Simple Summary
The genus Boulenophrys Fei, Ye & Jiang, 2016, consists of 69 recognized species and is known for its endemism and diversity. This study describes a new species from Yongzhou City, Southern Hunan Province, Central China: Boulenophrys dupanglingensis sp. nov. The species presents a monophyletic lineage that differs from its sister species B. yunkaiensis, possessing relatively shorter shanks, distinct supernumerary tubercles below the base of the I and II toes, rough dorsal skin with dense granules, and several tubercles on the flanks.
Abstract
A new species of Asian horned toad, Boulenophrys, is described from Yongzhou City, Hunan Province, China. The species is a phylogenetically sister to B. yunkaiensis, based on 16S rRNA and COI genes. The new species differs from its congeners, possessing the following combination of characters: (1) moderate body size: SVL 37.6–40.2 mm (38.9 ± 1.3, n = 7) in adult males and SVL 41.8–45.9 mm (43.6 ± 2.1, n = 3) in adult females; (2) tympanum boundary clear: TD/ED 0.48–0.57 in males and 0.47–0.57 in females; (3) the presence of a small horn-like tubercle at the edge of the upper eyelid; (4) vomerine ridge present and vomerine teeth absent; (5) margin of tongue rounded, not notched posteriorly; (6) rough dorsal skin: a discontinuous “V”-shaped ridge with two discontinuous dorsolateral ridges on two sides on the back, dense tubercles on the skin of the ventral surface of the dorsal shank and thigh, and spiny tubercles surrounding the cloaca; (7) slender hindlimbs with heels overlapping when the flexed hindlimbs are held at right angles to the body axis; tibio-tarsal articulation reaching forward between anterior margin of tympanum and posterior corner of eye when leg stretched forward; (8) relative finger length IV < II < I < III, with a subarticular tubercle present at the base of each finger; (9) distinct supernumerary tubercles below the base of I and II toes; (10) toes without lateral fringes and with rudimentary webbing (webbing formula: I1 − 1-II1 − 2-III2 − 3IV3- − 2V).
1. Introduction
The subfamily Megophryinae is endemic to Asia, with a distribution spanning the Himalayas, northeastern India, southern China, and extending southwards to the islands of Southeast Asia [1,2,3]. The classification of Megophryinae has been a subject of debate for decades. Molecular phylogenetic studies consistently indicate that it is a monophyletic group [4,5,6]. However, the generic relationships and divisions within this subfamily have went through multiple alternations in recent years [4,5,6,7,8,9,10]. Lyu et al. proposed a new classification system, identifying ten genera: Atympanophrys Tian and Hu, 1983; Brachytarsophrys Tian and Hu, 1983; Grillitschia Dubois, Ohler, and Pyron, 2021; Sarawakiphrys Lyu and Wang, 2023; Jingophrys Lyu and Wang, 2023; Xenophrys Günther, 1864; Megophrys Kuhl and Van Hasselt, 1822; Pelobatrachus Beddard, 1907; Ophryophryne Boulenger, 1903; Boulenophrys Fei, Ye, and Jiang, 2016 [7,10,11,12,13,14,15,16]. Among these, Boulenophrys is the most diverse genus, comprising 69 recognized species to date (Table S1).
Dupangling National Nature Reserve is located in the southwest of Yongzhou City, southern Hunan Province, Central China, within the Nanling Mountains. During herpetological surveys conducted in 2016 and 2022, we discovered a species of Boulenophrys from Dupangling National Nature Reserve in Daoxian County and Jiangyong County and Yongzhou County of Yongzhou city. The genus was diagnosed based on the diagnostic characters by Lyu et al. [10], these specimens were identified as belonging to the genus Boulenophrys. However, due to a unique combination of morphological characteristics, they could not be classified into any known species. Further molecular phylogenetic analysis revealed that these specimens represent a separate evolutionary lineage. Accordingly, we formally describe them here as a new species within the genus Boulenophrys.
2. Materials and Methods
2.1. Sampling
Field surveys were carried out at Dupangling National Nature Reserve, Hunan Province, China (Figure 1), in May 2016 and April 2022. A total of ten specimens were newly collected. Initially, nine specimens were fixed in 10% formalin and later transferred to 75% ethanol for long-term storage. Before formalin fixation, liver tissues from four specimens were sampled and stored in 95% ethanol for DNA extraction. Nine specimens were deposited in the Animal Museum of Life Sciences College of Hunan Normal University (HNNU), Changsha City, Hunan Province, China, while one specimen was stored in 75% ethanol and placed in Chengdu Institute of Biology (CIB), Chinese Academy of Sciences, Chengdu City, Sichuan Province, China.
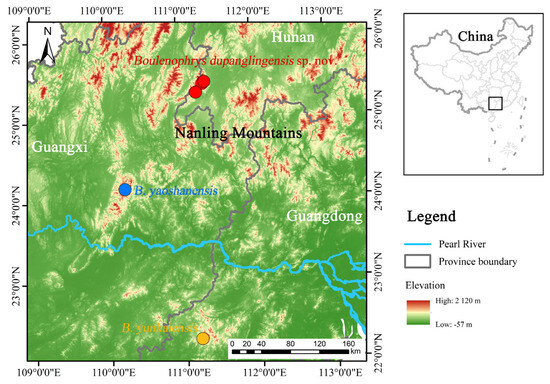
Figure 1.
Distribution map of Boulenophrys dupanglingensis sp. nov. and the type localities of two phylogenetically close species.
2.2. Molecular and Phylogenetic Analyses
Genomic DNA was extracted from liver tissues preserved in 95% ethanol using the TSINGKE DNA extraction kit. For sequencing, mitochondrial genes, specifically the partial 16S ribosomal RNA gene (16S rRNA) and partial cytochrome c oxidase 1 gene (COI), were targeted in four samples (HUNU 22SA05, HUNU 22SA07, HUNU 22SA08, and CIB 2016050802). Polymerase Chain Reaction (PCR) amplifications were conducted following Liu et al. [5]. The PCR products were sequenced using an ABI 3730 XL Genetic Analyzer at Sangon Biotech Co., Ltd. (Shanghai, China), and the obtained sequences were deposited in GenBank.
For the phylogenetic analyses, sequences were obtained from GenBank, including 68 known Boulenophrys species (except B.shuichengensis, for which no sequence information is available) and 2 outgroup species (Xenophrys glandulosa and X. mangshanensis) (Table S2). All sequences were aligned using the Clustal W algorithm with default parameters [17] and then trimmed using the partial gap deletion option in MEGA 11 [18]. Gaps in highly variable regions were removed. The best-fit evolutionary model (GTR+G+I) was determined using PartitionFinder v. 2.1.1 based on the AIC criterion [19]. Phylogenetic analyses were conducted using Bayesian inference (BI) with MrBayes 3.2 [20], and maximum likelihood (ML) trees were generated using PhyML v. 3.0 [21]. The BI analysis ran for 20,000,000 generations, with samples taken every 1000 generations, discarding the first 25% as burn-in, resulting in a potential scale reduction factor (PSRF) of <0.005. For the ML analysis, a bootstrap consensus tree was created from 1000 replicates.
2.3. Morphological Analysis
The morphological character definitions follow those established by Lyu et al. [10]. The following measurements were taken with digital calipers, with accuracy to the nearest 0.1 mm: SVL, snout–vent length (from the tip of the snout to the posterior of the vent); HDL, head length (from the snout tip to jaw articulation); HDW, head width (at the jaw commissure); SNT, snout length (from the snout tip to the anterior corner of the eye); IND, internasal distance (distance between nares); IOD, interorbital distance (minimum distance between the upper eyelids); ED, eye diameter (from the anterior corner to the posterior corner of the eye); TD, tympanum diameter (horizontal diameter of the tympanum); TED, tympanum–eye distance (from the anterior edge of the tympanum to the posterior corner of the eye); HND, hand length (from the proximal border of the outer palmar tubercle to the tip of digit III); RAD, radio–ulna length (from the flexed elbow to the proximal border of the outer palmar tubercle); FTL, foot length (from the distal end of the shank to the tip of digit IV); TIB, tibial length (from the outer surface of the flexed knee to the heel). The webbing was expressed using the webbed formula [22], with Roman numerals indicating fingers and toes. Sex determination was based on the direct observation of calls or the presence of internal vocal sac openings in males. The presence or absence of nuptial pads was examined under a dissecting microscope.
3. Results
3.1. Molecular Phylogenetic Analyses
A total of 540 base pairs (bps) of the 16S gene and 636 bps of the COI gene were concatenated into a single 1196 bp sequence. Phylogenetic analyses using both ML and BI methods yielded nearly identical results, with strong support for the major nodes in both tree types. The Bayesian tree is shown in Figure 2, where nodes with ML bootstrap values (BS) ≥ 70 and Bayesian posterior probabilities (BPP) ≥ 0.90 are considered to be strongly supported. In different analyses, the newly collected samples consistently formed a monophyletic group, displaying robust nodal support (BS 100, BPP 1.00). Additionally, based on the p-distances in the COI gene, genetic divergence among species within the Boulenophrys genus ranges from 2.3% to 18.9% (Table S3). The genetic distance between the candidate new species and its most close congener B.yunkaiensis is 4.3%, which is among interspecific level. Furthermore, these specimens are distinguishable from other congeners within the genus by a unique combination of morphological characters (see taxonomic account below).
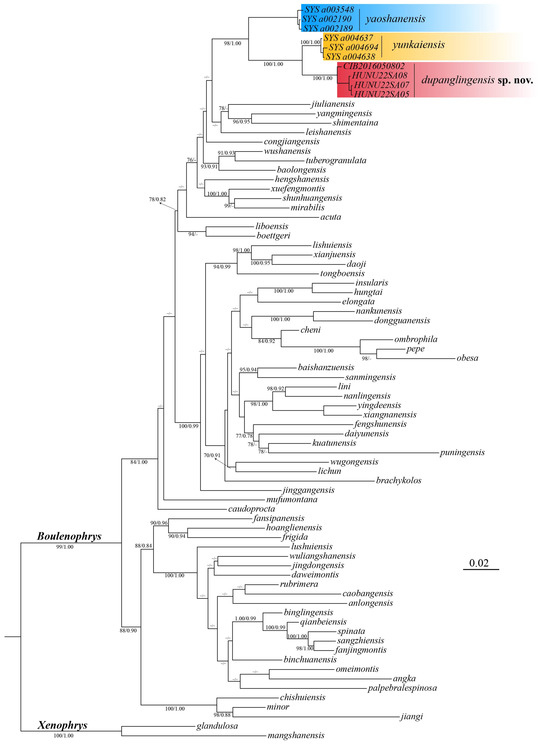
Figure 2.
Bayesian topology is based on the partial DNA sequences of the mitochondrial 16S rRNA and COI genes, with bootstrap support (BS)/Bayesian posterior probabilities (BPP) displayed at the tree nodes. A dash (‘−’) indicates BS value below 70 or BPP value below 90.
3.2. Morphological Comparisons
Morphological comparisons for the candidate new species with other recognized species of the Boulenophrys genus are listed in Table 1.

Table 1.
Diagnostic characters distinguishing all 69 species of Boulenophrys. For species with unavailable data, “/” is indicated.
The candidate new species is grouped within the same clade with closed congeners (B. congjiangensis, B. jiulianensis, B. leishanensis, B. shimentaina, B.yangmingensis, B.yaoshanensis, B.yunkaiensis) based on molecular analysis, and it further morphologically different from the latters by combination of following characters: (1) moderate body size of SVL 37.6–40.2 mm (n = 7) in adult males and SVL 41.8–45.9 mm in adult females (n = 3) (vs. smaller species B. congjiangensis (28.6–33.4, n = 15 in males, 38.4–40.2, n = 2 in females), B. jiulianensis (30.4–33.9, n = 10 in males, 34.1–37.5, n = 2 in females), and B. shimentaina (25.8–30.6, n = 20 in males, 34.5 in female)); (2) absence of lateral fringes on the toes (vs. B. congjiangensis and B. shimentaina, which have narrow lateral fringes); (3) nuptial pads bearing fine and dense black nuptial spines on the dorsal bases of fingers I and II in breeding adult males (vs. B.yangmingensis and B.yaoshanensis with villiform black nuptial spines); (4) dense tubercles on ventral shank and thigh, and spiny tubercles surrounding the cloaca (vs. B.leishanensis and B.yaoshanensis, with relatively smooth ventral skin); (5) unnotched tongue (vs. tongue of B. jiulianensis weakly notched posteriorly); (6) absence of vomerine teeth (vs. B. shimentaina with vomerine teeth present).
The candidate new species is phylogenetically most close to B. yunkaiensis. It differs from B. yunkaiensis in the following characters: (1) relatively shorter shanks: TIB/SVL 0.37–0.39 (0.38 ± 0.01, n = 7) in adult males (vs. TIB/SVL 0.40–0.48, 0.44 ± 0.04, n = 3) and 0.32–0.36 in adult females (0.34 ± 0.02, n = 3) (vs. TIB/SVL 0.42–0.46 in adult, 0.44 ± 0.02, n = 3 for B. yunkaiensis); (2) distinct supernumerary tubercles below the base of toes I and II (vs. indistinct tubercles under all toes in B. yunkaiensis); (3) rough dorsum skin with several dense small granules on the flanks (vs. sparse larger granules on the flanks) (Figure 3).

Figure 3.
Comparisons of Boulenophrys dupanglingensis sp. nov. (CIB2016050802) and B. yunkaiensis (SYS a004986) in preservative. White arrows indicate differences between closely related congeners. Boulenophrys dupanglingensis sp. nov.: (A) dorsolateral view; (B) plantar view of foot. B. yunkaiensis: (C) dorsolateral view; (D) plantar view of foot. Photo by Shengchao Shi (A,B) and Le-Qiang Zhu (C,D).
The candidate new species can be easily distinguished from 16 other congeners by its relatively longer shanks and overlapping of its heels when the flexed hindlimbs are held at right angles to the body axis, differing from B. acuta, B. brachykolos, B. daoji, B. dongguanensis, B. fengshunensis, B. hungtai, B. hengshanesis, B. insularis, B. kuatunensis, B.lichun, B. nankunensis, B. obesa, B. ombrophila, B. puningensis, B. wugongensis (vs. relatively shorter shanks with the heels not meeting), and B. xuefengmontis (vs. just meeting).
The candidate new species can be distinguished from nine congeners by its unnotched tongue, including B. baolongensis, B. binlingensis, B. boettgeri, B. cheni, B. lushuiensis, B. minor, B.mufumontana, B.qianbeiensis, and B. sanmingensis (vs. tongue notched posteriorly). By having a subarticular tubercle present at the base of each finger, it differs from the following seven congeners: B. angka, B. chishuiensis, B. leishanensis, B. lishuiensis, B. tuberogranulatu, B. wuliangshanensis, and B. yaoshanensis (vs. just the first and second finger present or not a finger in sight).
The candidate new species differs from 15 congeners by lacking vomerine teeth, with a vomerine ridge present. It differs from B. daiyunensis, B. daweimontis, B. elongata, B. fansipanensis, B. frigida, B. hoanglienensis, B. jinggangensis, B. jiulianensis, B. nanlingensis, B. palpebralespinosa, B. pepe, B. rubrimera, B. shimentaina, B. tongboensis, and B. yingdeensis (vs. presence of vomerine teeth). The candidate new species differs from the following nine congeners by lacking lateral fringes on its toes: B. anlongensis, B. baishanzuensis, B. binchuanensis, B. congjiangensis, B. lini, B. xiangnanensis, B. xianjuensis, B. yangmingensis (vs. presence of lateral fringes on the toes in these species), and B. wushanensis (vs. presence of wide lateral fringes on the toes in males, lacking in females).
Compared to other congeners, The candidate new species has a smaller body size, with an SVL of 37.6–40.2 mm (38.9 ± 1.3, n = 7) in adult males and an SVL of 41.8–45.9 mm (43.7 ± 2.1, n = 3) in adult females. It is significantly different from the nine congeners whose adult male and female SVL is ≥ 50 mm, including B. caudoprocta (81.3 mm in males), B.fangjingmontis (58.6–63.8 in males), B. jingdongensis (53.0–56.5 mm in males), B. liboensis (60.5–67.7 mm in males), B. mirabilis (55.8–61.4 mm in males), B. omeimontis (56.0–59.5 mm in males), B. sangzhiensis (53.0–60.8 mm in male), B. shuichengensis (102.0–118.3 mm in males), and B. spinata (47.2–54.4 mm in males). The candidate new species can be further distinguished from other congeners by possessing two subarticular tubercles at the base of each finger (vs. three metacarpal tubercles found in B.jiangi), and having tibio-tarsal articulation reaching forward between the anterior margin of the tympanum and the posterior corner of the eye (vs. tibio-tarsal articulation reaching to the tip of the snout in B. shunhuangensis, or the middle of the eye in B. caobangensis).
3.3. Taxonomic Account
Based on the results of these molecular phylogenetic and morphological comparisons, the specimen is distinct from all other congeners of Boulenophrys. The description of this new species is provided below.
Boulenophrys dupanglingensis Xiao & Mo, sp. nov.
Dupangling Horned Toad (in English)/(都庞岭角蟾 in Chinese)
Holotype. Adult male, HUNU 22SA01 (Figure 4), collected by Xiaoyang Mo on 20 April 2022 from Dajiangyuan Village (111.35931958° E, 25.46065718° N; 380 m a.s.l.), Dupangling National Nature Reserve, Daoxian County, Yongzhou City, Hunan Province, China.
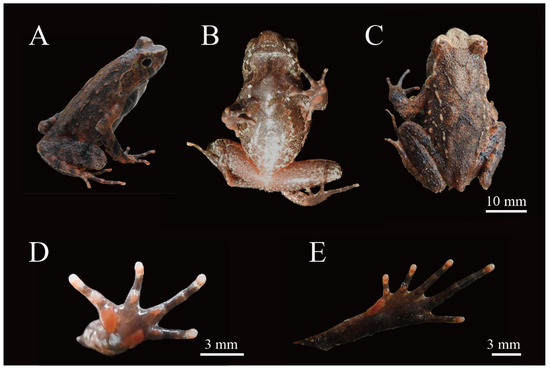
Figure 4.
Holotype of Boulenophrys dupanglingensis sp. nov. (HUNU 22SA01) in life: (A) lateral view; (B) ventral view; (C) dorsal view; (D) volar view of left hand; (E) plantar view of left foot. Photo by Jia-Yan Xi.

Figure 5.
Paratypes of Boulenophrys dupanglingensis sp. nov. in life. Male paratype (HUNU 22SA003): (A,C,E); female paratype (HUNU 22SA009): (B,D,F). Photos by Jia-Yan Xi.
Paratypes: Nine adults: five adult males (HUNU 22SA02–06) were collected from the same locality as the holotype on 21 April 2022 and 23 June 2016 by Xiaoyang Mo, Bin Wang, Jiayan Xi, Qi Li, Hui Li, Leqiang Zhu, Ayinuer Maimaiti, Yalan Xu, and Fan Zhou., one adult male CIB 2016050802 was collected from type locality on 8 May 2016 by Shengchao Shi; one adult female (HUNU 22SA07), which was collected from Daguyuan Village, Jiangyong County (111.33171300° E, 25.43785510° N; 442 m a.s.l.) on 23 April 2022 (Figure 5) by Xiaoyang Mo, Bin Wang, Jiayan Xi,; two adult females (HUNU 22SA08–09), which were collected from Daguyuan Village, Jiangyong County (112.24011945° E, 25.33178657° N; 451 m a.s.l.) on 24 April 2022 by Hui Li, Leqiang Zhu, Ayinuer Maimaiti.
Etymology: The specific epithet dupanglingensis refers to the type locality of the new species, Dupangling National Nature Reserve, Hunan Province, China. Dupangling Horned Toad is suggested as common name. 都庞岭角蟾 (dū pánɡ lǐnɡ jiǎo chán) is suggested as Chinese name.
Diagnosis: (1) moderate body size: SVL 37.6–40.2 mm (38.9 ± 1.3, n = 7) in adult males and SVL 41.8–45.9 mm (43.6 ± 2.1, n = 3) in adult females; (2) tympanum boundary clear: TD/ED 0.48–0.57 in males, 0.47–0.57 in females; (3) presence of small horn-like tubercle at the edge of the upper eyelid; (4) vomerine ridge present; vomerine teeth absent; (5) margin of tongue rounded, not notched posteriorly; (6) rough dorsal skin: a discontinuous “V”-shaped ridge with two discontinuous dorsolateral ridges on two sides on the back, dense tubercles on the skin of the ventral surface of the dorsal shank and thigh, and spiny tubercles surrounding the cloaca; (7) slender hindlimbs with heels overlapping when the flexed hindlimbs are held at right angles to the body axis; tibio-tarsal articulation reaching forward between anterior margin of tympanum and posterior corner of the eye when leg stretched forward; (8) relative finger length IV < II < I < III, with a subarticular tubercle present at the base of each finger; (9) distinct supernumerary tubercles below the base of toes I and II; (10) toes without lateral fringes and with only rudimentary webbing (webbing formula: I1 − 1-II1 − 2-III2 − 3IV3- − 2V) (Figure 6 and Figure 7A).
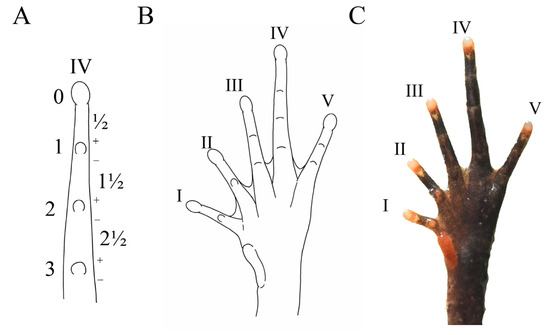
Figure 6.
Description of webbing formula according to Savage and Heyer (1997). (A) Pattern of the fourth toes finger, with phalangeal joint used as the measurement; (B) pattern of the ventral foot in B. dupanglingensis sp. nov.; (C) photograph of the ventral foot. Photo by Jia-Yan Xi.
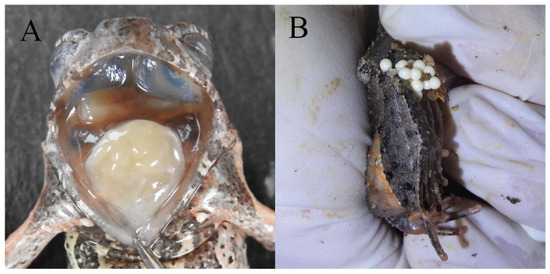
Figure 7.
(A) Edge of the tongue; (B) eggs of living paratype. Photo by Jia-Yan Xi.
Coloration of holotype in life. Forearms and hindlimbs are marked with dark-brown transverse bands; supratympanic ridges are light ivory with a dark spot on the posterior edge; a dark vertical band extends from the lower margin of the eye to the upper lip; numerous brown patches are scattered across the lateroventral surface of the flanks; the groin is red-orange; the ventral surface of the throat and chest is light salmon, with dark-brown patches and dark-orange blotches; distinct longitudinal stripe along the throat; ventral body surface is light salmon in color with brown patches and white spots; ventral surfaces of limbs are light salmon with dark-brown spots and blotches; hands and feet have brown ventral surfaces, with pale brown tips on the digits; metacarpal and metatarsal tubercles are reddish; pectoral glands and femoral glands appear white; iris is yellowish brown.
Coloration of holotype in preservative. Dark-brown coloration has faded to greyish brown dorsally. A triangular marking is visible between the eyes, accompanied by a “V”-shaped marking along the mid-dorsum; transverse bands on dorsal sides of the forearms and hindlimbs have become indistinct. The ventral surface has faded to a greyish white color, with all previously distinct bands and spots becoming less noticeable or completely indistinguishable.
Variation and sexual dimorphism. Measurement data for the type series are provided in Table 2. Morphological diagnostic characters observed in all paratypes were consistent with those of the holotype. However, there were variations in coloration and stripe patterns between individuals (Figure 4). For example, the adult male paratype (HUNU 22SA03) with a smaller body size (SVL 37.8 mm) has a light-brown back and a black-brown belly, the ventral surfaces of the limbs are primarily black-brown, and there is a prominent black spot at the right upper eyelid. In contrast, the adult female paratype (HUNU 22SA09) with a larger body size (SVL 41.8 mm) has a yellowish-brown dorsal coloration, the chest and anterior abdomen exhibit dark-orange blotches on a light-brown background, the ventral surfaces of the limbs are predominantly orange-red, and fewer markedly enlarged tubercles are present on the flanks.

Table 2.
Measurements (in mm) and ratios for the type series of Boulenophrys dupanglingensis sp. nov. “*” denotes the holotype.
Sexual size dimorphism: Females (SVL 43.6 ± 2.1 mm, n = 3) are significantly larger than males (SVL 38.8 ± 0.9 mm, n = 6). Adult males possess a single internal subgular vocal sac. During the breeding season, males develop prominent black nuptial spines at the bases of the first and second fingers on dorsal surfaces.
Reproductive characteristics: The eggs are creamy white, with an approximate diameter of 2.6 mm. The observed clutch size consisted of approximately 258 eggs (female, HUNU 22SA08, Figure 7B)
Distribution and habitats. Boulenophrys dupanglingensis sp. nov. is only found in the Dupangling National Nature Reserve, located in southern Hunan, China. The species was observed in evergreen secondary forest, typically near mountain streams, where it was found among the leaf litter on the forest floor. The elevation records of the new species range from 380 to 451 m (Figure 8). Calls of males were recorded between April and June, suggesting that the species’ breeding season including this period of time. Additionally, female specimens collected during this time were found to contain mature, creamy white eggs. No tadpoles were encountered during the field surveys.

Figure 8.
Habitat of Boulenophrys dupanglingensis sp. nov.(A) Landscape of montane forests at the type locality. (B) microhabitats of the new species, mountain stream (white box shown in the Fig 8A). Photo by Sheng-Chao Shi.
4. Discussion
The discover and description the new species once more highlight the diversity of Boulenophrys, which is currently classified into three primary subgroups: the B. boettgeri group, the B. minor group, and the B. omeimontis group [10,23,24]. The newly described species, Boulenophrys dupanglingensis, is classified within the B. boettgeri group. This new species brings the total number of recognized Boulenophrys species in Hunan Province to 10, including B. caudoprocta, B. dupanglingensis, B.hengshanensi, B. mufumontana, B.Sangzhiensis, B. shunhuangensis, B. tuberogranulatus, B. xiangnanensis, B. xuefengmontis, and B. yangmingensis [10,25,26], and it suggests that further investigation on this group might lead to more findings of hidden diversity.
Most species of Boulenophrys are endemic to small region [5,10], although B. dupanglingensis and B. yunkaiensis exhibit a close evolutionary relationship, yet their habitats are separated by low lands in southern Hunan province (Figure 1). The Nanling Mountain range spans the provinces of Guangxi, Hunan, Guangdong, and Jiangxi, forming a natural barrier separating the Yangtze River and Pearl River basins. This region exhibits species diversity, strong forest dependence, and overlapping distributions of the Boulenophry genus [27]. These patterns highlight the need for further research into speciation processes, niche differentiation, and coexistence mechanisms [28]. Moreover, climatic fluctuations, habitat diversity, sexual selection, and the dynamics of montane forests likely drive diversification within Boulenophrys. Geographic isolation often leads to allopatric speciation, while ecological factors can drive adaptive divergence, influencing the evolution of complex phenotypes. For further studies, it would be valuable to analysis skeletal and acoustic features, which could provide insights into the evolution and speciation of this group.
The new species was found to be abundant at its habitat in breeding seasons from 2015 to 2022 based on our field investigation. However, considering the endemism of the genus, the distribution area of this species is expected to restricted to small region bordering Hunan and Guangxi. And this species is expected to found in Guilin of northeastern Guangxi. The known distribution area is located in the Dupangling National Nature Reserve and the habitat is well protected. The conservation status for this species is suggested to be evaluated based on further investigation covering all its distribution area.
5. Conclusions
Combining the molecular phylogenetic and morphological analysis results, a new Boulenophry species, B. dupanglingensis sp. nov., is described from Yongzhou City, Hunan Province, China. This research brings the total number of recognized Boulenophrys species in Hunan Province to 10. This discovery highlights the biodiversity within the Nanling Mountain range and further emphasizes the importance of the region as a biodiversity hotspot.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15030440/s1, Table S1: Information on the morphological characters of 69 recognized species of Boulenophrys. Table S2: Localities, voucher information, and GenBank accession numbers for all samples used in this study. Table S3: Uncorrected p-distance (%) among Boulenophrys species inferred from mitochondrial COI gene [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
Author Contributions
Conceptualization, S.S. and X.M.; methodology, B.X. and J.X.; software, B.X.; J.X. and H.L.; validation, all authors; investigation, all authors; visualization, L.Z.; J.X.; S.S.; and B.X.; data curation, all authors; writing—original draft preparation, B.X.; J.X.; and X.M.; writing—review and editing, B.X.; J.X.; S.S.; B.W.; and X.M.; funding acquisition, B.W. and X.M.; project administration, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
Biodiversity Survey Project of Dupangling National Nature Reserve: 2021(299).
Institutional Review Board Statement
All animal protocols in this study were reviewed and approved by the Animal Ethical and Welfare Committee of Hunan Normal University (permit number: 2022580).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
We would especially like to thank Xuejian Deng of HUNU for the support and investigation assistance. We are grateful for the help of Lu Wang, Yinyong Wang, Shuo Qi, and Yinmeng Hou for their assistance with specimen examination and suggestions on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- AmphibiaWeb: Information on Amphibian Biology and Conservation, University of California, Berkeley, California. Available online: http://amphibiaweb.org/ (accessed on 18 November 2024).
- Bonaparte, C.L. Conspectus Systematum Herpetologiae et Amphibiologiae; Brill EJ: Leiden, The Netherlands, 1850. [Google Scholar]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Available online: https://amphibiansoftheworld.amnh.org (accessed on 18 November 2024).
- Chen, J.M.; Zhou, W.W.; Poyarkov, N.J.; Stuart, B.L.; Brown, R.M.; Lathrop, A.; Wang, Y.Y.; Yuan, Z.Y.; Jiang, K.; Hou, M.; et al. A novel multilocus phylogenetic estimation reveals unrecognized diversity in Asian horned toads, genus Megophrys sensu lato (Anura: Megophryidae). Mol. Phylogenetics Evol. 2017, 106, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Chen, G.L.; Zhu, T.Q.; Zeng, Z.C.; Lyu, Z.T.; Wang, J.; Messenger, K.; Greenberg, A.J.; Guo, Z.; Yang, Z.H.; et al. Prevalence of cryptic species in morphologically uniform taxa—Fast speciation and evolutionary radiation in Asian frogs. Mol. Phylogenetics Evol. 2018, 127, 723–731. [Google Scholar] [CrossRef]
- Mahony, S.; Foley, N.M.; Biju, S.D.; Teeling, E.C. Evolutionary History of the Asian Horned Frogs (Megophryinae): Integrative Approaches to Timetree Dating in the Absence of a Fossil Record. Mol. Biol. Evol. 2017, 34, 744–771. [Google Scholar] [CrossRef]
- Dubois, A.; Ohler, A.; Pyron, R.A. New concepts and methods for phylogenetic taxonomy and nomenclature in zoology, exemplified by a new ranked cladonomy of recent amphibians (Lissamphibia). Megataxa 2021, 5, 1–738. [Google Scholar] [CrossRef]
- Frost, D.R.; Grant, T.; Faivovich, J.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; De Sá, R.O.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006, 2006, 1–291. [Google Scholar] [CrossRef]
- Li, S.Z.; Lu, N.N.; Liu, J.; Wang, B. Description of a new Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from Guizhou Province, China. Zookeys 2020, 986, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.T.; Qi, S.; Wang, J.; Zhang, S.Y.; Zhao, J.; Zeng, Z.C.; Wan, H.; Yang, J.H.; Mo, Y.M.; Wang, Y.Y. Generic classification of Asian horned toads (Anura: Megophryidae: Megophryinae) and monograph of Chinese species. Zool Res. 2023, 44, 380–450. [Google Scholar] [CrossRef]
- Beddard, F.E. Contributions to the knowledge of the anatomy of the batrachian family Pelobatid. Proc. Zool. Soc. Lond. 1907, 77, 871–911. [Google Scholar] [CrossRef]
- Boulenger, G.A. Descriptions of three new batrachians from Tonkin. Ann. Mag. Nat. Hist. Ser. 7 1903, 12, 186–188. [Google Scholar] [CrossRef]
- Fei, L.; Ye, C.Y. Amphibians of China; Chengdu Institute of Biology, Chinese Academy of Sciences: Chengdu, China; Science Press: Beijing, China, 2016; Volume 1. [Google Scholar]
- Günther, A.C.L.G. The Reptiles of British India; Ray Society: London, UK, 1864; pp. 414–415. [Google Scholar]
- Kuhl, H.; Van Hasselt, J.C. Uittreksels uit brieven van de heeren Kuhl en Van Hasselt, aan de heeren CJ Temminck, Th. van Swinderen, W. de Haan, DJ van Ewyck, H. Boie en ZE den Minister voor het Openbaar Onderwijs, de Nationale Nijverheid en de Kolonien. Uittreksels uit brieven van de heeren Kuhl en Van Hasselt, aan de heeren CJ Temminck, Th. van Swinderen, W. de Haan, DJ van Ewyck, H. Boie en ZE den Minister voor het Openbaar Onderwijs, de Nationale Nijverheid en de Kolonien. Alg. Konst En Lett. Bode 1822, 7, 99–104. [Google Scholar]
- Tian, W.S.; Hu, Q.X. Taxonomic study on genus Megophrys, with descriptions of two new genera. Acta Herpetol. Sin. 1983, 2, 41–48. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Savage, J.M.; Heyer, W.R. Variation and distribution in the tree-frog genus Phyllomedusa. Beit. Neotrop. Fauna 1967, 5, 111–131. [Google Scholar] [CrossRef]
- Xiang, H.; Zhou, Q.; Li, W.; Shu, J.; Gu, Z.; Jiang, W. Insights into phylogenetic positions and distribution patterns: Complete mitogenomes of two sympatric Asian horned toads in Boulenophrys (Anura: Megophryidae). Ecol. Evol. 2024, 14, e11687. [Google Scholar] [CrossRef]
- Qi, S.; Lyu, Z.T.; Wang, J.; Mo, Y.M.; Zeng, Z.C.; Zeng, Y.J.; Dai, K.Y.; Li, Y.Q.; Grismer, L.L.; Wang, Y.Y. Three new species of the genus Boulenophrys (Anura, Megophryidae) from southern China. Zootaxa 2021, 5072, 401–438. [Google Scholar] [CrossRef]
- Gao, Z.W.; Qian, T.Y.; Jiang, J.P.; Hou, D.J.; Deng, X.J.; Yang, D.D. Species diversity and distribution of amphibians and reptiles in Hunan Province, China. Biodivers. Sci. 2022, 30, 21290. [Google Scholar] [CrossRef]
- Qian, T.Y.; Hu, K.; Mo, X.Y.; Gao, Z.W.; Zhang, N.; Yang, D.D. A new species of Boulenophrys from central Hunan Province, China (Anura: Megophryidae). Vertebr. Zool. 2023, 73, 915–930. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.Z.; Wei, G.; Xu, N.; Cheng, Y.L.; Wang, B.; Wu, J. A New Species of the Asian Toad Genus Megophrys sensu lato (Anura: Megophryidae) from Guizhou Province, China. Asian Herpetol. Res. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Y.; Wang, S.; Lu, X.; Wang, W.; Deng, H.; Zhou, J. A species of the genus Panophrys (Anura, Megophryidae) from southeastern Guizhou Province, China. Zookeys 2021, 1047, 27–60. [Google Scholar] [CrossRef]
- Li, Y.L.; Jin, M.J.; Zhao, J.; Liu, Z.Y.; Wang, Y.Y.; Pang, H. Description of two new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Heishiding Nature Reserve, Fengkai, Guangdong, China, based on molecular and morphological data. Zootaxa 2014, 3795, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Suwannapoom, C.; Poyarkov, N.A., Jr.; Pawangkhanant, P.; Xu, K.; Jin, J.Q.; Murphy, R.W.; Che, J. A new species of the genus Xenophrys Anura Megophryidae from northern Thailand. Zool. Res. 2019, 40, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.D.; Lyu, Z.T.; Wang, J.; Li, Y.L.; Liu, Z.Y.; Chen, H.H.; Rao, D.Q.; Jin, Z.F.; Zhang, C.Y.; et al. Review of the genus Brachytarsophrys (Anura: Megophryidae), with revalidation of Brachytarsophrys platyparietus and description of a new species from China. Zool. Res. 2020, 41, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.Y.; Fei, L.; Xie, F. A new species of Megophryidae—Megophyrys baolongensis from China (Amphibia, Anura). Acta Herpetol. Sin. 2007, 11, 38–41. [Google Scholar]
- Nguyen, T.Q.; Pham, C.T.; Nguyen, T.T.; Luong, A.M.; Ziegler, T. A new species of Megophrys (Amphibia: Anura: Megophryidae) from Vietnam. Zootaxa 2020, 4722, 401–422. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, D.D.; Mo, X.Y.; Li, H.H.; Chen, D. The Fauna of Hunan: Amphibia; Hunan Science and Technology Press: Changsha, China, 2014. [Google Scholar]
- Wang, Y.Y.; Zhao, J.; Yang, J.H.; Zhou, Z.; Chen, G.L.; Liu, Y. Morphology, molecular genetics, and bioacoustics support two new sympatric Xenophrys toads (Amphibia: Anura: Megophryidae) in southeast China. PLoS ONE 2014, 9, e93075. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Li, S.Z.; Liu, J.; Wei, G.; Wang, B. A new species of the horned toad Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 943, 119–144. [Google Scholar] [CrossRef]
- Lyu, Z.T.; Zeng, Z.C.; Wang, J.; Liu, Z.Y.; Huang, Y.Q.; Li, W.Z.; Wang, Y.Y. Four new species of Panophrys (Anura, Megophryidae) from eastern China, with discussion on the recognition of Panophrys as a distinct genus. Zootaxa 2021, 4927, 9–40. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, Z.T.; Liu, Z.Y.; Liao, C.K.; Zeng, Z.C.; Zhao, J.; Li, Y.L.; Wang, Y.Y. Description of six new species of the subgenus Panophrys within the genus Megophrys (Anura, Megophryidae) from southeastern China based on molecular and morphological data. Zookeys 2019, 851, 113–164. [Google Scholar] [CrossRef]
- Zeng, Z.C.; Wang, J.; Chen, H.H.; Xiao, W.W.; Zhan, B.B.; Li, Y.H.; Lin, S.S. A New Species of the Genus Boulenophrys; (Anura, Megophryidae) from Eastern Guangdong, China. Asian Herpetol. Res. 2024, 15, 12–21. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, L.; Ran, H.; Shen, Z.X. Megophrys binlingensis fanjingmontis: A New Subspecies of Megophryidae from Guizhou, China. Chin. J. Zool. 2012, 47, 135–138. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.; Nguyen, L.T.; Nguyen, C.T.; Harding, L.; Portway, C.; Van Luong, H.; Rowley, J.J. A new locality and elevation extension for Megophrys rubrimera (Tapley et al., 2017) in Bat Xat Nature Reserve, Lao Cai Province, northern Vietnam. Herpetol. Notes 2018, 11, 865–868. [Google Scholar]
- Wang, J.; Zeng, Z.C.; Lyu, Z.T.; Qi, S.; Liu, Z.Y.; Chen, H.H.; Lu, Y.H.; Xiao, H.W.; Lin, C.R.; Chen, K.; et al. Description of three new Boulenophrys species from eastern Guangdong, China, emphasizing the urgency of ecological conservation in this region (Anura, Megophryidae). Zootaxa 2022, 5099, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Tapley, B.; Cutajar, T.; Nguyen, L.T.; Portway, C.; Mahony, S.; Nguyen, C.T.; Harding, L.; Luong, H.V.; Rowley, J.L. A new potentially Endangered species of Megophrys (Amphibia: Megophryidae) from Mount Ky Quan San, north-west Vietnam. J. Nat. Hist. 2020, 54, 2543–2575. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.; Mahony, S.; Nguyen, C.T.; Dau, V.Q.; Luong, A.M.; Le, D.T.; Nguyen, T.T.; Nguyen, T.Q.; Portway, C.; et al. Two new and potentially highly threatened Megophrys Horned frogs (Amphibia: Megophryidae) from Indochina’s highest mountains. Zootaxa 2018, 4508, 301–333. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.Y.; Lyu, Z.T.; Zeng, Z.C.; Wang, Y.Y. A new species of the genus Xenophrys (Amphibia: Anura: Megophryidae) from an offshore island in Guangdong Province, southeastern China. Zootaxa 2017, 4324, 541–556. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, T.D.; Zhao, J.; Sung, Y.H.; Yang, J.H.; Pang, H.; Zhang, Z. Description of a new species of the genus Xenophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Jinggang, China, based on molecular and morphological data. Zootaxa 2012, 3546, 53–67. [Google Scholar] [CrossRef]
- Li, S.Z.; Xu, N.; Liu, J.; Jiang, J.P.; Wei, G.; Wang, B. A New Species of the Asian Toad Genus Megophrys sensu lato (Amphibia: Anura: Megophryidae) from Guizhou Province, China. Asian Herpetol. Res. 2018, 9, 224–239E. [Google Scholar] [CrossRef]
- Lin, S.S.; Chen, H.H.; Li, Y.H.; Peng, Z.N.; Zeng, Z.C.; Wang, J. A new Boulenophrys species (Anura, Megophryidae) from the coastal hills of eastern Fujian Province, China. Zookeys 2024, 1216, 1–15. [Google Scholar] [CrossRef]
- Shi, S.C.; Li, D.H.; Zhu, W.B.; Wang, J.; Jiang, J.P.; Ye, C.Y.; Fei, L.; Wang, B. Description of a new toad of Megophrys Kuhl & Van Hasselt, 1822 (Amphibia: Anura: Megophryidae) from western Yunnan Province, China. Zootaxa 2021, 4942, 351–381. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Li, G.; Xiao, N.; Li, J.Q.; Pan, T.; Wang, H.; Zhang, B.W.; Zhou, J. A New Species of the Genus Xenophrys (Amphibia: Anura: Megophryidae) from Libo County, Guizhou, China. Asian Herpetol. Res. 2017, 8, 75–85. [Google Scholar] [CrossRef]
- Wang, L.; Deng, X.J.; Liu, Y.; Wu, Q.Q.; Liu, Z. A new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Hunan, China. Zootaxa 2019, 4695, 301–330. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.T.; Li, Y.Q.; Zeng, Z.C.; Zhao, J.; Liu, Z.Y.; Guo, G.X.; Wang, Y.Y. Four new species of Asian horned toads (Anura, Megophryidae, Megophrys) from southern China. ZooKeys 2020, 942, 105–140. [Google Scholar] [CrossRef] [PubMed]
- Messenger, K.R.; Dahn, H.A.; Liang, Y.; Xie, P.; Wang, Y.; Lu, C. A new species of the genus Megophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Wuyi, China. Zootaxa 2019, 4554, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, S.S.; Gan, J.S.; Chen, H.H.; Yu, L.M.; Pan, Z.; Xiao, J.J.; Zeng, Z.C. A new species of the genus Boulenophrys from South China (Anura, Megophryidae). Zootaxa 2024, 5514, 451–468. [Google Scholar] [CrossRef]
- Su, H.J.; Shi, S.C.; Wu, Y.Q.; Li, G.R.; Yao, X.G.; Wang, B.; Li, S.Z. Description of a new horned toad of Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 974, 131–159. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.; Mahony, S.; Nguyen, C.T.; Dau, V.Q.; Nguyen, T.T.; Luong, H.V.; Rowley, J.J.L. The Vietnamese population of Megophrys kuatunensis (Amphibia: Megophryidae) represents a new species of Asian horned frog from Vietnam and southern China. Zootaxa 2017, 4344, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.P.; Ye, C.Y.; Fei, L. A New Horn Toad Megophrys sangzhiensis from Hunan, China (Amphibia, Anura). Zool. Res. 2008, 29, 219–222. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Gu, X.M.; Sun, A.Q. A new species of Xenophrys in China (Amphibia: Pelobatidae). Acta Zootaxonomica Sin. 2000, 25, 462–466. [Google Scholar]
- Mo, X.Y.; Shen, Y.H.; Li, H.H.; Wu, X.S. A new species of Megophrys (Amphibia: Anura: Megophryidae) from the northwestern Hunan Province, China. Curr. Zool. 2010, 56, 432–436. [Google Scholar] [CrossRef]
- Fei, L. Atlas of Amphibians in China. Field Edition; Henan Science and Technology Press: Zhengzhou, China; p. 432.
- Wang, B.; Wu, Y.Q.; Peng, J.W.; Shi, S.C.; Lu, N.N.; Wu, J. A new Megophrys Kuhl & Van Hasselt (Amphibia, Megophryidae) from southeastern China. Zookeys 2020, 904, 35–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).