Simple Summary
Rhodopseudomonas palustris (R. palustris) is a nutrient-rich bacterium with a protein content of up to 65% and abundant bioactive compounds. In this study, we evaluated its effects on the rumen microbiota of Leizhou goats. A 75-day feeding trial combined with high-throughput sequencing demonstrated that it increased the number of unique operational taxonomic units (OTUs), with the most pronounced effect observed in the low-concentration R. palustris (LRPRF) group. At the phylum level, the relative abundance of the Verrucomicrobiota community in the R. palustris group was significantly higher than that in the CONRF and PBMRF groups (p < 0.05). Compared with the CONRF group, the relative abundance of the Firmicutes and Euryarchaeota showed an upward trend, while that of the phylum Bacteroidota exhibited a downward trend. At the genus level, Prevotella remained dominant, whereas Methanobrevibacter abundance declined, and several probiotic taxa were significantly enriched. Functional prediction indicated that the rumen microbiota was primarily associated with carbohydrate and amino acid metabolism. These findings suggest that R. palustris supplementation can beneficially modulate the rumen microbial community, promote the absorption of nutrients, such as carbohydrates, and the degradation of coarse fibers, like lignocellulose, in the rumen, thereby enhancing the rumen digestive function of Leizhou goats while providing a theoretical basis for ecological and sustainable farming.
Abstract
This study investigated the effects of Rhodopseudomonas palustris (R. palustris) supplementation on the rumen microbiota of Leizhou goats and explored its potential mechanisms. Thirty healthy Leizhou goats of similar weight and age were selected and randomly assigned to five groups (six goats per group) using a completely randomized block design for a 75-day feeding trial. The control group (CONRF) was fed a basal diet, and the Photosynthetic Bacteria Medium (PBMRF) group was fed a basal diet + PBM solution. The low-concentration R. palustris (LRPRF), medium-concentration R. palustris (MRPRF), and high-concentration R. palustris (HRPRF) groups were fed a base diet supplemented with 20.0 mL, 40.0 mL, and 80.0 mL of R. palustris solution, respectively. All supplements were administered by mixing them into the feed. On day 75 of the trial, three goats were randomly selected from each group for slaughter and evisceration. Rumen contents were collected, immediately filtered, aliquoted, quick-frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis of rumen microbial diversity. Rumen microbial community structure was analyzed using high-throughput sequencing. The results showed that R. palustris enriched unique operational taxonomic units (OTUs), particularly in the LRPRF group. At the phylum level, Firmicutes and Bacteroidota were dominant; Firmicutes and Euryarchaeota abundance increased, while Bacteroidota decreased in the experimental groups. In addition, Verrucomicrobiota abundance was significantly elevated (p < 0.05). At the genus level, Prevotella was predominant, whereas Selenomonas abundance was significantly reduced (p < 0.01). Meanwhile, compared to the CONRF, PBMRF, and LRPRF groups, the MRPRF and HRPRF groups exhibited higher relative abundances of Christensenellaceae_R-7 group and Anaeroplasma. LEfSe analysis revealed a greater number of differential taxa in the experimental groups compared with the control, including enrichment of beneficial bacteria, such as Lactobacillus, which may contribute to optimizing the rumen environment by regulating immune and metabolic functions. Functional prediction indicated that rumen microorganisms were mainly involved in carbohydrate and amino acid metabolism. In conclusion, supplementation with R. palustris can beneficially modulate rumen microbial composition and function and promote rumen absorption of nutrients and degradation of crude fiber. This study provides a theoretical basis for green goat farming practices.
1. Introduction
The rumen, as a large and complex fermenter in ruminants, has a microbial community that is the core support of ruminant life activities and plays a decisive role in the enhancement of animal health and production performance [,]. The rumen is inhabited by trillions of microorganisms such as bacteria, fungi, protozoa, and archaea []. They form a highly synergistic ecosystem. These microorganisms can take plant polysaccharides, among other structures, such as cellulose and hemicellulose, which are indigestible by ruminants themselves [,] and are efficiently degraded to volatile fatty acids, among other metabolites and byproducts (NH3, CO2, CH4, H), providing over 70% of the host’s energy source [,]. At the same time, microbial industrial fermentation produces mycoprotein, which also becomes a high-quality protein supplement for animals []. In addition, rumen microorganisms are also involved in regulating the development of the host immune system, and through interactions with intestinal mucosal immune cells, they enhance animal resistance to pathogenic bacteria and maintain healthy homeostasis in the body [,,]. Therefore, in-depth exploration of the functional potential of rumen microorganisms and precise regulation of their flora structure has become a research hotspot in the field of ruminant nutrition and healthy breeding.
R. palustris, a purple non-sulfur bacterium with metabolic diversity, carbon source diversity, and metabolite diversity, has attracted attention for its unique metabolic properties and beneficial functions as a photosynthetic bacterium widely found in nature []. The bacterium can utilize a variety of carbon and nitrogen sources for growth and reproduction and can fix nitrogen, degrade harmful substances, and synthesize vitamins [,,]. It demonstrates good potential for applications in aquaculture, wastewater treatment, and livestock rearing [,,,]. Studies have shown that R. palustris can secrete a variety of digestive enzymes, such as cellulase and protease, which are directly involved in the degradation process of feed and accelerate the release of nutrients []. At the same time, its metabolism produces vitamins, amino acids, and other active substances, which can enhance the antioxidant capacity of the animal body and improve immunity [,]. In livestock and poultry breeding, R. palustris can promote animal growth and development and improve feed utilization by regulating the structure of intestinal flora, enhancing the antioxidant capacity and immune function of the body [,]. Studies on rumen digestion and fermentation in cows have revealed that R. palustris demonstrates significant potential in promoting rumen microbial growth and promoting microbial fermentation for non-sugar energy supply. This process relies on the maintenance of an anaerobic environment, a prerequisite for microbial homeostasis [].
As a characteristic local breed in Guangdong Province, the Leizhou goat occupies an important position in regional economic development []. However, in the process of large-scale farming, problems such as low feed conversion efficiency, high farming costs, and unstable product quality have gradually come to the forefront []. Given the potential advantages demonstrated by R. palustris in in vitro rumen studies of ruminants and the underdeveloped research field regarding its effects on the goat rumen microbiota, this study selected geographically representative Leizhou goats as subjects to investigate the effects of R. palustris on the rumen microbial diversity of Leizhou goats, aiming to elucidate its mechanisms in improving microecological balance, stabilizing pH levels, alleviating acidosis, and modulating the gut microbiota by augmenting beneficial populations and restricting pathogenic ones. These findings provide theoretical foundations and technical support for optimizing Leizhou goat farming practices and advancing green, efficient livestock development.
2. Materials and Methods
2.1. Animal Ethics Statement
The Animal Care Facility and Ethics Committee of Guangdong Ocean University approved this experiment. All animal experiments were approved by the Animal Ethics Committee of Guangdong Ocean University, with permit number GDOU (Guangdong)-2024N0930-23 (dated 30 September 2024).
2.2. Design and Housing
The experimental animals were provided by the Leizhou Goat Conservation Farm—Guangdong Ocean University Branch. Thirty healthy Leizhou goats were selected, all with similar body weights (approximately 16.0 ± 2.0 kg) and consistent ages (approximately 8–10 months). After a 5-day adaptation period, they were randomly assigned to 5 treatment groups. Each treatment group comprised six goats. All goats were housed in pens (3.5 m long × 5 m wide), each equipped with feeders and drinking troughs supplying fresh water. Each pen housed six goats to allow free movement. A 5-day adaptation period was provided during which pens were disinfected and goats underwent deworming, and they became familiar with the experimental environment, including feeders, basal diet, and waterers. After the 5-day adaptation period, goats’ health status and environmental suitability were carefully assessed, and the feeding trial commenced. The control group (CONRF) goats were fed a basal diet. The Photosynthetic Bacteria Medium (PBMRF) group received the basal diet supplemented with PBM solution. The low-concentration R. palustris (LRPRF), medium-concentration R. palustris (MRPRF), and high-concentration R. palustris (HRPRF) experimental groups received the basal diet supplemented with 20.0 mL, 40.0 mL, and 80.0 mL (concentration of 1 × 109 CFU/mL) of R. palustris solution per goat per day, respectively, administered via feed mixing. The experimental period was 75 days. The basal diet was formulated according to Nutritional Requirements for Meat Sheep (NY/T816-2021) [], and its composition is shown in Table 1.

Table 1.
Basic feed composition and nutritional levels (as-fed basis).
2.3. Preparation of R. palustris
Expand the commercial R. palustris stock solution using photosynthetic bacteria culture medium (both purchased from Bainuo Biotechnology Co., Ltd., Yancheng, China). Prepare the liquid medium by mixing the solid medium with purified water at a 1:100 ratio. Then, combine the stock solution with the liquid medium at a ratio of 1~1.5:5 to create the R. palustris bacterial suspension for expansion. After aliquoting the prepared bacterial solution, place it under conditions with abundant sunlight and a suitable temperature (25–35 °C) for expansion. The optimal expansion period is 4–5 days. During this time, shake the liquid evenly to ensure uniform light exposure (supplement with 60–100-watt bulbs at night or on cloudy days) to enhance the expansion rate and biomass.
2.4. Sample Collection
On the 75th day of the trial, prior to the morning feeding, three goats were randomly selected from both the control and experimental groups and euthanized via jugular vein exsanguination. The abdominal cavity was promptly opened to expose the rumen. A small incision was made in the dorsal sac of the rumen, avoiding major blood vessels, and approximately 200 g of rumen content was collected. The content was immediately filtered through sterile gauze and aliquoted into 50 mL sterile centrifuge tubes for rumen microbiota diversity analysis. Samples were rapidly frozen in liquid nitrogen and subsequently stored in an ultra-low temperature freezer at −80 °C for preservation.
2.5. DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
Total DNA of rumen microorganisms was extracted from frozen rumen samples using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the instructions, and the DNA concentration and purity were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), with the OD/OD280 ratio between 1.8 and 2.0 and the OD/OD80 ratio between 1.8 and 2.0. The OD260/OD280 ratio was required to be between 1.8 and 2.0, and the OD260/OD230 ratio was required to be greater than 2.0. The qualified DNA samples were diluted to 2.0 °C. The qualified DNA samples were diluted to 50 ng/μL and stored at −20 °C. The extracted DNA was used as a template to amplify and detect the full-length variable region (V1-V9) of the bacterial 16S rRNA gene, using primers 27F (AGRGTTTGATYNTGGCTCAG) and 1492R (TASGGHTACCTTGTTASGACTT). The PCR system was 25 μL, including 2 × Taq PCR Master Mix 12.5 μL, 0.5 μL each of upstream and downstream primers (10 μmol/L), 1 μL template DNA, and 10.5 μL ddH2O. The reaction conditions were as follows: 95 °C pre-denaturation, 0.5 μL each of upstream and downstream primers, 1 μL template DNA, 10.5 μL ddH2O, and 10.5 μL ddH2O. The reaction conditions were as follows: pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 30 s for 30 cycles, and final extension at 72 °C for 10 min. The amplified products were detected by electrophoresis on a 1.5% agarose gel, and the target fragment was recovered by cutting the gel. The recovered PCR products were sent to a professional sequencing company (e.g., UW Genetics) for double-end sequencing (PE 300) on the Illumina MiSeq platform, and sequencing libraries were constructed and sequenced according to standard procedures.
2.6. Statistical Analysis
This experiment employed a randomized complete block design (RCBD). Goats of similar weight and age were randomly assigned to different treatment groups: control, supplemented with photosynthetic bacterial culture medium, and added low, medium, and high concentrations of R. palustris. The experimental data were organized using Microsoft Excel 2016. SPSS 25.0 software was employed for data analysis. One-way analysis of variance (ANOVA) was used to investigate the effects of different treatment groups on α-diversity indices and microbial abundance at the phylum and genus levels. When ANOVA detected significant differences, Duncan’s multiple range test was applied for post hoc comparisons among treatment groups. The results are presented as mean ± SD. p < 0.05 indicates significant differences, while 0.05 ≤ p < 0.10 indicates a non-significant trend. Part of Figure 9 utilizes GenAI tools (doubao version 1.76.3_win) to generate relevant illustrations of goats and fungal strains.
Sequencing data were quality controlled and analyzed using QIIME 2 (v2020.8) software. First, the original bipartite sequencing data were spliced and filtered for low-quality sequences, and chimeras were removed to obtain high-quality, valid sequences. Then, the effective sequences were clustered with 97% similarity to obtain operational taxonomic units (OTUs). Based on the OTUs, the Chao1 abundance index, the Shannon diversity index, and the Simpson dominance index were calculated to analyze the abundance and diversity of the rumen flora. Principal coordinate analysis (PCoA) and analysis of differences between groups (ANOSIM) were performed using the R language (v4.0.3) to explore the differences in rumen flora structure between the two groups of goats. LEfSe (LDA Effect Size) analysis was used to screen species with significant differences (LDA value ≥ 4.0, p < 0.05) between groups, and evolutionary branching diagrams and bar charts were drawn to show the distribution of differential species.
3. Results
3.1. Analysis of Microbial OTUs in Goat Rumen
As shown in Figure 1, a total of 1099 operational taxonomic units (OTUs) were detected, comprising 649 OTUs in the CONRF group, 681 in the PBMRF group, 718 in the LRPRF group, 616 in the MRPRF group, and 666 in the HRPRF group. Shared OTUs numbered 314, accounting for 28.57% of the total OTUs. The number of OTUs unique to the CONRF, PBMRF, LRPRF, MRPRF, and HRPRF groups was 48, 58, 98, 43, and 43, respectively. The order of OTU abundance was LRPRF group > PBMRF group > CONRF group > MRPRF group and HRPRF group. A comprehensive analysis revealed that both the total OTU count and the unique OTU count in the LRPRF group exceeded those in the CONRF group and other experimental groups. This indicates that supplementing the diet with R. palustris at a concentration of 20 mL/head increases rumen-specific microbial diversity.
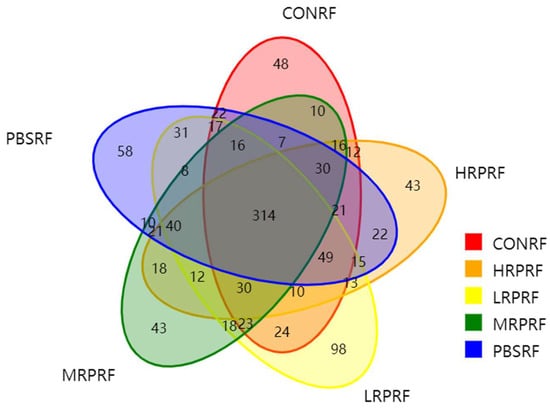
Figure 1.
Venn diagram showing the number of operational taxonomic units (OTUs). CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.
3.2. Effect of R. palustris on the Alpha Diversity of Microorganisms in the Gastric Rumen of Goats
Within a certain sequencing range, a steep increase in the rarefaction curve indicates the discovery of many new species, whereas a plateau suggests that additional sequencing yields few new species. In this study, the rarefaction curves (Figure 2a) showed a gradual increase with sequencing depth, without evident plateauing, indicating that new species continued to emerge, but the sequencing depth was sufficient to capture the majority of characteristic species. The Shannon index curve (Figure 2b) rose sharply at low sequencing depth and gradually approached saturation near 10,000 reads, suggesting that the sequencing volume was adequate to reliably assess rumen bacterial diversity. Rank-abundance curves (Figure 2c) were relatively broad and flat across all treatment groups, reflecting both high community richness and evenness. Overall, these results demonstrate that the sequencing depth was sufficient and that all treatment groups exhibited diverse and balanced rumen microbial communities.
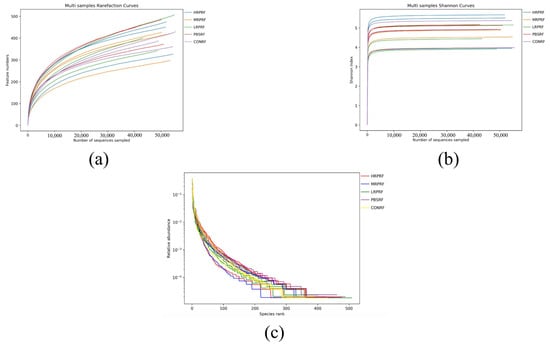
Figure 2.
Alpha diversity analysis of rumen microorganisms. (a) The number of features was observed to vary with sequencing depth (rarefaction curve). (b) It was observed that more species were found as the sequencing volume increased until the species volume saturated (Shannon index). (c) Relative distribution of species abundance (rank abundance curve). CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.
As shown in Table 2, the coverage index exceeded 99% across all groups, indicating that the sequencing data comprehensively covered the majority of species information within the samples. Compared to the CONRF group, the PBSRF and LRPRF groups showed a trend toward an increased Ace index, while the MRPRF and HRPRF groups exhibited a similar trend for the Chao1 index. Similarly, the phylogenetic diversity (PD) whole tree index values increased across these groups. However, as depicted in Figure 3, no significant differences (p > 0.05) were observed between groups for Simpson indices, Shannon indices, Chao1 indices, or Ace indices.

Table 2.
Alpha diversity index.
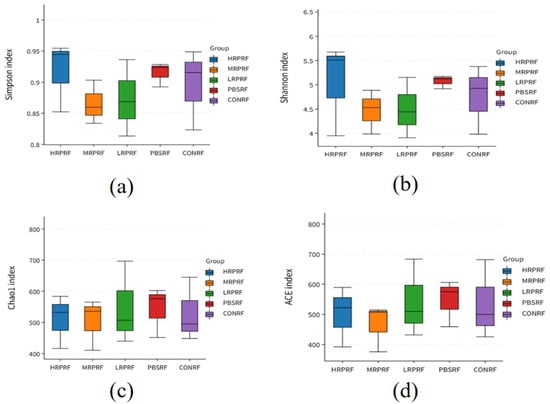
Figure 3.
In microbiome research, alpha diversity serves as a core metric for assessing both richness and evenness within individual samples. This box plot focuses on five treatment groups: HRPRF, MRPRF, LRPRF, PBMRF, and CONRF. Using four key indices, Simpson, Shannon, Chao1, and ACE, it systematically reveals the effects of different treatments on the internal diversity of microbial communities. (a) Simpson index boxplots for different grouped samples, illustrating community dominance patterns; (b) Boxplots of the Shannon index for different grouped samples, illustrating community diversity; (c) Boxplots of the Chao1 index for different grouped samples, illustrating community richness; (d) Boxplots of the ACE index for different grouped samples, illustrating community diversity. CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.
3.3. Effect of R. palustris on the Diversity of Microorganisms in the Gastric Rumen of Goats
Beta diversity refers to the variation in species composition between different communities along an environmental gradient, with greater distances between samples indicating greater divergence. PCoA analysis visually displays the bacterial community differences between control and experimental samples. The Bray–Jaccard distance algorithm was employed, and ANOSIM analysis was used for intergroup difference testing. PCoA analysis revealed a significant grouping effect (R2 = 0.264, p = 0.015), indicating that 13.27% and 11.91% of the variance were explained by PC1 and PC2, respectively, with statistically significant differences between groups (Figure 4a). NMDS analysis revealed differences in species composition between the control and experimental groups. A stress value of 0.1573 indicates reliable dimensionality reduction in the NMDS analysis, with the plot effectively reflecting differences in the original data (Figure 4b). Both analytical methods consistently revealed a distinct clustering tendency among samples from different treatment groups, confirming significant differences in microbial community structure between the HPPRF, MRPRF, LRPRF, PBSRF, and CONRF groups.
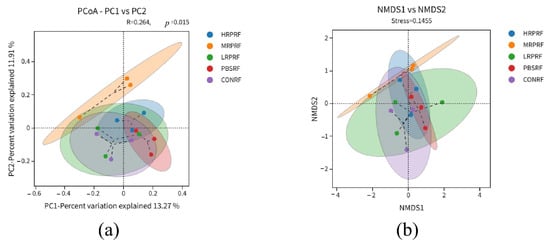
Figure 4.
Beta diversity analysis of goat rumen coordinates and metric multidimensional calibration analysis. (a) Principal Coordinate Analysis (PCoA) of microbial communities. (b) Non-metric multidimensional scaling (NMDS) of microbial communities. CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.
3.4. Effects of R. palustris on the Composition and Community Structure of Rumen Flora in Leizhou Goats
As shown in Figure 5a,b, a total of 10 phyla were detected at the phylum level, including Firmicutes, Bacteroidota, Spirochaetota Desulfobacterota, Proteobacteria, Proteobacteria, and Actinobacteriota. As can be seen from Table 3, the rumen flora of each group of bacterial phylum was dominated by Firmicutes and Bacteroidetes at the phylum level, which accounted for more than 90% of the total bacterial structure. The relative abundance of Firmicutes and Bacteroidetes in the RPRF group, although numerically higher than that in the CONRF group, showed no statistically significant difference compared with the control group (p = 0.98). Specifically, compared with the CONRF and PBMRF groups, the relative abundance of Verrucomicrobiota in the R. palustris group was significantly higher than that in the CONRF and PBMRF groups (p = 0.02).
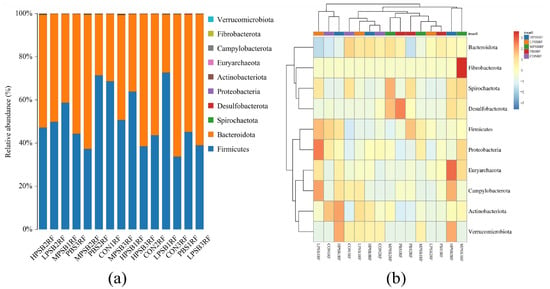
Figure 5.
Rumen microbial composition at the phylum level. (a) Bar chart displaying taxonomic abundance. (b) Clustered heatmap of abundance profiles. CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.

Table 3.
Effect of R. palustris on the relative abundance of rumen flora of Leizhou goats on the phylum.
As shown in Figure 6a,b, at the genus level, the major dominant bacteria included Prevotella, uncultured rumen bacterium, Selenomonas, and an unclassified group (UCG-OO4). The main dominant bacteria include Prevotella, uncultured_rumen_bacterium, Selenomonas, UCG-004, Christensenellaceae R-7 group, unclassified Selenomonadaceae, and Ruminococcus. As can be seen from Table 4, the secondary dominant bacteria included Erysipelotrichaceae UCG 009, Anaeroplasma, Mogibacterium, and Prevotellaceae UCG 003, among others. Compared with the CONRF group, the relative abundance of Selenomonas in the four experimental groups was highly significantly lower (p < 0.05); the relative abundance of the uncultured rumen bacteria was lower (p > 0.05), but the relative abundance of the Christensenellaceae R-7 group was relatively higher (p > 0.05) in the RPRF groups. Compared to the CONRF group, PBMRF group, and LRPRF group, the MRPRF group and HRPRF group showed a trend toward relatively increased relative abundance of Anaeroplasma and UCG-004.
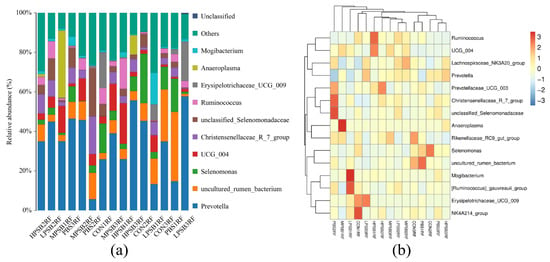
Figure 6.
Relative abundance of rumen at the genus level. (a) Bar chart of species abundance at the genus level. (b) Cluster heatmap of genus-level abundance. CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.

Table 4.
Effect of R. palustris on the relative abundance of rumen flora of Leizhou goats on the genus.
3.5. LEfSe Species Difference Analysis
As shown in Figure 7a, after screening with a linear discriminant analysis (LDA) threshold greater than 4, the main bacterial groups exhibiting significant differences in abundance in the rumen included f_Acholeplasmataceae, f_Selenomonadaceae, s_Clostridium_disporicum, and nine other groups. Compared to the CONRF group and the PBMRF group, the MRPRF and HRPRF groups had a higher number of significantly different bacterial groups. As shown in Figure 7b, the key microbial groups in the CONRF group were o_Vellionellales_Selenomonadales and f_Selenomonadaceae. In contrast, the key microbial groups in the MRPRF and HRPRF groups were g_Anaeroplasma and s_Lactobacillus amylovorus, among others. Compared to the CONRF group, the MRPRF and HRPRF groups exhibited a higher number of significantly contributing microbial groups.
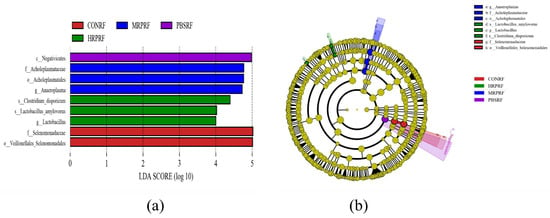
Figure 7.
Display LDA scores between different groups, where LDA > 4.0 and p < 0.05. (a) The figure displays biomarkers with LDA scores exceeding the threshold value (LDA > 4.0), indicating statistically significant differences between groups. (b) In the evolutionary tree diagram, concentric circles radiating outward represent taxonomic levels from kingdom (single circle) to genus (or species). CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.
3.6. Predictive Analysis of Colony Function (Picrust2)
As can be seen from Figure 8a, rumen microbial functional genes were mainly categorized as metabolic pathways. As can be seen from Figure 8b, the relative abundance of rumen microbial functional genes from high to low were global and overview mapping, carbohydrate metabolism, amino acid metabolism, metabolism of cofactors and vitamins, and nucleotide metabolism, respectively. It can be concluded that there are no significant differences in functional genes between different groups.
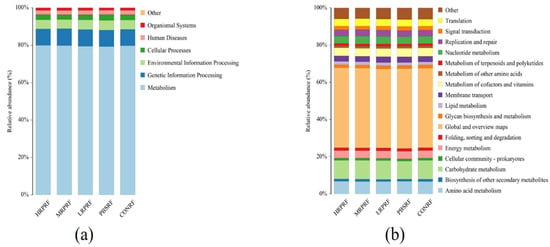
Figure 8.
Functional analysis of rumen microorganisms. (a) Distribution of relative abundance of level 1 in Picrust2 functional prediction across different samples; (b) Distribution of relative abundance of level 2 in Picrust2 functional prediction across different samples. CON = feeding only the basal diet; PBM (PBS) = basic diet and photosynthetic bacteria culture medium liquid; LRP = base diet supplemented with low R. palustris; MRP = base diet supplemented with medium R. palustris; HRP = base diet supplemented with high R. palustris; RF = rumen fluid.
4. Discussion
High-throughput sequencing has been widely used to study the microbiota in ruminants as a rapid and efficient method to determine the structure of rumen microbial communities []. Therefore, high-throughput sequencing methods can be used to show the overall effect of R. palustris in regulating the rumen microbiota of Leizhou goats. In this study, the sparse curves of the samples analyzed by high-throughput sequencing were relatively flat. This indicates that the data of our study are reasonable.
4.1. Influence of R. palustris on Rumen Microbial Diversity
As the largest compartment of the ruminant digestive system, the rumen is compartmentalized into various sacs. This environment sustains one of the most spectrally diverse ecosystems in nature, harboring a complex microbial community that includes a wide spectrum of bacteria, archaea, fungi, and ciliated protozoa. These microbial populations orchestrate the primary degradation of dietary plant polymers into monomers, culminating in the production of volatile fatty acids that supply the ruminant’s carbon and energy needs []. YY Chen et al. [] found in an in vitro rumen fermentation assay that the addition of R. palustris improved the viability of rumen microorganisms, thereby promoting microbial fermentation, with high potential to promote rumen microbial growth and enhance the supply of microbial fermentation to the energy of the non-biogenic sugars through the maintenance of an anaerobic environment for microbial equilibrium. Consistent with the results of this study, the addition of R. palustris increased the number of OUTs as well as unique OUTs in the rumen of Leizhou goats.
Species richness (estimated by the Ace and Chao indices) and community diversity (assessed by the Shannon and Simpson indices) were evaluated. It is noteworthy that elevated values of the Shannon and Simpson indices are indicative of higher community diversity. Alpha diversity analysis showed that the Ace, Chao1, and PD whole tree indices of the experimental group were higher than those of the control group, and although the differences between the groups were not significant (p > 0.05), there was a trend of increase, suggesting that R. palustris may optimize the abundance and phylogenetic diversity of microorganisms by enhancing the rumen function. In addition, the Shannon and Simpson indices of the experimental group were slightly higher than those of the control group, further confirming the positive effect of R. palustris on microbial community evenness.
4.2. Influence of R. palustris on the Structure of Rumen Microbial Communities
B ADehority et al. [] isolated 44 bacterial strains from the rumen contents of goats, of which Vibrio butyricus accounted for about 70% of the overall isolates, and Vibrio butyricus belongs to the Firmicutes phylum Trichoderma. Many studies have also shown that [,], at the phylum level, the most numerically dominant of the terrestrial mammalian gut microbiomes are the Firmicutes and the Bacteroidota. Dan Xue et al. [] found that the dominant phyla in the rumen microorganisms during the growth phase of ruminants were Firmicutes phylum and anaplasma phylum, and the results of the present study were consistent with them. The Firmicutes phylum is a key group of rumen microorganisms in the process of roughage utilization, which is mainly involved in the degradation and utilization of cellulose and hemicellulose. Firmicutes can increase the abundance of genes encoding enzymes related to energy metabolism, promote the digestibility of oligosaccharides, starch, and cellulose, and improve enzyme activity and rumen fermentation [,]. Bacteroidota is mainly involved in the decomposition and absorption of non-fiber plant components during rumen fermentation in ruminants []. It has been shown that an increase in the relative abundance of the Firmicutes and a decrease in the relative abundance of the Bacteroidota can promote the growth of goats []. The results of this experiment showed that the addition of R. palustris increased the relative abundance of Firmicutes and decreased the relative abundance of Bacteroidota in the rumen of Leizhou goats, thus promoting the digestion and absorption of roughage in goats. In this experiment, dietary addition of R. palustris increased the relative abundance of the Euryarchaeota as well as significantly increased the relative abundance of Verrucomicrobiota (p < 0.05). Archaea comprised only 3–4% of the rumen microbiome, whereas the Euryarchaea were the dominant archaea in the rumen []. It has been shown that [], in the rumen fluid of goats, Euryarchaeota accounts for 82% of the composition of methanogenic bacteria. Li et al. [] found that the Euryarchaeota were positively correlated with body weight; Broad Archaea accounted for a higher percentage of the obese group, and it may be one of the strongest predictors of obesity measures []. Vera Guerra [], in a study of the microbial community of the jejunum of grazing goats, found that Verrucomicrobiota and Bacillota, among others, belonged to the dominant flora. Some studies have claimed that [,] the Verrucomicrobiota is involved in the degradation of intestinal polysaccharides, such as fibrous disaccharides, etc. Verrucomicrobiota are considered highly suited for lignocellulose degradation in the rumen because they can encode various carbohydrate-degrading enzymes, peptidases, and sulfatases []. Concurrently, a metagenomic study has also revealed their unique role in rumen lignocellulose degradation []. Bamola mentioned in the report that [] the reduced abundance of Verrucomicrobiota may potentially affect the preservation of intestinal mucosal barrier and immunomodulatory functions. In this study, the increased relative abundance of Verrucomicrobiota suggests that R. palustris holds potential for improving ruminal degradation of lignocellulose and enhancing the intestinal immune barrier.
At the genus level, one of the major carbohydrate-degrading microorganisms, Prevotella, had the highest relative abundance [,], consistent with the results of this study. Bekele et al. found that [] Prevotella accounted for 56% or 60% of the rumen bacteria in goats. The results of the study showed that the addition of R. palustris to the ration increased the relative abundance of Prevotella in the rumen flora. The main function of Prevotella is protein degradation, but it is also involved in fiber degradation [,]. Prevotella is abundant in many ruminants; one of its fermentation products is propionic acid, and thus it has the potential to compete with methanogenic and archaeal bacteria for hydrogen utilization. Its abundance is negatively correlated with methane emissions, and Prevotella may have potential for use as an antimethanogenic agent []. Selenomonas crescentus plays a critical role in the rumen as a key consumer of lactic acid. Reducing lactic acid accumulation helps stabilize ruminal pH and creates a more favorable environment for other rumen microorganisms. Furthermore, this bacterium promotes the synthesis of propionic acid, thereby enhancing overall rumen fermentation efficiency []. It has been shown that [] lipopolysaccharide in the rumen is positively correlated with Selenomonas. Douglas B. Jordan et al. [] found in ruminant studies that Selenomonas can produce β-d-xylosidase in animals. Uncultured rumen bacterium may play a role in rice straw digestion []. In prior studies [], it has been shown that most fiber-associated bacterial communities are uncultured bacteria. It has been suggested that [] the role of uncultured rumen bacterium in the rumen of ruminants may be responsible for fiber digestion in the rumen. K. N. Joblin mentioned that [] all rumen mycoplasmas found so far have been categorized in the genus Astroplasma or anaerobic protozoa, which were produced to adapt to rumen mycoplasmas. The interaction between the gut microbiota and the gastrointestinal immune system is of particular importance for animal health, and important elements of the gastrointestinal immune response are the cytokines TGF-β and IgA antibodies. TGF-β is a potent immunomodulatory cytokine that suppresses adverse immune responses in the gut and induces IgA-like exchanges. Bacteria belonging to the Anaeroplasma, on the other hand, have the ability to enhance the level of mucosal IgA and induce the synthesis of TGF-β, a key regulatory cytokine, in T cells. Anaeroplasma categorized the anti-inflammatory cytokine TGF-β also fortifies the intestinal barrier by promoting mucosal IgA production [,]. Selenomonas is a functionally diverse and important bacterium in the rumen, exhibiting strong adaptability and the ability to survive under extreme nutritional fluctuations. Its primary function is not the direct degradation of complex polysaccharides, such as dietary fiber, but rather the efficient utilization of soluble carbohydrates produced by other bacteria through hydrolysis []. A study indicates that [] Selenomonas may participate in and stimulate the digestion of rumen fiber, with increased propionate production. However, Schingoethe’s report indicates that [] Selenomonas bacteria in the rumen of ruminants possess the ability to ferment starch and release lactic acid within the rumen, thereby increasing the risk of low rumen pH. In this study, the relative abundance of genus-level Selenomonas decreased, potentially weakening the rumen’s capacity to degrade carbohydrates and lignocellulose. However, this reduction also balanced the pH within the rumen environment, thereby reducing the risk of rumen acidosis caused by low pH levels.
4.3. Influence of R. palustris on Rumen Differential Flora
LEfSe analysis (Figure 7) showed that the number of differential flora in the test group was more than that in the CONRF group; especially, the MRPRF group had more significant differential flora, including Prevotella, g-Lactobacillus, and Selenomonadaceae. Alterations in these flora may directly affect rumen fermentation patterns and host metabolism. For example, Lactobacillus may stimulate an increase in the innate immune response; the use of Lactobacillus in food is widely distributed, and it has been studied for its control of intestinal infections, effects on cholesterol levels, and anticancer effects [,,,]. V Chiofalo et al. [] found in growth trials with lambs that the addition of Lactobacillus rhamnosus positively affected the development and maintenance of fermenting rumen activity, increasing feed utilization and dry matter intake; determined optimal pH conditions for pancreatic enzyme activity, which improved intestinal absorption of nutrients; and reduced protein-hydrolyzing microorganisms, which improved the nutritive properties of the intestinal mucosa in a secreted and absorbed manner. Lactobacillus rhamnosus strains isolated from goat’s milk can act as immunoactive modulators of intestinal and respiratory infections []. In vivo, G-Lactobacillus regulates the intestinal flora, thereby maintaining its homeostasis. It also protects against pathogenic bacteria by competing for adhesion sites [], and Lactobacillus spp. can inhibit the proliferation of pathogenic bacteria in the rumen by producing organic acids and antimicrobial peptides [,]. Lactic acid bacteria (LAB) produce various antimicrobial compounds—including lactic acid, short-chain fatty acids, hydrogen peroxide, and bacteriocin-like substances—that inhibit the growth of pathogens. Furthermore, they enhance immune function and lower the susceptibility to allergic responses [], whereas the enrichment of certain spore-producing bacteria (e.g., Turicibacter) may be associated with fat deposition. In a previous report [], Turicibacter emerged as a key regulator of host adipose biology, whose colonization effectively modulated host lipid metabolism, leading to reduced serum triglycerides and adipose tissue mass. However, Liu et al. [] indicated in their study that Turicibacter may be one of the elements that cause the inflammation that occurs in the epithelium of the hindgut mucosa.
4.4. Changes in the Environment of the Rumen
As shown in Figure 9, in the rumen microbial ecosystem of Leizhou goats, introducing R. palustris may optimize rumen fermentation function and promote microbial community health. Specifically, increased relative abundance of Firmicutes and Euryarchaeota indicates enhanced carbohydrate degradation capacity and methane metabolic activity, potentially improving feed energy utilization efficiency; increased abundance of Verrucomicrobiota and Prevotella suggests enhanced fiber degradation and protein metabolism processes, which are beneficial for roughage digestion and absorption. The enrichment of beneficial microorganisms, such as Christensenellaceae_R-7_group, further supports the trend toward a stable and healthy rumen microbial community structure; conversely, the reduced abundance of bacterial groups, like Selenomonas, may reflect an overall optimization of microbial metabolic functions toward greater efficiency.
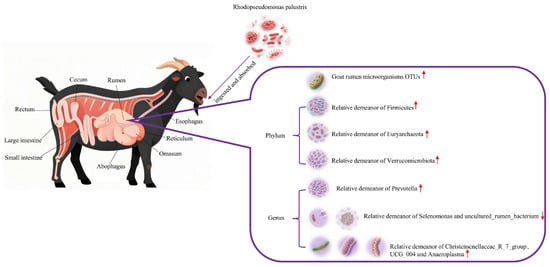
Figure 9.
The changes in rumen microflora and diversity that occurred after the ingestion and uptake of R. palustris in Leizhou goats in terms of OTUs and the distribution of the flora at the phylum level and the genus level, respectively, were used to determine whether this organism positively regulated the rumen microflora of Leizhou goats (doubao version 1.76.3_win).
5. Conclusions
Research indicates that supplementing the diet of Leizhou goats with low concentrations of R. palustris can increase the number of unique OTUs within their rumen microbiota. At the phylum level, Firmicutes and Bacteroidota were identified as the dominant bacterial phyla in the rumen of Leizhou goats. Compared with the CONRF group, the relative abundances of Verrucomicrobiota and Euryarchaeota in the R. palustris-supplemented groups showed a significant increase. At the genus level, the main dominant genera were Prevotella, uncultured rumen bacterium, and Selenomonas. Specifically, the relative abundance of Selenomonas was decreased, and that of the uncultured rumen bacterium was significantly decreased in the supplemented groups. In contrast, the relative abundances of the Christensenellaceae R-7 group, Anaeroplasma, and UCG-004 were increased, though the differences did not reach statistical significance. Furthermore, LEfSe analysis of species-level differences revealed that R. palustris also exerted a positive regulatory effect on the relative abundances of rumen microorganisms, such as Prevotella, g-Lactobacillus, and Selenomonadaceae, further confirming its role in optimizing rumen microbial community structure.
Author Contributions
Formal analysis, investigation, writing—original draft, and visualization, L.Z.; conceptualization, L.Z. and D.K.; methodology, L.Z.; resources and visualization, X.H.; validation, L.Z.; writing—review and editing, G.Z., D.K., F.Y. and S.G.; funding acquisition, project administration, and methodology, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the program for scientific research start-up funds of Guangdong Ocean University, grant numbers R19066 and R20045; Guangdong Province’s Special Fund for Rural Revitalization Strategy-Seed Industry Revitalization Project (2022-XDY-00-009); the Zhanjiang Science and Technology Plan Project-Technical Services and Promotion for Goat Farming (A23085); and the Quality Engineering Construction Project-Animal Science Major (PX-16223472).
Institutional Review Board Statement
This research was approved by the Animal Care and Use Committee of Guangdong Ocean University (No. GDOU-2024N0330-23; approved on 30 September 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset is available upon request from the authors.
Acknowledgments
During the preparation of this manuscript, the author(s) used doubao, version 1.76.3_win, for the purposes of generating the goat animation and fungal strain graphics shown in Figure 9. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krause, D.O.; Nagaraja, T.G.; Wright, A.D.G.; Callaway, T.R. Board-invited review: Rumen microbiology: Leading the way in microbial ecology1,2. J. Anim. Sci. 2013, 91, 331–341. [Google Scholar] [CrossRef]
- Russell, J.B.; Hespell, R.B. Microbial Rumen Fermentation. J. Dairy Sci. 1981, 64, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed]
- Béra-Maillet, C.; Devillard, E.; Cezette, M.; Jouany, J.P.; Forano, E. Xylanases and carboxymethylcellulases of the rumen protozoa Polyplastron multivesiculatum Eudiplodinium maggii and Entodinium sp. FEMS Microbiol. Lett. 2005, 244, 149–156. [Google Scholar]
- Akin, D.E.; Borneman, W.S. Role of Rumen Fungi in Fiber Degradation. J. Dairy Sci. 1990, 73, 3023–3032. [Google Scholar] [CrossRef]
- Russell, J.B.; Rychlik, J.L. Factors That Alter Rumen Microbial Ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Ke, S.; Chen, X.; Kenéz, Á.; Xu, W.; Cao, Y. Yak rumen microbiome elevates fiber degradation ability and alters rumen fermentation pattern to increase feed efficiency. Anim. Nutr. 2022, 11, 201–214. [Google Scholar] [CrossRef]
- Finnigan, T.; Mach, K.; Edlin, A. Mycoprotein: A healthy new protein with a low environmental impact. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2024; pp. 539–566. [Google Scholar]
- Zhang, X.; Li, X.; Wu, J.; Jiao, J.; He, Z.; Tan, Z.; Han, X. Rumen-protected glucose supplementation in transition dairy cows shifts fermentation patterns and enhances mucosal immunity. Anim. Nutr. 2021, 7, 1182–1188. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Zhu, W.; Mao, S. Dynamic changes in rumen fermentation and bacterial community following rumen fluid transplantation in a sheep model of rumen acidosis: Implications for rumen health in ruminants. FASEB J. 2019, 33, 8453–8467. [Google Scholar] [CrossRef]
- Trevisi, E.; Riva, F.; Filipe, J.F.S.; Massara, M.; Minuti, A.; Bani, P.; Amadori, M. Innate immune responses to metabolic stress can be detected in rumen fluids. Res. Vet. Sci. 2018, 117, 65–73. [Google Scholar] [CrossRef]
- Imhoff, J.F. Taxonomy and Physiology of Phototrophic Purple Bacteria and Green Sulfur Bacteria. In Anoxygenic Photosynthetic Bacteria; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–15. [Google Scholar]
- Hsu, S.H.; Shen, M.W.; Chen, J.C.; Lur, H.S.; Liu, C.T. The Photosynthetic Bacterium Rhodopseudomonas palustris Strain PS3 Exerts Plant Growth-Promoting Effects by Stimulating Nitrogen Uptake and Elevating Auxin Levels in Expanding Leaves. Front. Plant Sci. 2021, 12, 573634. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, F. Growth-Promoting Ability of Rhodopseudomonas palustris G5 and Its Effect on Induced Resistance in Cucumber Against Salt Stress. J. Plant Growth Regul. 2019, 38, 180–188. [Google Scholar] [CrossRef]
- Batool, K.; Zahra, T.F.; Rehman, Y. Arsenic-Redox Transformation and Plant Growth Promotion by Purple Nonsulfur Bacteria Rhodopseudomonas palustris CS2 and Rhodopseudomonas faecalis SS5. BioMed Res. Int. 2017, 1, 6250327. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, M.; Wang, Y.; Fu, L.; Li, W.; Deng, B.; Shen, W. Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J. Microbiol. Biotechnol. 2014, 30, 2523–2531. [Google Scholar] [CrossRef]
- Liu, J.; Xia, B.; Du, X.; Zeng, T.; Liu, Y.; Chen, L.; Li, C. Effects of water supplemented with Bacillus subtilis and photosynthetic bacteria on egg production, egg quality, serum immunoglobulins and digestive enzyme activity of ducks. J. Appl. Anim. Res. 2018, 46, 322–326. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, B.K. Mass production of Rhodopseudomonas palustris as diet for aquaculture. Aquac. Eng. 2000, 23, 281–293. [Google Scholar] [CrossRef]
- Peirong, Z.; Wei, L. Use of fluidized bed biofilter and immobilized Rhodopseudomonas palustris for ammonia removal and fish health maintenance in a recirculation aquaculture system. Aquac. Res. 2013, 44, 327–334. [Google Scholar] [CrossRef]
- Patterson, E.; Cryan, J.F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota, the pharmabiotics they produce and host health. Proc. Nutr. Soc. 2014, 73, 477–489. [Google Scholar] [CrossRef]
- Liu, R.; Wu, W.; Xu, X.; Wang, Y.; Yu, T.; Wang, J.; Wu, P. Rhodopseudomonas palustris in effluent enhances the disease resistance, TOR and NF-κB signalling pathway, intestinal microbiota and aquaculture water quality of Pelteobagrus vachelli. Aquac. Res. 2020, 51, 3959–3971. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Yan, H.; Liu, X.L.; Lv, L.; Yin, C.H.; Wang, P. Growth performance and meat quality of broiler chickens supplemented with Rhodopseudomonas palustris in drinking water. Br. Poult. Sci. 2014, 55, 360–366. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Wang, Y.; Li, W. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol. Biochem. 2010, 36, 501–509. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, Y.L.; Wang, W.K.; Zhang, Z.W.; Si, X.M.; Cao, Z.J.; Yang, H.J. Beneficial effect of Rhodopseudomonas palustris on in vitro rumen digestion and fermentation. Benef. Microbes 2020, 11, 91–100. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Han, X.; Liu, H.; Han, J.; Sun, W.; Zhou, H. Transcriptome analysis of muscle atrophy in Leizhou black goats: Identification of key genes and insights into limb-girdle muscular dystrophy. BMC Genom. 2025, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mao, H.; Wang, W.; Peng, W.; Mao, K.; Sun, W.; Zhou, H. Comparison of average daily gain, apparent digestibility, rumen fermentation parameters and bacterial communities, and serum antioxidant indices in Leizhou goats fed with or without rumen-protected fat. Front. Vet. Sci. 2024, 11, 1518826. [Google Scholar] [CrossRef] [PubMed]
- NY/T 816-2021; Feeding Standard of Meat-Producing Sheep and Goats. Agricultural Industry Standard of the People’s Republic of China: Beijing, China, 2021.
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; Mcsweeney, C.S. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Dehority, B.A.; Grubb, J.A. Characterization of the predominant bacteria occurring in the rumen of goats (Capra hircus). Appl. Environ. Microbiol. 1977, 33, 1030–1036. [Google Scholar] [CrossRef]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Singh, K.M.; Ahir, V.B.; Tripathi, A.K.; Ramani, U.V.; Sajnani, M.; Koringa, P.G.; Joshi, C.G. Metagenomic analysis of Surti buffalo (Bubalus bubalis) rumen: A preliminary study. Mol. Biol. Rep. 2012, 39, 4841–4848. [Google Scholar] [CrossRef]
- Xue, D.; Chen, H.; Luo, X.; Guan, J.; He, Y.; Zhao, X. Microbial diversity in the rumen, reticulum, omasum, and abomasum of yak on a rapid fattening regime in an agro-pastoral transition zone. J. Microbiol. 2018, 56, 734–743. [Google Scholar] [CrossRef]
- Guo, W.; Van Niekerk, J.K.; Zhou, M.; Steele, M.A.; Guan, L.L. Longitudinal assessment revealed the shifts in rumen and colon mucosal-attached microbiota of dairy calves during weaning transition. J. Dairy Sci. 2021, 104, 5948–5963. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Ding, X. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Atriaeenejad, S.; Ding, X.Z.; Han, J.L.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; Gurung, N.; Shange, R.; Solaiman, S. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 2019, 97, 3523–3534. [Google Scholar] [CrossRef]
- Kumar, S.; Indugu, N.; Vecchiarelli, B.; Pitta, D. Associative patterns among anaerobic fungi, methanogenic archaea, and bacterial communities in response to changes in diet and age in the rumen of dairy cows. Front. Microbiol. 2015, 6, 781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Elekwachi, C.O.; Jiao, J.; Wang, M.; Tang, S.; Zhou, C.; Forster, R.J. Investigation and manipulation of metabolically active methanogen community composition during rumen development in black goats. Sci. Rep. 2017, 7, 422. [Google Scholar] [CrossRef]
- Li, L.; Li, K.; Bian, Z.; Chen, Z.; Li, B.; Cui, K.; Wang, F. Association between body weight and distal gut microbes in Hainan black goats at weaning age. Front. Microbiol. 2022, 13, 951473. [Google Scholar] [CrossRef]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Da, B.; Marinello, K. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef]
- Guerra, V.; Tiago, I.; Aires, A.; Coelho, C.; Nunes, J.; Matiins, L.O.; Verissimo, A. The gastrointestinal microbiome of browsing goats (Capra hircus). PLoS ONE 2022, 17, e0276262. [Google Scholar] [CrossRef]
- Godoy-Vitorino, F.; Goldfarb, K.C.; Karaoz, U.; Leal, S.; Garcia-Amado, M.A.; Hugenholtz, P.; Dominguez-Bello, M.G. Comparative analyses of foregut and hindgut bacterial communities in hoatzins and cows. ISME J. 2012, 6, 531–541. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Brazel, D.M.; Swan, B.K.; Kai, Z.; Jacques, R. Capturing Single Cell Genomes of Active Polysaccharide Degraders: An Unexpected Contribution of Verrucomicrobia. PLoS ONE 2012, 7, e35314. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, J.; Sarikhan, S.; Han, J.L.; Ding, X.Z.; Salekdeh, G.H. Functional and phylogenetic analyses of camel rumen microbiota associated with different lignocellulosic substrates. NPJ Biofilms Microbiomes 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Bamola, V.D.; Ghosh, A.; Kapardar, R.K.; Lal, B.; Cheema, S.; Sarma, P.; Chaudhry, R. Gut microbial diversity in health and disease: Experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb. Ecol. Health Dis. 2017, 28, 1322447. [Google Scholar] [CrossRef] [PubMed]
- Mickdam, E.; Khiaosa-Ard, R.; Metzler-Zebeli, B.U.; Klevenhusen, F.; Chizzola, R.; Zebeli, Q. Rumen microbial abundance and fermentation profile during severe subacute ruminal acidosis and its modulation by plant derived alkaloids in vitro. Anaerobe 2016, 39, 4–13. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Tan, C.; López-Villalobos, N.; Padmanabha, J.; Wang, J.; Mcsweeney, C.S. Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation. Anim. Feed Sci. Technol. 2016, 216, 58–67. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Koike, S.; Kobayashi, Y. Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol. Lett. 2010, 305, 49–57. [Google Scholar] [CrossRef]
- Myer, P.R.; Wells, J.E.; Smith, T.P.L.; Kuehn, L.A.; Freetly, H.C. Cecum microbial communities from steers differing in feed efficiency1,2,3. J. Anim. Sci. 2015, 93, 5327–5340. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Gilbert, H.J. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef]
- Betancur-Murillo, C.L.; Aguilar-Marín, S.B.; Jovel, J. Prevotella: A Key Player in Ruminal Metabolism. Microorganisms 2023, 11, 1. [Google Scholar] [CrossRef]
- Cidrini, I.A.; Ferreira, I.M.; Oliveira, K.; Granja-Salcedo, Y.; Lage, J.F.; Siqueira, G.; Resende, F. PSX-B-9 Effect of trace mineral sources in the supplement for grazing cattle on ruminal bacteria diversity. J. Anim. Sci. 2021, 99, 453–454. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liu, Y.J.; Yin, Y.Y.; Jin, W.; Mao, S.Y.; Liu, J.H. Response of rumen microbiota, and metabolic profiles of rumen fluid, liver and serum of goats to high-grain diets. Animal 2019, 13, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.B.; Wagschal, K. Properties and applications of microbial β-D-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium. Appl. Microbiol. Biotechnol. 2010, 86, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Nyonyo, T.; Shinkai, T.; Mitsumori, M. Improved culturability of cellulolytic rumen bacteria and phylogenetic diversity of culturable cellulolytic and xylanolytic bacteria newly isolated from the bovine rumen. FEMS Microbiol. Ecol. 2014, 88, 528–537. [Google Scholar] [CrossRef]
- Koike, S.; Yoshitani, S.; Kobayashi, Y.; Tanaka, K. Phylogenetic analysis of fiber-associated rumen bacterial community and PCR detection of uncultured bacteria. FEMS Microbiol. Lett. 2003, 229, 23–30. [Google Scholar] [CrossRef]
- Koike, S.; Kobayashi, Y. Fibrolytic Rumen Bacteria: Their Ecology and Functions. Asian-Australas. J. Anim. Sci. 2009, 22, 131–138. [Google Scholar] [CrossRef]
- Joblin, K.N.; Naylor, G.E. The Ruminal Mycoplasmas: A Review. J. Appl. Anim. Res. 2002, 21, 161–179. [Google Scholar] [CrossRef]
- Goetze, V.V. Direct Induction of TGF-β Signaling by the Gut Commensal Anaeroplasma; Technische Universitaet: Berlin, Germany, 2024. [Google Scholar]
- Beller, A.; Kruglov, A.; Durek, P.; Goetze, V.V.; Hoffmann, U.; Maier, R.; Chang, H.D. P104 Anaeroplasma, a potential anti-inflammatory probiotic for the treatment of chronic intestinal inflammationP104. Ann. Rheum. Dis. 2019, 78, A45–A46. [Google Scholar] [CrossRef]
- Ricke, S.C.; Martin, S.A.; Nisbet, D.J. Ecology, metabolism, and genetics of ruminal selenomonads. Crit. Rev. Microbiol. 1996, 22, 27–65. [Google Scholar] [CrossRef]
- Sawanon, S.; Koike, S.; Kobayashi, Y. Evidence for the possible involvement of Selenomonas ruminantium in rumen fiber digestion. FEMS Microbiol. Lett. 2011, 325, 170–179. [Google Scholar] [CrossRef]
- Schingoethe, D.J. A 100-Year Review: Total mixed ration feeding of dairy cows. J. Dairy Sci. 2017, 100, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Yadav, H.; Sinha, P.R. Stimulation of Innate Immunity by Oral Administration of Dahi Containing Probiotic Lactobacillus casei in Mice. J. Med. Food 2008, 11, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Taranto, M.P.; Medici, M.; Perdigon, G.; Holgado, A.R.; Valdez, G.F.L. Evidence for Hypocholesterolemic Effect of Lactobacillus reuteri in Hypercholesterolemic Mice. J. Dairy Sci. 1998, 81, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Perdigon, G.; De Macias, M.E.N.; Alvarez, S.; De Ruiz Holgado, A.A.P.; Oliver, G. Prevention of gastrointestinal infection using immunobiological methods with milk fermented with Lactobacillus casei and Lactobacillus acidophilus. J. Dairy Res. 1990, 57, 255–264. [Google Scholar] [CrossRef]
- Commane, D.; Hughes, R.; Shortt, C.; Rowland, I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 591, 276–289. [Google Scholar] [CrossRef]
- Chiofalo, V.; Liotta, L.; Chiofalo, B. Effects of the administration of Lactobacilli on body growth and on the metabolic profile in growing Maltese goat kids. Reprod. Nutr. Dev. 2004, 44, 449–457. [Google Scholar] [CrossRef]
- Salva, S.; Villena, J.; Alvarez, S. Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: Impact on intestinal and respiratory infections. Int. J. Food Microbiol. 2010, 141, 82–89. [Google Scholar] [CrossRef]
- Foksowicz-Flaczyk, J.; Wójtowski, J.A.; Danków, R.; Mikołajczak, P.; Pikul, J.; Gryszczyńska, A.; Stanisławski, D. The Effect of Herbal Feed Additives in the Diet of Dairy Goats on Intestinal Lactic Acid Bacteria (LAB) Count. Animals 2022, 12, 255. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Jiang, X.; Zhang, H.; Mehmood, K.; Zhang, L.; Li, J. Probiotic Potential of Leuconostoc pseudomesenteroides and Lactobacillus Strains Isolated from Yaks. Front. Microbiol. 2018, 9, 2987. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef]
- Manning, T.S.; Gibson, G.R. Microbial-gut interactions in health and disease. Prebiotics Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, D.; Zhang, D.; Huang, K.; Zhang, Y.; Li, X.; Wang, W. Exploring the cecal microbial community associated with fat deposition in sheep and its possible pathways of action. Microbiol. Spectr. 2025, 13, e01488-24. [Google Scholar] [CrossRef]
- Liu, J.; Xu, T.; Zhu, W.; Mao, S. High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats. Br. J. Nutr. 2014, 112, 416–427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).