slag Carbonation Researchers
4. PSD Utilization in Mineral Carbonation Weathering and Conversion Models
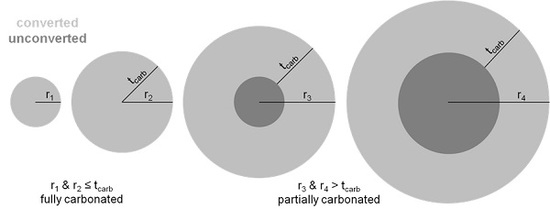
4.1. Carbonation Weathering Rate Model
4.2. Modeling Mineral Carbonation Conversion Based on PSD and CWR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bodor, M.; Santos, R.M.; Van Gerven, T.; Vlad, M. Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—A review. Cent. Eur. J. Eng. 2013, 3, 566–584. [Google Scholar] [CrossRef]
- De Crom, K.; Chiang, Y.W.; Van Gerven, T.; Santos, R.M. Purification of slag-derived leachate and selective carbonation for high-quality precipitated calcium carbonate synthesis. Chem. Eng. Res. Des. 2015, 104, 180–190. [Google Scholar] [CrossRef]
- Santos, R.M.; Knops, P.C.M.; Rijnsburger, K.L.; Chiang, Y.W. CO2 Energy Reactor—Integrated Mineral Carbonation: Perspectives on Lab-Scale Investigation and Products Valorization. Front. Energy Res. 2016, 4, 5. [Google Scholar] [CrossRef]
- Bodor, M.; Santos, R.M.; Cristea, G.; Salman, M.; Cizer, Ö.; Iacobescu, R.I.; Chiang, Y.W.; Van Balen, K.; Vlad, M.; Van Gerven, T. Laboratory investigation of carbonated BOF slag used as partial replacement of natural aggregate in cement mortars. Cem. Concr. Compos. 2016, 65, 55–66. [Google Scholar] [CrossRef]
- Santos, R.M.; Van Bouwel, J.; Vandevelde, E.; Mertens, G.; Elsen, J.; Van Gerven, T. Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: Effect of process parameters on geochemical properties. Int. J. Greenhouse Gas Control 2013, 17, 32–45. [Google Scholar] [CrossRef]
- Gopinath, S.; Mehra, A. Carbon sequestration during steel production: Modelling the dynamics of aqueous carbonation of steel slag. Chem. Eng. Res. Des. 2016, 115, 173–181. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Ling, T.-C.; Park, A.-H.A.; Chiang, P.-C. An Overview: Reaction Mechanisms and Modelling of CO2 Utilization via Mineralization. Aerosol Air Qual. Res. 2018, 18, 829–848. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Liu, H.-L.; Chang, E.-E.; Kim, H.; Chen, Y.-H.; Chiang, P.-C. Multiple model approach to evaluation of accelerated carbonation for steelmaking slag in a slurry reactor. Chemosphere 2016, 154, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Yu, H.; Yu, B.; Zhang, R.; French, D.; Grigore, M.; Wang, X.; Chen, Z.; Zhao, S. Insights into Carbonation Kinetics of Fly Ash from Victorian Lignite for CO2 Sequestration. Energy Fuels 2018, 32, 4569–4578. [Google Scholar] [CrossRef]
- Georgakopoulos, E.; Santos, R.M.; Chiang, Y.W.; Manovic, V. Influence of process parameters on carbonation rate and conversion of steelmaking slags – Introduction of the ‘carbonation weathering rate’. Greenh. Gases Sci. Technol. 2016, 6, 470–491. [Google Scholar] [CrossRef]
- Santos, R.M.; Van Gerven, T. Process intensification routes for mineral carbonation. Greenh. Gases Sci. Technol. 2011, 1, 287–293. [Google Scholar] [CrossRef]
- Żegleń, K.; Grygier, D.; Ambroziak, A.; Tulej, M. Particle size distribution determination methods comparison based on sieve analysis and laser method. Interdiscip. J. Eng. Sci. 2016, 4, 19–23. [Google Scholar]
- Eshel, G.; Levy, G.J.; Mingelgrin, U.; Singer, M.J. Critical evaluation of the use of laser diffraction for particle-size distribution analysis. Soil Sci. Soc. Am. J. 2004, 68, 736–743. [Google Scholar] [CrossRef]
- Di Stefano, C.; Ferro, V.; Mirabile, S. Comparison between grain-size analyses using laser diffraction and sedimentation methods. Biosyst. Eng. 2010, 106, 205–215. [Google Scholar] [CrossRef]
- Fisher, P.; Aumann, C.; Chia, K.; O’Halloran, N.; Chandra, S. Adequacy of laser diffraction for soil particle size analysis. PLoS ONE 2017, 12, e0176510. [Google Scholar] [CrossRef] [PubMed]
- Goossens, D. Techniques to measure grain-size distributions of loamy sediments: A comparative study of ten instruments for wet analysis. Sedimentology 2008, 55, 65–96. [Google Scholar] [CrossRef]
- Roberson, S.; Weltje, G.J. Inter-instrument comparison of particle-size analysers. Sedimentology 2014, 61, 1157–1174. [Google Scholar] [CrossRef]
- Worlitschek, J.; de Burh, J. Crystallization Studies with Focused Beam Reflectance Measurement and Multimax. Application Note, AutoChem, MultiMax; Mettler Toledo: Schwerzenbach, Switzerland, 2005; pp. 1–13. [Google Scholar]
- Kail, N.; Briesen, H.; Marquardt, W. Analysis of FBRM measurements by means of a 3D optical model. Powder Technol. 2008, 185, 211–222. [Google Scholar] [CrossRef]
- Geers, H.; Witt, W. Ultrasonic Extinction for in-line Measurement of Particle size and Concentration of Suspensions and Emulsions; Particulate Systems Analysis 2003: Harrogate, UK, 2003; pp. 1–5. [Google Scholar]
- DosRamos, J.G. Acoustic attenuation spectroscopy for process control of dispersed systems. IOP Conf. Ser. Mater. Sci. Eng. 2012, 42, 012023. [Google Scholar] [CrossRef]
- Santos, R.M.; François, D.; Mertens, G.; Elsen, J.; Van Gerven, T. Ultrasound-intensified mineral carbonation. Appl. Therm. Eng. 2013, 57, 154–163. [Google Scholar] [CrossRef]
- Santos, R.M.; Ceulemans, P.; Van Gerven, T. Synthesis of pure aragonite by sonochemical mineral carbonation. Chem. Eng. Res. Des. 2012, 90, 715–725. [Google Scholar] [CrossRef]
- Santos, R.M.; Bodor, M.; Dragomir, P.N.; Vraciu, A.G.; Vlad, M.; Van Gerven, T. Magnesium chloride as a leaching and aragonite-promoting self-regenerative additive for the mineral carbonation of calcium-rich materials. Miner. Eng. 2014, 59, 71–81. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J.; Witkamp, G.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chen, C.H.; Chen, Y.H.; Pan, S.Y.; Chiang, P.C. Performance evaluation for carbonation of steel-making slags in a slurry reactor. J. Hazard. Mater. 2011, 186, 558–564. [Google Scholar] [CrossRef] [PubMed]
- van Zomeren, A.; van der Laan, S.R.; Kobesen, H.B.A.; Huijgen, W.J.J.; Comans, R.N.J. Changes in mineralogical and leaching properties of converter steel slag resulting from accelerated carbonation at low CO2 pressure. Waste Manag. 2011, 31, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R.; Stramazzo, A.; Zingaretti, D. Accelerated Carbonation of Steel Slags Using CO2 Diluted Sources: CO2 Uptakes and Energy Requirements. Front. Energy Res. 2016, 3, 56. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chiu, A.C.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. Carbon sequestration via steel slag accelerated carbonation: The influence of operating conditions on process evolution and yield. In Proceedings of the 5th International Conference on Accelerated Carbonation for Environmental and Material Engineering (ACEME), New York, NY, USA, 21–24 January 2015; pp. 1–30. [Google Scholar]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Tai, C.I.; Su, C.Y.; Chen, Y.T.; Shih, S.M.; Chien, W.C. Carbonation of natural rock and steel slag using supercritical carbon dioxide. In Proceedings of the 11th European Meeting on Supercritical Fluids, Barcelona, Spain, 4–7 May 2008; pp. 1–6. [Google Scholar]
- Baciocchi, R.; Costa, G.; Di Bartolomeo, E.; Polettini, A.; Pomi, R. Wet versus slurry carbonation of EAF steel slag. Greenh. Gases Sci. Technol. 2011, 1, 312–319. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Di Bartolomeo, E.; Polettini, A.; Pomi, R. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valorization 2010, 1, 467–477. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Chu, H.W.; Wang, C.F.; Chiang, P.C. CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor. J. Hazard. Mater. 2011, 195, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cappai, G.; De Giudici, G.; Medas, D.; Muntoni, A.; Nieddu, A.; Orrù, G.; Piredda, M. Carbon dioxide sequestration by accelerated carbonation of Waelz slag. In Proceedings of the 5th International Conference on Accelerated Carbonation for Environmental and Material Engineering (ACEME), New York, NY, USA, 21–24 June 2015; pp. 1–34. [Google Scholar]
- Santos, R.M.; Ling, D.; Sarvaramini, A.; Guo, M.; Elsen, J.; Larachi, F.; Beaudoin, G.; Blanpain, B.; Van Gerven, T. Stabilization of basic oxygen furnace slag by hot-stage carbonation treatment. Chem. Eng. J. 2012, 203, 239–250. [Google Scholar] [CrossRef]
- Santos, R.M. Sustainable Materialization of Residues from Thermal Processes into Carbon Sinks. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2013. [Google Scholar]
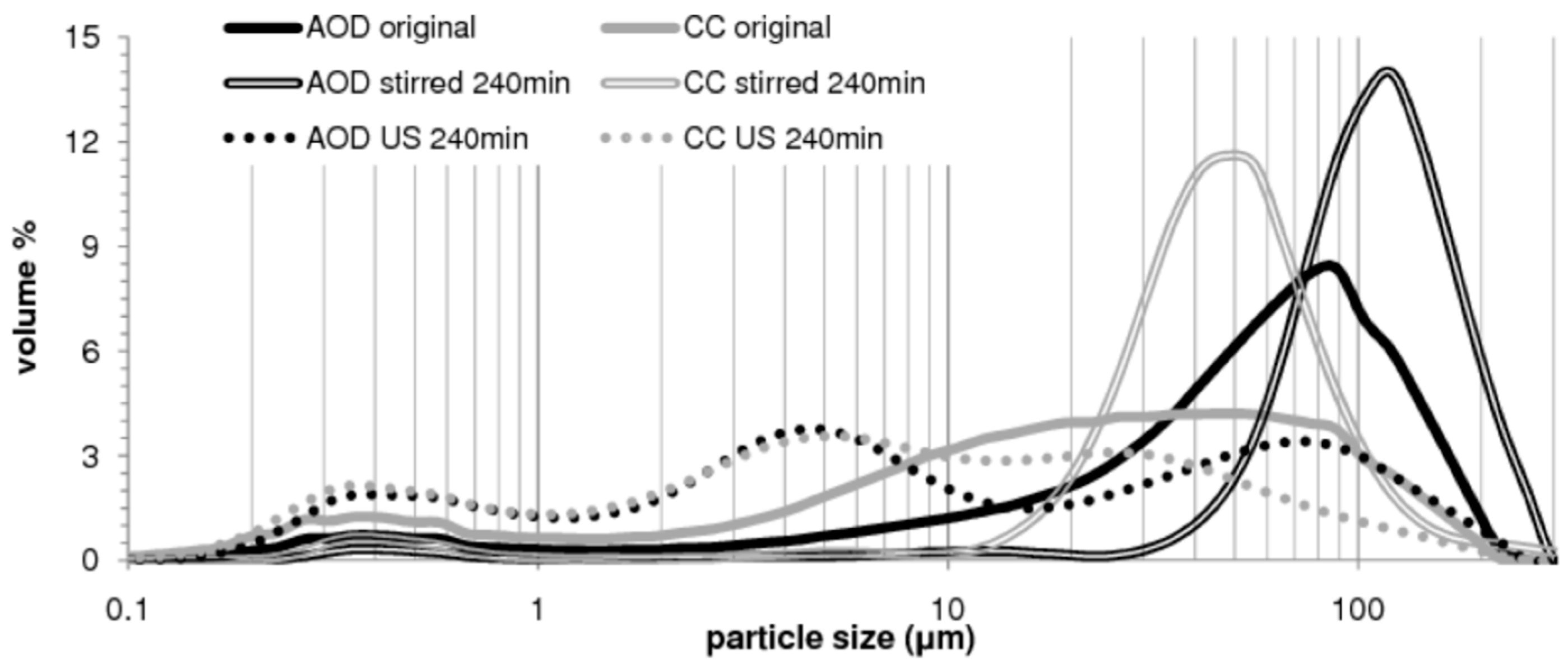


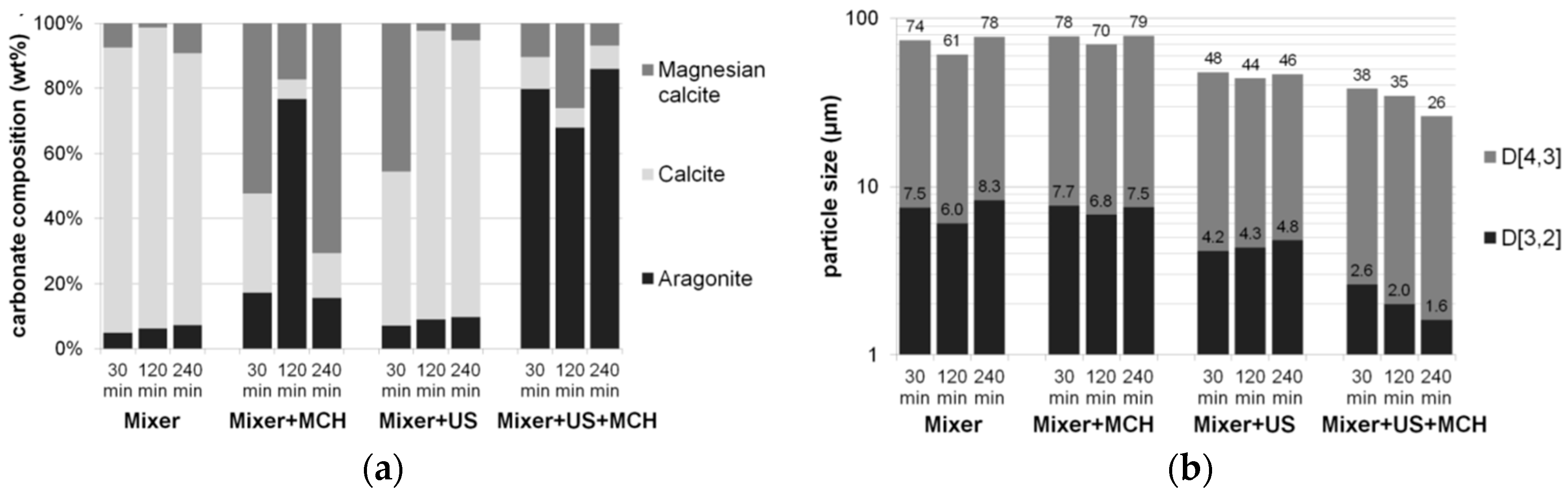

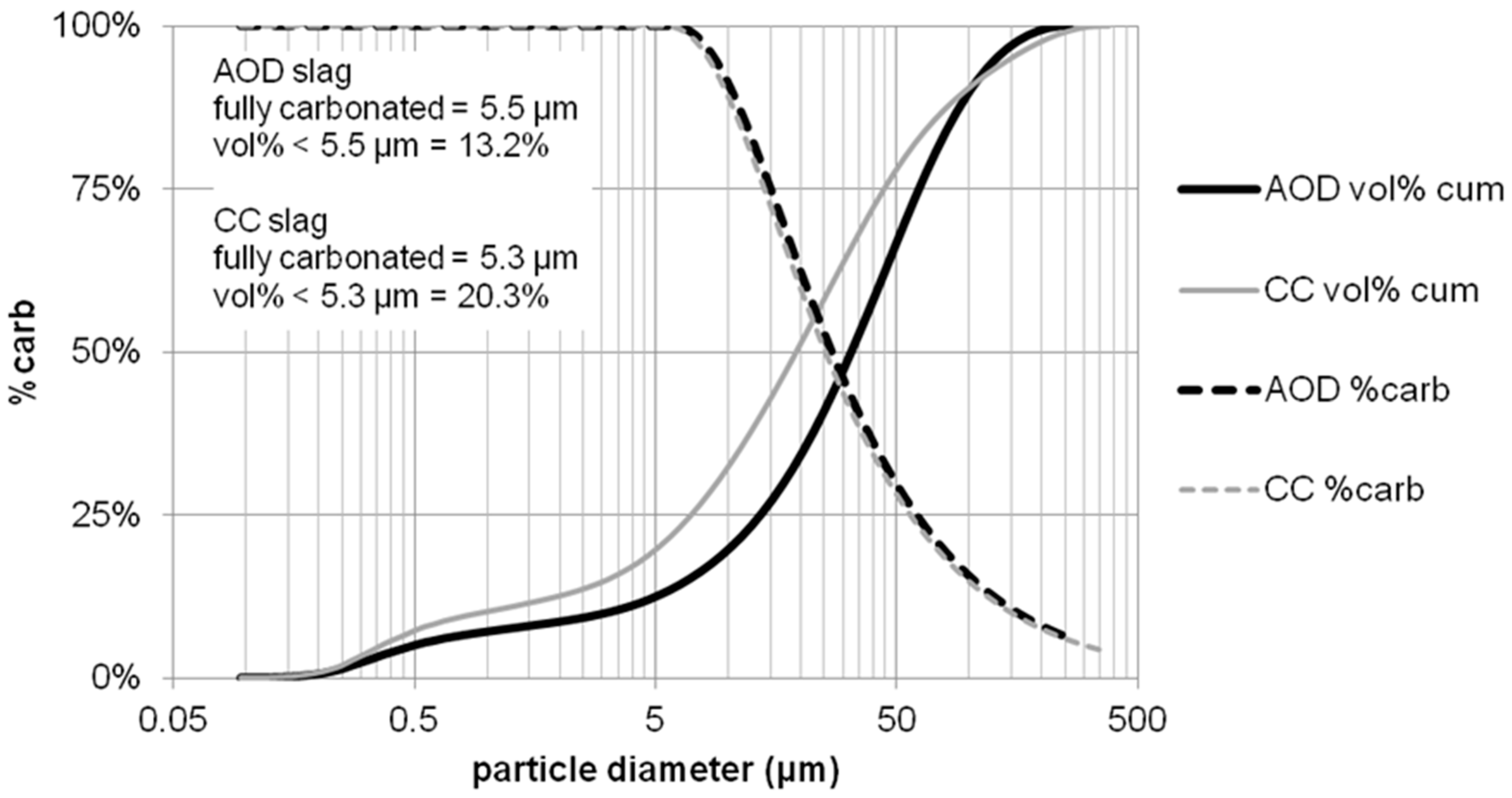
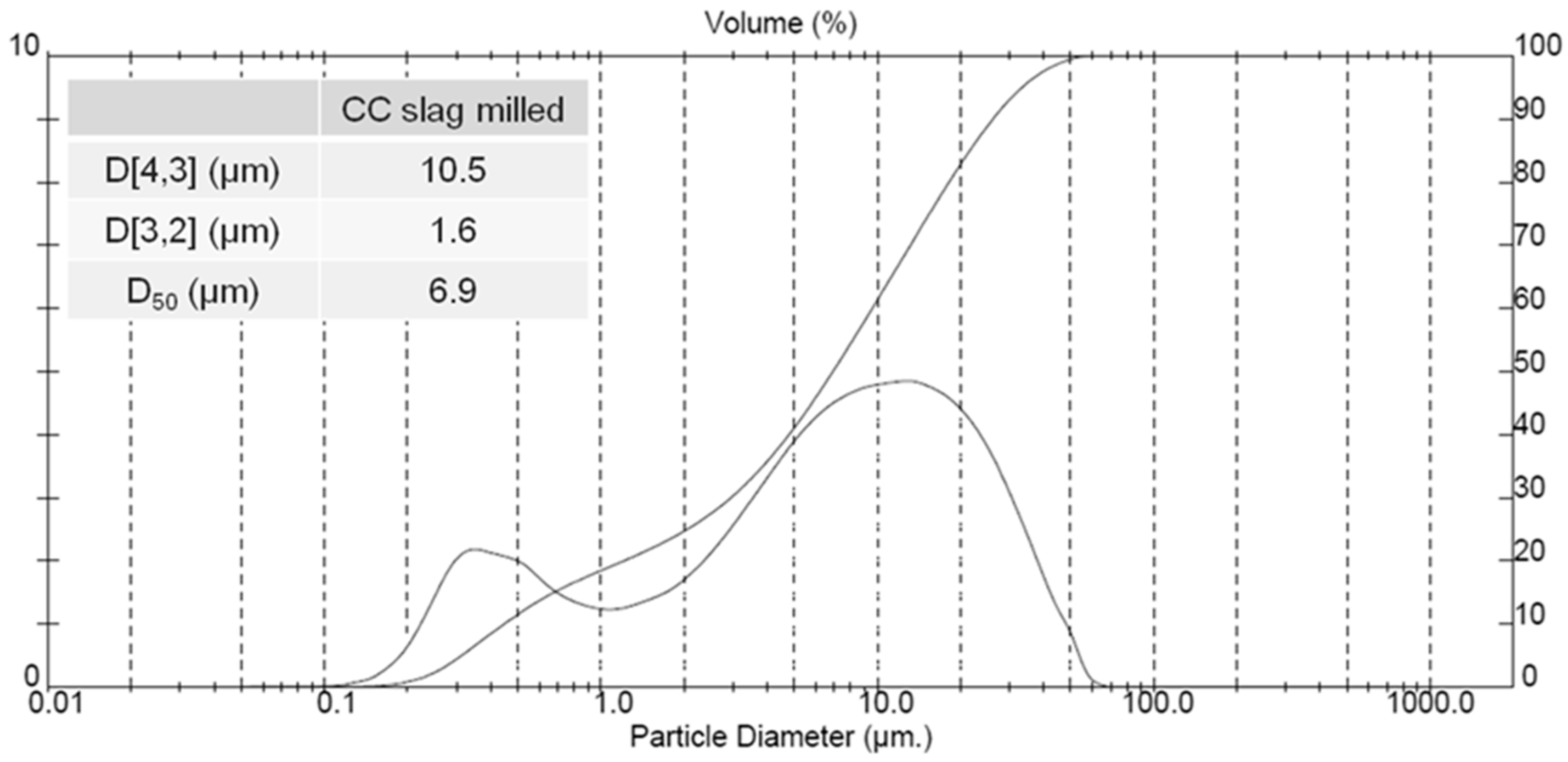
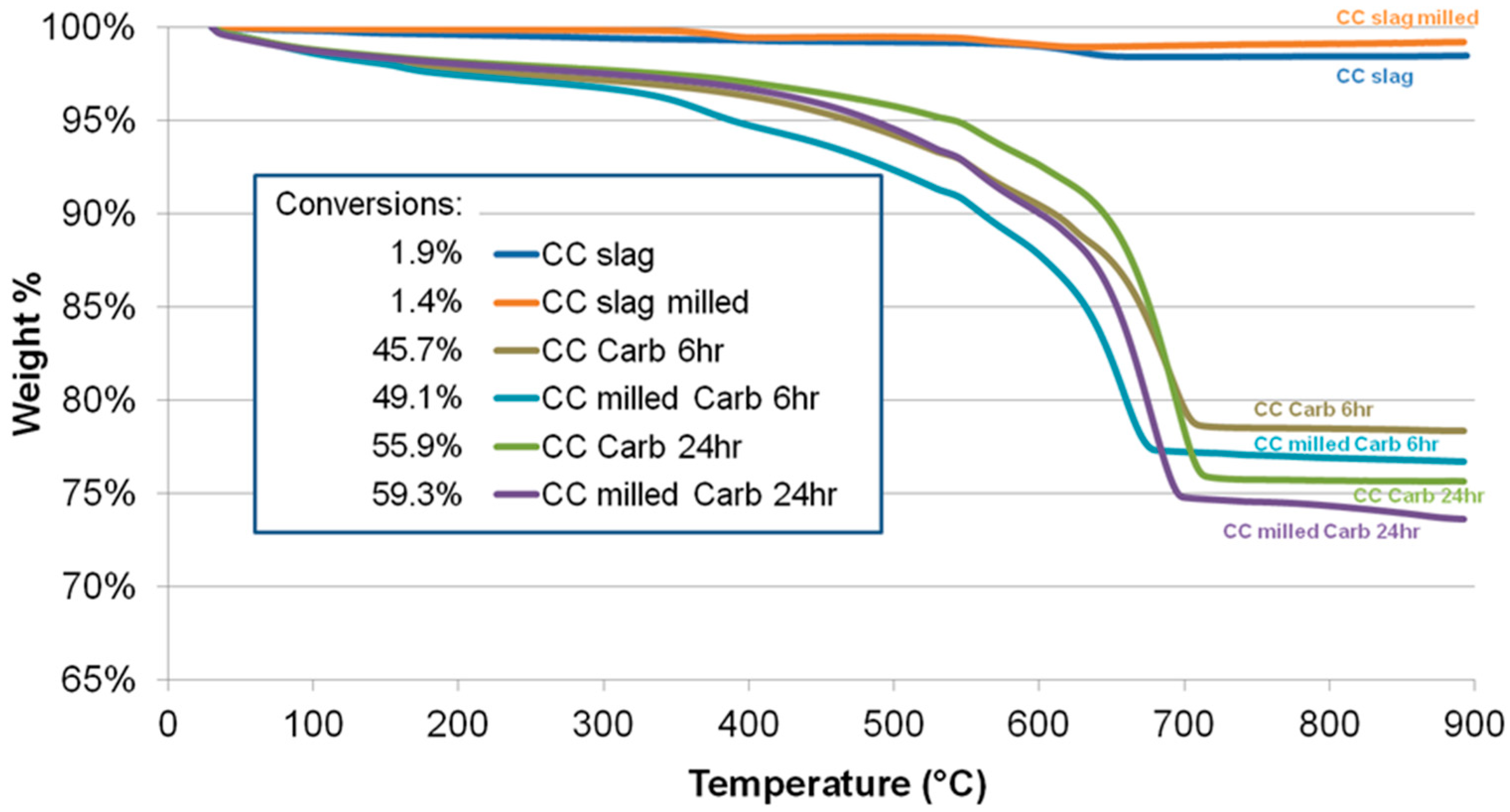
| Powder | Original D(3,2) (μm) | Sonicated D(3,2) (μm) | Reduction (%) |
|---|---|---|---|
| CaCO3 <200 μm | 5.6 | 4.8 | –14% |
| CaCO3 200–500 μm | 25.7 | 6.4 | –75% |
| Ca(OH)2 | 1.8 | 0.9 | –51% |
| CC slag <200 μm | 2.6 | 2.5 | –5% |
| Temperature | D50 | D(3,2) | D(4,3) | Aragonite |
|---|---|---|---|---|
| °C | μm | μm | μm | % |
| 70 °C | 4.15 | 1.31 | 6.48 | 99.5% |
| 55 °C | 1.33 | 0.78 | 1.87 | 98.7% |
| 40 °C | 0.98 | 0.65 | 1.27 | 98.6% |
| 30 °C | 0.85 | 0.69 | 1.23 | 99.3% |
| 24 °C | 0.84 | 0.79 | 1.09 | 98.6% |
| Fed-batch (30 °C) | 1.74 | 0.87 | 2.22 | 96.7% |
| Type of Slag | Method of Particle Size Determination | How Particle Size/PSD Data Was Used | |
|---|---|---|---|
| Huijgen et al. [25] | Basic Oxygen Furnace (BOF) | Sieving was used for particle size classification: ground slag was sieved into fractions: <38 µm, <106 µm, <200 µm, <500 µm, <2000 µm. Particle size distribution and volume-based mean particle size (D(4,3)) and d0.5 of samples were measured, pre-carbonation, by laser diffraction (Malvern Mastersizer 2000) using ethanol (96%) as a dispersing agent. | It was noticed from PSD that all sample fractions had wide size distribution and all included very small particles <1 µm. It was suggested that since slag particles are non-porous, the specific surface area can be estimated using the D(4,3) value, rather than performing BET analysis. Carbonation conversion was inversely proportional to particle size, and it was found that the D(4,3) value can be fitted to the conversion data to yield a predictive exponential relationship. |
| Chang et al. [26] | BOF | Sieving was used for particle size classification: ground slag was sieved, with only materials passing a 44 µm mesh being used. SEM imaging was used, post-carbonation, to visualize particle size and shape. | SEM imaging qualitatively showed that particles shrank after carbonation, and that cubic particles of approximate 1–2 µm size, thought to be calcium carbonate, adhered to the surface of the remnants of slag particles. This was used as a sign that shrinking core model, with a protective carbonate layer around reacting particles, can describe the slag carbonation mechanism. The particle size (i.e., sieve mesh size) was used in a kinetic modeling equation fitted to the experimental data. |
| van Zomeren et al. [27] | BOF | Sieving was used for particle size classification: slags were air-cooled, broken and sieved to obtain the particle fraction in the range of 2–3.3 mm. Samples of carbonated slags were sieved, to <106 µm, before TGA analysis. | The size range of the pre-carbonation sieved slags corresponded to the size desired for aggregates, the intended application of carbonated slags. No discussion is made relating the particle size to experimental results. |
| Baciocchi et al. [28] | BOF | Sieving was used for particle size classification: slag was either directly sieved to <125 µm, or ball-milled followed by sieving to <150 µm. | No discussion is made relating to the particle size. Particle size, before and after grinding, appears in an equation to calculate the energy requirement to sequester one tonne of CO2. |
| Chang et al. [29] | BOF | Sieving was used for particle size classification: dried slag was sieved to <88 µm. The particle size distribution of the slag, pre-carbonation, in tap water was obtained by laser diffraction (Malvern, Hydro 2000MU), which was adapted from the ISO 13320-1 method, with a range of 0.02–2000 µm. SEM imaging was used, post-carbonation, to visualize particle size and shape. | The value of the average particle size, determined from the PSD, was reported and compared to other studies. Particle size appears in an equation to model the reaction kinetics according to shrinking core model. SEM images showed small crystals of CaCO3 on the surface of the reacting slag particles. |
| Chang et al. [30] | BOF | Sieving was used for particle size classification: dried slag was sieved to <44 µm. The particle size distribution of the slag, pre-carbonation, in tap water was obtained by laser diffraction (Malvern, Hydro 2000 MU), which was adapted from the ISO 13320-1 method, with a range of 0.02–2000 µm. SEM imaging was used, post-carbonation, to visualize particle size and shape. | The value of the average particle size, determined from the PSD, was only reported. SEM images showed small crystals of CaCO3 on the surface of the reacting slag particles. |
| Polettini et al. [31] | BOF | The slag was separated through dry sieving into various size fractions, but only the 63−100 μm size class was investigated. | The choice of particle size fraction used was based on previous work, where the fraction chosen exhibited the highest carbonation yield. |
| Baciocchi et al. [32] | Stainless steel (SS) | Sieving was used for particle size classification: 0.425–2 mm (class A), 0.177–0.425 mm (class B), 0.105–0.177 mm (class C), < 0.105 mm (class D). Particle size distribution was determined, pre-carbonation, according to ASTM D422 standard procedure (withdrawn January 2016). The distribution of particle sizes larger than 75 μm (retained on the no. 200 sieve) was to be determined by sieving, while the distribution of particle sizes smaller than 75 μm was to be determined by a sedimentation process, using a hydrometer. | The particle size distribution curve indicated that the slag could be classified as sandy granular material. Loss on ignition (LOI) was performed on each size fraction, and it was found that finer fraction had greater LOI due to presence of hydroxide and carbonate species. Elemental composition was found to vary moderately depending on particle size, while pH only varied for the coarsest fraction, and mineralogy and leaching were similar for all fractions. Higher CO2 uptake was achieved with smaller particle size, and this was attributed to increased surface area (not measured). |
| Tai et al. [33] | BOF | It is not mentioned how the reported particle size of the slag, 63–90 μm, was determined nor how this size fraction was obtained. | The particle size is only reported, with no further discussion or justification. |
| Baciocchi et al. [34] | Electric arc furnace (EAF) | The EAF slag was milled in a corundum ball mill to a particle size below 150 μm; the method of classification is not specified. | Particle size of two previous studies is also mentioned, but there is no discussion regarding particle size. |
| Baciocchi et al. [35] | Argon Oxygen Decarburization (AOD) | The AOD slag, as received, as sieved to a particle size <150 μm, with 90% of the slag passing this mesh size. This study also used the same slag samples (SS and EAF) that were prepared by Baciocchi et al. [32] and by Baciocchi et al. [34]. | Particle size was related to the elemental composition (heavy metals) of the different slags and fractions, and to their initial carbonate content, which was higher in the finer materials. Greater CO2 uptake was found for finer SS slag fractions, and it was confirmed that milling of the coarsest SS fraction increases its CO2 uptake. Maximum calcium conversions were estimated for each sieved size fraction, being highest for the finest fraction. |
| Chang et al. [36] | Blast Furnace (BF) | It is not mentioned how the reported particle size of the slag, 44 μm, was determined nor how this size fraction was obtained. SEM imaging was used, post-carbonation, to visualize particle size and shape. | Similar comments to those made in Chang et al. [29,30] regarding SEM images are made. A comparative table shows particle size of other studies, but no discussion about particle size is made. |
| Cappai et al. [37] | Waelz | The slag was crushed to a final particle size below 4 mm; the method of size classification is not mentioned. | Low carbonation conversion was associated to the coarse particle size used and, in turn, the low surface area of the slag, which is concluded to not favor the dissolution kinetics of reactive species. It is suggested that a pre-treatment stage based on particle size reduction could contribute to an optimized process. |
| Type of Slag | rx (μm) | Conversion, C% (%) | tcarb (μm) | Reaction Time, τreact (min) | CWR (μm/min) | |
|---|---|---|---|---|---|---|
| Huijgen et al. [25] | BOF | 19 | 74 | 6.87 | 30 | 0.229 |
| Chang et al. [26] | BOF | 22 | 68 | 6.95 | 60 | 0.116 |
| van Zomeren et al. [27] | BOF | 1000–1650 | 4.67 | 16.95–27.97 | 3600 | 0.004–0.007 |
| Santos et al. [38] | BOF | 40 | 36 | 5.53 | 30 | 0.184 |
| Baciocchi et al. [28] | BOF(slurry) | 75 | 40 | 11.74 | 1440 | 0.008 |
| BOF(wet) | 62.5 | 20 | 4.48 | 1440 | 0.003 | |
| Chang et al. [29] | BOF | 31 | 93.5 | 18.54 | 30 | 0.618 |
| Chang et al. [30] | BOF | 22 | 89.4 | 11.59 | 120 | 0.097 |
| Polettini et al. [31] | BOF | 31.5–50 | 53.6 | 7.11–11.29 | 240 | 0.030–0.047 |
| Baciocchi et al. [32] | SS | 52.5 | 27.2 | 5.26 | 480 | 0.011 |
| Tai et al. [33] | BOF | 31.5–45 | 65 | 9.30–13.29 | 60 | 0.155–0.221 |
| Baciocchi et al. [34] | EAF(wet) | 75 | 34.3 | 9.80 | 1440 | 0.007 |
| EAF(slurry) | 75 | 25.4 | 7.00 | 240 | 0.029 | |
| Baciocchi et al. [35] | AOD | 75 | 69.9 | 50.73 | 1440 | 0.017 |
| Santos et al. [22] | AOD(mechanical) | 30–115 | 30.5 | 3.43–13.14 | 240 | 0.0143–0.0547 |
| CC(mechanical) | 30–115 | 61.6 | 8.20–31.41 | 240 | 0.0341–0.1309 | |
| AOD(sonication) | 30–115 | 48.5 | 5.95–22.82 | 240 | 0.025–0.095 | |
| CC(sonication) | 30–115 | 73.2 | 10.66–40.86 | 240 | 0.044–0.170 | |
| Santos et al. [5] | AOD(wet) | 23.05 | 24.2 | 2.03 | 8640 | 0.000235 |
| CC(wet) | 19.65 | 37 | 2.81 | 8640 | 0.000325 | |
| AOD(slurry) | 23.05 | 44 | 4.05 | 60 | 0.068 | |
| CC(slurry) | 19.65 | 57 | 4.82 | 60 | 0.080 | |
| Chang et al. [36] | BF | 22 | 68.3 | 7.00 | 720 | 0.010 |
| Cappai et al. [37] | Waelz | 2000 | 18.3 | 130.46 | 14400 | 0.009 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudhaiya, A.; Santos, R.M. How Characterization of Particle Size Distribution Pre- and Post-Reaction Provides Mechanistic Insights into Mineral Carbonation. Geosciences 2018, 8, 260. https://doi.org/10.3390/geosciences8070260
Dudhaiya A, Santos RM. How Characterization of Particle Size Distribution Pre- and Post-Reaction Provides Mechanistic Insights into Mineral Carbonation. Geosciences. 2018; 8(7):260. https://doi.org/10.3390/geosciences8070260
Chicago/Turabian StyleDudhaiya, Aashvi, and Rafael M. Santos. 2018. "How Characterization of Particle Size Distribution Pre- and Post-Reaction Provides Mechanistic Insights into Mineral Carbonation" Geosciences 8, no. 7: 260. https://doi.org/10.3390/geosciences8070260
APA StyleDudhaiya, A., & Santos, R. M. (2018). How Characterization of Particle Size Distribution Pre- and Post-Reaction Provides Mechanistic Insights into Mineral Carbonation. Geosciences, 8(7), 260. https://doi.org/10.3390/geosciences8070260