Simple Summary
Sea urchins are valued for their edible reproductive organs, called gonads or roe, which are considered a delicacy in many cultures. However, in some areas, overpopulation of sea urchins has led to overgrazing of kelp forests, creating underwater deserts called urchin barrens. While sea urchins have great commercial value, those from barrens yield poor-quality gonads due to nutritional deficiencies, rendering them commercially unviable. This review examines how short-term sea urchin ranching using specially designed feeds could generate commercially viable gonads. We analyzed research on different feed ingredients to determine what helps sea urchins grow larger, better-colored gonads. We found that seaweed-based feeds work best but are expensive and environmentally challenging to obtain in large quantities with standard quality. Plant-based proteins like soybean meal show promise as more sustainable and affordable alternatives. The right amount of fats in the diet is also critical for gonad development. Farming sea urchins from barren areas could help restore kelp forests while producing valuable seafood, but more research is needed on developing economically viable and sustainable formulated feeds with new supplements.
Abstract
This review analyzes current research on short-term culture of sea urchin from barrens through formulated feed, addressing the need for sustainable aquaculture practices and ecological restoration of kelp forests. We compare the results of multiple studies to identify the optimal feed composition to induce gonad growth and coloration. Our analysis suggests that macroalgae are the best feed ingredients to improve gonad growth and coloration; however, environmental and economic challenges persist in expanding sea urchin production with these types of ingredients. Plant-based protein sources like soy have emerged as a potential cost-effective alternative to fish products; nevertheless, the presence of antinutritional factors in soy products limits their inclusion in formulated feed. Regarding the composition and amount of lipids, we found that they are critical macronutrients in gonad development. The review also explores the potential of sea urchin aquaculture in mitigating urchin barrens and restoring kelp forests, highlighting the interplay between ecological and economic factors. We identify key knowledge gaps and propose future research directions, including large-scale economic viability assessments, novel feed additives, and integrated multitrophic aquaculture systems. These findings have significant implications for developing sustainable and economically viable sea urchin aquaculture, potentially transforming urchin barrens into productive ecosystems while meeting market demand for roe.
1. Introduction
The global market for sea urchin gonads, known as uni or roe, has experienced substantial growth driven by expanding consumer demand and high market value [1]. Sea urchin gonads are preferred for their large size [2], bright orange or yellow color [3] (Figure 1b,c), high content of sweet-tasting alanine, strong umami, and low content of bitter-tasting arginine [4]. In addition, Takagi et al. [4] show the role of urchin diet in relation to odor-active compounds from butyl acetate and amino acids for umami as essential flavor components of high-quality gonads. The annual average price of sea urchin gonads at the Tokyo Metropolitan Central Wholesale Market, the largest sea urchin market in the world, has nearly doubled over the past two decades, with uni reaching USD 131/kg in 2018 [5]. However, the growing demand for uni has resulted in significant pressure on wild sea urchin populations (e.g., in the Mediterranean region), leading to the depletion of natural stocks and disrupting marine ecosystem balance [6,7,8].
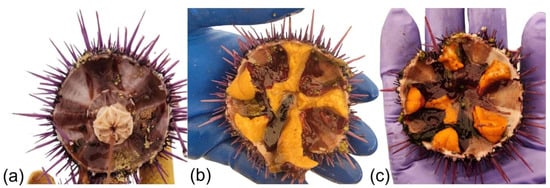
Figure 1.
Purple sea urchin, Strongylocentrotus purpuratus, gonads. (a) Sea urchin collected from urchin barren with no marketable gonads; (b) yellow and (c) orange gonads. All images constitute original content from the authors and are used as examples for coloration.
Paradoxically, in other coastal areas, climate change-related processes combined with the overharvesting or disappearance of sea urchin predators have led to the proliferation of sea urchin populations, creating “urchin barrens”—areas of low biodiversity dominated by sea urchins that have overgrazed macroalgae [9,10,11,12,13]. In these barrens, urchin densities can reach hundreds of individuals m−2 in three-dimensional aggregations that affect kilometers of coastline [14]. While sea urchins have great commercial value, those from barrens yield poor-quality gonads or even no gonads at all (Figure 1a) due to nutritional deficiencies, rendering them commercially unviable [15,16,17,18,19,20].
The expansion of urchin barrens over the past five decades has been causing significant ecological consequences, particularly in temperate rocky ecosystems dominated by kelp forests [4,10,13,21,22,23,24,25,26,27]. These macroalgal forests, primarily comprising brown algae of the order Laminariales, red algae, and green algae, are among the most productive marine biomes [28,29,30]. However, kelp forests have experienced an alarming 40–60% decline worldwide due to complex abiotic and biotic stressors, with many regions transitioning to urchin barrens [22,30].
The transition from kelp forests to urchin barrens has been documented globally, including areas in the U.S.A, Mexico, Australia, Tasmania, New Zealand, Korea, Japan, and Norway [21,22,23,27,31,32,33,34,35,36,37,38]. Once established, urchin barrens can persist for years or even decades due to positive feedback mechanisms, making the transition back to kelp forests difficult [39]. One short-term strategy to restore kelp forests is to significantly reduce urchin abundances through removal efforts [21]. Nonetheless, the scalability and economic feasibility of such removals require innovative biotechnological solutions to add value to sea urchins obtained from barrens.
Aquaculture represents an opportunity to aid in the removal of sea urchins from barrens and simultaneously address market demand for high-quality uni. While full life-cycle aquaculture exists, its profitability has been hindered by the relatively slow growth of sea urchins, taking up to 3 years to reach market size [4,40,41,42]. In contrast, gonad enhancement of wild-caught sea urchins can be achieved in just 6–12 weeks [17,18,20,43]. The latter approach, known as sea urchin ranching, has been proposed as a restorative aquaculture strategy to generate economic benefits for coastal communities and induce the production of sustainable food while restoring macroalgae in local removal areas [7,44,45].
For example, the Norwegian coastline has experienced extensive kelp forest decline due to green sea urchin (Strongylocentrotus droebachiensis) overpopulation, with urchin barrens covering hundreds of kilometers of formerly productive kelp habitat. In response, Norway has pioneered both mechanical removal programs and experimental quicklime (CaO) treatments to reduce urchin densities and facilitate macroalgal recovery [35]. Within 2 years post-removal, macroalgal cover increased from 0% to 40–80% at depths of 2 and 5 m, respectively. As pioneers, Norwegian sea urchin ranching operations have successfully enhanced wild-caught S. droebachiensis to commercial quality within 9 weeks using formulated feeds [46]. This case study indicates the potential of sea urchin ranching as an integrative strategy to increase the economic and long-term viability of kelp forest restoration programs.
Today, sea urchin ranching operations remain limited but are growing, with pilot projects and small-scale commercial companies operating in Japan, Norway, Canada, New Zealand, and the United States [7]. Feed is the most important factor in determining the success of sea urchin ranching. Macroalgae are the natural food of sea urchins; however high prices, unstable supply, temporal and seasonal variation in quality and/or quantity, negative environmental effects from large-scale harvesting of wild populations, and the scarcity in affected barren areas make macroalgae unlikely to be commercially viable for scaling up operations [47,48,49,50]. Therefore, formulated feed is crucial for the ongoing expansion and sustainable development of this industry.
Formulated feed has the advantage of balanced and precise nutrition, convenient storage, stable supply, and effectiveness in providing functional substances and bioactive compounds. Additionally, formulated feed can promote higher gonad indices in sea urchins with better conversion ratios than macroalgae [17,51,52]. In comparison, in the South African abalone (a gastropod herbivore from the genus Haliotis) industry, the use of formulated feed is generally less expensive than the use of fresh macroalgae due to availability, accessibility, transport, and food conversion ratios [53]. In that sense, one of the most pressing goals in sea urchin aquaculture is the development of formulated feed that can effectively mimic the nutritional complexity and variability of natural diets while maintaining palatability, consumability and digestibility [7,54]. The challenge extends beyond simply matching the macronutrient profiles of macroalgae, but also involves the intricate balance of pigments and other bioactive compounds to achieve gonad enhancement and quality while simultaneously ensuring the cost-effectiveness and sustainability of the feed [50,55,56].
Despite the progress in formulated feed development, challenges persist in optimizing nutrient utilization and mitigating the potential negative effects of plant-based ingredients. Biotechnological approaches, particularly the use of functional feed additives such as probiotics, prebiotics, phytobiotics, and enzymes, offer promising solutions to these challenges [57]. Probiotics (e.g., Bacillus spp.) can enhance nutrient utilization, improve growth performance, strengthen immune response, and enhance reproductive performance in aquatic species; moreover, probiotics can improve water quality and reduce the use of antibiotics in aquatic systems [58,59,60,61,62,63]. Prebiotics (e.g., inulin, fructooligosaccharides and transgalactooligosaccharides) may improve gut health and feed efficiency [64]. Phytobiotics (e.g., alkaloids, terpenoids, tannins, saponins, glycosides, flavonoids, phenolics, steroids or essential oils), derived from plants, have shown potential in reducing oxidative stress, increasing antimicrobial activity, and enhancing growth performance [65]. Enzymes (e.g., phytase, protease, carbohydrase, and lipase), particularly those targeting antinutritional factors in plant-based ingredients, can significantly improve nutrient availability and reduce the environmental impacts of aquaculture [66]. These biotechnological interventions may play a crucial role in developing more efficient and sustainable feed formulations for sea urchin ranching, potentially addressing both nutritional and ecological concerns.
The economic imperative for proper coloration becomes particularly acute in the context of sea urchin ranching from barren areas, where urchins typically present with pale, commercially unviable gonads due to nutritional deficiencies. Without feed formulations capable of reliably producing market-preferred colors within the short culture period (6–12 weeks) necessary for economic feasibility, the restorative aquaculture model cannot succeed commercially. Thus, understanding the nutritional factors—particularly the carotenoid content, composition, and lipids necessary for their absorption—that determine gonad coloration is as critical as optimizing for gonad size and growth rate.
The objective of this review is to analyze the effects of formulated feed on sea urchin gonad enhancement and coloration across multiple sea urchin barren-forming species. By synthesizing current research, we aim to evaluate the sustainability of feed composition and its impact on gonadosomatic index growth rate and marketable coloration qualities. This comprehensive analysis will provide insights into the nutritional strategies, economic and ecological considerations, and biotechnological frontiers of short-term sea urchin culture, with implications for both the aquaculture industry and marine ecosystem management.
2. Sea Urchin Species with Ranching Potential
The varied ecological contexts and several sea urchin species that form barrens demand a diverse approach to sea urchin ranching. Each species has unique characteristics that influence their suitability for ranching operations (Table 1), underscoring the need for species-specific strategies in feed formulation.
The most extensively ranched species is the green sea urchin S. droebachiensis. Its prominence is largely due to decades of research and development in Norway, which has established a strong foundation for commercial ranching practices [46,49,67,68,69,70,71]. Moreover, S. droebachiensis possesses several characteristics that make it particularly suitable for ranching operations (Table 1): (1) It has a wide temperature tolerance range with an Arctic boreal distribution that facilitates ranching initiatives across multiple regions [70]; (2) it forms extensive barrens, causing significant ecological impacts that ranching can help mitigate [72]; (3) it exhibits rapid gonad development under optimal feeding conditions [52]; and (4) its roe generally meets good market quality standards [49].
Another species with outstanding potential is the purple sea urchin, Strongylocentrotus purpuratus. Its wide distribution along the North American Pacific coast, temperature range (12–20 °C), and rapid gonad growth (8 weeks) under optimal feed condition make it an attractive option for ranching (Table 1). Additionally, it forms extensive barrens, resulting in a >90% decline in kelp forests in some areas [22], impacting valuable fisheries like the red sea urchin Mesocentrotus franciscanus, which is worrisome for small-scale fishing communities that depend on these resources [73,74]. Moreover, the full-year open fishery for S. purpuratus in Baja California, Mexico [75], provides a critical advantage for continuous ranching operations, enhancing the economic viability and scalability of such initiatives in the region.
Other sea urchin species that show promise for ranching are shown in Table 1. These include M. franciscanus [76], Evechinus chloroticus [77,78], Tripneustes gratilla [47,79], Heliocidaris erythrogramma [16,56,80,81,82], Paracentrotus lividus [83,84,85], and Mesocentrotus nudus [4,5,6,18,20,86,87]. It is important to note that different sea urchin species may respond differently to formulated feed in terms of gonad enhancement and quality. Therefore, the results and interpretations presented in this review should be approached with caution, and further species-specific research is warranted to optimize feed formulation for each target species in sea urchin ranching operations.

Table 1.
Characteristics of sea urchin species with potential for commercial ranching and gonad enhancement.
Table 1.
Characteristics of sea urchin species with potential for commercial ranching and gonad enhancement.
| Species | Temperature Range (°C) | Key Distribution Areas | Wild Densities (ind/m2) | Max Size (mm) | GI (%) | Market Demand | ERP | Key Considerations for Ranching | References |
|---|---|---|---|---|---|---|---|---|---|
| Strongylocentrotus droebachiensis | −1–20 | Arctic boreal distribution | 0.1–70 (barrens up to 300) | 80 | 21 with FF in 9 weeks. | High | High | Adapts well to various feed types; potential for kelp forest restoration. | [32,52,71,88] |
| Strongylocentrotus purpuratus | 12–20 | Northeastern Pacific from Alaska, U.S.A. to Isla Cedros, Baja California, Mexico | 0.5–50 (barrens up to 200) | 100 | 24 with FF vs. 11.7 with kelp Macrocystis pyrifera in 9 weeks. | High | High | Forms extensive barrens; good candidate for restorative aquaculture. | [17,22,89,90,91,92,93] |
| Mesocentrotus nudus (formerly Strongylocentrotus nudus) | 5–25 | East Asia (China, Russia, Korea, Japan) Range extension towards the Pacific coast of Japan-Akkeshi Bay) | 0.5–10 (barrens up to 100) | 80 | 8.9 to 24 in 6 weeks fed sporophylls of Undaria pinnatifida 9.1 to 23.2 with formulated feed. 16 with Saccharina ochotensis. Up to 38.5 in an Eisenia bicyclis bed and 14.7 in barrens. | Highest prices | High | Valuable species in Asian markets; responsive to both formulated and macroalgae diets. | [5,20,48,50,86,94,95,96] |
| Mesocentrotus franciscanus (formerly Strongylocentrotus franciscanus) | 8–14 | Northeastern Pacific from Alaska to Baja California | 0.1–30 (barrens up to 40) | 180 | 20 with FF and kelp Nereocystis luetkaena in 12 weeks vs. 11 from the wild organisms from the fishery in the same season. | High | Medium | Valuable fishery species; bigger gonads but slower growth rates than S. purpuratus. | [76,90,91,93,97,98,99,100,101] |
| Evechinus chloroticus | 10–18 | Aotearoa New Zealand | 0.1–20 (barrens up to 50) | 170 | 12.3 with FF in 8 weeks. | Medium | Medium | Important in New Zealand fisheries; requires specific feed formulations. | [77,102,103,104] |
| Tripneustes gratilla | 24–30 | Indo-Pacific with ranching in South Africa | 0.1–10 | 150 | 22 with FF and 12 with green Ulva rigida in 12 weeks. | Medium | Low | Fast growth; potential for tropical aquaculture. | [47,79,105,106,107] |
| Heliocidaris erythrogramma | 12–23 | Southern Australia | 1–4 (barrens 10–192) | 100 | 21.7 with FF in 12 weeks. | Medium | Medium | Adapted to temperate waters; good response to formulated feed. | [31,82,108,109,110,111] |
| Paracentrotus lividus | 10–25 | Northeastern Atlantic and Mediterranean. | 1–50 (barrens > 15) | 70 | 19 with FF in 17 weeks. | High | Medium | Important in European markets; sensitive to handling. | [84,112,113,114,115,116] |
Note: population densities and maximum gonadosomatic indices (GIs) can vary significantly depending on geographical location, season, and environmental conditions. The values presented here are representative ranges based on the available literature but may not encompass all possible scenarios. Gonad enhancement results are from controlled studies and may differ in commercial settings. Feed responses may vary based on specific formulations and environmental factors. Market demand and ecological restoration potential are general assessments and may change over time or differ by region. Readers are encouraged to consult the most recent and location-specific literature for precise figures and up-to-date information. ERP = ecological restoration potential; FF = formulated feed.
3. Sea Urchin Formulated Feed Development
3.1. Sea Urchin Natural Diets
The natural diet of sea urchins, primarily composed of various macroalgae species, plays a crucial role in both the nutritional health of these echinoderms and broader ecosystem dynamics [12]. The complex dietary profile of macroalgae, as evidenced in Table 2, underscores the challenge faced in developing formulated feed for gonad enhancement. The wide ranges observed in protein (2.5–44%), carbohydrate (23–75%), and lipid (0.1–4%) content across different macroalgae species reflect the diverse and dynamic nutritional landscape that sea urchins have adapted to over evolutionary time.
This nutritional variability also provides valuable insights into the profile that formulated feed should aim to replicate. Importantly, the proximate analyses of these macroalgae species point towards a medium-protein, high-carbohydrate, and low-lipid profile, aligning with our findings, which will be discussed in subsequent sections.

Table 2.
Nutritional composition of macroalgae species in the natural diet of various sea urchin species.
Table 2.
Nutritional composition of macroalgae species in the natural diet of various sea urchin species.
| Sea Urchin Species | Macroalgae | Type | Protein Range (%) | Carbohydrates Range (%) | Lipid Range (%) | References |
|---|---|---|---|---|---|---|
| Strongylocentrotus droebachiensis | Laminaria spp. | Kelp | 3–21 | 35.8–60.2 | 0.5–3.9 | [117,118,119,120] |
| Saccharina spp. | Kelp | 3–21 | 39.3–68.4 | 0.4–3.6 | ||
| Strongylocentrotus purpuratus | Macrocystis pyrifera | Kelp | 3–13.2 | 47.2–75.3 | 0.7–1.6 | [121,122,123] |
| Nereocystis luetkeana | Kelp | 2.5–15.28 | 23–54 | 1.4–4.4 | [76,89,124] | |
| Saccharina latissima | Kelp | 5.1–21 | 39.3–68.4 | 0.2–3.6 | [89,117,118,119] | |
| Heliocidaris erythrogramma | Ecklonia radiata | Kelp | 4.1–7.8 | 58–71.8 | 0.1–1.7 | [125] |
| Undaria pinnatifida | Kelp | 11–24 | 38.7–53.2 | 1.4 –3.5 | [118] | |
| Paracentrotus lividus | Posidonia oceanica | Seagrass | 4.1–4.8 | 56–60 | 2.1–3.2 | [126,127] |
| Rissoella verruculosa | Red | 3–12.6 | 30.5–41.7 | 0.8–1.7 | [127] | |
| Evechinus chloroticus | Ecklonia radiata | Kelp | 4.1–7.8 | 58–71.8 | 0.1–1.7 | [125] |
| Carpophyllum spp. | Brown | 4–8 | 36.8–65 | 1.8–2.5 | [128] | |
| Ulva spp. | Green | 4–44 | 38.5–61.5 | 0.1–2.9 | [118,120,129,130] | |
| Mesocentrotus nudus | Saccharina japonica | Kelp | 3–21 | 39.3–68.4 | 0.4–3.6 | [117,118,119,120,131] |
| Undaria pinnatifida | Kelp | 11–24 | 38.7–53.2 | 1.4 –3.5 | [118] | |
| Tripneustes gratilla | Sargassum spp. | Brown | 9–20 | 37.4–49.8 | 1.2–2.9 | [118] |
| Kappaphycus alvarezii | Red | 6.2–6.8 | 42.1–54.3 | 0.9–1.0 | [132] | |
| Mesocentrotus franciscanus | Macrocystis pyrifera | Kelp | 5.1–12.7 | 38.6–59.4 | 0.7–1.1 | [121,122,123] |
| Nereocystis luetkeana | Kelp | 2.5–15.3 | 23–54 | 1.4–4.4 | [76,89,124] | |
| Eisenia arborea | Kelp | 5.5–11.7 | 43.3–54.3 | 0.45–0.66 | [133] |
Note that the protein, carbohydrate, and lipid values are approximate and may vary depending on factors such as geographical location, season, and the specific part of the macroalgae analyzed.
3.2. Nutritional and Economic Considerations of Formulated Feed for Sea Urchins
The development of formulated feed for sea urchin gonad enhancement presents significant ecological and economic challenges, particularly in sourcing sustainable and cost-effective ingredients (Table 3). Traditionally, the development of aquaculture feed, including sea urchin feed, has relied primarily on fish meal and fish oil as primary sources of protein and essential fatty acids [134,135]. However, these ingredients depend on exploiting wild fish stocks, contributing to overfishing and potentially disrupting marine food chains [136,137]. Moreover, the fishing and processing methods used to produce these ingredients can produce a substantial carbon footprint, contributing to climate change [138,139]. In addition, the high costs associated with the incorporation of fish products into formulated feed impair the economic feasibility of large-scale sea urchin ranching with these ingredients [135]. Fish meal is always subject to price volatility due to the increasing demand from various sectors, including human nutrition and other aquaculture operations [140,141,142]. Finally, fish meal is associated with lower quality taste (bitterness and oiliness) in sea urchin gonads [143,144].
While sustainability and cost concerns have driven exploration of alternatives to fish meal, its nutritional attributes deserve recognition. Fish meal provides highly digestible protein (>60% digestibility), an amino acid profile closely matching aquatic animal requirements (Table 3), and essential omega-3 fatty acids (EPA, DHA) [135]. These attributes explain its historical dominance in aquaculture feeds. The challenge lies not in fish meal’s nutritional inadequacy, but rather in the economic and environmental constraints of scaling its use to meet expanding aquaculture demands globally. Thus, substitution efforts should be viewed as responses to supply and sustainability limitations rather than rejections of fish meal’s nutritional value.
To address these challenges, researchers have explored alternative ingredients, with particular focus on macroalgae and plant-based products. Yet, the nutritional composition and economic considerations of these ingredients play crucial roles in determining their viability for sea urchin feed (Table 3).
Macroalgae are a natural component of the sea urchin diet; therefore, they are often incorporated as a main ingredient of their formulated feed. Brown (kelps S. japonica and U. pinnatifida), green (Ulva spp.), and red (Palmaria palmata) macroalgae have been used in feed with good results [17,47,52]. These macroalgae offer unique nutritional profiles with bioactive compounds linked to gonad quality [48]. For instance, kelp species contain high levels of β-carotene (13–30 μg/g), fucoxanthin (0.002–6 μg/g), and lutein (0.01–0.3 μg/g), which are linked to development and high-quality colors in sea urchin gonads [49,85,145,146]. In contrast, these compounds are absent or present in much lower quantities in soybean and fish meal (Table 3), suggesting that even small inclusions of macroalgae in formulated feed could significantly enhance gonad growth and coloration [5,17,47,52,147].
The free amino acids (FAAs) resulting from sea urchins consuming macroalgae are closely associated with the taste of their gonads [4,18,20]. Kaneko et al. [148] categorized the FAAs into three groups: umami-tasting (aspartic acid and glutamic acid), sweet-tasting (alanine, glycine, serine, proline, and threonine), and bitter-tasting (arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, tyrosine, and valine). The FAA composition in gonads is influenced by both the amino acid profile of dietary proteins and the sea urchins’ metabolic processes during protein digestion and amino acid metabolism [4,5]. This relationship between feed protein sources and resulting gonad FAA profiles presents challenges when incorporating different compounds, like fish and soybean meal, into sea urchin formulated feed while maintaining a balance to obtain marketable-quality gonads [5,78,149] (Table 3).
Using macroalgae as main ingredients in formulated feed leads to several hurdles. Large-scale harvesting of wild macroalgae may worsen the current ecological problem and increase diet costs [150,151]. Cultivating macroalgae specifically for feed production could be another option, but premium macroalgae often fetch high prices in human food, cosmetic, and pharmaceutical markets, making their use economically challenging for mass feed production [152] (Table 3). Moreover, the prices of cultivated macroalgae will vary greatly depending on where it is used, making it unviable in regions without large-scale culture.
Given these challenges, plant-based ingredients, particularly soybean products, have emerged as a promising alternative. Soybean products could offer advantages, including lower cost, constant availability, and a relatively high protein content [135] (Table 3). Moreover, soybean meal (400–500 USD/ton) is significantly cheaper than fish meal (1500–2000 USD/ton) and macroalgae (>1000 USD/ton) (Table 3). While soybean products offer potential advantages as alternative protein sources in sea urchin feed, their implementation faces several key challenges that require careful consideration.
The presence of antinutritional factors (ANFs) in soybean products can affect nutrient absorption and sea urchins’ health, limiting its supplementation in formulated feed [135,153]. Specifically, trypsin inhibitors and toxic oligosaccharides can reduce physiological functions and sea urchin survival [154]. Pearce et al. [52] obtained 15% mortality in S. droebachiensis fed a diet with 32% macroalgae (P. palmata and Ascophyllum nodosum) and an inclusion of 27% soybean meal. Similar mortality results have been observed when substituting 30% of fish meal with soy protein concentrate and soybean meal in diets for Totoaba mcdonaldi [155] and Oreochromis niloticus [61], respectively. These mortalities have been associated with increased enzymatic production in hepatopancreas related to trypsin inhibitors, resulting in inflammation and intestinal inflammation due to the oligosaccharides present in soy products. On the other hand, Cyrus et al. [47] obtained a mortality rate of 7% in T. gratilla using a plant-based diet (Maize, wheat bran, lucerne meal, and premium HP 300 soy concentrate without ANFs) with 20% Ulva spp. inclusion. In this sense, it is critical to develop new feed additives to improve the absorption and utilization of plant-based nutrients, especially proteins and carbohydrates. Moreover, exploring methods to reduce or neutralize ANFs (probiotics, prebiotics, phytobiotics, and enzymes) in soybean products is key for improving feed efficiency and reducing mortality rates [155,156,157]. Finally, soybean products may present amino acid imbalance, particularly in methionine and lysine, compared to brown macroalgae (Table 3).
Despite these challenges, soybean products represent a more economically viable and environmentally sustainable option for scaling up sea urchin ranching in the short term. Still, it must be carefully balanced to meet nutritional efficacy and health requirements. Future research should focus on optimizing soybean-based formulations that can meet the dietary needs of sea urchins without compromising health, growth rates, and gonad quality.
Alternative protein sources from agricultural byproducts are another option being explored to develop sustainable sea urchin feed. These include preprocessed grain residues, fermented plant proteins, and agricultural processing wastes that could provide cost-effective nutrient sources while supporting circular economy principles [17,20,46]. However, these alternatives must be carefully evaluated for their effects on gonad quality, particularly taste and color, as well as their potential impact on sea urchin health and growth performance.
The economic challenges of macroalgae-based feeds could be partially addressed through innovative sourcing strategies. One potential approach involves utilizing byproducts from human-grade algae processing. These offcuts, which would otherwise be considered waste material, often retain significant nutritional value and beneficial compounds that could enhance gonad quality. For example, sporophylls of U. pinnatifida have shown success in enhancing gonad quality in M. nudus [17,20]. Additionally, fouling algae, particularly Ulva species growing naturally on oyster and mussel farms, represents an untapped resource that could be harvested and incorporated into sea urchin feed formulations. This approach not only provides a sustainable feed source but also creates additional value streams for existing aquaculture operations while potentially reducing their maintenance costs [47].
Nonetheless, these alternatives, while interesting from sustainability and circular economy perspectives, require substantial research and development before they can be implemented at commercial scales. The priority for advancing sea urchin aquaculture remains the development of standardized formulated feeds that balance economic viability with consistent production of high-quality gonads. Such feeds must be readily available, cost-effective, and capable of producing marketable gonads within the 6–12-week culture period that makes sea urchin ranching commercially attractive [17,47,52,79]. While exploration of alternative protein sources should continue, the immediate focus should be on optimizing existing formulations that have demonstrated success in gonad enhancement and quality, perhaps incorporating these alternative ingredients incrementally as their efficacy is proven through rigorous testing.

Table 3.
Comparative analysis of nutritional composition, bioactive compounds, and estimated costs of key ingredients used in sea urchin formulated feeds.
Table 3.
Comparative analysis of nutritional composition, bioactive compounds, and estimated costs of key ingredients used in sea urchin formulated feeds.
| Costs and Macronutrients | SBM [158,159] | Wakame [160,161] | Rockweed [162,163] | Sea Lettuce [164,165] | Kombu [161,166] | Fish Meal [167] |
|---|---|---|---|---|---|---|
| Costs (USD/ton) | 400–500 [168] | 2000–4000 [169] | 1000–1500 [170] | 2000–5000 [169] | 3000–6000 [169] | 1500–2000 [168] |
| Macronutrients (%) | ||||||
| Protein | 48 | 16.3 | 8.7 | 15.7 | 6.2 | 70 |
| Carbohydrates | 34 | 37 | 42.6 | 33.22 | 35 | - |
| Lipids | 0.7 | 1 | 2.14 | 3.8 | 2 | 12 |
| Carotenoids (μg/g) | ||||||
| β-carotene | 0.69 | 13 | 0.096 | 0.5 | 29.9 | - |
| Fucoxanthin | 0 | 0.8 | 2.52 | 0.69 | 1.12 | - |
| Zeaxanthin | 0.002 | 0 | 0.58 | 9.47 | 6 | - |
| Lutein | 0.0193 | 0.015 | 0.052 | 10.23 | - | - |
| Amino acids (g/100 g protein DW) | ||||||
| Phenylalanine + Tyrosine | 2.64 | 12.11 | 0.5 | 8.79 | 3.56 | 3.8 |
| Methionine + Cysteine | 0.67 | 5.99 | 0.15 | 4.32 | 3.45 | 3.6 |
| Threonine | 1.87 | 7.33 | 0.36 | 6.31 | 3.41 | 4.1 |
| Valine | 2.45 | 16.84 | 0.35 | 9.15 | 6.01 | 4.9 |
| Isoleucine | 2.35 | 7.91 | 0.29 | 4.76 | 2.61 | 4.3 |
| Leucine | 2.69 | 13.70 | 0.53 | 8.25 | 4.45 | 7 |
| Lysine | 2.69 | 11.12 | 0.43 | 6.50 | 4.77 | 7.5 |
| Histidine | 1.2 | 5.25 | 0.12 | 1.39 | 2.38 | 2.2 |
| Alanine | 2.16 | 27.20 | 0.65 | 9.16 | 4.51 | 7.7 |
| Arginine | 3.74 | 8.41 | 0.31 | 6.19 | 2.96 | 5.8 |
| Aspartic acid | 5.66 | 10.18 | 0.84 | 12.90 | 4.69 | 10 |
| Glutamic acid | 9.84 | 10.65 | 1.71 | 12.94 | 3.86 | 20.8 |
| Glycine | 2.11 | 8.76 | 0.41 | 6.65 | 3.31 | 4.1 |
| Tyrosine | 1.87 | 4.31 | 0.16 | 5.99 | 1.74 | 5.1 |
| Serine | 2.5 | 5.76 | 0.37 | 6.94 | 2.45 | 4.8 |
Note: Values presented in this table are representative averages based on the available literature and may vary significantly depending on factors such as geographical origin, season, processing methods, and market conditions. Prices are subject to fluctuation based on supply and demand, transportation costs, and global economic factors. The content of bioactive compounds (e.g., carotenoids, amino acids) can vary substantially based on species, growing conditions, harvesting time, and post-harvest processing. Nutritional composition may also differ depending on the species or the specific part of the ingredient analyzed (e.g., whole algae vs. specific tissues). Readers should consider these values as indicative rather than absolute and are encouraged to consult the most recent and relevant sources for specific applications. The absence of a value does not necessarily indicate that the compound is not present but may reflect a lack of data in the reviewed literature. SBM = soybean meal; DW = dry weight; USD = United States dollars.
4. Effect of Formulated Feed on Sea Urchin Gonad Enhancement and Coloration
4.1. Literature Review and Data Extraction
To analyze the effects of macronutrients’ proximate composition and feed ingredients on sea urchin gonad growth rate and coloration, we conducted a comprehensive literature review using Web of Science, Scopus, and Google Scholar databases. Our search employed keywords such as “sea urchin,” “gonad enhancement,” “formulated feed,” “gonad production,” “gonad size,” “protein requirement,” “optimum protein,” “dietary protein,” and “gonad color” and specific sea urchin species names, including the former names Strongylocentrotus franciscanus and Strongylocentrotus nudus. We included studies reporting feed proximal, nutrient profile, and gonad enhancement results using the gonadosomatic index (GI) and color values. We extracted data on protein, carbohydrate, and lipid content. To standardize values between studies, when data was provided with moisture, we converted to dry weight using the following formula:
We also extracted fish meal, soybean meal, and macroalgae meal percentage when available. Data from 26 experimental treatments across 15 studies were compiled, covering 8 sea urchin species: S. purpuratus, S. droebachiensis, E. chloroticus, T. gratilla, M. franciscanus, H. erythrogramma, M. nudus, and P. lividus. The studies spanned from 1997 to 2025, with experimental durations ranging from 42 to 120 days or 6 to 17 weeks (Appendix A Table A1 and Table A2).
4.2. Data Analysis and Visualization
To standardize comparison across studies with different durations, we calculated the weekly GI increase (WGII, %):
where GI final is the final gonadosomatic index, GI initial is the initial gonadosomatic index, and the number of weeks is the duration of the study converted to weeks. This calculation method allows for a straightforward comparison of gonad growth rates across studies with different lengths and different initial GIs.
For color analysis, we employed a two-pronged approach. When studies provided CIE Lab* color values, we converted these to RGB values and positioned them on a standardized color table https://colordesigner.io/ (accessed on 12 April 2025). We used these directly for studies that provided qualitative color descriptions. We then categorized colors into a qualitative range from best to worst market quality: orange, orange/yellow, yellow, pale orange, pale yellow, and pale yellow/brown. This approach allowed us to integrate quantitative and qualitative color data from diverse studies into a unified analysis framework, facilitating comparisons of gonad color quality across different experiments. Lastly, to explore the relationship between feed composition, nutrient profile, and gonad enhancement outcomes, we developed a series of interactive 3D scatter plots using R (version 4.4.1) with the tidyverse, plotly, and htmlwidgets packages. Compiled data on formulated feed composition and gonad enhancement results for various sea urchin species are available in Appendix A Table A1. Data on feed ingredients and color analysis results are available in Appendix A Table A2.
While our analysis encompasses the available peer-reviewed literature meeting our inclusion criteria, the relatively modest sample size (26 treatments, 15 studies, 8 species) represents a limitation. Additional controlled feeding trials across more species, environmental conditions, and feed formulations would strengthen confidence in the identified optimal composition ranges and ingredient recommendations. Particularly needed are (1) standardized multi-species comparisons using identical feed formulations, (2) studies on additional barren-forming species from underrepresented regions, and (3) longer-term trials evaluating effects beyond single growth cycles. The patterns identified in this review provide a foundation for such expanded research efforts.
4.3. Effect of Proximate Macronutrient Composition on Gonad Growth Rate and Color
Our analysis revealed interesting relationships between macronutrient composition and sea urchin gonad growth rate (Figure 2a) and color (Figure 2b). The highest growth rates (1.66–1.73% WGII) and optimal colors were achieved with diets containing 21–28% protein, 48–53% carbohydrates, and 1.2–4% lipids. These findings provide valuable insights into optimal feed formulations for sea urchin gonad enhancement.
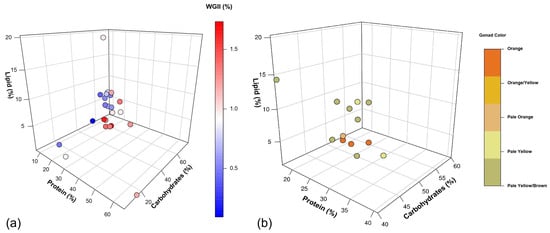
Figure 2.
The effect of macronutrients (protein, carbohydrates and lipids) in formulated feed in sea urchin gonad enhancement. (a) Weekly gonadosomatic index increase (WGII, %) of sea urchin fed with different proximate compositions (protein, carbohydrates, and lipids, %). The color gradient represents the WGII, ranging from low (blue) to high (red) values. (b) Relationship between proximate composition and marketable gonad color. The color of each point represents the resulting gonad color, ranging from orange (best) to pale yellow/brown (worst) quality. The best values used for discussion are from [17,47,52], respectively. For more information, refer to Appendix A, Table A1 and Table A2, where all the data is available.
4.4. Protein Composition
Proteins are fundamental components of all multicellular organisms, playing crucial roles in structure, function, and metabolism [171]. Sea urchins in their natural habitats must consume and digest large quantities of food with relatively low protein content to meet their dietary requirements [172]. This is particularly relevant when developing formulated feed as protein is typically the most expensive macronutrient in aquaculture feed.
Protein content in diets greatly influences gonad growth rates in sea urchins, as it provides energy and essential amino acids for growth and reproduction [43,55,68,134,173]. Interestingly, diets with higher protein content tend to result in lower consumption rates, while low-protein diets stimulate increased feed intake [50,55,134]. This relationship was demonstrated by Hammer et al. [134] in their study with Lytechinus variegatus. They found that sea urchins fed a diet containing 20% protein showed superior performance across multiple parameters—including consumption, survival, specific growth rate, and gonad production efficiency—compared to those fed diets with either 9% or 31% protein. Moreover, the 20% protein diet resulted in the highest protein efficiency ratio, indicating optimal protein utilization.
These findings align well with our analysis, which identified highest gonad growth rates at intermediate protein levels of 21 to 28% (Figure 2a). This range appears to reach a balance between providing sufficient protein for growth and avoiding the reduced feed intake associated with high-protein diets. The consistency between our findings and previous studies strengthens the case for optimizing protein levels in sea urchin feed. However, it is important to note that gonad growth is influenced not only by the quantity of dietary protein but also by its source, digestibility, and absorption efficiency [80]. Different protein sources, like fish meal and soybean products, can vary significantly in their amino acid profiles and bioavailability, which in turn affects their nutritional value for sea urchins’ development (Table 3).
Importantly, Takagi et al. [50] suggest that protein content has been overestimated in diets for sea urchins. They evaluated protein leaching in formulated diets after immersion in sea water and concluded that leaching is important even in diets that have not disintegrated. They suggested wheat gluten as a binder due to its viscoelasticity and reported that the optimum level of protein in gluten-based formulated feed for M. nudus was 11–13%. This is lower than previous studies, yet as edible sea urchins prefer Laminariales kelp with 4–16% protein, this result seems reasonable and should be further investigated.
The optimization of protein content in sea urchin feed presents an opportunity to balance nutritional efficacy with economic viability. Future research should focus on identifying protein sources that offer the best combination of nutritional value, digestibility, and cost-effectiveness for sea urchin gonad enhancement. Additionally, investigating the potential for protein-sparing effects through the optimization of non-protein energy sources (e.g., carbohydrates and lipids) could further improve feed efficiency and reduce costs.
4.5. Carbohydrate Composition
Carbohydrate content in sea urchin diets plays a crucial role in overall nutrition and gonad enhancement. One of its primary functions is the protein-sparing effect, where carbohydrates serve as the main energy source, allowing proteins to be reserved for tissue growth and repair rather than being catabolized for energy [134]. This emphasizes the importance of optimizing carbohydrate absorption to enhance protein utilization and to reduce the reliance on more expensive and environmentally impactful protein sources, such as fish meal [135,155]. Thus, by improving carbohydrate utilization, some other economic and environmental advantages in aquaculture can be obtained [144].
In natural diets, carbohydrates range from 23 to 75.3% (Table 2), suggesting that sea urchins are adapted to utilize varying levels of carbohydrates efficiently. Our analysis suggests that high carbohydrates (48–53%), combined with medium protein (21–28%) and low lipids (1.2–4%), produced the highest gonad growth rates (Figure 2a) and colors (Figure 2b). Interestingly, the carbohydrate levels in potential feed ingredients like soybean meal and various macroalgae species (33–43%, Table 3) align well. This suggests that blending these ingredients could achieve the desired carbohydrate levels while improving overall nutrient profiles [162].
The relationship between carbohydrates and gonad quality extends beyond growth rates. Pearce et al. [144] suggested that the balance of macronutrients, including carbohydrates, can significantly affect the taste of S. droebachiensis gonads. They found that diets with high protein levels were associated with bitter-tasting gonads and that this could be mitigated by adjusting the carbohydrate content, as glycogen and its breakdown products may contribute to the sweet taste of high-quality sea urchin gonads. However, an excessive level of carbohydrate in diets can cause undesirable changes in the composition and taste of the gonad, highlighting the importance of balancing macronutrients for gonad growth rates and quality while maintaining economic and ecological sustainability.
Using plant ingredients in formulated feed for sea urchin has challenges related to the cellulose content, as sea urchins have limited expression of cellulase genes [174] and cellulose activity [175]. Future research should focus on (1) optimizing carbohydrate types and levels in formulated feed to maximize the protein-sparing effect without compromising gonad quality; (2) investigating the role of different carbohydrate sources (e.g., from various macroalgae or plant-based ingredients) on gonad quality; (3) exploring the potential of carbohydrate-degrading enzymes or probiotics to enhance carbohydrate utilization in sea urchins, particularly in soybean products that contain less digestible complex carbohydrates; and (4) examining the interaction between carbohydrates and other feed components, such as pigments, which may influence gonad coloration and market value.
4.6. Lipid Composition
The role of lipids in sea urchin nutrition and gonad development is complex. Our analysis revealed a threshold for lipid content in formulated feed, with growth rates and color declining when dietary lipid content exceeded 4% (Figure 2a,b). This finding aligns with the natural diet of sea urchins, which typically contains low lipid levels (0.1–4%) (Table 2). Our results are in accordance with those of Suckling et al. [146] and Gibbs et al. [176], who documented that an increment in lipid levels from 2.2% to 8.4% and 1% to 8.8%, respectively, resulted in reduced gonad growth rates. Additionally, Warren-Myers et al. [56] also obtained lower gonad growth rates in H. erythrogramma when fed high (131 g of lipids per kg of feed) and medium (101 g/kg), compared to low (73 g/kg), lipid levels.
This decline in gonad growth rate and color with higher level of lipids could be attributed to several factors: (1) sea urchins’ limited capacity to metabolize high levels of dietary lipids [177]; (2) lipids potentially leading to reduced feed intake or impaired nutrient absorption [178]; (3) high lipid levels potentially disrupting the balance of other essential nutrients, potentially leading to suboptimal nutrient utilization [179]; and (4) lipid oxidation in high-fat diets potentially producing harmful byproducts, potentially causing metabolic stress that could reduce gonad growth rate [180].
Angwin et al. [17] found that despite varying dietary lipid levels from 0.04% to 1.24%, the final gonad lipid composition was between 3.5 to 3.9% in S. purpuratus. Similarly, Liyana-Pathirana et al. [71] reported that S. droebachiensis, whether fed on macroalgae-rich diets or living in the wild, typically had gonad lipid compositions of approximately 3.5–4%. This consistency in lipids concentration suggests tightly regulated lipid homeostasis in sea urchin gonad development [181]. In this sense, our results support these findings, indicating that dietary lipid levels exceeding 4% may affect gonad growth rate (Figure 2a) and color (Figure 2b). These results are in agreement with González-Durán et al. [177], who found that while dietary lipids influenced fatty acid profiles in sea urchin tissues, there are physiological limits to lipid accumulation, particularly in gonads. They noted that excessive dietary lipids could lead to reduced feed intake or impaired nutrient absorption.
The low optimal lipid content for sea urchin feed (1.5–4%) contrasts sharply with the high lipid content of fish meal (~12%, Table 3), which has been used as a major component of sea urchin feed. This discrepancy underscores the need to reconsider the use of fish meal in sea urchin feed, not only for ecological and economic reasons but also for nutritional optimization. Interestingly, both plant-based ingredients like soybean meal and various macroalgae species have lower lipid content (0.7–3.8%, Table 3) compared to fish meal, aligning more closely with the optimal lipid levels for sea urchin gonad enhancement, further supporting the potential of plant-based and macroalgae-derived ingredients in formulated sea urchin feed.
5. Effect of Feed Ingredients on Gonad Growth Rate and Color
Feed ingredients play a crucial role in sea urchin gonad enhancement, influencing both growth rates and marketable color qualities. Our analysis reveals interesting differences in performance between macroalgae-based, plant-based, and fish meal-based diets.
5.1. Macroalgae- and Plant-Based Diets
The highest growth rates (1.66–1.73%) in WGII (Figure 3a) and marketable color qualities (Figure 3b) were observed in diets containing more than 20% of macroalgae [17,47,52], aligning with evolutionary adaptation of sea urchins to efficiently assimilate nutrients from macroalgae [12]. The peak growth rate (1.73% WGII) was achieved with a diet primarily composed of human-grade Kombu, S. japonica [17]. While the exact formulation is proprietary, the absence of fish meal and soybean products suggests a high macroalgae content. However, the high costs associated with human-grade macroalgae could hinder large-scale production and industry scalability.
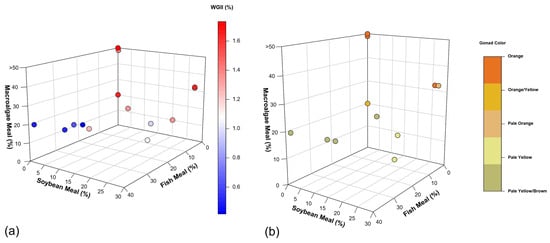
Figure 3.
(a) Weekly gonadosomatic index increase (WGII, %) relationship between fish meal (%), soybean meal (%), and macroalgae meal (%) in diets. The color gradient represents the WGII, ranging from low (blue) to high (red) values. (b) Relationship with marketable gonad colors. The color of each point represents the resulting gonad color, ranging from orange (best quality) to pale yellow/brown (worst quality). The best values used for discussion are from [17,47,52], respectively. For more information, refer to Appendix A, Table A1 and Table A2, where all the data is available.
Pearce et al. [52] reported the second-highest growth rate (1.67% WGII) using a diet containing 32% macroalgae (Ascophyllum nodosum and P. palmata) and 27% soybean meal. This formulation represents a significant step towards cost reduction through the partial substitution of expensive macroalgae with more affordable plant protein. However, it faces challenges including ecological concerns related to wild harvesting of A. nodosum [182] and potential cost increases due to the growing popularity of P. palmata for human and animal consumption [183,184].
Cyrus et al. [47] also achieved a 1.67% weekly growth rate using plant-based feed (maize, wheat bran, soy HP 300 protein, and lucerne meal) with 20% Ulva spp. inclusion. While this formulation achieved the same growth rate with a lower mortality rate (7%) than the formulation used by Pearce et al. [52,155], who obtained a 15% mortality rate using 27% soybean meal, it still faces economic challenges due to the cost of cultivated Ulva spp. and premium HP 300 soy protein without ANFs. This approach, however, demonstrates the potential for diversifying plant-based ingredients and exploring methods to reduce or neutralize ANFs to enhance overall performance in sea urchin feed.
5.2. Fish Meal
Fish meal exceeding 13% in sea urchin feed appears to have adverse effects on gonad growth rate (Figure 3a and Figure 4a) and gonad coloration (Figure 3b and Figure 4b). This negative impact may be attributed to the high levels of animal-derived nitrogen in fish meal. Olmos et al. [155] found that excessive levels of fish meal in aquaculture diets can disrupt nitrogen homeostasis in the cultivated species. Additionally, high levels of fish meal can lead to increased ammonia excretion, potentially causing water quality issues in aquaculture systems. In the case of sea urchins, this excess of nitrogen and poor water quality could potentially interfere with their metabolic processes, leading to reduced gonad growth rate and color quality. In addition, the high lipid content in fish meal (~12%) limits its inclusion in sea urchin feed compared to macroalgae- and plant-based alternatives (0.1–4%; Table 3).
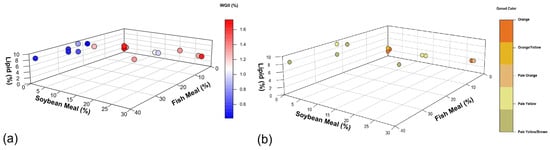
Figure 4.
(a) Weekly gonadosomatic index increase (WGII, %) relationship between fish meal (%), soybean meal (%), and lipids (%) in formulated feed. The color gradient represents the WGII, ranging from low (blue) to high (red) values. (b) Relationship with marketable gonad colors. The color of each point represents the resulting gonad color, ranging from orange (best quality) to pale yellow/brown (worst quality). The best values used for discussion are from [17,47,52], respectively. For more information, refer to Appendix A, Table A1 and Table A2, where all the data is available.
Contrary to 13% fish meal, 27% inclusion of soybean meal does not appear to have a negative effect on growth and color (Figure 4a,b), suggesting promise for plant-based diets [52]. However, Liyana-Pathirana, Shahidi, and Whittick [71] and Woods et al. [103] found that despite a well-balanced proximate composition in a plant-based diet, the resulting gonad protein content was only 8% in S. droebachiensis and E. chloroticus, respectively. These results highlight the need for further research to enhance plant-based protein assimilation efficiency in sea urchin diets to reach the 12% found in wild sea urchin gonads [17,185].
Further incorporation of plant-based ingredients aligns with the aquaculture industry’s trend towards reducing animal-derived protein use due to sustainability concerns and rising costs [141]. However, future studies must optimize blends of macroalgae and soybean to balance performance with cost-effectiveness and sustainability. Moreover, it is crucial to investigate methods to improve digestibility and nutritional value of plant-based proteins for sea urchins, especially soybean [61,62,63,155]. Finally, we should adopt a holistic approach, considering not only growth rates and coloration but also gonad taste, texture, and market acceptability balanced with feed costs and sustainability [2].
5.3. Effect of Feed Ingredients on Gonad Coloration
Gonad coloration in sea urchins is fundamentally determined by the accumulation and metabolic transformation of dietary carotenoids, with market value directly correlating to pigmentation intensity and hue. Since sea urchins cannot synthesize carotenoids de novo, gonadal accumulation depends exclusively on dietary carotenoid concentration and bioavailability. The predominant gonadal carotenoid is 9′-cis-echinenone, accounting for 90–100% of total carotenoids in high-quality, orange-colored gonads, produced through enzymatic transformation of dietary β-carotene. Echinenone is synthesized via β-carotene oxidation in the gut, then transported by low-density lipoproteins to the gonads [3,113]. This lipid-dependent transport explains the observed relationship between dietary lipid levels (1.2–4%) and optimal gonad coloration (Figure 2b), as insufficient lipids impair carotenoid absorption while excessive lipids may cause metabolic imbalances.
5.4. Metabolic Pathways and Compartmentalization
Nutritive phagocytes (NPs) within gonadal tissue mediate carotenoid accumulation and storage through a three-step pathway: (1) dietary β-carotene undergoes enzymatic oxidation by β-carotene ketolase in the gut, introducing a keto group at the C-4 position to form echinenone (4-keto-β-carotene); (2) all-trans-echinenone isomerizes to 9′-cis-echinenone, the thermodynamically stable configuration favored in the lipid-rich environment; and (3) 9′-cis-echinenone is transported in lipoprotein droplets and deposited in NP, creating the characteristic orange coloration valued commercially. Comparative studies across multiple species demonstrate that β-echinenone is the major gonadal carotenoid in 19 of the 20 examined species [186,187,188]. Individuals fed β-echinenone-supplemented diets showed five-fold higher echinenone concentrations versus controls, indicating efficient metabolite accumulation [186,187].
5.5. Alternative Carotenoid Pathways and Biological Functions
While α-carotene, lutein, astaxanthin, fucoxanthin, and other xanthophylls accumulate at minor concentrations in gonads [71,187,189,190,191], they may serve as protective antioxidant sources. Lutein absorption and retention appear less efficient than β-carotene absorption and retention, possibly due to its more polar structure, though it may contribute to yellow gonad tones and antioxidant protection [192].
Although fucoxanthin, the major carotenoid in brown algae (Laminaria spp., U. pinnatifida, E. bicyclis) constituting natural sea urchin diets [160,166], and its metabolite fucoxanthinol were detected in P. depressus viscera, neither accumulated in the gonads, suggesting that sea urchins lack mechanisms to incorporate these xanthophylls into gonadal tissue [188]. Despite absent gonadal accumulation, fucoxanthin significantly enhanced phagocytic activities (three-fold higher phagocytic index versus controls) and egg ovulation numbers. Remarkably, ovulated egg numbers exceeded those in β-carotene-fed groups, suggesting that fucoxanthin exerts beneficial effects during critical reproductive stages [188].
Astaxanthin presents complex, species-specific accumulation patterns. While P. depressus accumulated minimal astaxanthin from supplemented diets [188], Arbacia lixula demonstrated remarkable accumulation capacity in eggs when fed astaxanthin precursor-containing diets. Farmed A. lixula showed egg astaxanthin concentrations of 27.0 μg/mg, fifteen-fold higher than wild specimens (1.5 μg/mg), with astaxanthin as the sole detected pigment [193]. Total astaxanthin reached approximately 2.7% of egg dry weight in cultivated individuals [193], suggesting variable metabolic capabilities among species regarding xanthophyll biotransformation.
5.6. Proposed Mechanisms for Carotenoid Effects on Gonad Development and Quality
Carotenoids’ influence on gonad development extends beyond pigmentation to encompass critical biological defense functions. Carotenoids, particularly β-carotene and echinenone, significantly enhance biological defense mechanisms, including phagocytic activities [188]. Echinenone demonstrated the strongest phagocytic enhancement, with a phagocytic index 7.4-fold higher than controls, surpassing even vitamin A and vitamin E [188]. Phagocytic ratios increased 2.5-fold for echinenone and 2.1-fold for β-carotene versus controls [188].
This immunoenhancement likely occurs through protection of phagocytic cells from autooxidative damage by quenching singlet oxygen and reactive oxygen species generated during phagocytosis [194,195]. Sea urchin phagocytic cells produce reactive oxygen species when engulfing foreign materials [196], and while these products aid bacterial killing, they can damage DNA and cell membranes. Carotenoids protect phagocytic cells from this autooxidative damage through antioxidant properties [194,195].
Carotenoid deficiency consequences were demonstrated through disease characterized by spine loss and underdeveloped gonads in sea urchins fed carotenoid-free diets, with mortality reaching 30% in control groups versus zero in carotenoid-supplemented groups [188]. These symptoms were attributed to decreased biological defense from carotenoid deficiency [188].
Feeding trials revealed that gonads increased significantly in size regardless of diet type, indicating that carotenoids at the tested concentrations did not directly influence gonad growth [187]. However, absolute carotenoid amounts in gonad tissue increased significantly over time despite decreasing concentrations, suggesting either that gonad growth exceeded carotenoid deposition rates or dietary carotenoid amounts were suboptimal [187].
5.7. Applications in Formulated Feed Development
While formulated feeds effectively promote gonadal development, achieving optimal color quality remains challenging when combining algae and plant meals [194]. To address this, researchers have evaluated natural and synthetic β-carotene inclusion in feed formulations [49,85,101,103,146,187]. Robinson et al. [49] demonstrated that 250 mg β-carotene kg−1 dry feed produces desirable S. droebachiensis gonad coloration. However, the high costs and estimated 50–75% pigment degradation during pellet manufacture and storage have limited commercial production [146,191,197,198,199,200], prompting exploration of dried or fresh macro- and microalgae as more cost-effective, stable natural carotenoid sources.
Macro- and microalgae inclusion has yielded promising results for gonad growth rates (Figure 3a), palatability, and coloration (Figure 3b) [17,47,52,54]. McLaughlin and Kelly [201] demonstrated that 70% Phaeodactylum microalgae incorporation in wet diets significantly improved P. miliaris gonad coloration. Our analysis indicates that >20% macroalgae inclusion positively affects gonad coloration in multiple species, including S. droebachiensis [52], S. purpuratus [17], and T. gratilla [47] (Figure 3b). However, these findings require cautious interpretation due to metabolism, temperature, light exposure, season [56,71], diet ingredients [187], maturation stage [189], and sex effects on gonad coloration [113,190,202].
Temperature is the most influential environmental factor affecting sea urchin metabolism, feed consumption, and gonad development rates. Each species exhibits an optimal temperature range for growth and gonad production, with performance declining outside these bounds. For example, S. droebachiensis shows maximal feed consumption at 12–14 °C, with consumption declining at both lower (6–8 °C) and higher (16–18 °C) temperatures [70]. Also, S. purpuratus demonstrates optimal feeding activity at 16 °C, with reduced consumption below 12 °C [89]. Additionally, M. nudus exhibits temperature-dependent feeding, with peak consumption at 15 °C, declining with lower (10 °C) or higher (20 °C) temperatures [36]. These temperature–consumption relationships create a fundamental challenge: at temperatures below optima, sea urchins consume insufficient feed to support rapid gonad growth, while at temperatures above optima, consumption may decline due to metabolic stress despite elevated metabolic demands.
Temperature affects not only the quantity of feed consumed but also the efficiency of nutrient utilization. Optimal protein retention efficiency typically occurs at mid-range temperatures within species tolerance. At low temperatures, reduced metabolic rates may improve feed conversion ratios but slow absolute growth rates. At high temperatures, increased maintenance energy requirements reduce the proportion of nutrients allocated to gonad production [203]. Additionally, digestive enzyme production and activity show temperature-dependent patterns, potentially affecting the digestibility of carbohydrates which are crucial for thermoregulation and movement [78]. Moreover, higher temperatures generally increase lipid catabolism for energy [204], potentially reducing lipid deposition in gonads. Plant-based ingredients with lower inherent digestibility may be particularly sensitive to temperature effects. This temperature sensitivity has direct implications for commercial ambient water operations that must account for seasonal variation in growth rates and the relation with feed efficiency.
Sex-related coloration differences have been well-documented, with females generally exhibiting more attractive marketable colors due to differential nutrient and pigment allocation to gonads [200,202]. Hagen et al. [202] suggested that synthetic carotenoid supplementation may be inefficient for male S. droebachiensis, which naturally produce paler gonads than females. Lourenço et al. [200] evaluated the combined effects of sex, fishmeal, macroalgae, plant-based ingredients, and 100 mg kg−1 β-carotene on P. lividus gonad coloration, finding that (1) carotenoid source and concentration were insufficient to promote gonadal pigment accumulation, potentially due to feed formulation degradation; (2) carotenoid content decreased with increasing gonad weight; and (3) sex significantly influenced both color development and carotenoid content, with females consistently achieving superior results.
The environmental variability discussed above—combined with species-specific physiological differences, regional variations in natural diet composition, and seasonal fluctuations in nutritional demands— introduces heterogeneity into our data synthesis. While our approach of standardizing growth rates using the WGII partially controls for different culture durations, it cannot eliminate the confounding effects of temperature, photoperiod, salinity, or developmental stage variations across studies. Consequently, the optimal macronutrient composition ranges identified in our analysis (21–28% protein, 48–53% carbohydrates, 1.2–4% lipids) should be interpreted as robust general trends emerging across diverse experimental conditions rather than precise prescriptions applicable to all species, regions, and seasons. The patterns we identify represent central tendencies that persist despite environmental heterogeneity, suggesting fundamental nutritional requirements common across barren-forming sea urchins. However, practitioners implementing these findings should recognize that finetuning feed formulations for specific species, geographic locations, seasonal conditions, and target market specifications will likely require region-specific optimization trials. Our results provide a scientifically grounded starting framework for such optimization efforts rather than universal optima applicable without local adaptation.
5.8. Integrated Mechanistic Model and Future Directions
Developing sustainable feeds for optimal gonad growth and coloration may benefit from plant-based ingredients, particularly soybean meal, which contains notably higher carotenoid levels than fish meal (Table 3). Functional feed formulations including probiotics, prebiotics, phytobiotics, and specialized enzymes present promising approaches to enhance plant-based nutrient assimilation and carotenoid metabolic conversion into gonad pigmentation, potentially reducing reliance on expensive macro- and microalgae while maintaining high-quality gonad coloration standards.
Based on current evidence, we propose an integrated mechanistic model for carotenoid effects on sea urchin gonad development and quality, encompassing four interconnected pathways.
First, the digestive bioconversion pathway establishes that β-carotene from plant-based ingredients undergoes enzymatic ketolation by β-carotene ketolase in the gut wall to form echinenone before gonadal transport [186,187,188], with nutritive phagocytes in gonads functioning primarily in storage and accumulation.
Second, the lipoprotein transport mechanism conveys echinenone through coelomic fluid via low-density lipoproteins to gonadal tissue [3,113], explaining the critical relationship between dietary lipid levels (1.2–4%) and optimal carotenoid accumulation.
Third, immunological enhancement and biological defense mechanisms show that carotenoids, particularly echinenone, protect developing gametes and somatic tissues through enhanced phagocytic defense (7.4-fold increased phagocytic index) and antioxidant activity quenching reactive oxygen species during immune responses [188,193,194,196], explaining spine loss disease and underdeveloped gonads in carotenoid-deficient sea urchins.
Fourth, alternative xanthophyll pathways reveal that carotenoid benefits may operate through systemic effects on maternal physiology and immunity, not solely through gonadal pigment accumulation. Fucoxanthin and other xanthophylls from brown algae, while not accumulating in gonads, enhance reproduction (increased egg ovulation exceeding β-carotene-fed groups) and immunity through metabolic action in digestive tissues or via undetected metabolites [188]. Astaxanthin demonstrates species-specific accumulation patterns, with some species like A. lixula achieving high-level egg accumulation (2.7% dry weight) while others show minimal incorporation [188,193], differences that may reflect phylogenetic constraints, ecological adaptations, or life-history correlates requiring systematic comparative investigation.
This approach offers multiple sustainability advantages: reducing feed costs through premium algal ingredient substitution, decreasing dependence on environmentally problematic fish meal, and alleviating wild macroalgae overharvesting. Future research should prioritize optimizing synergistic combinations of plant-based ingredients and functional additives to develop economically viable feed formulations consistently producing market-color-quality gonads while supporting long-term sea urchin aquaculture sustainability.
Based on our comprehensive review of sea urchin gonad enhancement through formulated feed, several key areas for future research and development emerge. The economic feasibility and scalability of formulated feed in commercial settings require thorough trials, alongside efforts to develop cost-effective alternatives to premium ingredients while maintaining optimal gonad quality. Optimizing soybean-based feed remains a priority, alongside investigating methods to reduce or neutralize ANFs and exploring optimal inclusion levels that balance growth performance, gonad quality, and cost-effectiveness [58,59,60,61,62,63]. Developing and testing feed formulations that combine soybean products with strategic inclusions of macroalgae could leverage their unique nutritional properties, such as carotenoids for gonad coloration.
Ecological impact studies should document the broader effects of sea urchin ranching, emphasizing its potential for restoring kelp forests and enhancing biodiversity in urchin barren areas. Long-term studies are necessary to understand the environmental implications of restorative aquaculture practices fully. The potential of Integrated multitrophic aquaculture (IMTA) systems, incorporating sea urchins with complementary species such as seaweed and sea cucumbers, warrants exploration to maximize resource use, minimize environmental impact, and potentially improve sea urchin gonad quality.
Finally, collaboration with policymakers is crucial to develop regulations that support sustainable sea urchin ranching practices while protecting wild populations and ecosystems. Investigating potential incentives or support systems for sea urchin ranchers adopting sustainable, plant-based feed formulations could accelerate the industry’s transition towards more environmentally friendly practices. These multifaceted research directions aim to address the complex challenges in sea urchin aquaculture, balancing economic viability with ecological sustainability and product quality.
6. Conclusions
This review provides a critical framework in optimizing formulated feed for sea urchin gonad enhancement, with implications for both aquaculture development and ecosystem restoration. The results obtained reveal that the best feed compositions balance is moderate protein (21–28%), high carbohydrate (48–53%), and low lipid (1.2–4%) levels, with a preference for macroalgae- and plant-based sources.
Macroalgae-based feed demonstrated superior performance in terms of gonad growth rates and marketable colors. However, their economic and ecological viability on a commercial scale remains a challenge, particularly when using premium ingredients. Therefore, for advancing the sea urchin ranching industry, we need innovative feed formulations that strike a balance between performance, gonad quality, cost-effectiveness, sustainability, and scalability.
Our findings suggest that partial substitution of macroalgae with soybean meal could be a solution to reduce feed costs and ecological impacts while maintaining adequate growth and gonad development. However, further research is crucial to determine optimal inclusion levels, as well as the development of biotechnological alternatives (probiotics, prebiotics, phytobiotics, and enzymes) to mitigate antinutritional factors and avoid health problems. As the sea urchin ranching industry evolves, refinement of feed formulations based on plant ingredients will be crucial. This approach has the potential to transform sea urchin ranching into a viable tool for restorative aquaculture, contributing to both the blue economy and ecosystem preservation.
Author Contributions
J.B. Wrote original manuscript, literature research, visualization, and analyses. J.O. Conceptualization, revision, literature research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by SECIHTI (CVU 860611).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data needed is available in the study.
Acknowledgments
We acknowledge SECIHTI for the postdoctoral grant given to J.B.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GI | Gonadosomatic index |
| WGII | Weekly gonadosomatic index increase |
| ERP | Ecological restoration potential |
| FAA | Free amino acids |
| FF | Formulated feed |
| ANFs | Anti-nutritional factors |
| DW | Dry weight |
| IMTA | Integrated multi-trophic aquaculture |
Appendix A
Appendix A.1

Table A1.
Formulated feed composition, experimental conditions, and gonad enhancement results for various sea urchin species. Proximate composition of the diets (protein, carbohydrates, and lipids in dry weight %), time of the studies, initial size, weight and gonadosomatic indexes (GI) from the different sea urchin species and studies. Pro = protein; Carb = carbohydrates; Lip = lipids; D = days; W = weeks; IGI = initial GI; FGI = final GI; TGII = total GI increase; WGII = weekly GI increase.
Table A1.
Formulated feed composition, experimental conditions, and gonad enhancement results for various sea urchin species. Proximate composition of the diets (protein, carbohydrates, and lipids in dry weight %), time of the studies, initial size, weight and gonadosomatic indexes (GI) from the different sea urchin species and studies. Pro = protein; Carb = carbohydrates; Lip = lipids; D = days; W = weeks; IGI = initial GI; FGI = final GI; TGII = total GI increase; WGII = weekly GI increase.
| Pro (%) | Carb (%) | Lip (%) | D | W | Size (mm) | Weight (g) | IGI (%) | FGI (%) | TGII (%) | WGII (%) | Sea Urchin Species | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 53 | 1.2 | 63 | 9 | 52.5 | 54 | 6.8 | 19.56 | 187.6471 | 1.417778 | Strongylocentrotus purpuratus | [17] |
| 24 | 55 | 1.5 | 63 | 9 | 52.5 | 54 | 6.8 | 22.4 | 229.4118 | 1.733333 | Strongylocentrotus purpuratus | [17] |
| 25 | 54 | 9 | 84 | 12 | 58.2 | 82.4 | 9.7 | 24 | 147.4227 | 1.191667 | Strongylocentrotus purpuratus | [89] |
| 40.8 | 26.2 | 20 | 56 | 8 | 75.6 | 174.8 | 4.3 | 12.3 | 186.0465 | 1 | Evechinus choloritus | [103] |
| 24.5 | 49.6 | 9 | 56 | 8 | 75.6 | 174.8 | 4.3 | 10.1 | 134.8837 | 0.725 | Evechinus choloritus | [103] |
| 65.2 | 16 | 0.3 | 56 | 8 | 54.3 | 68.1 | 8.3 | 17.9 | 115.6627 | 1.2 | Strongylocentrotus droebachiensis | [205] |
| 28 | 48 | 2.6 | 63 | 9 | 60 | 99 | 7 | 22 | 214.2857 | 1.666667 | Tripneutes gratilla | [47] |
| 28 | 48 | 2.6 | 84 | 12 | NP | 177 | 6 | 22.2 | 270 | 1.35 | Tripneutes gratilla | [79] |
| 23 | 14 | 0.1 | 120 | 17 | NP | 248 | 3.4 | 20 | 488.2353 | 0.968333 | Mesocentrotus franciscanus | [76] |
| 22 | 50 | 3.1 | 42 | 6 | 55.8 | 74.2 | 4.9 | 12.7 | 159.1837 | 1.3 | Strongylocentrotus droebachiensis | [52] |
| 22 | 50 | 3.3 | 42 | 6 | 45.8 | 42.2 | 4 | 14 | 250 | 1.666667 | Strongylocentrotus droebachiensis | [52] |
| 39 | 43 | 7.3 | 84 | 12 | 44 | 46.3 | 2.7 | 14.9 | 451.8519 | 1.016667 | Heliocidaris erythogramma | [111] |
| 39 | 43 | 7.3 | 84 | 12 | 47 | 53.7 | 2 | 15.3 | 665 | 1.108333 | Heliocidaris erythogramma | [111] |
| 39 | 43 | 7.3 | 84 | 12 | 44 | 46.3 | 2.7 | 15.9 | 488.8889 | 1.1 | Heliocidaris erythogramma | [111] |
| 39 | 43 | 7.3 | 84 | 12 | 47 | 53.7 | 2 | 13.6 | 580 | 0.966667 | Heliocidaris erythogramma | [111] |
| 25 | 55 | 4.6 | 84 | 12 | 40.18 | 29.62 | 6 | 18 | 200 | 1 | Paracentrotus lividus | [85] |
| 16 | 77 | 4.3 | 63 | 9 | 33 | 43.7 | 9.1 | 21.3 | 134.0659 | 1.355556 | Strongylocentrotus droebachiensis | [71] |
| 28 | 6.3 | 4.3 | 84 | 12 | 45.73 | 39.52 | 13.32 | 19.16 | 43.84384 | 1.46 | Paracentrotus lividus | [84] |
| 23 | 53 | 9 | 84 | 12 | NP | 53.9 | 9.3 | 19.3 | 107.5269 | 0.833333 | Strongylocentrotus purpuratus | [206] |
| 23 | 53 | 6 | 84 | 12 | NP | 53.9 | 9.3 | 17 | 106 | 0.64 | Strongylocentrotus purpuratus | [206] |
| 30 | 47 | 7 | 63 | 9 | NP | 35.9 | 3.3 | 8.5 | 157.5758 | 0.577778 | Strongylocentrotus purpuratus | [207] |
| 23 | 50 | 8 | 63 | 9 | NP | 35.9 | 3.3 | 8.2 | 169.697 | 0.544444 | Strongylocentrotus purpuratus | [207] |
| 17 | 64 | 7 | 63 | 9 | NP | 35.9 | 3.3 | 8 | 142.4242 | 0.522222 | Strongylocentrotus purpuratus | [207] |
| 30 | 41 | 8 | 84 | 12 | NP | 45 | 8.7 | 14.7 | 68.96552 | 0.5 | Strongylocentrotus purpuratus | [208] |
| 17 | 53 | 8 | 84 | 12 | NP | 45 | 8.7 | 13.7 | 57.47126 | 0.416667 | Strongylocentrotus purpuratus | [208] |
| 47 | 40 | 6.17 | 84 | 12 | NP | 53.2 | 2.9 | 18.5 | 537.931 | 1.3 | Heliocidaris erythogramma | [56] |
| 47 | 40 | 6.17 | 84 | 12 | NP | 58.2 | 6.6 | 21.7 | 228.7879 | 1.258333 | Heliocidaris erythogramma | [56] |
| 6.45 | 56.74 | 0.49 | 84 | 12 | 49.8 | 54.6 | 8.1 | 9.2 | 13.58025 | 0.091667 | Mesocentrotus nudus | [5] |
Appendix A.2

Table A2.
Color analysis, feed composition, and ingredient inclusion levels in sea urchin gonad enhancement studies. Values of Lab, converted RGB, color range obtained, and the amount of fish meal (FM, %), soybean meal (SBM, %), and macroalgae (M, %) in different diets per study. NP = not presented.
Table A2.
Color analysis, feed composition, and ingredient inclusion levels in sea urchin gonad enhancement studies. Values of Lab, converted RGB, color range obtained, and the amount of fish meal (FM, %), soybean meal (SBM, %), and macroalgae (M, %) in different diets per study. NP = not presented.
| Sea Urchin Species | Lab | RGB | Color | FM (%) | SBM (%) | M (%) | References |
|---|---|---|---|---|---|---|---|
| Strongylocentrotus purpuratus | NP | NP | Orange | 0 | 0 | >50 | [17] |
| Strongylocentrotus purpuratus | NP | NP | Orange | 0 | 0 | >50 | [17] |
| Strongylocentrotus purpuratus | 50.1, 7, 31 | rgb (147, 114, 66) | Pale Yellow/Brown | NP | NP | NP | [89] |
| Evechinus choloritus | 51.4, 12.9, 28 | rgb (158, 113, 75) | Pale Yellow/Brown | NP | NP | NP | [103] |
| Evechinus choloritus | 51.7, 12.2, 25.1 | rgb (156, 115, 81) | Pale Yellow/Brown | NP | NP | NP | [103] |
| Strongylocentrotus droebachiensis | NP | NP | NP | 89.05 | 0 | 8.91 | [205] |
| Tripneutes gratilla | 62, 14, 34 | rgb (191, 139, 90) | Orange/Yellow | 0 | 0 | 20 | [47] |
| Tripneutes gratilla | 56, 7, 30 | rgb (162, 129, 82) | Pale Yellow/Brown | 12 | 12 | 20 | [79] |
| Strongylocentrotus droebachiensis | 41, 24, 34 | rgb (145, 80, 41) | Orange | 0 | 27 | 32 | [52] |
| Heliocidaris erythogramma | NP | NP | Pale Yellow | 14 | 20 | 5 | [111] |
| Heliocidaris erythogramma | NP | rgb (208, 206, 174) | Pale Yellow | 14 | 20 | 5 | [111] |
| Heliocidaris erythogramma | NP | rgb (208, 206, 174) | Pale Yellow | 14 | 21 | 15 | [111] |
| Strongylocentrotus droebachiensis | NP | NP | NP | 0 | 20 | 10 | [71] |
| Strongylocentrotus purpuratus | 60, 15.8, 37.9 | rgb (189, 133, 78) | Pale Yellow | 22 | 0 | 11 | [206] |
| Strongylocentrotus purpuratus | 57, 15.4, 34.8 | rgb (179, 125, 76) | Pale Yellow/Brown | 22 | 0 | 11 | [206] |
| Strongylocentrotus purpuratus | NP | NP | NP | 26 | 0 | 10 | [207] |
| Strongylocentrotus purpuratus | NP | NP | NP | 26 | 0 | 10 | [207] |
| Strongylocentrotus purpuratus | NP | NP | NP | 26 | 0 | 10 | [207] |
| Strongylocentrotus purpuratus | 51, 14, 34 | rgb (160, 111, 63) | Pale Yellow/Brown | 38 | 0 | 19 | [208] |
| Strongylocentrotus purpuratus | 49, 14, 32 | rgb (154, 106, 62) | Pale Yellow/Brown | 18 | 0 | 9 | [208] |
| Heliocidaris erythogramma | NP | NP | NP | 15 | 0 | 5 | [56] |
| Heliocidaris erythogramma | NP | NP | NP | 15 | 0 | 5 | [56] |
References
- Sun, J.; Chiang, F.S. Use and Exploitation of Sea Urchins. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015; Volume 1, pp. 25–45. [Google Scholar]
- Walker, C.; Bottger, S.A.; Unuma, T.; Watts, S.; Harris, L.; Lawrence, A.; Eddy, S. Enhancing the Commercial Quality of Edible Sea Urchin Gonads-Technologies Emphasizing Nutritive Phagocytes. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 263–286. [Google Scholar]
- Kelly, M.S.; Symonds, R.C. Carotenoids in Sea Urchins; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, ISBN 9780123964915. [Google Scholar]
- Takagi, S.; Murata, Y.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Production of High Quality Gonads in the Sea Urchin Mesocentrotus nudus (A. Agassiz, 1864) from a Barren by Feeding on the Kelp Saccharina japonica at the Late Sporophyte Stage. J. Appl. Phycol. 2019, 31, 4037–4048. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Koshiishi, T.; Agatsuma, Y. The Amino Acids Glutamic Acid and Alanine in Feed Increase the Alanine Content in Gonads of the Sea Urchin Mesocentrotus nudus. Front. Mar. Sci. 2020, 7, 593. [Google Scholar] [CrossRef]
- Pais, A.; Serra, S.; Meloni, G.; Saba, S.; Ceccherelli, G. Harvesting Effects on Paracentrotus lividus Population Structure: A Case Study from Northwestern Sardinia, Italy, before and after the Fishing Season. J. Coast. Res. 2012, 28, 570–575. [Google Scholar] [CrossRef]
- Rubilar, T.; Cardozo, D. Blue Growth: Sea Urchin Sustainable Aquaculture, Innovative Approaches. Rev. Biol. Trop. 2021, 69, S474–S486. [Google Scholar] [CrossRef]
- Pinna, F.; Fois, N.; Mura, F.; Ruiu, A.; Ceccherelli, G. Predation Risk of the Sea Urchin Paracentrotus lividus Juveniles in an Overfished Area Reveal System Stability Mechanisms and Restocking Challenges. PLoS ONE 2024, 19, e0301143. [Google Scholar] [CrossRef]
- Chapman, A.R.O. Stability of Sea Urchin Dominated Barren Grounds Following Destructive Grazing of Kelp in St. Margaret’s Bay, Eastern Canada. Mar. Biol. 1981, 62, 307–311. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Scheibling, R.E. Sea Urchin Barrens as Alternative Stable States of Collapsed Kelp Ecosystems. Mar. Ecol. Prog. Ser. 2014, 495, 1–25. [Google Scholar] [CrossRef]
- Ling, S.D. Range Expansion of a Habitat-Modifying Species Leads to Loss of Taxonomic Diversity: A New and Impoverished Reef State. Oecologia 2008, 156, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S. Regular Sea Urchins as Drivers of Shallow Benthic Marine Community Structure. In Developments in Aquaculture and Fisheries Science; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 43, pp. 255–279. [Google Scholar]
- Eger, A.M.; Blain, C.O.; Brown, A.L.; Chan, S.S.W.; Miller, K.I.; Vergés, A. Kelp Forests versus Urchin Barrens: A Comparison of Ecosystem Functions and Services Provided by Two Alternative Stable Marine Habitats. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241539. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Hennigar, A.W.; Balch, T. Destructive Grazing, Epiphytism, and Disease: The Dynamics of Sea Urchin-Kelp Interactions in Nova Scotia. Can. J. Fish. Aquat. Sci. 1999, 56, 2300–2314. [Google Scholar] [CrossRef]
- Miller, K.I.; Balemi, C.A.; Blain, C.O.; Spyksma, A.J.P.; Shears, N.T. Sea Urchin Roe Quality within Urchin Barrens and Improvement through Kelp Restoration. Ecosphere 2024, 15, e4911. [Google Scholar] [CrossRef]
- Pert, C.G.; Swearer, S.E.; Dworjanyn, S.; Kriegisch, N.; Turchini, G.M.; Francis, D.S.; Dempster, T. Barrens of Gold: Gonad Conditioning of an Overabundant Sea Urchin. Aquac. Environ. Interact. 2018, 10, 345–361. [Google Scholar] [CrossRef]
- Angwin, R.E.; Hentschel, B.T.; Anderson, T.W. Gonad Enhancement of the Purple Sea Urchin, Strongylocentrotus purpuratus, Collected from Barren Grounds and Fed Prepared Diets and Kelp. Aquac. Int. 2022, 30, 1353–1367. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Inomata, E.; Endo, H.; Aoki, M.N.; Agatsuma, Y. Improvement of Gonad Quality of the Sea Urchin Mesocentrotus nudus Fed the Kelp Saccharina japonica during Offshore Cage Culture. Aquaculture 2017, 477, 50–61. [Google Scholar] [CrossRef]
- Agatsuma, Y.; Sato, M.; Taniguchi, K. Factors Causing Brown-Colored Gonads of the Sea Urchin Strongylocentrotus Nudus in Northern Honshu, Japan. Aquaculture 2005, 249, 449–458. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Agatsuma, Y. Feeding the Sporophyll of Undaria pinnatifida Kelp Shortens the Culture Duration for the Production of High-Quality Gonads of Mesocentrotus nudus Sea Urchins from a Barren. Aquaculture 2020, 528, 735503. [Google Scholar] [CrossRef]
- Miller, K.I.; Balemi, C.A.; Bell, D.R.; Blain, C.O.; Caiger, P.E.; Hanns, B.J.; Kulins, S.E.; Peleg, O.; Spyksma, A.J.P.; Shears, N.T. Large-scale One-off Sea Urchin Removal Promotes Rapid Kelp Recovery in Urchin Barrens. Restor. Ecol. 2023, 32, e14060. [Google Scholar] [CrossRef]
- Rogers-Bennett, L.; Catton, C.A. Marine Heat Wave and Multiple Stressors Tip Bull Kelp Forest to Sea Urchin Barrens. Sci. Rep. 2019, 9, 15050. [Google Scholar] [CrossRef]
- Kerr, V.C.; Grace, R.V.; Shears, N.T. Estimating the Extent of Urchin Barrens and Kelp Forest Loss in Northeastern Aotearoa, New Zealand. N. Z. J. Mar. Freshw. Res. Res. 2024, 59, 441–462. [Google Scholar] [CrossRef]
- Rogers-Bennett, L.; Catton, C. Cascading Impacts of a Climate-Driven Ecosystem Transition Intensifies Population Vulnerabilities and Fishery Collapse. Front. Clim. 2022, 4, 908708. [Google Scholar] [CrossRef]
- Smith, J.G.; Malone, D.; Carr, M.H. Consequences of Kelp Forest Ecosystem Shifts and Predictors of Persistence through Multiple Stressors. Proc. R. Soc. B Biol. Sci. 2024, 291, 20232749. [Google Scholar] [CrossRef]
- Bauer, J.; Beas-Luna, R.; Malpica-Cruz, L.; Abadía-Cardoso, A.; Filz, P.; Bonilla, J.C.; Lorda, J. Community-Led Management Maintains Higher Predator Biomass Supporting Kelp Forests Persistence in Baja California. Sci. Rep. 2025, 15, 23253. [Google Scholar] [CrossRef]
- Bauer, J.; Lorda, J.; Beas-Luna, R.; Malpica-Cruz, L.; Abadía-Cardoso, A.; Paz-Lacavex, A.; Olmos, J. Extreme Marine Heatwaves Drive Divergent Kelp Forest Trajectories and Alternative Stable States. Front. Mar. Sci. 2025, 12, 1691156. [Google Scholar]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp Forest Ecosystems: Biodiversity, Stability, Resilience and Future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial Role of Macroalgae in Marine Carbon Sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Beas-luna, R.; Blain, C.O.; Blamey, L.K.; Byrnes, J.E.K.; Carnell, P.E.; Choi, C.G.; Hessing-Lewis, M.; Kim, K.Y.; et al. The Value of Ecosystem Services in Global Marine Kelp Forests. Nat. Commun. 2023, 14, 1894. [Google Scholar] [CrossRef]
- Keesing, J.K. Heliocidaris Erythrogramma. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 38, pp. 369–379. [Google Scholar]
- Steneck, R.S. Sea Urchins as Drivers of Shallow Benthic Marine Community Structure. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 192–212. [Google Scholar]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The Tropicalization of Temperate Marine Ecosystems: Climate-Mediated Changes in Herbivory and Community Phase Shifts. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Beas-Luna, R.; Micheli, F.; Woodson, C.B.; Carr, M.; Malone, D.; Torre, J.; Boch, C.; Caselle, J.E.; Edwards, M.; Freiwald, J.; et al. Geographic Variation in Responses of Kelp Forest Communities of the California Current to Recent Climatic Changes. Glob. Change Biol. 2020, 26, 6457–6473. [Google Scholar] [CrossRef] [PubMed]
- Strand, H.K.; Christie, H.; Fagerli, C.W.; Mengede, M.; Moy, F. Optimizing the Use of Quicklime (CaO) for Sea Urchin Management—A Lab and Field Study. Ecol. Eng. X 2020, 143, 100018. [Google Scholar] [CrossRef]
- Seo, J.; Koo, B.J. Temperature-Dependent Food Consumption Rates of the Sea Urchin Mesocentrotus nudus and Top Shell Turbo Sazae: Potential Impacts on Seaweed Beds. Animals 2023, 13, 3436. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.D.; Keane, J.P. Climate-Driven Invasion and Incipient Warnings of Kelp Ecosystem Collapse. Nat. Commun. 2024, 15, 400. [Google Scholar] [CrossRef]
- Przeslawski, R.; Chick, R.C.; Davis, T.; Day, J.K.; Glasby, T.M.; Knott, N.; Byrne, M. A Review of Urchin Barrens and the Longspined Sea Urchin (Centrostephanus rodgersii) in New South Wales, Australia. Mar. Freshw. Res. 2025, 76, MF24149. [Google Scholar] [CrossRef]
- Ling, S.D.; Scheibling, R.E.; Rassweiler, A.; Johnson, C.R.; Shears, N.; Connell, S.D.; Salomon, A.K.; Norderhaug, K.M.; Pérez-Matus, A.; Hernández, J.C.; et al. Global Regime Shift Dynamics of Catastrophic Sea Urchin Overgrazing. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130269. [Google Scholar] [CrossRef]
- James, P.; Siikavuopio, S.I.; Mortensen, A. Sea Urchin Aquaculture in Norway. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 1, pp. 147–173. ISBN 9781119005810. [Google Scholar]
- Unuma, T.; Sakai, Y.; Agatsuma, Y.; Kayaba, T. Sea Urchin Aquaculture in Japan. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 1, pp. 75–126. [Google Scholar]
- Williamson, J.E. Sea Urchin Aquaculture in Australia. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 1, pp. 211–224. [Google Scholar]
- Heflin, L.E.; Raubenheimer, D.; Simpson, S.J.; Watts, S.A. Balancing Macronutrient Intake in Cultured Lytechinus variegatus. Aquaculture 2016, 450, 295–300. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Christie, H.; Fagerli, C.W.; Fujita, D.; Gonzalez, A.P.; Hong, S.W.; Kim, J.H.; Lee, L.C.; McHugh, T.A.; et al. Global Kelp Forest Restoration: Past Lessons, Present Status, and Future Directions. Biol. Rev. 2022, 97, 1449–1475. [Google Scholar] [CrossRef]
- Miller, K.I.; Blain, C.O.; Shears, N.T. Sea Urchin Removal as a Tool for Macroalgal Restoration: A Review on Removing “the Spiny Enemies”. Front. Mar. Sci. 2022, 9, 831001. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Dale, T.; Carlehög, M. Sensory Quality of Gonads from the Green Sea Urchin, Strongylocentrotus droebachiensis, Fed Different Diets. J. Shellfish Res. 2007, 26, 637–643. [Google Scholar] [CrossRef]
- Cyrus, M.D.; Bolton, J.J.; De Wet, L.; Macey, B.M. The Development of a Formulated Feed Containing Ulva (Chlorophyta) to Promote Rapid Growth and Enhanced Production of High Quality Roe in the Sea Urchin Tripneustes gratilla (Linnaeus). Aquac. Res. 2013, 45, 159–176. [Google Scholar] [CrossRef]
- Inomata, E.; Murata, Y.; Matsui, T.; Agatsuma, Y. Gonadal Production and Quality in the Sea Urchin Mesocentrotus nudus Fed a High-Protein Concentrated Red Alga Pyropia yezoensis. Aquaculture 2016, 454, 184–191. [Google Scholar] [CrossRef]
- Robinson, S.M.C.; Castell, J.D.; Kennedy, E.J. Developing Suitable Colour in the Gonads of Cultured Green Sea Urchins (Strongylocentrotus droebachiensis). Aquaculture 2002, 206, 289–303. [Google Scholar] [CrossRef]
- Takagi, S.; Takahashi, K.; Kaneta, T.; Sugawara, A.; Narita, M.; Kato, S.; Akino, M.; Takeda, H.; Hasegawa, N.; Machiguchi, Y.; et al. Modest Dietary Protein Requirement for Sea Urchin Gonad Production Demonstrated by Feeding Trials with Consideration of Protein Leaching. Aquac. Nutr. 2022, 2022, 3140222. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Olave, S.; Otaiza, R.; Lawrence, A.L.; Bustos, E. Enhancement of Gonad Production in the Sea Urchin Loxechinus Albus in Chile Fed Extruded Feeds. J. World Aquac. Soc. 1997, 28, 91–96. [Google Scholar] [CrossRef]
- Pearce, C.M.; Daggett, T.L.; Robinson, S.M.C. Effect of Urchin Size and Diet on Gonad Yield and Quality in the Green Sea Urchin (Strongylocentrotus droebachiensis). Aquaculture 2004, 233, 337–367. [Google Scholar] [CrossRef]
- Troell, M.; Robertson-Andersson, D.; Anderson, R.J.; Bolton, J.J.; Maneveldt, G.; Halling, C.; Probyn, T. Abalone Farming in South Africa: An Overview with Perspectives on Kelp Resources, Abalone Feed, Potential for on-Farm Seaweed Production and Socio-Economic Importance. Aquaculture 2006, 257, 266–281. [Google Scholar] [CrossRef]
- Cyrus, M.D.; Bolton, J.J.; Scholtz, R.; Macey, B.M. The Advantages of Ulva (Chlorophyta) as an Additive in Sea Urchin Formulated Feeds: Effects on Palatability, Consumption and Digestibility. Aquac. Nutr. 2015, 21, 578–591. [Google Scholar] [CrossRef]
- Hammer, H.S.; Powell, M.L.; Jones, W.T.; Gibbs, V.K.; Lawrence, A.L.; Lawrence, J.M.; Watts, S.A. Effect of Feed Protein and Carbohydrate Levels on Feed Intake, Growth, and Gonad Production of the Sea Urchin, Lytechinus variegatus. J. World Aquac. Soc. 2012, 43, 145–158. [Google Scholar] [CrossRef]
- Warren-Myers, F.; Turchini, G.; Swearer, S.E.; Francis, D.; Dempster, T. The Balancing Act: Protein, Lipid and Seaweed Dietary Levels to Maximize Gonad Quantity in a Wild-Caught Sea Urchin. Aquac. Nutr. 2021, 27, 1019–1030. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial Roles of Feed Additives as Immunostimulants in Aquaculture: A Review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Leonel Ochoa-Solano, J.; Olmos-Soto, J. The Functional Property of Bacillus for Shrimp Feeds. Food Microbiol. 2006, 23, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Olmos, J. Bacillus Subtilis A Potential Probiotic Bacterium to Formulate Functional Feeds for Aquaculture. J. Microb. Biochem. Technol. 2014, 6, 361–365. [Google Scholar] [CrossRef]
- Olmos Soto, J. Bacillus Probiotic Enzymes: External Auxiliary Apparatus to Avoid Digestive Deficiencies, Water Pollution, Diseases, and Economic Problems in Marine Cultivated Animals. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 80, pp. 15–35. [Google Scholar]
- Macias, L.; Mercado, V.; Olmos, J. Assessment of Bacillus Species Capacity to Protect Nile Tilapia from A. hydrophila Infection and Improve Growth Performance. Front. Cell. Infect. Microbiol. 2024, 14, 1354736. [Google Scholar] [CrossRef]
- Olmos, J.; Acosta, M.; Mendoza, G.; Pitones, V. Bacillus subtilis, an Ideal Probiotic Bacterium to Shrimp and Fish Aquaculture That Increase Feed Digestibility, Prevent Microbial Diseases, and Avoid Water Pollution. Arch. Microbiol. 2020, 202, 427–435. [Google Scholar] [CrossRef]
- Olmos, J.; Ochoa, L.; Paniagua-Michel, J.; Contreras, R. Functional Feed Assessment on Litopenaeus vannamei Using 100% Fish Meal Replacement by Soybean Meal, High Levels of Complex Carbohydrates and Bacillus Probiotic Strains. Mar. Drugs 2011, 9, 1119–1132. [Google Scholar] [CrossRef]
- Ringø, E.; Olsen, R.E.; Gifstad, T.; Dalmo, R.A.; Amlund, H.; Hemre, G.I.; Bakke, A.M. Prebiotics in Aquaculture: A Review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of Plant Extracts in Fish Aquaculture as an Alternative to Chemotherapy: Current Status and Future Perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Phytate and Phytase in Fish Nutrition. J. Anim. Physiol. Anim. Nutr. 2012, 96, 335–364. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; James, P.; Sæther, B.S. Long-Term Growth Study of Male and Female Green Sea Urchins, Strongylocentrotus droebachiensis, under Constant Light and Temperature Regime. J. World Aquac. Soc. 2014, 45, 481–486. [Google Scholar] [CrossRef]
- de Jong-Westman, M.; March, B.E.; Carefoot, T.H. The Effect of Different Nutrient Formulations in Artificial Diets on Gonad Growth in the Sea Urchin Strongylocentrotus droebachiensis. Can. J. Zool. 1995, 73, 1495–1502. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Christiansen, J.S.; Sæther, B.S.; Dale, T. Seasonal Variation in Feed Intake under Constant Temperature and Natural Photoperiod in the Green Sea Urchin (Strongylocentrotus droebachiensis). Aquaculture 2007, 272, 328–334. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Christiansen, J.S.; Dale, T. Effects of Temperature and Season on Gonad Growth and Feed Intake in the Green Sea Urchin (Strongylocentrotus droebachiensis). Aquaculture 2006, 255, 389–394. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F.; Whittick, A. The Effect of an Artificial Diet on the Biochemical Composition of the Gonads of the Sea Urchin (Strongylocentrotus droebachiensis). Aquaculture 2002, 79, 461–472. [Google Scholar] [CrossRef]
- Fagerli, C.W.; Stadniczeñko, S.G.; Pedersen, M.F.; Christie, H.; Fredriksen, S.; Norderhaug, K.M. Population Dynamics of Strongylocentrotus droebachiensis in Kelp Forests and Barren Grounds in Norway. Mar. Biol. 2015, 162, 1215–1226. [Google Scholar] [CrossRef]
- Medellín-Ortiz, A.; Montaño-Moctezuma, G.; Alvarez-Flores, C.; Santamaria-Del-Angel, E. Retelling the History of the Red Sea Urchin Fishery in Mexico. Front. Mar. Sci. 2020, 7, 167. [Google Scholar] [CrossRef]
- Medellín–Ortiz, A.; Montaño–Moctezuma, G.; Álvarez–Flores, C.; Santamaría-del-Ángel, E.; García–Nava, H.; Beas–Luna, R.; Cavanaugh, K. Understanding the Impact of Environmental Variability and Fisheries on the Red Sea Urchin Population in Baja California. Front. Mar. Sci. 2022, 9, 987242. [Google Scholar] [CrossRef]
- Olivares-Bañuelos, N.C.; Enríquez-Paredes, L.M.; Ladah, L.B.; De La Rosa-Vélez, J. Population Structure of Purple Sea Urchin Strongylocentrotus purpuratus along the Baja California Peninsula. Fish. Sci. 2008, 74, 804–812. [Google Scholar] [CrossRef]
- Mcbride, S.C.; Pinnix, W.D.; Lawrence, J.M.; Lawrence, A.L.; Mulligan, T.M. The Effect of Temperature on Production of Gonads by the Sea Urchin Strongylocentrotus franciscanus Fed Natural and Prepared Diets. J. World Aquac. Soc. 1997, 28, 357–365. [Google Scholar] [CrossRef]
- James, P.J.; Heath, P.; Unwin, M.J. The Effects of Season, Temperature and Initial Gonad Condition on Roe Enhancement of the Sea Urchin Evechinus chloroticus. Aquaculture 2007, 270, 115–131. [Google Scholar] [CrossRef]
- Phillips, K.; Hamid, N.; Silcock, P.; Sewell, M.A.; Barker, M.; Weaver, A.; Then, S.; Delahunty, C.; Bremer, P. Effect of Manufactured Diets on the Yield, Biochemical Composition and Sensory Quality of Evechinus chloroticus Sea Urchin Gonads. Aquaculture 2010, 308, 49–59. [Google Scholar] [CrossRef]
- Onomu, A.J.; Vine, N.G.; Cyrus, M.D.; Macey, B.M.; Bolton, J.J. The Effect of Fresh Seaweed and a Formulated Diet Supplemented with Seaweed on the Growth and Gonad Quality of the Collector Sea Urchin, Tripneustes gratilla, under Farm Conditions. Aquac. Res. 2020, 51, 4087–4102. [Google Scholar] [CrossRef]
- Senaratna, M.; Evans, L.H.; Southam, L.; Tsvetnenko, E. Effect of Different Feed Formulations on Feed Efficiency, Gonad Yield and Gonad Quality in the Purple Sea Urchin Heliocidaris erythrogramma. Aquac. Nutr. 2005, 11, 199–207. [Google Scholar] [CrossRef]
- Warren-Myers, F.; Swearer, S.E.; Overton, K.; Dempster, T. Stocking Density and Rearing Environment Affect External Condition, Gonad Quantity and Gonad Grade in Onshore Sea Urchin Roe Enhancement Aquaculture. Aquaculture 2020, 515, 734591. [Google Scholar] [CrossRef]
- Warren-Myers, F.; Swearer, S.; Francis, D.; Turchini, G.; Dempster, T. Solving Key Industry Bottlenecks for Sea Urchin Roe Enhancement; AgriFutures: Wagga Wagga, Australia, 2021; ISBN 9781760532246. [Google Scholar]
- Fernandez, C.; Pergent, G. Effect of Different Formulated Diets and Rearing Conditions on Growth Parameters in the Sea Urchin Paracentrotus lividus. J. Shellfish Res. 1998, 17, 1571–1581. [Google Scholar]
- Prato, E.; Chiantore, M.; Kelly, M.S.; Hughes, A.D.; James, P.; Ferranti, M.P.; Biandolino, F.; Parlapiano, I.; Sicuro, B.; Fanelli, G. Effect of Formulated Diets on the Proximate Composition and Fatty Acid Profiles of Sea Urchin Paracentrotus lividus Gonad. Aquac. Int. 2018, 26, 185–202. [Google Scholar] [CrossRef]
- Shpigel, M.; McBride, S.C.; Marciano, S.; Ron, S.; Ben-Amotz, A. Improving Gonad Colour and Somatic Index in the European Sea Urchin Paracentrotus lividus. Aquaculture 2005, 245, 101–109. [Google Scholar] [CrossRef]
- Takagi, S.; Hasegawa, N. Potential of Sea Urchin Mesocentrotus nudus as a Target Catch Species in the Pacific Ocean off Eastern Hokkaido, Japan. Animals 2024, 14, 1740. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Pronounced Effects of the Basal Frond Portion of the Kelp Saccharina japonica on Gonad Qualities of the Sea Urchin Mesocentrotus nudus from a Barren. Aquaculture 2020, 516, 734623. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Hennigar, A.W. Recurrent Outbreaks of Disease in Sea Urchins Strongylocentrotus droebachiensis in Nova Scotia: Evidence for a Link with Large-Scale Meteorologic and Oceanographic Events. Mar. Ecol. Prog. Ser. 1997, 152, 155–165. [Google Scholar] [CrossRef]
- Azad, A.K.; Pearce, C.M.; McKinley, R.S. Effects of Diet and Temperature on Ingestion, Absorption, Assimilation, Gonad Yield, and Gonad Quality of the Purple Sea Urchin (Strongylocentrotus purpuratus). Aquaculture 2011, 317, 187–196. [Google Scholar] [CrossRef]
- Tegner, M. The Ecology of Strongylocentrotus Franciscanus and Strongylocentrotus purpuratus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 32, pp. 307–331. [Google Scholar]
- Rogers-Bennett, L. The Ecology of Strongylocentrotus franciscanus and Strongylocentrotus purpuratus. In Edible Sea Urchins; Elsevier B.V.: Amsterdam, the Netherlands, 2007; pp. 393–426. [Google Scholar]
- Gardner, L.; Lindsey, H.; Neylan, K.; Chang, W.; Herrmann, K.; Rintoul, M.; Roy, K. Preliminary Feasibility Assessment of Purple Sea Urchin, Strongylocentrotus purpuratus, Roe Enhancement. Bull. Jap. Fish. Res. Edu. Agen. 2021, 50, 47–53. [Google Scholar]
- Rogers-Bennett, L.; Okamoto, D. Mesocentrotus franciscanus and Strongylocentrotus purpuratus, 4th ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 43, ISBN 9780128195703. [Google Scholar]
- Agatsuma, Y.; Endo, H.; Taniguchi, K. Inhibitory Effect of 2,4-Dibromophenol and 2,4,6-Tribromophenol on Larval Survival and Metamorphosis of the Sea Urchin Strongylocentrotus nudus. Fish. Sci. 2008, 74, 837–841. [Google Scholar] [CrossRef]
- Feng, W.; Nakabayashi, N.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Impacts of Water Temperature on the Physiology and Behaviours of the Sea Urchins Heliocidaris crassispina and Mesocentrotus nudus That Reflect Their Range Extension and Disappearance in the Oga Peninsula, Northern Honshu, Japan. Can. J. Fish. Aquat. Sci. 2021, 78, 580–588. [Google Scholar] [CrossRef]
- Sano, M.; Omori, M.; Taniguchi, K.; Seki, T. Age Distribution of the Sea Urchin Strongylocentrotus (A. Agassiz) in Relation to Algal Zonation in a Rocky Area on Oshika Peninsula, Northern Japan. Fish. Sci. 2001, 67, 628–639. [Google Scholar] [CrossRef]
- Kalvass, P.E.; Hendrix, J.M. The California Red Sea Urchin, Strongylocentrotus franciscanus, Fishery: Catch, Effort, and Management Trends. Mar. Fish. Rev. 1997, 59, 1–17. [Google Scholar]
- Warren, E. Optimizing Sea Urchin Gonad Enhancement and Gastrointestinal Parameters with Newly. Master’s Thesis, University Prince Edward Island, Charlottetown, PE, Canada, 2022. [Google Scholar]
- Tegner, M. The Feasibility of Enhancing Red Sea Urchin, Strongylocentrotus franciscanus, Stocks in California: An Analysis of the Options. Mar. Fish. Rev. 1989, 51, 1–22. [Google Scholar]
- Warren, E.M.; Pearce, C.M. Effect of Transport Method on Subsequent Survivorship and Gonad Yield/Quality in the Red Sea Urchin Mesocentrotus franciscanus. N. Am. J. Aquac. 2020, 82, 371–376. [Google Scholar] [CrossRef]
- McBride, S.C.; Price, R.J.; Tom, P.D.; Lawrence, J.M.; Lawrence, A.L. Comparison of Gonad Quality Factors: Color, Hardness and Resilience, of Strongylocentrotus franciscanus between Sea Urchins Fed Prepared Feed or Algal Diets and Sea Urchins Harvested from the Northern California Fishery. Aquaculture 2004, 233, 405–422. [Google Scholar] [CrossRef]
- Lamare, M.D.; Barker, M.F. In Situ Estimates of Larval Development and Mortality in the New Zealand Sea Urchin Evechinus chloroticus (Echinodermata: Echinoidea). Mar. Ecol. Prog. Ser. 1999, 180, 197–211. [Google Scholar] [CrossRef]
- Woods, C.M.C.; James, P.J.; Moss, G.A.; Wright, J.; Siikavuopio, S. A Comparison of the Effect of Urchin Size and Diet on Gonad Yield and Quality in the Sea Urchin Evechinus chloroticus Valenciennes. Aquac. Int. 2008, 16, 49–68. [Google Scholar] [CrossRef]
- Barker, M.F. Evechinus Chloroticus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 355–368. [Google Scholar]
- Juinio-Meñez, M.A.; Bangi, H.G.; Malay, M.C.; Pastor, D. Enhancing the Recovery of Depleted Tripneustes gratilla Stocks through Grow-out Culture and Restocking. Rev. Fish. Sci. 2008, 16, 35–43. [Google Scholar] [CrossRef]
- Juinio-Meñez, M.A.; Grace, H.; Bangi, P.; Celia, M.; Malay, D. Effect of Type of Feed, Stocking Density and Grow-out Site on Gonad Index, Growth and Survivorship of Cultured Sea Urchin (Tripneustes gratilla). Philipp. Agric. Sci. 2008, 91, 439–449. [Google Scholar]
- Sheppard Brennand, H.; Soars, N.; Dworjanyn, S.A.; Davis, A.R.; Byrne, M. Impact of Ocean Warming and Ocean Acidification on Larval Development and Calcification in the Sea Urchin Tripneustes gratilla. PLoS ONE 2010, 5, e11372. [Google Scholar] [CrossRef]
- Wolfe, K.; Dworjanyn, S.A.; Byrne, M. Effects of Ocean Warming and Acidification on Survival, Growth and Skeletal Development in the Early Benthic Juvenile Sea Urchin (Heliocidaris erythrogramma). Glob. Change Biol. 2013, 19, 2698–2707. [Google Scholar] [CrossRef]
- Valentine, J.P.; Johnson, C.R. Persistence of Sea Urchin (Heliocidaris erythrogramma) Barrens on the East Coast of Tasmania: Inhibition of Macroalgal Recovery in the Absence of High Densities of Sea Urchins. Bot. Mar. 2005, 48, 106–115. [Google Scholar] [CrossRef]
- Pederson, H.G.; Johnson, C.R. Growth and Age Structure of Sea Urchins (Heliocidaris erythrogramma) in Complex Barrens and Native Macroalgal Beds in Eastern Tasmania. ICES J. Mar. Sci. 2008, 65, 1–11. [Google Scholar] [CrossRef]
- Warren-Myers, F.; Swearer, S.E.; Francis, D.S.; Turchini, G.M.; Overton, K.; Dempster, T. Algal Supplements in Formulated Feeds: Effects on Sea Urchin Gonad Quality. Aquaculture 2022, 548, 737673. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Ecology of Paracentrotus lividus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 31, pp. 177–216. [Google Scholar]
- Symonds, R.C.; Kelly, M.S.; Caris-Veyrat, C.; Young, A.J. Carotenoids in the Sea Urchin Paracentrotus lividus: Occurrence of 9′-Cis-Echinenone as the Dominant Carotenoid in Gonad Colour Determination. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Ceccherelli, G.; Pais, A.; Pinna, S.; Sechi, N.; Chessa, L.A. Human Impact on Paracentrotus lividus: The Result of Harvest Restrictions and Accessibility of Locations. Mar. Biol. 2011, 158, 845–852. [Google Scholar] [CrossRef]
- Pais, A.; Chessa, L.A.; Serra, S.; Ruiu, A.; Meloni, G.; Donno, Y. The Impact of Commercial and Recreational Harvesting for Paracentrotus lividus on Shallow Rocky Reef Sea Urchin Communities in North-Western Sardinia, Italy. Estuar. Coast. Shelf Sci. 2007, 73, 589–597. [Google Scholar] [CrossRef]
- Privitera, D.; Chiantore, M.; Mangialajo, L.; Glavic, N.; Kozul, W.; Cattaneo-Vietti, R. Inter- and Intra-Specific Competition between Paracentrotus lividus and Arbacia lixula in Resource-Limited Barren Areas. J. Sea Res. 2008, 60, 184–192. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The Seasonal Variation in the Chemical Composition of the Kelp Species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Peteiro, C.; Freire, Ó. Biomass Yield and Morphological Features of the Seaweed Saccharina latissima Cultivated at Two Different Sites in a Coastal Bay in the Atlantic Coast of Spain. J. Appl. Phycol. 2013, 25, 205–213. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Rodríguez-Montesinos, Y.; Hernández-Carmona, G. Seasonal and Geographic Variations of Macrocystis pyrifera Chemical Composition at the Western Coast of Baja California. Cienc. Mar. 2021, 17, 91–107. [Google Scholar] [CrossRef]
- Martínez-Milián, G.; Olvera-Novoa, M.A. Evaluation of Potential Feed Ingredients for the Juvenile Four-Sided Sea Cucumber, Isostichopus badionotus. J. World Aquac. Soc. 2016, 47, 712–719. [Google Scholar] [CrossRef]
- Biancacci, C.; Visch, W.; Callahan, D.L.; Farrington, G.; Francis, D.S.; Lamb, P.; McVilly, A.; Nardelli, A.; Sanderson, J.C.; Schwoerbel, J.; et al. Optimisation of At-Sea Culture and Harvest Conditions for Cultivated Macrocystis Pyrifera: Yield, Biofouling and Biochemical Composition of Cultured Biomass. Front. Mar. Sci. 2022, 9, 951538. [Google Scholar] [CrossRef]
- Barta, E.S.; Branen, A.L.; Leung, H.K. Nutritional Analysis of Puget Sound Bull Kelp (Nereocystis luetkeana). J. Food Sci. 1981, 46, 494–497. [Google Scholar] [CrossRef]
- Nepper-Davidsen, J.; Glasson, C.R.K.; Lawton, R.J.; Magnusson, M. High Spatial and Temporal Variation in Biomass Composition of the Novel Aquaculture Target Ecklonia radiata. J. Appl. Phycol. 2023, 35, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Boudouresque, C.F.; Maggiore, F. Proximate Constituents, Biomass, and Energy in Posidonia oceanica (Potamogetonaceae). Mar. Ecol. 1989, 10, 263–270. [Google Scholar] [CrossRef]
- Frantzis, A.; Gremare, A.; Vetion, G. Growth Rates and RNA: DNA Ratios in Paracentrotus lividus (Echinodermata: Echinoidea) Fed on Benthic Macrophytes. J. Exp. Mar. Biol. Ecol. 1992, 156, 125–138. [Google Scholar] [CrossRef]
- Hrstich-Manning, G.; Aguirre, J.D. Nutritional Composition of Common, Coastal Seaweeds from Northeastern New Zealand. N. Z. J. Mar. Freshw. Res. 2024, 59, 485–500. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva lactuca and Durvillaea antarctica. Food. Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Rasyid, A. Evaluation of Nutritional Composition of the Dried Seaweed Ulva lactuca from Pameungpeuk Waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119–125. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Inomata, E.; Endo, H.; Aoki, M.N.; Agatsuma, Y. Dietary Effect of Kelp (Saccharina japonica) on Gonad Quantity and Quality in Sea Urchins (Mesocentrotus nudus) Collected from a Barren before the Fishing Season. J. Shellfish Res. 2018, 37, 659–669. [Google Scholar] [CrossRef]
- Xiren, G.K.; Aminah, A. Proximate Composition and Total Amino Acid Composition of Kappaphycus alvarezii Found in the Waters of Langkawi and Sabah, Malaysia. Int. Food. Res. J. 2017, 24, 1253–1260. [Google Scholar]
- Hernández-Carmona, G.; Carrillo-Domínguez, S.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Castillo-Domínguez, R.M. Monthly Variation in the Chemical Composition of Eisenia arborea J.E. Areschoug. J. Appl. Phycol. 2009, 21, 607–616. [Google Scholar] [CrossRef]
- Hammer, H.; Watts, S.; Lawrence, A.; Lawrence, J.; Desmond, R. The Effect of Dietary Protein on Consumption, Survival, Growth and Production of the Sea Urchin Lytechinus variegatus. Aquaculture 2006, 254, 483–495. [Google Scholar] [CrossRef]
- Olmos, J.; Paniagua-Michel, J.D.J.; Lopez, L.; Ochoa, L. Functional Feeds in Aquaculture. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1303–1319. [Google Scholar] [CrossRef]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The Contribution of Fisheries and Aquaculture to the Global Protein Supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. N-3 Oil Sources for Use in Aquaculture Alternatives to the Unsustainable Harvest of Wild Fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef]
- Parker, R.W.R.; Tyedmers, P.H. Uncertainty and Natural Variability in the Ecological Footprint of Fisheries: A Case Study of Reduction Fisheries for Meal and Oil. Ecol. Indic. 2012, 16, 76–83. [Google Scholar] [CrossRef]
- Ghamkhar, R.; Hicks, A. Comparative Environmental Impact Assessment of Aquafeed Production: Sustainability Implications of Forage Fish Meal and Oil Free Diets. Resour. Conserv. Recycl. 2020, 161, 104849. [Google Scholar] [CrossRef]
- Hodar, A.R.; Vasava, R.; Joshi, N.H. Fish Meal and Fish Oil Replacement for Aqua Feed Formulation by Using Alternative Sources: A Review. J. Exp. Zool. India 2020, 23, 13–21. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Global Overview on the Use of Fish Meal and Fish Oil in Industrially Compounded Aquafeeds: Trends and Future Prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish Oil Replacement in Finfish Nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Hoshikawa, H. The Effects of Fish Meal Feeding on the Gonad Quality of Cultivated Sea Urchin, Strongylocentrotus nudus (A. Agassiz). Sci. Rep. Hokkaido Fish Exp. Stn. 1998, 52, 17–24. [Google Scholar]
- Pearce, C.M.; Daggett, T.L.; Robinson, S.M.C. Effect of Protein Source Ratio and Protein Concentration in Prepared Diets on Gonad Yield and Quality of the Green Sea Urchin, Strongylocentrotus droebachiensis. Aquaculture 2002, 214, 307–332. [Google Scholar] [CrossRef]
- Shpigel, M.; Schlosser, S.C.; Ben-Amotz, A.; Lawrence, A.L.; Lawrence, J.M. Effects of Dietary Carotenoid on the Gut and the Gonad of the Sea Urchin Paracentrotus lividus. Aquaculture 2006, 261, 1269–1280. [Google Scholar] [CrossRef]
- Suckling, C.C.; Symonds, R.C.; Kelly, M.S.; Young, A.J. The Effect of Artificial Diets on Gonad Colour and Biomass in the Edible Sea Urchin Psammechinus miliaris. Aquaculture 2011, 318, 335–342. [Google Scholar] [CrossRef]
- Schlosser, S.C.; Lupatsch, I.; Lawrence, J.M.; Lawrence, A.L.; Shpigel, M. Protein and Energy Digestibility and Gonad Development of the European Sea Urchin Paracentrotus lividus (Lamarck) Fed Algal and Prepared Diets during Spring and Fall. Aquac. Res. 2005, 36, 972–982. [Google Scholar] [CrossRef]
- Kaneko, K.; Shirai, T.; Tanaka, M.; Kamei, M.; Matsumoto, H.; Osako, K. Taste Characteristics of the Gonad of Longspine Black Urchin Diadema setosum. Nippon Suisan Gakkaishi 2009, 75, 689–694. [Google Scholar] [CrossRef]
- Phillips, K.; Bremer, P.; Silcock, P.; Hamid, N.; Delahunty, C.; Barker, M.; Kissick, J. Effect of Gender, Diet and Storage Time on the Physical Properties and Sensory Quality of Sea Urchin (Evechinus chloroticus) Gonads. Aquaculture 2009, 288, 205–215. [Google Scholar] [CrossRef]
- Stagnol, D.; Renaud, M.; Davoult, D. Effects of Commercial Harvesting of Intertidal Macroalgae on Ecosystem Biodiversity and Functioning. Estuar. Coast. Shelf Sci. 2013, 130, 99–110. [Google Scholar] [CrossRef]
- Stagnol, D.; Michel, R.; Davoult, D. Unravelling the Impact of Harvesting Pressure on Canopy-Forming Macroalgae. In Proceedings of the Marine and Freshwater Research; CSIRO: Campbell, Australia, 2016; Volume 67, pp. 153–161. [Google Scholar] [CrossRef]
- Pereira, L. Seaweed: Ecology, Nutrient Composition, and Medicinal Uses; Pomin, V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; ISBN 9781614708780. [Google Scholar]
- Pan, L.; Farouk, M.H.; Qin, G.; Zhao, Y.; Bao, N. The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals. Int. J. Mol. Sci. 2018, 19, 554. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Lawrence, A.L.; Watts, S.A. Feeding, Digestion and Digestibility of Sea Urchins; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, ISBN 9780123964915. [Google Scholar]
- Olmos, J.; López, L.M.; Gorriño, A.; Galaviz, M.A.; Mercado, V. Bacillus subtilis Effects on Growth Performance and Health Status of Totoaba macdonaldi Fed with High Levels of Soy Protein Concentrate. Animals 2022, 12, 3422. [Google Scholar] [CrossRef] [PubMed]
- Adorian, T.J.; Jamali, H.; Farsani, H.G.; Darvishi, P.; Hasanpour, S.; Bagheri, T.; Roozbehfar, R. Effects of Probiotic Bacteria Bacillus on Growth Performance, Digestive Enzyme Activity, and Hematological Parameters of Asian Sea Bass, Lates calcarifer (Bloch). Probiotics Antimicrob. Proteins 2019, 11, 248–255. [Google Scholar] [CrossRef]
- López, L.M.; Olmos Soto, J.; Trejo Escamilla, I.; Flores Ibarra, M.; Ochoa, L.; Drawbridge, M.; Peres, H. Evaluation of Carbohydrate-to-Lipid Ratio in Diets Supplemented with Bacillus subtilis Probiotic Strain on Growth Performance, Body Composition and Digestibility in Juvenile White Seabass (Atractoscion nobilis, Ayres 1860). Aquac. Res. 2016, 47, 1864–1873. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef]
- Monma, M.; Ito, M.; Saito, M.; Chikuni, K. Carotenoid Components in Soybean Seeds Varying with Seed Color and Maturation Stage. Biosci. Biotechnol. Biochem. 1994, 58, 926–930. [Google Scholar] [CrossRef]
- Salomone, V.N.; Riera, M. Proximal Composition of Undaria pinnatifida from San Jorge Gulf (Patagonia, Argentina). Biol. Trace Elem. Res. 2020, 196, 252–261. [Google Scholar] [CrossRef]
- Kolb, N.; Vallorani, L.; Milanovic, N.; Stocchi, V. Evaluation of Marine Algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata japonica) as Food Supplements. Food Technol. Biotechnol. 2004, 42, 57–61. [Google Scholar]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs. 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Tabakaeva, O.V.; Razgonova, M.P.; Tabakaev, A.V.; Kapusta, S.V.; Zinchenko, Y.N. Qualitative and Quantitative Composition of Carotenoids in Extracts of the Brown Alga Ascophyllum nodosum. Chem. Nat. Compd. 2023, 59, 999–1001. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein Content, Amino Acid Composition and Nitrogen-to-Protein Conversion Factors of Ulva rigida and Ulva capensis from Natural Populations and Ulva lactuca from an Aquaculture System, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Pirian, K.; Piri, K.; Sohrabipour, J.; Blomster, J. Three Species of Ulva (Ulvophyceae) from the Persian Gulf as Potential Sources of Protein, Essential Amino Acids and Fatty Acids. Phycol. Res. 2018, 66, 149–154. [Google Scholar] [CrossRef]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The Allenic Carotenoid Fucoxanthin, a Novel Marine Nutraceutical from Brown Seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Fish Meal—Nutritive Value. J. Anim. Physiol. Anim. Nutr. 2011, 95, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Indexmundi Commodity Prices. Available online: https://www.indexmundi.com/commodities/ (accessed on 25 September 2024).
- IMARC Group Seaweed Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024–2032. Available online: https://www.imarcgroup.com/seaweed-market (accessed on 1 September 2024).
- National Fisherman Northeast. Available online: https://www.nationalfisherman.com/northeast/maine-prosecuting-illegal-rockweed-harvesters (accessed on 1 September 2024).
- Vologodskii, A. The Basics of Molecular Biology; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Miller, R.J.; Mann, K.H. Ecological Energetics of the Seaweed Zone in a Marine Bay on the Atlantic Coast of Canada. III. Energy Transformations by Sea Urchins. Mar. Biol. 1973, 18, 99–114. [Google Scholar] [CrossRef]
- Heflin, L.E.; Gibbs, V.K.; Powell, M.L.; Makowsky, R.; Lawrence, J.M.; Lawrence, A.L.; Watts, S.A. Effect of Dietary Protein and Carbohydrate Levels on Weight Gain and Gonad Production in the Sea Urchin Lytechinus variegatus. Aquaculture 2012, 358–359, 253–261. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Hidalgo, F.; Villanueva, D.; Furné, M.; Díaz-Casado, M.E.; Merino, R.; Sanz, A. Study of the Enzymatic Digestive Profile in Three Species of Mediterranean Sea Urchins. Aquaculture 2012, 344–349, 174–180. [Google Scholar] [CrossRef]
- Obrietan, K.; Drinkwine, M.; Williams, D.C. Marine Biology Amylase, Cellulase and Protease Activities in Surface and Gut Tissues of Dendraster Excentricus, Pisaster Ochraceus and Strongylocentrotus droebachiensis (Echinodermata). Mar. Biol. 1991, 109, 53–57. [Google Scholar] [CrossRef]
- Gibbs, V.K.; Watts, S.A.; Lawrence, A.L.; Lawrence, J.M. Dietary Phospholipids Affect Growth and Production of Juvenile Sea Urchin Lytechinus variegatus. Aquaculture 2009, 292, 95–103. [Google Scholar] [CrossRef]
- González-Durán, E.; Castell, J.D.; Robinson, S.M.C.; Blair, T.J. Effects of Dietary Lipids on the Fatty Acid Composition and Lipid Metabolism of the Green Sea Urchin Strongylocentrotus droebachiensis. Aquaculture 2008, 276, 120–129. [Google Scholar] [CrossRef]
- Fan, Z.; Li, J.; Zhang, Y.; Wu, D.; Zheng, X.; Wang, C.; Wang, L. Excessive Dietary Lipid Affecting Growth Performance, Feed Utilization, Lipid Deposition, and Hepatopancreas Lipometabolism of Large-Sized Common Carp (Cyprinus carpio). Front. Nutr. 2021, 8, 694426. [Google Scholar] [CrossRef]
- De Smet, S. Meat, Poultry, and Fish Composition: Strategies for Optimizing Human Intake of Essential Nutrients. Anim. Front. 2012, 2, 10–16. [Google Scholar] [CrossRef]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Castell, J.D.; Kennedy, E.J.; Robinson, S.M.C.; Parsons, G.J.; Blair, T.J.; Gonzalez-Duran, E. Effect of Dietary Lipids on Fatty Acid Composition and Metabolism in Juvenile Green Sea Urchins (Strongylocentrotus droebachiensis). Aquaculture 2004, 242, 417–435. [Google Scholar] [CrossRef]
- Seeley, R.H.; Schlesinger, W.H. Sustainable Seaweed Cutting? The Rockweed (Ascophyllum nodosum) Industry of Maine and the Maritime Provinces. Ann. N. Y. Acad. Sci. 2012, 1249, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the Human Consumption of the Red Seaweed Dulse (Palmaria palmata (L.) Weber & Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Carro, M.D.; Roleda, M.Y.; Weisbjerg, M.R.; Lind, V.; Novoa-Garrido, M. In Vitro Ruminal Fermentation and Methane Production of Different Seaweed Species. Anim. Feed Sci. Technol. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Rocha, F.; Rocha, A.C.; Baião, L.F.; Gadelha, J.; Camacho, C.; Carvalho, M.L.; Arenas, F.; Oliveira, A.; Maia, M.R.G.; Cabrita, A.R.; et al. Seasonal Effect in Nutritional Quality and Safety of the Wild Sea Urchin Paracentrotus lividus Harvested in the European Atlantic Shores. Food Chem. 2019, 282, 84–94. [Google Scholar] [CrossRef]
- Tsushima, M.; Matsuno, T. Comparative Biochemical Studies of Carotenoids in Sea-Urchins—I. In Comparative Biochemistry and Physiology Part B: Comparative Biochemistry; Elsevier: Amsterdam, The Netherlands, 1990; Volume 96, pp. 801–810. [Google Scholar] [CrossRef]
- Plank, L.R.; Lawrence, J.M.; Lawrence, A.L.; Olvera, R.M. The Effect of Dietary Carotenoids on Gonad Production and Carotenoid Profiles in the Sea Urchin Lytechinus variegatus. J. World Aquac. Soc. 2002, 33, 127–137. [Google Scholar] [CrossRef]
- Kawakami, T.; Tsushima, M.; Katabami, Y.; Mine, M.; Ishida, A.; Matsuno, T. Effect of β,β-Carotene, β-Echinenone, Astaxanthin, Fucoxanthin, Vitamin A and Vitamin E on the Biological Defense of the Sea Urchin Pseudocentrotus depressus. J. Exp. Mar. Biol. Ecol. 1998, 226, 165–174. [Google Scholar] [CrossRef]
- Borisovets, E.E.; Zadorozhny, P.A.; Kalinina, M.V.; Lepskaya, N.V.; Yakush, E.V. Changes of Major Carotenoids in Gonads of Sea Urchins (Strongylocentrotus intermedius and S. nudus) at Maturation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Symonds, R.C.; Kelly, M.S.; Suckling, C.C.; Young, A.J. Carotenoids in the Gonad and Gut of the Edible Sea Urchin Psammechinus miliaris. Aquaculture 2009, 288, 120–125. [Google Scholar] [CrossRef]
- Baião, L.F.; Rocha, C.; Lima, R.C.; Marques, A.; Valente, L.M.P.; Cunha, L.M. Sensory Profiling, Liking and Acceptance of Sea Urchin Gonads from the North Atlantic Coast of Portugal, Aiming Future Aquaculture Applications. Food Res. Int. 2021, 140, 109873. [Google Scholar] [CrossRef]
- Na, J.C.; Song, J.Y.; Lee, B.D.; Lee, S.J.; Lee, C.Y.; An, G.H. Effect of Polarity on Absorption and Accumulation of Carotenoids by Laying Hens. Anim. Feed Sci. Technol. 2004, 117, 305–315. [Google Scholar] [CrossRef]
- Cirino, P.; Brunet, C.; Ciaravolo, M.; Galasso, C.; Musco, L.; Fernández, T.V.; Sansone, C.; Toscano, A. The Sea Urchin Arbacia Lixula: A Novel Natural Source of Astaxanthin. Mar. Drugs 2017, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Theron, A.J. Aspects of Some Vitamins, Minerals and Enzymes in Health and Disease. World Rev. Nutr. Diet. 1990, 62, 27–58. [Google Scholar] [PubMed]
- Weitberg, A.B.; Weitzman, S.A.; Clark, E.P.; Stossel, T.P. Effects of Antioxidants on Oxidant-Induced Sister Chromatid Exchange Formation. J. Clin. Investig. 1985, 75, 1835–1841. [Google Scholar] [CrossRef]
- Ito, T.; Matsutani, T.; Mori, K.; Nomurat, T. Phagocytosis and Hydrogen Peroxide Production by Phagocytes of the Sea Urchin Strongylocentrotus nudus. Dev. Comp. Immunol. 1992, 16, 287–294. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Rickman, J.C.; Bruhn, C.M.; Barrett, D.M. Nutritional Comparison of Fresh, Frozen, and Canned Fruits and Vegetables II. Vitamin A and Carotenoids, Vitamin E, Minerals and Fiber. J. Sci. Food Agric. 2007, 87, 1185–1196. [Google Scholar] [CrossRef]
- Jintasataporn, O.; Yuangsoi, B. Stability of Carotenoid Diets during Feed Processing and under Different Storage Conditions. Molecules 2012, 17, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.; Raposo, A.; Cunha, B.; Pinheiro, J.; Santos, P.M.; Gomes, A.S.; Ferreira, S.; Gil, M.M.; Costa, J.L.; Pombo, A. Temporal Changes in Sex-Specific Color Attributes and Carotenoid Concentration in the Gonads (Roe) of the Purple Sea Urchin (Paracentrotus lividus) Provided Dry Feeds Supplemented with β-Carotene. Aquaculture 2022, 560, 738608. [Google Scholar] [CrossRef]
- McLaughlin, G.; Kelly, M.S. Effect of Artificial Diets Containing Carotenoid-Rich on Gonad Growth and Color in the Sea Urchin Psammechinus miliaris (GMELIN). J. Shellfish Res. 2001, 20, 377–382. [Google Scholar]
- Hagen, N.T.; Jørgensen, I.; Egeland, E.S. Sex-Specific Seasonal Variation in the Carotenoid Content of Sea Urchin Gonads. Aquat. Biol. 2008, 3, 227–235. [Google Scholar] [CrossRef]
- Secor, S.M. Specific Dynamic Action: A Review of the Postprandial Metabolic Response. J. Comp. Physiol. B 2009, 179, 1–56. [Google Scholar] [CrossRef]
- Venter, L.; Loots, D.T.; Vosloo, A.; Jansen van Rensburg, P.; Lindeque, J.Z. Abalone Growth and Associated Aspects: Now from a Metabolic Perspective. Rev. Aquac. 2018, 10, 451–473. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Siikavuopio, S.I. The Relationship between Feed Intake and Gonad Growth of Single and Stocked Green Sea Urchin (Strongylocentrotus droebachiensis) in a Raceway Culture. Aquaculture 2007, 262, 163–167. [Google Scholar] [CrossRef]
- Cuesta-Gomez, D.M.; Lazo, J.P.; Sánchez-Saavedra, M.d.P. Effects of Dietary Fish Oil and Soya Bean Lecithin on Gonad Index, Colour and Biochemical Composition of the Purple Sea Urchin, Strongylocentrotus purpuratus (Stimpson 1857). Aquac. Res. 2020, 51, 3384–3402. [Google Scholar] [CrossRef]
- Cuesta-Gomez, D.M.; Sánchez-Saavedra, M.d.P. Effects of Protein and Carbohydrate Levels on Survival, Consumption and Gonad Index in Adult Sea Urchin Strongylocentrotus purpuratus (Stimpson 1857) from Baja California, Mexico. Aquac. Res. 2017, 48, 1596–1607. [Google Scholar] [CrossRef]
- Cuesta-Gomez, D.M.; Sánchez-Saavedra, M.d.P. Effects of Dietary Protein and Carbohydrate Levels on Gonad Index, Composition, and Color in the Purple Sea Urchin Strongylocentrotus purpuratus. N. Am. J. Aquac. 2018, 80, 193–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).