Abstract
Groundwater is a critical resource in the Upper Chao Phraya basin, providing consistent water supplies for agricultural, domestic, and industrial activities, especially during the dry season. This study utilized tritium and carbon-14 dating techniques to investigate groundwater age, analyzing 273 samples collected in 2021 from various wells and depths across the basin. Tritium and carbon-14 were measured using liquid scintillation counting (LSC). The results revealed a wide range of groundwater ages, including recently recharged water with tritium concentrations up to 2.4 TU, corresponding to groundwater ages ranging from a few months to 44.17 years BP (Before Present), with an average of 18.26 years BP. Older groundwater was identified with carbon-14 concentrations as low as 3.22 pMC, indicating ages of up to 22,899 years, with a mean age of 6687 years BP. Correlation analysis showed a positive relationship between tritium and carbon-14 concentrations (r = 0.52). Spatial distribution patterns indicated that tritium concentrations were higher in northern and mountainous areas, identifying these as critical recharge zones. In contrast, lower carbon-14 concentrations in the central and southern areas suggested the presence of older groundwater, emphasizing the need for careful management of these ancient water reserves. The spatial variation in tritium and carbon-14 concentrations highlights differences in groundwater circulation and recharge patterns, enabling the identification of key recharge zones in the northern and highland regions. This highlights the importance of conserving these areas from pollution and over-extraction. The presence of old groundwater in the central and southern areas further emphasizes the need for ongoing monitoring to sustainably manage these long-term water resources. This study enhances the understanding of groundwater dynamics in the Upper Chao Phraya basin and provides valuable insights for improving water resource management strategies.
1. Introduction
Groundwater plays a key role in serving all the water demands of humans and ecosystems, especially in areas where surface water sources are scarce or polluting. The Upper Chao Phraya River basin, located in northern Thailand, is a major hydrological area that sustains extensive agricultural practices and supplies water for domestic and industrial purposes [1,2,3]. The basin is characterized by its heterogeneous geology and complicated aquifer systems, which feature shallow and deep groundwater sources. Effectively managing these water resources is essential to ensuring a sustainable water supply in the region, particularly during the dry season when surface water availability is limited [1,4,5].
Previous studies have investigated the hydrochemical properties of groundwater in the Chao Phraya River basin [6,7], focusing on aspects such as source identification [6,7,8], distribution patterns [9,10,11], and quality assessment [12,13]. Stable isotope fingerprinting techniques were employed to assess the spatial and temporal distribution of rainfall and the interactions between surface water and groundwater in the region [14,15]. Their findings revealed that groundwater is primarily recharged by river water, contributing 54% of the groundwater, with the remaining 46% derived from precipitation. These works highlighted the importance of seasonal variations in precipitation, with distinct isotopic compositions observed between wet and dry seasons. Additional studies have further corroborated these findings, demonstrating that groundwater in the basin is heavily influenced by river water infiltration, particularly during the wet season, and that isotope hydrology is an essential tool for tracing groundwater recharge sources and understanding the dynamics of water flow in this region [5,13]. However, there remains a need for further research on the mechanisms controlling groundwater age and recharge patterns, particularly in the Upper Chao Phraya basin. Understanding these factors is essential for developing effective management strategies that ensure the long-term availability and quality of groundwater resources.
Several approaches have been employed to evaluate groundwater age in complex aquifers, including geophysical techniques [16,17], hydrochemical analysis [18], and radioisotope methods [19,20,21]. Among these, radioactive dating has proven to be a valuable tool for understanding hydrological processes such as groundwater recharge, mixing, and residence time [22,23,24,25,26]. It makes use of radiation that occurs naturally [27,28,29,30]. Tritium (H-3) and carbon-14 (C-14) are frequently employed for this objective [28,30,31,32]. Tritium, with a half-life of 12.32 years, is capable of reliably identifying the age of young groundwater, particularly up to 50 years old [32]. Nuclear bomb testing undertaken in the mid-twentieth century significantly increased the presence of this chemical [33]. This makes it a useful instrument for tracking water that has existed since the 1950s [34]. Carbon-14, which has a half-life of 5730 years, is used to date older groundwater up to about 50,000 years [35]. It is produced in the atmosphere when solar neutrinos absorb nitrogen-14. Wet deposition produces precipitation that seeps into the groundwater by dissolving carbonates and reacting with organic materials.
In recent years, studies utilizing tritium and carbon-14 for groundwater age dating have provided significant advancements in understanding groundwater dynamics across various geological settings, particularly those analogous to the Upper Chaophraya basin [29,30,31,36]. Research conducted in regions such as Romania, New Zealand, and Southern Africa demonstrates that the combined use of tritium and carbon-14 allows for a more comprehensive and accurate determination of groundwater recharge rates, the extent of mixing between different water bodies, and the delineation of modern versus ancient water sources within an aquifer. For instance, in Romania, the integration of carbon-14 and tritium data facilitated the identification of the contributions from modern precipitation to groundwater recharge while also accounting for the impact of historical mining activities on groundwater quality and flow patterns [29]. Similarly, studies in New Zealand have shown how tritium and carbon-14 data can be used to quantify the age gradients in exploited aquifers, revealing the extent of anthropogenic impacts on groundwater systems [31]. These studies highlight the importance of considering multiple isotopic tracers in heterogeneous aquifers, like those found in the Upper Chaophraya basin, where groundwater systems are often complex due to variations in geology, recharge conditions, and human activities.
This study utilizes tritium and carbon-14 dating techniques to investigate the groundwater age in the Upper Chao Phraya basin. Tritium, with a half-life of 12.32 years, is particularly useful for dating young groundwater (up to 50 years old), while carbon-14, with a half-life of 5730 years, is used to date older groundwater (up to 50,000 years). By examining the concentrations of these isotopes in groundwater samples, this research aims to answer the following questions: (1) What are the concentrations of tritium and carbon-14 in groundwater samples collected from the Upper Chao Phraya basin? (2) How do these concentrations relate to other variables, such as groundwater depth? (3) What insights can isotope data provide regarding the replenishment and residence time of groundwater in the research area? The outcomes of this study will enhance our understanding of groundwater dynamics in the Upper Chao Phraya basin and offer significant insights into regional water resource management. By identifying key recharge zones and understanding groundwater age distribution, this research will contribute to the development of sustainable groundwater management practices in the region.
2. Materials and Methods
2.1. Study Area and Hydrogeological Conditions
The Upper Chao Phraya basin is in northern Thailand. Figure 1a illustrates the basin, which covers an area of 103,833 square kilometers. The region experiences a tropical monsoon climate, characterized by distinct wet and dry seasons [37,38,39,40]. The average annual precipitation ranges from 1021 to 1275 mm (during 2014–2023), with the majority occurring during the monsoon season from May to October (Figure 1b). These precipitation fluctuations significantly influence river flow and groundwater recharge within the basin (Figure 1c). The basin is subdivided into four main sub-basins: Ping, Wang, Yom, and Nan, each with unique hydrological characteristics that impact the underlying aquifers [14].
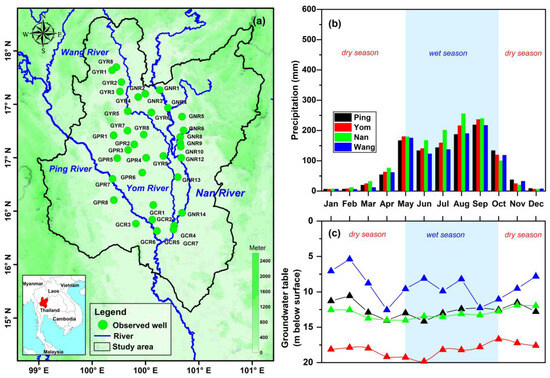
Figure 1.
(a) Location of the Upper Chao Phraya River Basin and isotope sampling sites (circles) (b) Mean monthly (2014–2023) rainfall (mm) over the Upper Chao Phraya River basin. (c) Mean monthly (2014–2023) groundwater table (m below surface) in the Upper Chao Phraya River basin.
The Ping Basin has a watershed area of 33,989 square kilometers and receives an average annual rainfall of 1021 mm. Groundwater levels in this basin typically range from 10 to 15 m below the surface. Table 1 clarifies that the Ping Basin has an average elevation of 1524 m above sea level (msl). The Wang Basin, covering 10,791 square kilometers, experiences an average annual rainfall of 1065 mm. Groundwater levels in this basin range from 8 to 15 m, and the average elevation is 1228 msl (with a range from 120 msl to 2004 msl). In the Yom Basin, which spans 24,089 square kilometers, the average annual rainfall is 1115 mm. Groundwater levels here range from 14 to 20 m deep, with an average elevation of 1134 msl (ranging from 14 msl to 1763 msl). The Nan Basin receives the highest average annual rainfall, at 1275 mm, and covers a watershed area of 34,964 square kilometers (Table 1). Groundwater levels in the Nan Basin range from 11 to 14 m, with an average elevation of 1500 msl (ranging from 14 msl to 2100 msl). These variations in groundwater levels, elevation, and precipitation have significant implications for water resource management in the basins.

Table 1.
Detailed information on the catchment characteristics.
In regions where groundwater is relatively close to the surface, typically around 10 m deep, there is a higher potential for groundwater recharge due to the shallow depth. This proximity allows for more efficient infiltration of rainwater and surface runoff, which is particularly beneficial in areas with intensive agricultural activity. Conversely, in areas where groundwater is deeper, around 20 m, the recharge process is slower, making these zones more susceptible to water scarcity during dry periods. Higher elevation areas, averaging 1524 m above sea level, typically experience cooler temperatures and potentially greater precipitation [39], which can contribute to increased recharge rates in these regions.
The hydrogeology of the Upper Chao Phraya basin is complex, consisting of several aquifer systems that vary in depth and composition (Figure 2). These aquifers are categorized into shallow and deep types based on their depth and characteristics [11]. The shallow aquifers are generally located at depths from the surface down to approximately 50 m. These aquifers are composed of alluvial deposits (Qfd) and young terraces (Qyt), primarily located in the central part of the basin [2]. These alluvial sediments, consisting of clay, sand, silt, and gravel, have a thickness of 5 to 10 m and are encountered at depths of 15 to 50 m. The pumping rates for these aquifers range from 3 to 8 cubic meters per hour. The young terraces (Qyt) consist of clay sequences interspersed with silts and very fine-grained sandstones. These terraces are 20 to 50 m thick. Due to the high clay content and the fine grain size of the sands, the pumping rates of these aquifers are limited to between 3 and 5 cubic meters per hour. Due to their shallow depth, these unconsolidated aquifers are highly vulnerable to pollution from surface activities [13].
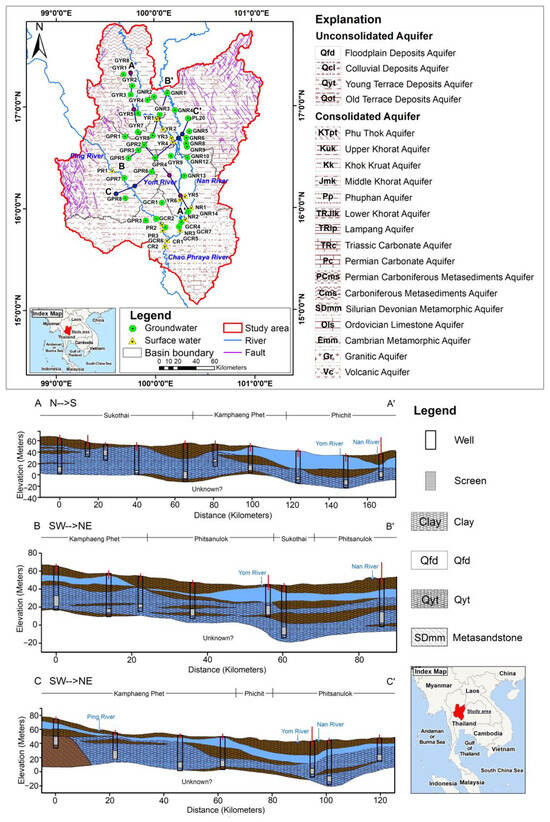
Figure 2.
Hydrogeological settings and geological cross sections in the Upper Chao Phraya River basin. Crossection A-A’ shows geological structure from north (N) to south (S). Crossection B-B’ and C-C’ show geological structure from southwest (SW) to northeast (NE).
The deep aquifers, composed of old terraces (Qot), are located at depths ranging from 50 to over 200 m. These aquifers are composed of more consolidated materials, such as sandstone and limestone, and are the most productive in the basin, with well pumping rates between 5 and 10 cubic meters per hour [7]. These deep groundwater sources are often confined.
2.2. Laboratory Analysis of Samples
Groundwater samples were collected from 214 wells in the Upper Chao Phraya basin, including both shallow and deep wells, during 2021 (Figure 1). Wells were selected to ensure representative coverage of varying depths and geographical locations. Groundwater was pumped at approximately 18 L/min using a submersible pump, and samples were taken after sufficient flushing of the wells, as determined by stable electrical conductivity (EC) readings. Samples were stored in 1 L high-density polyethylene (HDPE) bottles, which were pre-cleaned and rinsed with the sampled groundwater prior to collection. For C-14 analysis, samples were collected by adding excess BaCl2 to 120 L of water, pre-adjusted to a pH of ≥12 using CO2-free NaOH, resulting in the precipitation of BaCO3 within 1 L HDPE bottles. To prevent contamination, samples were filtered through 0.45-micron filters and refrigerated until analysis [41]. Detailed records of sampling dates, locations, and depths were meticulously maintained to ensure accurate data analysis.
Tritium analysis was performed using liquid scintillation counting (LSC, Model Quantulus 1220 from Perkin Elmer) following an electrolytic enrichment process [42]. Groundwater samples were distilled in the laboratory to concentrate the tritium. The distilled water was then mixed with a scintillation cocktail, and the samples were analyzed in the LSC to quantify tritium activity. Tritium concentrations were measured in tritium units (TU), where each TU corresponds to one tritium atom per 1018 hydrogen atoms. The method had a detection limit of approximately 0.1 TU, representing the smallest measurable concentration. Additionally, tritium data from rainfall observations at the Bangkok station, part of the Global Network of Isotopes in Precipitation (GNIP) campaign, provided critical reference values for determining recharge water concentration, which was then used in groundwater age calculations.
For carbon-14 (C-14) analysis, LSC was employed after the direct absorption (DA) method [43]. The initial phaseinvolved precipitating carbonate of groundwater sample. The groundwater carbonatewas then converted to carbondioxide (14CO2) and then using DA method for analysis via LSC to determine the C-14activity. Results were expressed as percent modern carbon (pMC), indicating the proportion of C-14 relative to a modern standard. The minimum detectable quantity of C-14 was approximately 2.0 pMC. Additionally, δ13C values of Total Dissolved Inorganic Carbon (TDIC) were measured in permil (‰) relative to the Pee Dee Belemnite (PDB) standard. These δ13C values serve as part of the correction method, helping to account for potential carbonate dissolution from rock formations or soil CO2. Accurate measurement of δ13C provides essential insights for refining groundwater age estimates and understanding geochemical processes within the aquifers.
2.3. Groundwater Dating Using Tritium and Carbon-14
2.3.1. Tritium (H-3) Dating
Tritium, a radioactive isotope of hydrogen with a half-life of approximately 12.32 years, is commonly used to determine the age of young groundwater, typically less than 50 years old (Table 2). Its presence in groundwater often indicates recent recharge, especially due to the significant fluctuations in tritium concentrations in precipitation over the past few decades, primarily driven by nuclear testing. By measuring the tritium concentration in groundwater samples, the residence time of the water can be estimated. In this study, tritium dating was applied to samples from shallow and intermediate aquifers (<50 m depth) using the following equation:
where groundwater age is expressed in years BP (Before Present), and λ is the decay constant (0.0563 year−1). The natural logarithm (ln) is dimensionless. A0 represents the initial tritium concentration in tritium units (TU). In this study, the initial tritium concentration (A0) in precipitation, obtained from the Global Network of Isotopes in Precipitation (GNIP), was 2.4 TU [44]. At is the measured tritium concentration in the groundwater sample, also expressed in TU, and was determined through laboratory analysis.

Table 2.
Tritium units (TU) ranges for groundwater age.
2.3.2. Carbon-14 (C-14) Dating
Carbon-14 is suitable for dating groundwater up to 50,000 years old due to its half-life of 5730 years [35]. Groundwater can acquire carbon-14 through the dissolution of atmospheric CO2 and soil carbonates (CO3). The decay of carbon-14 in groundwater allows for estimating the time that has passed since the water last interacted with the atmosphere [46]. However, accurate groundwater age calculations require corrections for the reservoir effect, which may be influenced by the dissolution of rock carbonates or soil CO2. This necessitates the inclusion of additional data, such as carbon-13 (C-13) isotope values and chemical analyses.
In this study, the corrected groundwater dating method was applied using the Pearson model [47], which relies on an isotopic mixing balance to determine A0, the initial carbon-14 concentration in the recharge area. The model considers the mixing of carbon derived from soil gas CO2 and dissolved calcite. The δ13C of TDIC (Total Dissolved Inorganic Carbon) was also measured. The δ13C value of soil gas CO2 was −23‰, while the δ13C of solid calcite was 2‰, attributable to recrystallization or the influence of organic matter. [47,48]. This correction method accounts for the influence of dissolved carbonates and ensures more accurate age estimations by adjusting the apparent carbon-14 activity in the groundwater. The Pearson model applies the following equations for correction:
where Ag and δg are the carbon-14 activity (100 pMC) and δ13C value (−23‰) of soil gas CO2, respectively. The As (0 pMC) and δs (2‰ of typical marine carbonate sources.) are the corresponding values for dissolved calcite, indicating that the carbon contribution from the dissolution of old calcite does not add any modern carbon-14 to the system. The model thus estimates A0 (pMC), the corrected initial carbon-14 concentration, based on the measured δ13C of TDIC, δt (‰).
Here, λ is the decay constant for carbon-14 (0.0001209 year−1). At is the current carbon-14 concentration (expressed as a percent of modern carbon, pMC) in the groundwater, and A0 is the corrected initial carbon-14 concentration (pMC) in the recharge area [47,49].
3. Results and Discussion
3.1. Tritium and Carbon-14 Concentrations in Groundwater
Groundwater samples were analyzed for tritium and carbon-14 concentrations and δ13C (Table A1). The mean tritium concentration was 1.08 tritium units (TU), with values ranging from 0.10 to 2.40 TU. Carbon-14 concentrations averaged 39.78 percent modern carbon (pMC), varying from 3.22 to 83.03 pMC. These isotopic values indicate a broad spectrum of groundwater ages, from recent recharge to water that has been isolated from atmospheric contact for thousands of years. The δ13C (‰ PDB) values from groundwater samples provided critical insights into the geochemical processes affecting carbon isotopic composition. These values ranged from −19.98‰ to −10.42‰, with an average of −16.24‰ ± 2.13‰ (Table A1), indicating variability in the sources of dissolved inorganic carbon (DIC) within the aquifers [27,36]. The most negative δ13C values, around −19.98‰, suggest contributions from biogenic carbon, typically associated with organic matter degradation. In contrast, less negative values, approaching −10.42‰, reflect the influence of carbonate dissolution, indicative of interactions with carbonate minerals present in the aquifers.
Several variables significantly influence the variation in tritium and carbon-14 concentrations across the region [30]. These factors include climatic conditions, geohydrological characteristics, and land use practices. The watershed experiences diverse rainfall patterns [38,39] (Figure 1b), which affect recharge rates (Figure 1c) and contribute to variable tritium levels. Furthermore, the complexity of the aquifer systems, which include deeper consolidated aquifers and shallower unconsolidated aquifers (Figure 2), contributes to the observed variability in isotopic concentrations. Agricultural activities, urbanization, and industrial processes also introduce contaminants that alter the concentrations of both isotopes, further contributing to this variability [13,49].
3.2. Tritium Concentration in Precipitation
Significant temporal variability was observed in the recorded tritium concentrations in rainfall at the Bangkok station between 1984 and 1991. An average concentration of 2.4 TU, with an average uncertainty of 0.3 TU, was observed during this period, with tritium levels ranging from 2.2 to 5.7 TU (Figure 3). The peak tritium concentration of 5.7 TU, recorded in June 1984, directly reflects the residual impact of historical atmospheric nuclear testing, primarily conducted during the mid-20th century [33]. These tests, particularly those from the 1950s and 1960s, introduced substantial amounts of tritium into the atmosphere [34]. Following these events, tritium was incorporated into the global hydrological cycle, resulting in elevated levels in precipitation and groundwater.
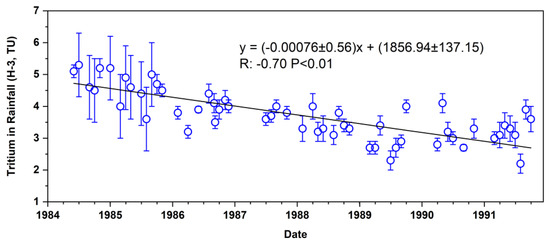
Figure 3.
Tritium concentrations in rainfall in Bangkok, Thailand.
The elevated tritium levels recorded in the mid-1980s, including the peak in 1984, represent the lingering effects of atmospheric contamination. Although large-scale atmospheric nuclear testing had ceased by this time, the tritium released in earlier decades remained in the atmosphere, continuing to be deposited via precipitation. The gradual decline in tritium concentrations observed from the late 1980s onwards can be attributed to two primary factors: the natural radioactive decay of tritium and the cessation of atmospheric nuclear testing. As tritium decayed, its levels in the atmosphere and hydrosphere diminished, leading to a corresponding reduction in tritium concentrations in precipitation and groundwater.
The initial tritium level of recharge water is determined by the tritium concentration in precipitation [32]. Based on the fitted trend line in Figure 3, the lowest tritium value observed (2.4 TU) was used as the initial tritium concentration of recharge water in this study. This baseline is critical for estimating the age of groundwater in the Upper Chao Phraya basin. Lower tritium levels in groundwater indicate ancient water that has undergone radioactive decay, while higher levels correspond to more recent recharge events [50].
3.3. Correlation Analysis
The relationship between tritium and carbon-14 concentrations in groundwater samples was explored through a plotted analysis (Figure 4a). The correlation coefficient (r = 0.52) indicates a moderately positive correlation, suggesting that tritium and carbon-14 exhibit certain shared behavioral patterns within the hydrological system, despite their distinct environmental origins and residence times. This correlation implies that some groundwater samples contain both tritium and carbon-14 in measurable quantities, indicating the presence of waters that have experienced a mix of recent and older recharge events [51]. Such a phenomenon can occur in aquifers where waters from different recharge periods mix or in areas with complex flow patterns that allow partial renewal of older groundwater with newer inputs. The positive correlation suggests that processes contributing to recent recharge also facilitate the incorporation of atmospheric carbon, likely through mechanisms such as precipitation infiltration, which introduces both tritium and carbon-14 into the groundwater system [52]. Therefore, using multiple isotopic tracers is critical to capturing the full range of recharge dynamics. Incorporating both tritium and carbon-14 data can offer a more comprehensive understanding of groundwater age distributions and flow patterns.
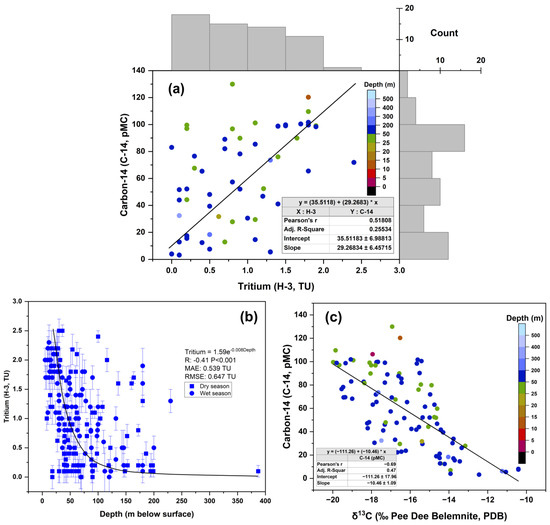
Figure 4.
Scatter plot of (a) tritium (TU) vs. carbon-14 concentrations (pMC) in the groundwater; (b) tritium vs. groundwater depth (m); (c) carbon-14 vs. δ13C (‰ PDB).
A negative correlation (r = −0.41) was observed between tritium concentrations and groundwater depth (Figure 4b). This relationship suggests that shallower groundwater tends to have higher tritium concentrations, indicating more recent recharge. In contrast, deeper groundwater exhibits lower tritium concentrations, reflecting reduced exposure to recent atmospheric inputs and indicating older water.
Additionally, Figure 4c shows a negative correlation (r = −0.69) between carbon-14 concentrations and δ13C values. This strong negative correlation suggests that as carbon-14 concentrations decrease, δ13C values become more positive. This relationship reflects geochemical processes within the aquifer. Groundwater with lower carbon-14 concentrations tends to show more positive δ13C values, indicating a greater influence of biogenic carbon sources, such as the decomposition of organic matter [53]. Conversely, groundwater with higher carbon-14 concentrations typically exhibits more negative δ13C values, suggesting a stronger influence of inorganic carbon sources, such as carbonate dissolution [28].
Similar correlations between tritium and carbon-14 concentrations have been documented in other studies [27,29,31]. Likewise, the negative correlation between isotopic concentrations and groundwater depth has been observed in various regions [28,30,50]. However, the strong correlation between carbon-14 and δ13C underscores the significance of site-specific geochemical conditions in influencing isotopic signatures. Different geological settings and carbon sources can substantially affect the retention, mixing, and movement of isotopes within an aquifer.
3.4. Spatial Distribution of Tritium and Carbon-14
The spatial distribution of carbon-14 and tritium concentrations in the Upper Chao Phraya River Basin was analyzed. As shown in Figure 5a, the northern and upland regions of the basin have experienced more recent recharge, with higher tritium concentrations observed in these areas. In contrast, lower carbon-14 concentrations were noted in the central and southern parts of the basin (Figure 5b), indicating a longer residence time for groundwater in these regions, where atmospheric carbon has been absent from the system for extended periods [49].
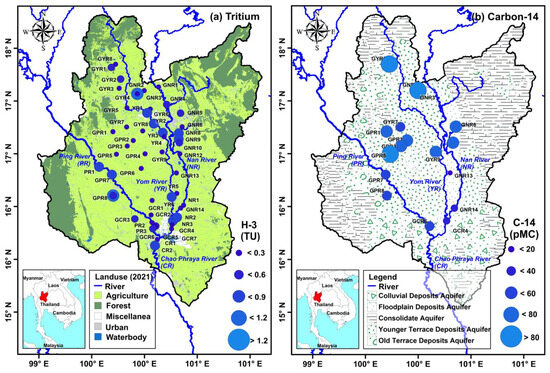
Figure 5.
Spatial distribution of tritium and carbon-14 concentrations.
The observed spatial patterns in tritium and carbon-14 distribution can be explained by the hydrogeological characteristics of the Upper Chao Phraya River Basin. The basin is primarily composed of alluvial deposits (Figure 5b), which facilitate the rapid recharge and movement of shallow groundwater. The presence of unconsolidated sediments allows for vertical percolation, explaining the higher tritium concentrations in shallow zones [54]. In deeper zones, however, vertical recharge is restricted by less permeable geological formations, resulting in lower tritium and carbon-14 levels, suggesting that groundwater in these areas is likely older [55].
The basin features diverse land use types (Figure 5a), including agriculture, urban development, and natural vegetation, each exerting distinct influences on groundwater quality and recharge [13]. Agricultural activities, particularly irrigation and fertilizer application, can enhance water infiltration into the soil, potentially increasing tritium levels in surrounding areas. In urban zones, where impervious surfaces dominate, recharge rates are typically lower, leading to isotopic signatures that differ from those in rural areas. Undisturbed natural vegetation zones offer valuable reference points for studying isotopic concentrations under natural recharge conditions [2,14].
Prioritizing the conservation of northern and upland areas in land use planning is essential for the long-term sustainability of groundwater resources in the Upper Chao Phraya River Basin. These regions are crucial to natural recharge processes due to their higher elevations, which typically receive more precipitation and contain geological formations that support vertical percolation. Consequently, these areas serve as primary recharge zones, where rainwater and surface runoff infiltrate the ground, replenishing aquifers that provide water for downstream use.
Maintaining the integrity of these recharge zones is critical to ensuring continuous and efficient aquifer replenishment. Land use planning should focus on conserving northern and upland areas to protect these vital zones from degradation caused by urbanization, deforestation, and agricultural expansion. Preserving natural vegetation and minimizing impervious surfaces, such as roads and buildings, can maximize water infiltration into the soil, thereby supporting the natural recharge processes that are essential for sustaining groundwater levels.
3.5. Groundwater Age and Recharge Patterns
Tritium concentrations in the Upper Chao Phraya River Basin indicate shallow groundwater ages, reflecting recent recharge events. A histogram (Figure 6a) shows groundwater ages for shallow depths (less than 50 m), ranging from a few months to 44.17 years BP (Before Present), with an average age of 18.26 years BP. A negative correlation between tritium levels and groundwater age was observed (Figure 6b), as expected given tritium’s half-life of 12.32 years [26].
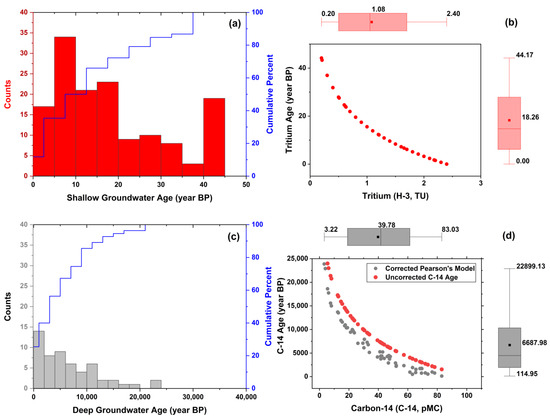
Figure 6.
Groundwater dating using (a,b) tritium and (c,d) carbon-14.
The corrected carbon-14 ages for deep groundwater (greater than 50 m) show significant variability. A histogram of deep groundwater ages (Figure 6c) reveals a range from 115 to 22,899 years BP, with a mean age of 6688 years BP and a standard deviation of 5797 years BP. This wide distribution suggests complex recharge histories and prolonged residence times for deep groundwater. Many deep groundwater samples fall between 5000 and 15,000 years BP, indicating long periods of isolation from atmospheric carbon, consistent with the basin’s hydrogeological features (Figure 2). The carbon-14 content is inversely correlated with groundwater age (Figure 6d), with younger samples having higher carbon-14 levels and older samples showing significant decay over time [46]. This relationship validates carbon-14 as a tracer for long-term groundwater dating. The distribution of carbon-14 is influenced by geological and geochemical factors in the basin, where lower permeability at greater depths limits recharge, leading to older groundwater.
Corrected carbon-14 ages, calculated using the Pearson model, differ significantly from uncorrected values (Figure 6d). These differences highlight the importance of accounting for local geochemical conditions in isotopic age estimations. Groundwater’s dissolved inorganic carbon, sourced from the atmosphere, gradually decreases in carbon-14 concentration as it moves through subsurface environments due to radioactive decay [31]. Geochemical processes, such as carbonate dissolution and precipitation, further alter carbon-14 levels, affecting the accuracy of age determinations [11,46]. Interactions with carbonate-rich formations can introduce additional carbon-14, leading to artificially high concentrations and skewed age estimates, suggesting more recent recharge than occurred. Conversely, carbonate precipitation can reduce carbon-14 levels, giving the appearance of older groundwater than is accurate. The variability in environmental conditions—such as pH, temperature, and carbonate solubility—complicates groundwater age estimations based solely on carbon-14 concentrations.
Table 3 illustrates the progression of groundwater ages with depth in the Upper Chao Phraya River Basin. Groundwater at depths of 50 to 100 m has an average age of 2515 years BP. This age increases to 5854 years BP at depths of 100 to 150 m and rises to 8121 years BP at depths of 150 to 200 m. At depths of 200 to 250 m, the average groundwater age reaches 9542 years BP, and at depths of 250 to 300 m, it can reach up to 16,513 years BP. This trend highlights the impact of depth on groundwater residence time and the limited influence of recent recharge at greater depths. The geochemical evolution of groundwater is driven by processes such as mineral dissolution, ion exchange, and radioactive decay, which alter water composition over time [26,28].

Table 3.
Groundwater age variability in the Upper Chao Phraya River Basin.
Radiocarbon dating in groundwater systems is complex, especially in aquifers with intricate hydrogeological conditions. One challenge is the mixing of waters with different carbon-14 levels, leading to “apparent ages” that do not represent the true recharge history. Mixing older groundwater with low carbon-14 levels and younger water with higher concentrations can result in intermediate ages that obscure the actual dynamics of the aquifer. Variations in initial carbon-14 activity, due to changes in atmospheric carbon or localized geochemical processes, further complicate age determinations [46]. These variations create different baseline concentrations, making it difficult to establish a consistent reference for age estimates across the aquifer.
4. Conclusions
The study identified tritium concentrations ranging from 0.10 to 2.40 TU, with a mean of 1.08 TU, and carbon-14 concentrations ranging from 3.22 to 83.03 pMC, with an average of 39.78 pMC. These values suggest a wide range of groundwater ages, from recent recharge to water that has been isolated from atmospheric carbon for thousands of years. A positive correlation (R = 0.52) was observed between tritium and carbon-14 concentrations, indicating a relationship influenced by various factors, including well depth and geological properties. Tritium concentrations point to a shallow groundwater age distribution in the basin, with ages ranging from a few months to 44.17 years BP (Before Present) and an average of 18.26 years BP, indicating recent recharge, particularly in the northern and upland areas where tritium levels are higher. The corrected carbon-14 age using Pearson’s Model for deep groundwater (greater than 50 m) shows significant variability, with ages ranging from 114.95 years BP to 22,899 years BP and a mean age of 6688 years BP. A significant portion of the deep groundwater, with ages ranging from 5000 to 15,000 years, indicates long-term isolation from atmospheric carbon, consistent with the region’s hydrogeological characteristics.
Tritium and carbon-14 tracers effectively identify key recharge zones and assess long-term water reserves, providing critical insights into groundwater age and flow patterns. However, the dynamic nature of groundwater systems, driven by climate variability and human activities, necessitates continuous monitoring. Ongoing isotope tracing is essential for detecting changes and guiding management decisions. Strategies such as protected recharge areas, sustainable land use incentives, and public awareness campaigns can further enhance groundwater conservation and ensure long-term resource sustainability.
Author Contributions
Conceptualization, J.L. and P.J.; methodology, J.L., K.K. and P.J.; software, K.K., M.Y. and C.P.; validation, J.L., C.P., C.S. and P.J.; formal analysis, J.L., K.K. and P.C. (Patchareeya Chanruang); investigation, J.L., M.Y., C.P., C.S. and K.K.; resources, J.L., P.J., C.S. and K.K.; data curation, M.Y., C.P., P.C. (Patchareeya Chanruang), C.S., N.U. and K.K.; original manuscript writing, J.L. and K.K.; manuscript review and editing, J.L., M.Y., C.P., C.S. and K.K.; visualization, P.C. (Peerapat Charoonchat), P.C. (Patchareeya Chanruang) and N.U.; supervision, P.J.; project administration, P.C. (Peerapat Charoonchat), M.Y. and C.P.; funding acquisition, J.L., K.K. and P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was mainly funded by the Thailand Institute of Nuclear Technology (Public Organization) through the Thailand Science Research and Innovation (TSRI) Fund (Project Research Code: 181061) and partially supported by the Global and Frontier Research Fund, Naresuan University (grant R2567C001).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the Thailand Institute of Nuclear Technology (public organization) and the Department of Groundwater for supporting the supplementary data and their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Supplementary data for groundwater dating using tritium and carbon-14.
Table A1.
Supplementary data for groundwater dating using tritium and carbon-14.
| No | Easting | Northing | Sampling Date | Developing Depth (m) | H-3 (TU) | Stdev H-3 | C-14 (pMC) | Stdev C-14 | δ13C (‰ PDB) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 602295 | 1839904 | 4-Jan-2021 | 14 | 1.4 | 0.2 | |||
| 2 | 600407 | 1842006 | 4-Jan-2021 | 50 | 0.9 | 0.2 | |||
| 3 | 616923 | 1851267 | 4-Jan-2021 | 30 | 0.1 | 0.2 | |||
| 4 | 613255 | 1851023 | 4-Jan-2021 | 67 | 0.2 | 0.2 | |||
| 5 | 608606 | 1848613 | 4-Jan-2021 | 30 | 0.1 | 0.2 | |||
| 6 | 606620 | 1843865 | 4-Jan-2021 | 15 | 1.2 | 0.2 | |||
| 7 | 605476 | 1847506 | 4-Jan-2021 | 40 | 0.9 | 0.2 | 89.84 | 1.30 | −18.05 |
| 8 | 601125 | 1851004 | 4-Jan-2021 | 59 | 0.4 | 0.3 | 12.39 | 1.33 | −14.22 |
| 9 | 605204 | 1850300 | 5-Jan-2021 | 56 | 0.4 | 0.3 | |||
| 10 | 609197 | 1850982 | 5-Jan-2021 | 52 | 0.1 | 0.1 | |||
| 11 | 616346 | 1855177 | 5-Jan-2021 | 30 | 1.1 | 0.2 | 101.18 | 1.06 | −18.94 |
| 12 | 614400 | 1857277 | 5-Jan-2021 | 76 | 0.3 | 0.2 | |||
| 13 | 615984 | 1856135 | 5-Jan-2021 | 25 | 1.8 | 0.3 | |||
| 14 | 619555 | 1853641 | 8-Jan-2021 | 23 | 1.9 | 0.3 | |||
| 15 | 611270 | 1855997 | 8-Jan-2021 | 80 | 0.7 | 0.3 | |||
| 16 | 608074 | 1856158 | 8-Jan-2021 | 42 | 0.3 | 0.4 | 67.58 | 0.96 | −16.76 |
| 17 | 602538 | 1855320 | 8-Jan-2021 | 60 | 0.4 | 0.2 | |||
| 18 | 603576 | 1859119 | 8-Jan-2021 | 90 | 0.0 | 0.3 | |||
| 19 | 606887 | 1858930 | 9-Jan-2021 | 18 | 1.2 | 0.3 | |||
| 20 | 610687 | 1858179 | 9-Jan-2021 | 18 | 0.0 | 0.2 | |||
| 21 | 623533 | 1846509 | 9-Jan-2021 | 18 | 1.8 | 0.2 | |||
| 22 | 609873 | 1841337 | 9-Jan-2021 | 40 | 0.4 | 0.3 | |||
| 23 | 609873 | 1841337 | 9-Jan-2021 | 51 | 1.8 | 0.4 | |||
| 24 | 602317 | 1851835 | 9-Jan-2021 | 70 | 0.0 | 0.3 | |||
| 25 | 620689 | 1845633 | 9-Jan-2021 | 8 | 1.0 | 0.2 | |||
| 26 | 617375 | 1850958 | 8-Jan-2021 | 70 | 101.92 | 1.99 | −19.07 | ||
| 27 | 612589 | 1843856 | 8-Jan-2021 | 30 | 96.87 | 1.77 | −18.01 | ||
| 28 | 494565 | 2090158 | 17-Mar-2021 | 124 | 23.70 | 4.10 | −16.23 | ||
| 29 | 494565 | 2090158 | 18-Mar-2021 | 387 | 0.1 | 0.1 | 32.40 | 4.20 | −17.49 |
| 30 | 502177 | 2086173 | 19-Mar-2021 | 58 | 0.5 | 0.2 | |||
| 31 | 506367 | 2083422 | 20-Mar-2021 | 90 | 0.1 | 0.2 | |||
| 32 | 513656 | 2077663 | 19-Mar-2021 | 103 | 33.90 | 4.50 | −13.77 | ||
| 33 | 513656 | 2077663 | 19-Mar-2021 | 44 | 0.2 | 0.2 | 44.20 | 1.90 | −15.06 |
| 34 | 515698 | 2069886 | 19-Mar-2021 | 186 | 0.4 | 0.2 | |||
| 35 | 515698 | 2069886 | 19-Mar-2021 | 129 | 46.10 | 4.70 | −17.31 | ||
| 36 | 492321 | 2067271 | 13-Mar-2021 | 62 | 1.1 | 0.1 | 85.40 | 3.90 | −16.46 |
| 37 | 492321 | 2067271 | 13-Mar-2021 | 120 | 90.20 | 2.60 | −15.51 | ||
| 38 | 478978 | 2028598 | 13-Mar-2021 | 140 | 41.70 | 2.20 | −14.42 | ||
| 39 | 478969 | 2028628 | 13-Mar-2021 | 130 | 63.20 | 2.40 | −17.93 | ||
| 40 | 502240 | 2062079 | 15-Mar-2021 | 78 | 69.30 | 4.50 | −16.95 | ||
| 41 | 502241 | 2062080 | 15-Mar-2021 | 148 | 0.1 | 0.1 | 44.00 | 3.00 | −15.55 |
| 42 | 507631 | 2056658 | 15-Mar-2021 | 52 | 19.00 | 2.50 | −14.65 | ||
| 43 | 507631 | 2056658 | 15-Mar-2021 | 136 | 1.6 | 0.2 | |||
| 44 | 491602 | 2072337 | 14-Mar-2021 | 195 | 0.2 | 0.2 | |||
| 45 | 481127 | 2056059 | 15-Mar-2021 | 62 | 2.1 | 0.2 | |||
| 46 | 481127 | 2056059 | 16-Mar-2021 | 116 | 2.0 | 0.2 | |||
| 47 | 488740 | 2054271 | 17-Mar-2021 | 134 | 21.10 | 2.70 | −14.48 | ||
| 48 | 488736 | 2054268 | 18-Mar-2021 | 48 | 1.0 | 0.1 | |||
| 49 | 494401 | 2047786 | 15-Mar-2021 | 199 | 0.1 | 0.1 | |||
| 50 | 494065 | 2048101 | 15-Mar-2021 | 243 | 6.20 | 2.30 | −11.25 | ||
| 51 | 494406 | 2047788 | 16-Mar-2021 | 73 | 0.2 | 0.2 | |||
| 52 | 497379 | 2043209 | 20-Mar-2021 | 194 | 0.1 | 0.1 | |||
| 53 | 497379 | 2043209 | 21-Mar-2021 | 119 | 0.2 | 0.1 | |||
| 54 | 497379 | 2043209 | 22-Mar-2021 | 152 | 0.7 | 0.2 | |||
| 55 | 497379 | 2043209 | 23-Mar-2021 | 52 | 0.8 | 0.2 | |||
| 56 | 467857 | 2040043 | 24-Mar-2021 | 122 | 0.5 | 0.2 | |||
| 57 | 473326 | 2033863 | 25-Mar-2021 | 172 | 0.2 | 0.1 | |||
| 58 | 480200 | 2026500 | 26-Mar-2021 | 140 | 41.70 | 2.20 | −15.68 | ||
| 59 | 480200 | 2026500 | 27-Mar-2021 | 102 | 47.13 | 2.40 | −18.22 | ||
| 60 | 607206 | 1812732 | 23-Jan-2021 | 60 | 0.2 | 0.2 | |||
| 61 | 612462 | 1813254 | 24-Jan-2021 | 39 | 0.4 | 0.1 | |||
| 62 | 631393 | 1809331 | 24-Jan-2021 | 30 | 0.1 | 0.1 | |||
| 63 | 640001 | 1802824 | 25-Jan-2021 | 32 | 2.0 | 0.2 | |||
| 64 | 651576 | 1800782 | 26-Jan-2021 | 18 | 0.1 | 0.1 | |||
| 65 | 607339 | 1824512 | 23-Jan-2021 | 42 | 0.1 | 0.1 | |||
| 66 | 617885 | 1825744 | 24-Jan-2021 | 60 | 0.1 | 0.1 | |||
| 67 | 631745 | 1823245 | 24-Jan-2021 | 60 | 0.1 | 0.2 | |||
| 68 | 622514 | 1839511 | 23-Jan-2021 | 46 | 0.1 | 0.1 | |||
| 69 | 640387 | 1809586 | 26-Jan-2021 | 56 | 0.2 | 0.2 | |||
| 70 | 640378 | 1802037 | 24-Jan-2021 | 48 | 0.4 | 0.1 | |||
| 71 | 666284 | 1808527 | 24-Jan-2021 | 42 | 0.2 | 0.1 | |||
| 72 | 560033 | 1828331 | 12-Sep-2021 | 142 | 1.6 | 0.1 | |||
| 73 | 598284 | 1820327 | 12-Sep-2021 | 148 | 0.8 | 0.1 | |||
| 74 | 630620 | 1822037 | 12-Oct-2021 | 80 | 2.4 | 0.1 | 71.90 | 2.00 | −17.77 |
| 75 | 642749 | 1857853 | 12-Oct-2021 | 56 | 99.40 | 3.40 | −17.74 | ||
| 76 | 628401 | 1859572 | 12-Oct-2021 | 60 | 0.5 | 0.1 | 48.10 | 1.70 | −18.12 |
| 77 | 615894 | 1842075 | 12-Nov-2021 | 60 | 0.9 | 0.1 | 78.20 | 3.20 | −19.46 |
| 78 | 606460 | 1854500 | 12-Nov-2021 | 149 | 29.50 | 2.60 | −15.37 | ||
| 79 | 606450 | 1854524 | 12-Nov-2021 | 30 | 1.1 | 0.1 | 29.50 | 2.60 | −16.54 |
| 80 | 565464 | 1797140 | 13-Dec-2021 | 104 | 22.90 | 1.40 | −16.45 | ||
| 81 | 565464 | 1797139 | 13-Dec-2021 | 84 | 0.2 | 0.1 | 17.50 | 3.70 | −14.48 |
| 82 | 565466 | 1797138 | 13-Dec-2021 | 46 | 0.9 | 0.1 | |||
| 83 | 588931 | 1803041 | 13-Dec-2021 | 102 | 1.2 | 0.1 | 46.80 | 1.70 | −14.61 |
| 84 | 588923 | 1803030 | 13-Dec-2021 | 98 | 0.2 | 0.1 | 52.20 | 4.20 | −15.36 |
| 85 | 575680 | 1803513 | 13-Dec-2021 | 30 | 0.7 | 0.1 | 12.83 | 1.06 | −14.43 |
| 86 | 569101 | 1835841 | 14-Dec-2021 | 45 | 0.8 | 0.1 | |||
| 87 | 587830 | 1837715 | 14-Dec-2021 | 70 | 0.2 | 0.1 | |||
| 88 | 587753 | 1837671 | 14-Dec-2021 | 32 | 1.7 | 0.1 | |||
| 89 | 570318 | 1892276 | 14-Dec-2021 | 96 | 1.0 | 0.1 | 30.50 | 4.10 | −17.84 |
| 90 | 600424 | 1878180 | 15-Dec-2021 | 70 | 1.1 | 0.1 | 14.60 | 2.50 | −13.44 |
| 91 | 601285 | 1874112 | 15-Dec-2021 | 186 | 0.2 | 0.1 | |||
| 92 | 592532 | 1878578 | 15-Dec-2021 | 168 | 1.3 | 0.1 | 5.50 | 1.30 | −11.06 |
| 93 | 624927 | 1888363 | 16-Dec-2021 | 162 | 33.10 | 4.00 | −16.78 | ||
| 94 | 624930 | 1888358 | 16-Dec-2021 | 52 | 1.8 | 0.1 | 99.60 | 3.40 | −17.74 |
| 95 | 596811 | 1891242 | 17-Jan-2021 | 52 | 8.10 | 1.60 | −10.42 | ||
| 96 | 590809 | 1906861 | 18-Jan-2021 | 52 | 82.80 | 2.45 | −18.40 | ||
| 97 | 568164 | 1914424 | 18-Jan-2021 | 20 | 99.10 | 3.80 | −19.92 | ||
| 98 | 615890 | 1946465 | 18-Jan-2021 | 30 | 79.79 | 3.37 | −14.90 | ||
| 99 | 606000 | 1945234 | 18-Jan-2021 | 42 | 59.72 | 4.83 | −14.61 | ||
| 100 | 618401 | 1903197 | 20-Jan-2021 | 30 | 47.94 | 2.81 | −17.27 | ||
| 101 | 613402 | 1883313 | 21-Jan-2021 | 42 | 51.94 | 1.92 | −14.67 | ||
| 102 | 637176 | 1831754 | 21-Jan-2021 | 60 | 61.41 | 5.18 | −15.24 | ||
| 103 | 621900 | 1834170 | 22-Jan-2021 | 113 | 30.18 | 4.51 | −13.81 | ||
| 104 | 621900 | 1834170 | 22-Jan-2021 | 56 | 68.26 | 2.16 | −19.00 | ||
| 105 | 605416 | 1798633 | 16-Jan-2021 | 50 | 63.16 | 2.75 | −14.75 | ||
| 106 | 638241 | 1798138 | 22-Jan-2021 | 65 | 71.93 | 5.52 | −14.97 | ||
| 107 | 667092 | 1807303 | 22-Jan-2021 | 30 | 81.63 | 2.20 | −18.37 | ||
| 108 | 653553 | 1782015 | 23-Jan-2021 | 48 | 6.99 | 1.60 | −13.92 | ||
| 109 | 616797 | 1711149 | 27-Jan-2021 | 42 | 80.40 | 2.40 | −15.45 | ||
| 110 | 618191 | 1732662 | 26-Jan-2021 | 78 | 69.37 | 2.54 | −19.44 | ||
| 111 | 661351 | 1771511 | 23-Jan-2021 | 67 | 81.96 | 2.16 | −16.45 | ||
| 112 | 606054 | 1765319 | 26-Jan-2021 | 89 | 0.1 | 0.2 | 51.65 | 2.20 | −16.31 |
| 113 | 609659 | 1774818 | 26-Jan-2021 | 40 | 0.5 | 0.2 | |||
| 114 | 575150 | 1780492 | 16-Jan-2021 | 32 | 1.2 | 0.2 | 52.43 | 2.24 | −14.75 |
| 115 | 604589 | 1753438 | 2-May-2021 | 21 | 0.6 | 0.2 | 31.70 | 1.45 | −15.40 |
| 116 | 569524 | 1806608 | 24-Apr-2021 | 33 | 0.1 | 0.2 | |||
| 117 | 555708 | 1823182 | 24-Apr-2021 | 42 | 2.2 | 0.3 | |||
| 118 | 596115 | 1917118 | 26-Apr-2021 | 39 | 1.7 | 0.2 | 89.90 | 1.90 | −16.56 |
| 119 | 631390 | 1870695 | 30-Apr-2021 | 33 | 0.2 | 0.2 | |||
| 120 | 640586 | 1845299 | 30-Apr-2021 | 42 | 0.6 | 0.2 | |||
| 121 | 622871 | 2039417 | 14-Sep-2021 | 32 | 0.2 | 0.3 | 97.00 | 2.00 | −19.66 |
| 122 | 623253 | 2038825 | 14-Sep-2021 | 72 | 1.5 | 0.3 | 99.77 | 2.32 | −17.67 |
| 123 | 621272 | 2036557 | 14-Sep-2021 | 12 | 2.3 | 0.3 | |||
| 124 | 621832 | 2034942 | 14-Sep-2021 | 36 | 1.7 | 0.2 | |||
| 125 | 621233 | 2046692 | 14-Sep-2021 | 14 | 1.8 | 0.2 | 120.30 | 10.41 | −16.52 |
| 126 | 621317 | 2046591 | 14-Sep-2021 | 4 | 106.35 | 5.32 | −17.94 | ||
| 127 | 621317 | 2046591 | 15-Sep-2021 | 20 | 2.1 | 0.2 | |||
| 128 | 621586 | 2046636 | 15-Sep-2021 | 180 | 0.2 | 0.2 | 15.50 | 2.39 | −13.70 |
| 129 | 627648 | 2043450 | 15-Sep-2021 | 76 | 1.8 | 0.2 | 65.50 | 1.95 | −17.74 |
| 130 | 626116 | 2050358 | 15-Sep-2021 | 36 | 0.5 | 0.2 | |||
| 131 | 627180 | 2046388 | 15-Sep-2021 | 9 | 2.0 | 0.2 | |||
| 132 | 626816 | 2047687 | 15-Sep-2021 | 30 | 0.6 | 0.2 | |||
| 133 | 625751 | 2043475 | 15-Sep-2021 | 40 | 0.8 | 0.2 | |||
| 134 | 626165 | 2042467 | 15-Sep-2021 | 45 | 1.4 | 0.2 | 76.02 | 1.59 | −15.57 |
| 135 | 626165 | 2042467 | 15-Sep-2021 | 10 | 2.2 | 0.2 | |||
| 136 | 625092 | 2041359 | 16-Sep-2021 | 90 | 1.7 | 0.2 | 100.45 | 0.86 | −15.42 |
| 137 | 625827 | 2041013 | 16-Sep-2021 | 180 | 1.6 | 0.2 | |||
| 138 | 626211 | 2038685 | 16-Sep-2021 | 30 | 1.0 | 0.2 | |||
| 139 | 626211 | 2038685 | 16-Sep-2021 | 132 | 0.3 | 0.2 | 76.53 | 2.50 | −19.54 |
| 140 | 626090 | 2034632 | 16-Sep-2021 | 132 | 1.0 | 0.2 | |||
| 141 | 624927 | 2035484 | 16-Sep-2021 | 90 | 0.1 | 0.2 | 3.22 | 0.92 | −12.45 |
| 142 | 624927 | 2035484 | 16-Sep-2021 | 69 | 0.1 | 0.2 | 3.99 | 0.33 | −13.92 |
| 143 | 624927 | 2035484 | 16-Sep-2021 | 64 | 0.2 | 0.2 | |||
| 144 | 627022 | 2032091 | 17-Sep-2021 | 63 | 0.8 | 0.3 | 57.33 | 1.91 | −17.01 |
| 145 | 627380 | 2031290 | 17-Sep-2021 | 123 | 0.1 | 0.1 | |||
| 146 | 627184 | 2031357 | 17-Sep-2021 | 18 | 1.6 | 0.2 | |||
| 147 | 627385 | 2030987 | 17-Sep-2021 | 60 | 1.0 | 0.2 | |||
| 148 | 607872 | 2023187 | 17-Sep-2021 | 27 | 1.6 | 0.2 | |||
| 149 | 607872 | 2023187 | 17-Sep-2021 | 12 | 2.3 | 0.2 | |||
| 150 | 607872 | 2023187 | 17-Sep-2021 | 16 | 1.9 | 0.3 | |||
| 151 | 621846 | 2024819 | 17-Sep-2021 | 29 | 1.8 | 0.2 | 109.64 | 2.97 | −19.85 |
| 152 | 623843 | 2023736 | 17-Sep-2021 | 47 | 1.0 | 0.2 | 102.45 | 2.63 | −16.85 |
| 153 | 625848 | 2025284 | 17-Sep-2021 | 12 | 1.7 | 0.2 | |||
| 154 | 624088 | 2029109 | 17-Sep-2021 | 30 | 1.3 | 0.2 | |||
| 155 | 624385 | 2028802 | 17-Sep-2021 | 48 | 1.7 | 0.2 | |||
| 156 | 628901 | 2029253 | 18-Sep-2021 | 33 | 1.2 | 0.2 | |||
| 157 | 625938 | 2025702 | 18-Sep-2021 | 53 | 1.7 | 0.2 | |||
| 158 | 621317 | 2034606 | 18-Sep-2021 | 50 | 1.5 | 0.2 | 99.07 | 1.54 | −17.31 |
| 159 | 621317 | 2034606 | 18-Sep-2021 | 18 | 1.7 | 0.2 | |||
| 160 | 620954 | 2036112 | 18-Sep-2021 | 5 | 2.0 | 0.2 | |||
| 161 | 624512 | 2034019 | 18-Sep-2021 | 30 | 1.9 | 0.3 | 99.11 | 2.09 | −18.10 |
| 162 | 624512 | 2034019 | 18-Sep-2021 | 60 | 1.4 | 0.3 | 41.06 | 0.76 | −17.39 |
| 163 | 624195 | 2034007 | 18-Sep-2021 | 46 | 0.8 | 0.2 | 27.84 | 2.00 | −13.16 |
| 164 | 624195 | 2034010 | 18-Sep-2021 | 23 | 1.8 | 0.2 | |||
| 165 | 624865 | 2041542 | 18-Sep-2021 | 45 | 0.8 | 0.2 | 129.93 | 1.85 | −16.93 |
| 166 | 625276 | 2040475 | 18-Sep-2021 | 78 | 1.3 | 0.2 | |||
| 167 | 623572 | 2033733 | 18-Sep-2021 | 6 | 1.7 | 0.2 | |||
| 168 | 625764 | 2034591 | 18-Sep-2021 | 30 | 0.8 | 0.2 | 96.78 | 1.64 | −17.79 |
| 169 | 639889 | 20266232 | 18-Sep-2021 | 60 | 1.2 | 0.2 | |||
| 170 | 638076 | 2026324 | 18-Sep-2021 | 10 | 1.1 | 0.2 | |||
| 171 | 634709 | 2026451 | 18-Sep-2021 | 200 | 0.5 | 0.2 | 18.35 | 0.79 | −14.04 |
| 172 | 632112 | 2024152 | 18-Sep-2021 | 19 | 2.0 | 0.2 | |||
| 173 | 632112 | 2024152 | 18-Sep-2021 | 198 | 0.4 | 0.2 | 65.35 | 1.47 | −16.16 |
| 174 | 630702 | 2023828 | 18-Sep-2021 | 50 | 0.5 | 0.1 | 39.39 | 0.84 | −14.30 |
| 175 | 634864 | 2022890 | 20-Sep-2021 | 230 | 1.3 | 0.2 | 73.62 | 1.26 | −17.65 |
| 176 | 634864 | 2022890 | 20-Sep-2021 | 8 | 1.7 | 0.2 | |||
| 177 | 636183 | 2024199 | 20-Sep-2021 | 7 | 2.0 | 0.2 | |||
| 178 | 636183 | 2024199 | 20-Sep-2021 | 198 | 0.2 | 0.2 | 17.11 | 0.66 | −13.77 |
| 179 | 623236 | 2022710 | 20-Sep-2021 | 30 | 1.4 | 0.2 | |||
| 180 | 624372 | 2019089 | 20-Sep-2021 | 44 | 0.2 | 0.3 | 99.56 | 1.63 | −19.98 |
| 181 | 624377 | 2019080 | 20-Sep-2021 | 150 | 0.1 | 0.2 | 83.03 | 1.34 | −19.05 |
| 182 | 626117 | 2019793 | 21-Sep-2021 | 72 | 1.8 | 0.3 | 101.68 | 2.72 | −15.61 |
| 183 | 622460 | 2024278 | 21-Sep-2021 | 27 | 1.8 | 0.3 | |||
| 184 | 627971 | 2019533 | 21-Sep-2021 | 30 | 1.8 | 0.2 | |||
| 185 | 63877 | 2020145 | 21-Sep-2021 | 100 | 1.4 | 0.8 | 98.70 | 1.74 | −19.34 |
| 186 | 623797 | 2023797 | 21-Sep-2021 | 4 | 1.2 | 0.3 | |||
| 187 | 624442 | 2021436 | 21-Sep-2021 | 70 | 1.4 | 0.3 | 98.85 | 2.66 | −19.80 |
| 188 | 627414 | 2019269 | 21-Sep-2021 | 24 | 1.1 | 0.2 | |||
| 189 | 630972 | 2020641 | 21-Sep-2021 | 30 | 0.9 | 0.2 | |||
| 190 | 632484 | 2021391 | 21-Sep-2021 | 180 | 1.9 | 0.3 | 98.33 | 2.00 | −15.66 |
| 191 | 627953 | 1999735 | 22-Sep-2021 | 100 | 1.2 | 0.3 | |||
| 192 | 628202 | 1999506 | 22-Sep-2021 | 100 | 0.9 | 0.2 | 52.09 | 1.18 | −15.00 |
| 193 | 628202 | 1999506 | 22-Sep-2021 | 87 | 0.6 | 0.2 | |||
| 194 | 628129 | 1999875 | 22-Sep-2021 | 99 | 0.5 | 0.3 | 7.55 | 0.43 | −10.42 |
| 195 | 628059 | 1999959 | 22-Sep-2021 | 78 | 0.8 | 0.4 | |||
| 196 | 628476 | 1998685 | 22-Sep-2021 | 120 | 0.5 | 0.3 | |||
| 197 | 624664 | 2004721 | 22-Sep-2021 | 86 | 0.7 | 0.2 | 82.02 | 2.22 | −17.31 |
| 198 | 625402 | 2005049 | 22-Sep-2021 | 60 | 0.1 | 0.2 | |||
| 199 | 623993 | 2002952 | 22-Sep-2021 | 79 | 0.1 | 0.3 | |||
| 200 | 622338 | 2004296 | 22-Sep-2021 | 112 | 0.1 | 0.4 | 12.82 | 0.55 | −14.46 |
| 201 | 622195 | 2005224 | 22-Sep-2021 | 120 | 0.1 | 0.3 | |||
| 202 | 620634 | 2006310 | 22-Sep-2021 | 56 | 1.2 | 0.2 | |||
| 203 | 620634 | 2006310 | 22-Sep-2021 | 64 | 1.2 | 0.2 | |||
| 204 | 620634 | 2006310 | 22-Sep-2021 | 98 | 0.7 | 0.2 | 89.03 | 2.41 | −17.18 |
| 205 | 618553 | 2006553 | 22-Sep-2021 | 77 | 0.5 | 0.2 | |||
| 206 | 617397 | 2006751 | 22-Sep-2021 | 100 | 1.2 | 0.2 | |||
| 207 | 618770 | 2006243 | 22-Sep-2021 | 65 | 1.0 | 0.2 | |||
| 208 | 624442 | 2021436 | 22-Sep-2021 | 102 | 42.93 | 1.32 | −18.20 | ||
| 209 | 627414 | 2019269 | 22-Sep-2021 | 143 | 26.11 | 0.89 | −13.34 | ||
| 210 | 630972 | 2020641 | 22-Sep-2021 | 136 | 22.35 | 1.17 | −16.66 | ||
| 211 | 632484 | 2021391 | 22-Sep-2021 | 96 | 42.33 | 1.19 | −15.94 | ||
| 212 | 627953 | 1999735 | 22-Sep-2021 | 94 | 47.61 | 0.93 | −16.70 | ||
| 213 | 628202 | 1999506 | 22-Sep-2021 | 65 | 69.54 | 2.02 | −19.36 | ||
| 214 | 628202 | 1999506 | 22-Sep-2021 | 51 | 87.63 | 1.74 | −16.60 |
References
- Pratoomchai, W.; Kazama, S.; Hanasaki, N.; Ekkawatpanit, C.; Komori, D. A projection of groundwater resources in the Upper Chao Phraya River basin in Thailand. Hydrol. Res. Lett. 2014, 8, 20–26. [Google Scholar] [CrossRef]
- Vasconcelos, V.V.; Koontanakulvong, S.; Suthidhummajit, C.; Junior, P.P.M.; Hadad, R.M. Analysis of spatial–temporal patterns of water table change as a tool for conjunctive water management in the Upper Central Plain of the Chao Phraya River Basin, Thailand. Appl. Water Sci. 2017, 7, 245–262. [Google Scholar] [CrossRef]
- Putthividhya, A.; Laonamsai, J. Assessment of Surface and Ground-Water Interactions Using Stable Isotope Fingerprinting Technique in Thailand. In Proceedings of the World Environmental and Water Resources Congress 2015, Austin, TX, USA, 17–21 May 2015; pp. 464–474. [Google Scholar]
- Sillberg, C.V.; Kullavanijaya, P.; Chavalparit, O. Water quality classification by integration of attribute-realization and support vector machine for the Chao Phraya River. J. Ecol. Eng. 2021, 22, 70–86. [Google Scholar] [CrossRef]
- Tanachaichoksirikun, P.; Seeboonruang, U. Groundwater vulnerability of thailand’s lower chao phraya basin. GEOMATE J. 2020, 18, 88–96. [Google Scholar] [CrossRef]
- Rahman, A.T.M.S.; Kono, Y.; Hosono, T. Self-organizing map improves understanding on the hydrochemical processes in aquifer systems. Sci. Total Environ. 2022, 846, 157281. [Google Scholar] [CrossRef]
- Kamdee, K.; Nantasin, P.; Chotpantarat, S.; Saengkorakot, C.; Chanruang, P.; Polee, C.; Khaweerat, S.; Uapoonphol, N.; Fungklin, R.; Sriwiang, W. Assessment of groundwater dynamics in Quaternary aquifers of the Phrae Basin, northern Thailand, using isotope techniques. Hydrogeol. J. 2022, 30, 1091–1109. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Hao, Q.; Xiao, D.; Zhu, Y.; Yin, S.; Zhang, Y. Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed, China. Catena 2022, 212, 106020. [Google Scholar] [CrossRef]
- Heydarizad, M.; Pumijumnong, N.; Mansourian, D.; Anbaran, E.D.; Minaei, M. The deterioration of groundwater quality by seawater intrusion in the Chao Phraya River Basin, Thailand. Environ. Monit. Assess. 2023, 195, 424. [Google Scholar] [CrossRef]
- Kumari, R. Seawater Intrusion and Salinity Mapping in Coastal Aquifers: A Geospatial Approach. Adv. Remote Sens. Nat. Resour. Monit. 2021, 1, 323–345. [Google Scholar] [CrossRef]
- Laonamsai, J.; Julphunthong, P.; Chipthamlong, P.; Pawana, V.; Chomchaewchan, P.; Kamdee, K.; Tomun, N.; Kimmany, B. Hydrochemical characteristics and salt intrusion in groundwater of the lower Chao Phraya river basin: Insights from stable isotopes and hydrochemical analysis. Groundw. Sustain. Dev. 2023, 23, 101044. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Luo, Y.; Yan, L.; Peng, K.; Liu, Z.; Wang, Y. Groundwater salinization in a subtropical region, Beihai, southern China: Insights from hydrochemistry and multiple isotopes (H, O, S, Sr). Appl. Geochem. 2022, 141, 105323. [Google Scholar] [CrossRef]
- Laonamsai, J.; Pawana, V.; Chipthamlong, P.; Chomcheawchan, P.; Kamdee, K.; Kimmany, B.; Julphunthong, P. Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use. Geosciences 2023, 13, 195. [Google Scholar] [CrossRef]
- Laonamsai, J.; Putthividhya, A. Preliminary Assessment of Groundwater and Surface Water Characteristics in the Upper Chao Phraya River Basin Land Using a Stable Isotope Fingerprinting Technique. In Proceedings of the World Environmental and Water Resources Congress 2016, West Palm Beach, FL, USA, 22–26 May 2016; pp. 367–386. [Google Scholar]
- Putthividhya, A.; Laonamsai, J. Hydrological assessment using stable isotope fingerprinting technique in the Upper Chao Phraya river basin. Lowl. Technol. Int. Off. J. Int. Assoc. Lowl. Technol. 2017, 19, 27–40. [Google Scholar]
- Eissa, M.A.; Mahmoud, H.H.; Shouakar-Stash, O.; El-Shiekh, A.; Parker, B. Geophysical and geochemical studies to delineate seawater intrusion in Bagoush area, Northwestern coast, Egypt. J. Afr. Earth Sci. 2016, 121, 365–381. [Google Scholar] [CrossRef]
- Himi, M.; Tapias, J.; Benabdelouahab, S.; Salhi, A.; Rivero, L.; Elgettafi, M.; El Mandour, A.; Stitou, J.; Casas, A. Geophysical characterization of saltwater intrusion in a coastal aquifer: The case of Martil-Alila plain (North Morocco). J. Afr. Earth Sci. 2017, 126, 136–147. [Google Scholar] [CrossRef]
- Sae-Ju, J.; Chotpantarat, S.; Thitimakorn, T. Hydrochemical, geophysical and multivariate statistical investigation of the seawater intrusion in the coastal aquifer at Phetchaburi Province, Thailand. J. Asian Earth Sci. 2020, 191, 104165. [Google Scholar] [CrossRef]
- Maurya, P.; Kumari, R.; Mukherjee, S. Hydrochemistry in integration with stable isotopes (δ18O and δD) to assess seawater intrusion in coastal aquifers of Kachchh district, Gujarat, India. J. Geochem. Explor. 2019, 196, 42–56. [Google Scholar] [CrossRef]
- Kanagaraj, G.; Elango, L.; Sridhar, S.G.D.; Gowrisankar, G. Hydrogeochemical processes and influence of seawater intrusion in coastal aquifers south of Chennai, Tamil Nadu, India. Environ. Sci. Pollut. Res. 2018, 25, 8989–9011. [Google Scholar] [CrossRef]
- Behera, A.K.; Chakrapani, G.J.; Kumar, S.; Rai, N. Identification of seawater intrusion signatures through geochemical evolution of groundwater: A case study based on coastal region of the Mahanadi delta, Bay of Bengal, India. Nat. Hazards 2019, 97, 1209–1230. [Google Scholar] [CrossRef]
- Yurtsever, Y.; Gat, J.R. Stable isotope hydrology: Deuterium and oxygen-18 in the water cycle. Atmos. Waters 1981, 1, 103–142. [Google Scholar]
- Longinelli, A.; Edmond, J.M. Isotope geochemistry of the Amazon basin: A reconnaissance. J. Geophys. Res. Ocean. 1983, 88, 3703–3717. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Carreira, P.M.; Nunes, D.; Marques, J.M.; do Rosário Carvalho, M.; da Silva, M.A.; Costa, A. Environmental isotopes (δ2H, δ13C, δ18O, 3H, and 14C) as a diagnostic tool in the appraisal of mineral water management and protection: Two case studies—Portugal. Sustain. Water Resour. Manag. 2023, 9, 126. [Google Scholar] [CrossRef]
- Irvine, D.J.; Wood, C.; Cartwright, I.; Oliver, T. Depth to water table correction for initial carbon-14 activities in groundwater mean residence time estimation. Hydrol. Earth Syst. Sci. 2021, 25, 5415–5424. [Google Scholar] [CrossRef]
- Papp, D.C. Analysis of the radiogenic Carbon-14 record of groundwater at the Zlatna post-mining site (Romania). Carpathian J. Earth Environ. Sci. 2021, 16, 413–422. [Google Scholar] [CrossRef]
- Stewart, M.K.; van der Raaij, R.W. Response of the Christchurch groundwater system to exploitation: Carbon-14 and tritium study revisited. Sci. Total Environ. 2022, 817, 152730. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Trasvina, A.; Rogiers, B.; Beerten, K.; Pärn, J.; Wouters, L.; Walraevens, K. Using helium-4, tritium, carbon-14 and other hydrogeochemical evidence to evaluate the groundwater age distribution: The case of the Neogene aquifer, Belgium. J. Hydrol. X 2022, 17, 100132. [Google Scholar] [CrossRef]
- Khamanek, K.; Khuntong, S.; Saenboonruang, K.; Toyen, D.; Chantarot, C.; Yongprawat, M.; Saengkorakot, C.; Phattanasub, A.; Krisanangkura, P.; Hazama, R. Assessing tritium contamination in Thailand’s rainwater: A study of environmental monitoring and nuclear surveillance. J. Environ. Radioact. 2023, 262, 107151. [Google Scholar] [CrossRef]
- Jessee, E.J. Radiation Ecologies: Bombs, Bodies, and Environment during the Atmospheric Nuclear Weapons Testing Period, 1942–1965; Montana State University: Bozeman, MT, USA, 2013. [Google Scholar]
- Fujii, Y. The role of atmospheric nuclear explosions on the stagnation of global warming in the mid 20th century. J. Atmos. Sol. Terr. Phys. 2011, 73, 643–652. [Google Scholar] [CrossRef]
- Olsson, I.U. Radiocarbon dating history: Early days, questions, and problems met. Radiocarbon 2009, 51, 1–43. [Google Scholar] [CrossRef]
- Carreira, P.M.; Lobo de Pina, A.; da Mota Gomes, A.; Marques, J.M.; Monteiro Santos, F. Radiocarbon dating and stable isotopes content in the assessment of groundwater recharge at Santiago Island, Republic of Cape Verde. Water 2022, 14, 2339. [Google Scholar] [CrossRef]
- Laonamsai, J.; Ichiyanagi, K.; Kamdee, K. Geographic effects on stable isotopic composition of precipitation across Thailand. Isot. Environ. Health Stud. 2020, 56, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Laonamsai, J.; Ichiyanagi, K.; Kamdee, K.; Putthividhya, A.; Tanoue, M. Spatial and temporal distributions of stable isotopes in precipitation over Thailand. Hydrol. Process. 2021, 35, e13995. [Google Scholar] [CrossRef]
- Laonamsai, J.; Ichiyanagi, K.; Patsinghasanee, S. Isotopic temporal and spatial variations of tropical rivers in Thailand reflect monsoon precipitation signals. Hydrol. Process. 2021, 35, e14068. [Google Scholar] [CrossRef]
- Laonamsai, J.; Ichiyanagi, K.; Patsinghasanee, S.; Kamdee, K. Controls on Stable Isotopic Characteristics of Water Vapor over Thailand. Hydrol. Process. 2021, 35, e14202. [Google Scholar] [CrossRef]
- Ferraro, A.; de Sario, S.; Attanasio, A.; di Capua, F.; Gorgoglione, A.; Fratino, U.; Mascolo, M.C.; Pirozzi, F.; Trancone, G.; Spasiano, D. Phosphorus recovery as struvite and hydroxyapatite from the liquid fraction of municipal sewage sludge with limited magnesium addition. J. Environ. Qual. 2023, 52, 584–595. [Google Scholar] [CrossRef]
- Plastino, W.; Chereji, I.; Cuna, S.; Kaihola, L.; de Felice, P.; Lupsa, N.; Balas, G.; Mirel, V.; Berdea, P.; Baciu, C. Tritium in water electrolytic enrichment and liquid scintillation counting. Radiat. Meas. 2007, 42, 68–73. [Google Scholar] [CrossRef]
- Vita-Finzi, C.; Leaney, F. The direct absorption method of 14C assay—Historical perspective and future potential. Quat. Sci. Rev. 2006, 25, 1073–1079. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Araguás-Araguás, L.J.; Groening, M.; Kulkarni, K.M.; Kurttas, T.; Newman, B.D.; Vitvar, T. Global hydrological isotope data and data networks. In Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping; Springer: Dordrecht, The Netherlands, 2010; pp. 33–50. [Google Scholar]
- Lindsey, B.D.; Jurgens, B.C.; Belitz, K. Tritium as an Indicator of Modern, Mixed, and Premodern Groundwater Age; 2328-0328; US Geological Survey: Reston, VA, USA, 2019. [Google Scholar]
- Geyh, M.A. An overview of 14C analysis in the study of groundwater. Radiocarbon 2000, 42, 99–114. [Google Scholar] [CrossRef]
- Ingerson, E.; Pearson, F.J. Estimation of age and rate of motion of groundwater by the 14C-method. Recent Res. Fields Atmos. Hydrosphere Nucl. Geochem. 1964, 1, 263–283. [Google Scholar]
- Cartwright, I.; Fifield, L.K.; Morgenstern, U. Using 3H and 14C to constrain the degree of closed-system dissolution of calcite in groundwater. Appl. Geochem. 2013, 32, 118–128. [Google Scholar] [CrossRef]
- Tanachaichoksirikun, P.; Seeboonruang, U. Distributions of Groundwater Age under Climate Change of Thailand’s Lower Chao Phraya Basin. Water 2020, 12, 3474. [Google Scholar] [CrossRef]
- Andries, C.W.; Kanyerere, T.; Israel, S.; Butler, M. The application of environmental isotopes to conceptualize groundwater recharge in a coastal aquifer system: Case study of the West Coast Aquifer System, South Africa. Phys. Chem. Earth Parts A/B/C 2021, 124, 102995. [Google Scholar] [CrossRef]
- Love, A.H.; Zdon, A. Use of radiocarbon ages to narrow groundwater recharge estimates in the southeastern Mojave Desert, USA. Hydrology 2018, 5, 51. [Google Scholar] [CrossRef]
- Hansen, J.; Johnson, D.; Lacis, A.; Lebedeff, S.; Lee, P.; Rind, D.; Russell, G. Climate impact of increasing atmospheric carbon dioxide. Science 1981, 213, 957–966. [Google Scholar] [CrossRef]
- Wassenaar, L.; Aravena, R.; Fritz, P.; Barker, J. Isotopic composition (13C, 14C, 2H) and geochemistry of aquatic humic substances from groundwater. Org. Geochem. 1990, 15, 383–396. [Google Scholar] [CrossRef]
- Kamdee, K.; Corcho Alvarado, J.A.; Occarach, O.; Hunyek, V.; Wongsit, A.; Saengkorakot, C.; Chanruang, P.; Polee, C.; Khaweerat, S.; Matiatos, I.; et al. Application of isotope techniques to study groundwater resources in the unconsolidated aquifers along the Ping River (Thailand). Isot. Environ. Health Stud. 2020, 56, 95–110. [Google Scholar] [CrossRef]
- Kamdee, K.; Corcho Alvarado, J.A.; Yongprawat, M.; Occarach, O.; Hunyek, V.; Wongsit, A.; Saengkorakot, C.; Chanruang, P.; Polee, C.; Uapoonphol, N.; et al. Using 81Kr and isotopic tracers to characterise old groundwater in the Bangkok metropolitan and vicinity areas. Isot. Environ. Health Stud. 2023, 59, 426–453. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).