Genome-Wide Egg Hunt: Unhiding Candidate Genes for Egg Component Traits in Layers of an F2 Resource Population
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Birds and Performance Data Collection
2.2. Phenotypic Characteristics and Their Analyses
2.3. Sampling and DNA Extraction
2.4. Genotyping and Quality Control of SNPs
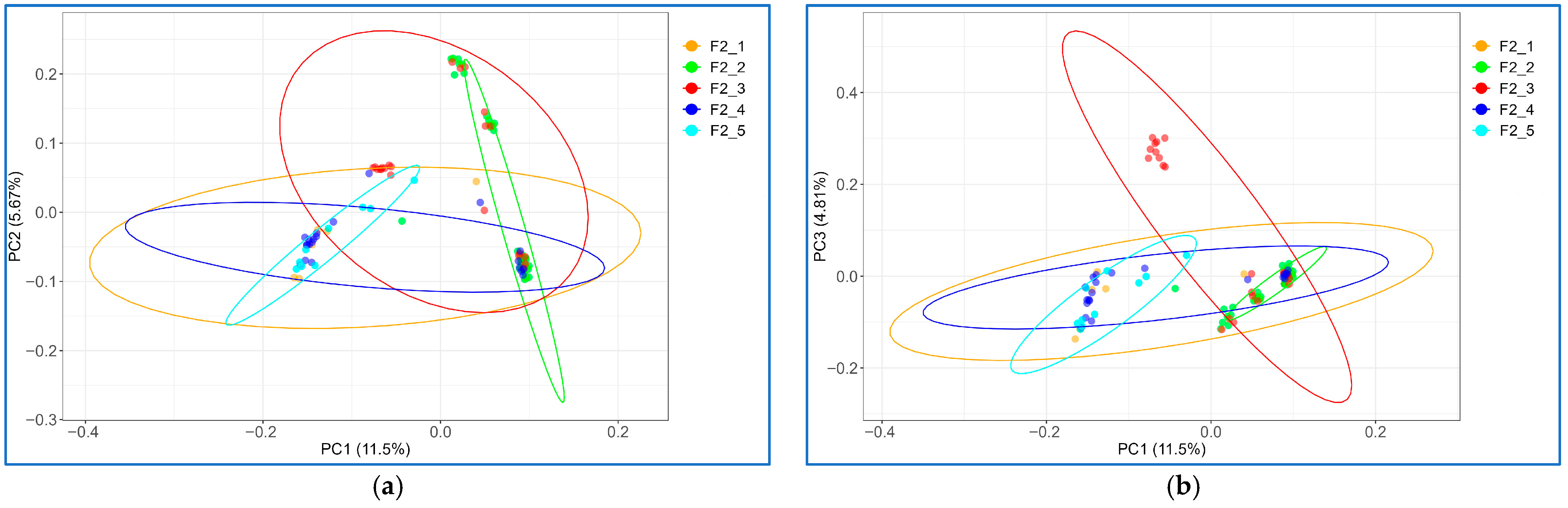
2.5. Principal Component Analysis
2.6. GWAS Scan
3. Results
3.1. Analyses of Phenotypic Data on EW Parameters and Population Stratification
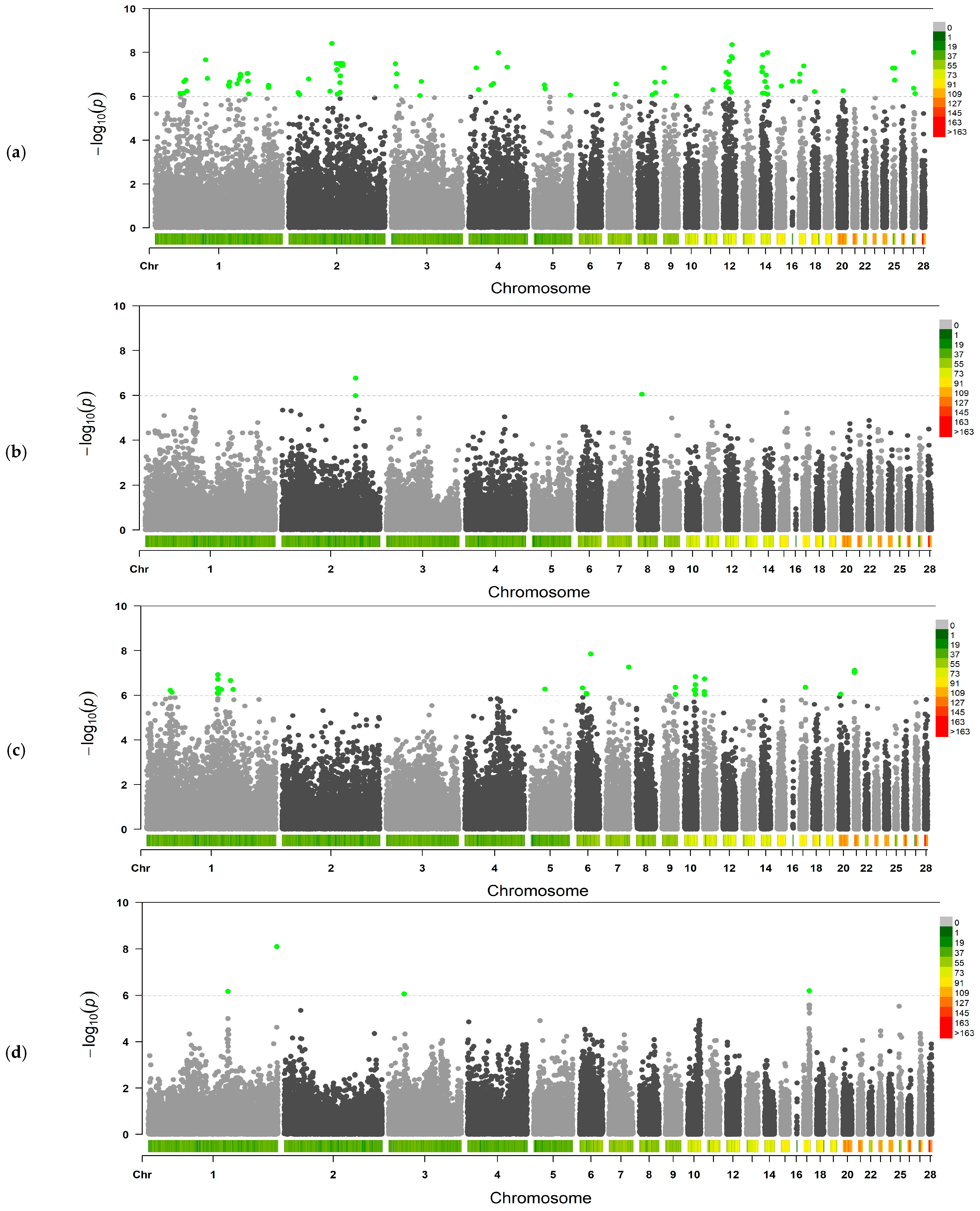
3.2. GWAS Analysis
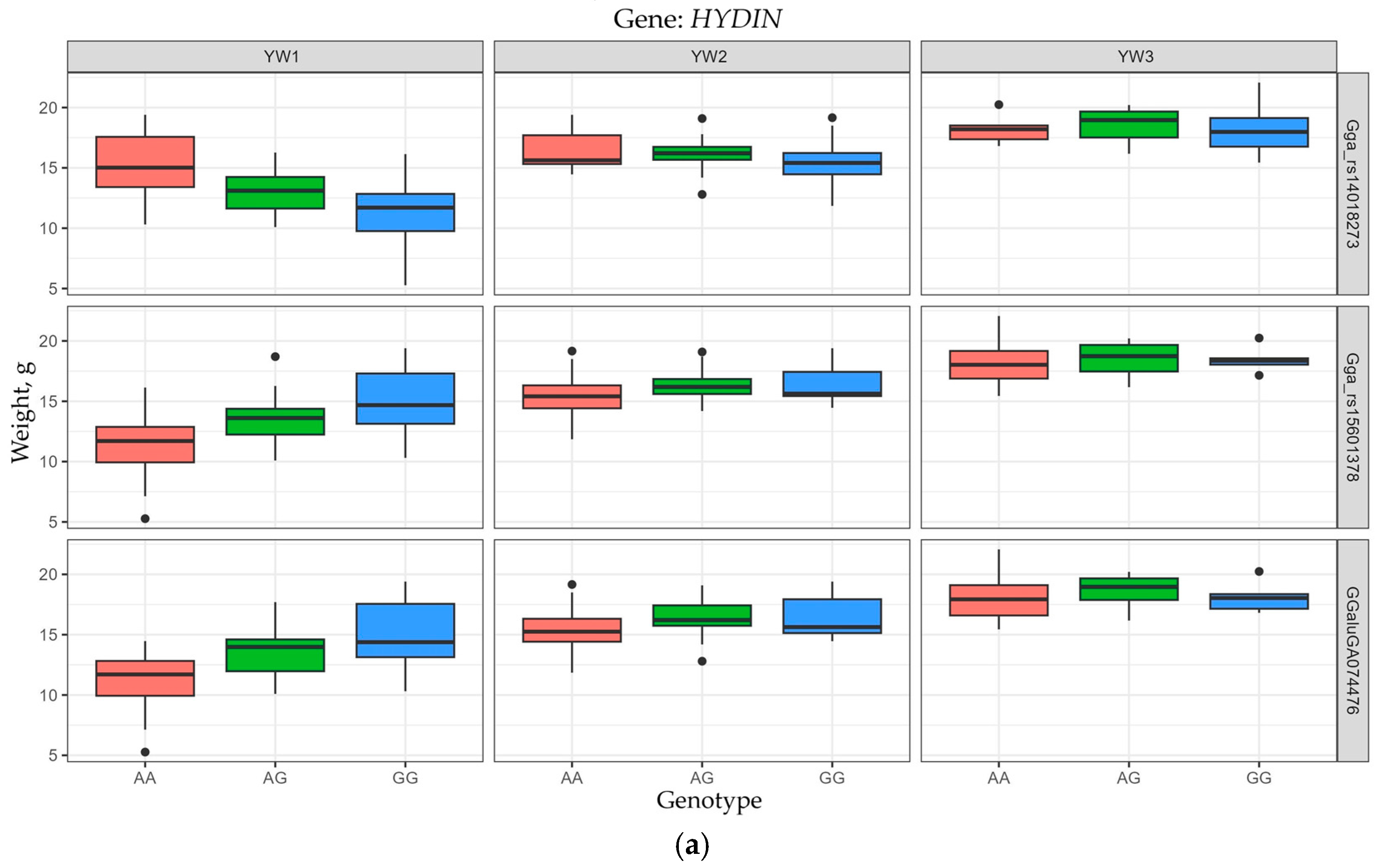
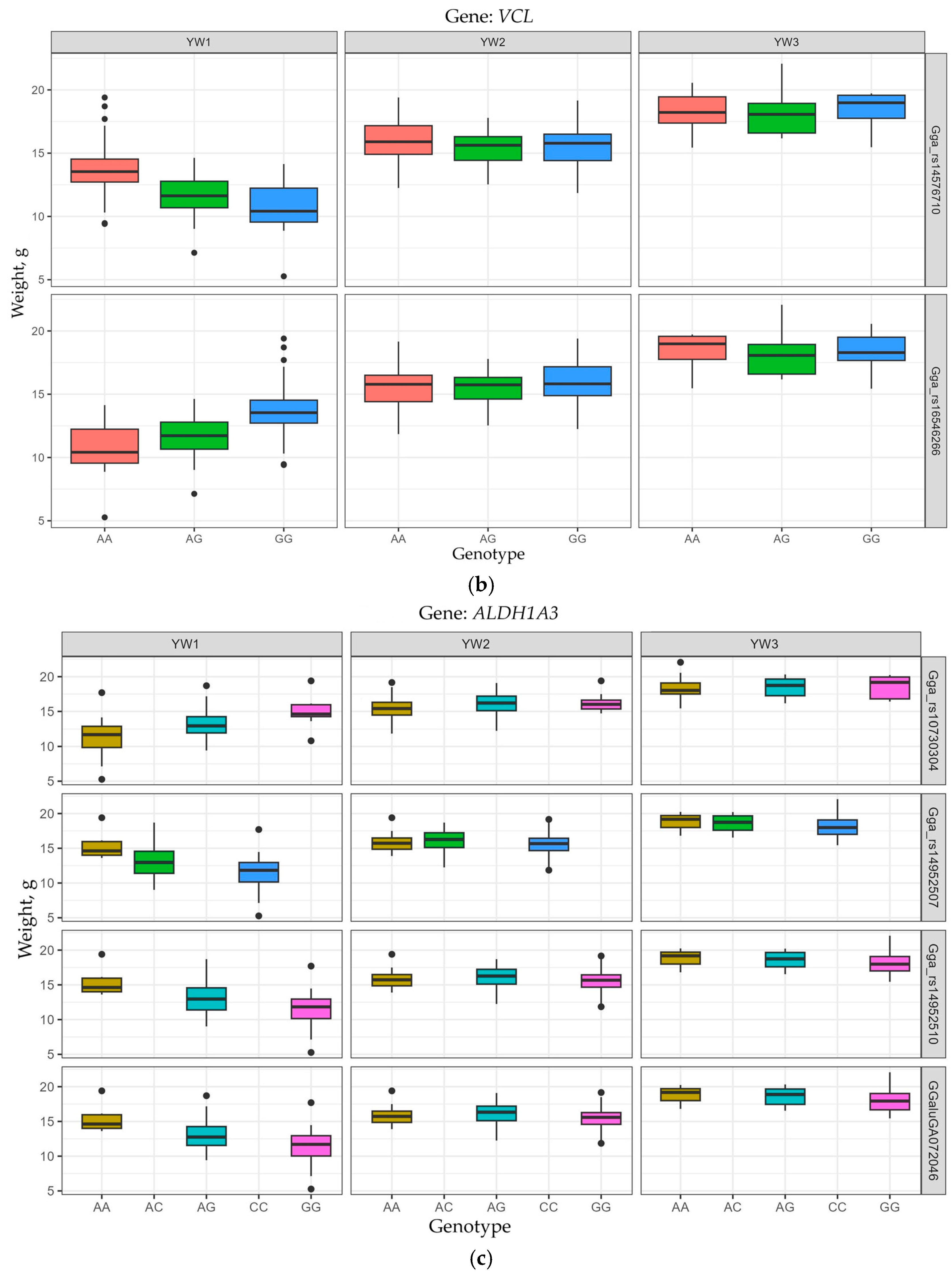
3.3. Identification of Candidate Genes
3.4. Allelic Variants of Genes Determining EW Trait Manifestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jez, C.; Beaumont, C.; Magdelaine, P. Poultry production in 2025: Learning from future scenarios. Worlds Poult. Sci. J. 2011, 67, 105–114. [Google Scholar] [CrossRef]
- Neeteson, A.-M.; Avendaño, S.; Koerhuis, A.; Duggan, B.; Souza, E.; Mason, J.; Ralph, J.; Rohlf, P.; Burnside, T.; Kranis, A.; et al. Evolutions in commercial meat poultry breeding. Animals 2023, 13, 3150. [Google Scholar] [CrossRef]
- Tereshchenko, O.V. The state of the poultry industry and the prospects for its scientific support. In Poultry Industry’2013, Materials of the IX International Conference; n.p.: Kharkiv, Ukraine, 2013; pp. 10–15. [Google Scholar]
- Bist, R.B.; Bist, K.; Poudel, S.; Subedi, D.; Yang, X.; Paneru, B.; Mani, S.; Wang, D.; Chai, L. Sustainable poultry farming practices: A critical review of current strategies and future prospects. Poult. Sci. 2024, 103, 104295. [Google Scholar] [CrossRef]
- Podisi, B.K.; Knott, S.A.; Burt, D.W.; Hocking, P.M. Comparative analysis of quantitative trait loci for body weight, growth rate and growth curve parameters from 3 to 72 weeks of age in female chickens of a broiler–layer cross. BMC Genet. 2013, 14, 22. [Google Scholar] [CrossRef]
- Khvostyk, V.; Tereshchenko, O.; Zakharchenko, O.; Bondarenko, Y. Influence of «adding blood» of cocks of foreign crosses upon economically beneficial attributes of meat-egg hens of domestic selection. Bull. Agric. Sci. 2017, 95, 44–48. [Google Scholar] [CrossRef]
- Mamontova, I.; Moiseeva, I. Results of using Moscow chickens of M5 line for crossing with White Leghorns of Highsex cross. Izv. Timiryazev Agric. Acad. 1981, 6, 136–142. Available online: https://eurekamag.com/research/016/935/016935374.php (accessed on 30 September 2025).
- Bondarenko, Y.V.; Ostapenko, V.I.; Ali, O.H.; Bulchenko, I.A.; Shubin, P.I. Sexual dimorphism and sex determination of broilers of the Ross 308 cross. Poult. Farm. 2013, 69, 51–54. [Google Scholar]
- Kochish, I.I.; Romanov, M.N.; Nikonov, I.N.; Ilyina, L.A.; Laptev, G.Y. Determination of Intestinal Microbiocenoses in Hens of Egg-type Crosses. In Proceedings of the World and Russian Trends in the Development of Poultry Industry: The Realities and Challenges of the Future, Proceedings of the 19th International Conference; Sergiev Posad, Russia, 15–17 May 2018, World’s Poultry Science Association (WSPA) Russian Branch, All-Russian Poultry Research and Technological Institute: Sergiyev Posad, Russia, 2018; pp. 240–243. Available online: https://kar.kent.ac.uk/89487 (accessed on 30 September 2025).
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef]
- Chomchuen, K.; Tuntiyasawasdikul, V.; Chankitisakul, V.; Boonkum, W. Genetic Evaluation of body weights and egg production traits using a multi-trait animal model and selection index in Thai native synthetic chickens (Kaimook e-san2). Animals 2022, 12, 335. [Google Scholar] [CrossRef]
- Shtele, A.L. Problem of egg productivity in hens and its early prediction. Agric. Biol. 2014, 6, 26–35. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and egg-derived foods: Effects on human health and use as functional foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Fernandez, M.L. The health benefits of egg protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and amino acid content in four brands of commercial table eggs in retail markets in relation to human requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef]
- Gyawali, D.; Goto, T. Assessing the genetic and environmental factors on egg amino acid traits in chickens: A review. Animals 2025, 15, 1554. [Google Scholar] [CrossRef]
- Layman, D.K.; Rodriguez, N.R. Egg protein as a source of power, strength, and energy. Nutr. Today 2009, 44, 43–48. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Herman, M.; Starostecka, E. Hen’s egg as a source of valuable biologically active substances. Postepy Hig. Med. Dosw. 2016, 70, 751–759. [Google Scholar] [CrossRef]
- Kutnyuk, P.I.; Bondarenko, Y.V.; Moiseyeva, I.G. Biochemical polymorphism of proteins and its significance in genetics and breeding of poultry. In Genetics and Selection in Ukraine at the Turn of the Millennium; Logos: Kyiv, Ukraine, 2001; p. 8. [Google Scholar]
- Sun, C.; Liu, J.; Yang, N.; Xu, G. Egg quality and egg albumen property of domestic chicken, duck, goose, turkey, quail, and pigeon. Poult. Sci. 2019, 98, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- van der Wagt, I.; de Jong, I.C.; Mitchell, M.A.; Molenaar, R.; van den Brand, H. A review on yolk sac utilization in poultry. Poult. Sci. 2020, 99, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Şahan, U.; Ipek, A.Y.D.I.N.; Sozcu, A.R.D.A. Yolk sac fatty acid composition, yolk absorption, embryo development, and chick quality during incubation in eggs from young and old broiler breeders. Poult. Sci. 2014, 93, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Yair, R.; Uni, Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult. Sci. 2011, 90, 1523–1531. [Google Scholar] [CrossRef]
- Artemenko, O.B.; Tagirov, M.T.; Baydevlyatova, O.M.; Shomina, N.V.; Tereshchenko, A.V. Study of the dynamics of changes in fatty acid composition of egg yolks during embryonic development of hens of different productivity types. Poult. Farm. 2014, 71, 7–17. [Google Scholar]
- Ionov, I.A.; Katerinich, O.O.; Kuchmistov, V.O.; Anisimova, O.V.; Griffin, D.K.; Romanov, M.N.; Zhukova, I.O. Vitamin E and A availability in goose embryos and goslings and improvement of reproduction traits depending on the starting temperature regime of egg incubation. Poultry 2023, 2, 305–319. [Google Scholar] [CrossRef]
- Hasan, M.K.; Khaliduzzaman, A. Egg formation and embryonic development: An overview. In Informatics in Poultry Production: A Technical Guidebook for Egg and Poultry Education, Research and Industry; Khaliduzzaman, A., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2022; pp. 13–32. [Google Scholar] [CrossRef]
- Guyot, N.; Réhault-Godbert, S.; Slugocki, C.; Harichaux, G.; Labas, V.; Helloin, E.; Nys, Y. Characterization of egg white antibacterial properties during the first half of incubation: A comparative study between embryonated and unfertilized eggs. Poult. Sci. 2016, 95, 2956–2970. [Google Scholar] [CrossRef] [PubMed]
- Milisits, G.; Kovács, E.; Pőcze, O.; Ujvári, J.; Taraszenkó, Z.; Jekkel, G.; Locsmándi, L.; Bázár, G.; Szabó, A.; Romvári, R.; et al. Effect of egg composition on hatchability and on growth and slaughter characteristics of meat-type chicks. Br. Poult. Sci. 2010, 51, 289–295. [Google Scholar] [CrossRef]
- Rezaee, M.S.; Liebhart, D.; Hess, C.; Hess, M.; Paudel, S. Bacterial infection in chicken embryos and consequences of yolk sac constitution for embryo survival. Vet. Pathol. 2021, 58, 71–79. [Google Scholar] [CrossRef]
- Sirri, F.; Zampiga, M.; Berardinelli, A.; Meluzzi, A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018, 97, 1818–1823. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. Comptes Rendus Palevol 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Solomon, S.E. The eggshell: Strength, structure and function. Br. Poult. Sci. 2010, 51 (Suppl. 1), 52–59. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Eggshell structure, measurements, and quality-affecting factors in laying hens: A review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef]
- Narushin, V.G.; Chausov, M.G.; Shevchenko, L.V.; Pylypenko, A.P.; Davydovych, V.A.; Romanov, M.N.; Griffin, D.K. Shell, a naturally engineered egg packaging: Estimated for strength by non-destructive testing for elastic deformation. Biosyst. Eng. 2021, 210, 235–246. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; D’alba, L.; Dunn, I.C.; Rehault-Godbert, S.; Rodriguez-Navarro, A.B.; Hincke, M.T. Properties, genetics and innate immune function of the cuticle in egg-laying species. Front. Immunol. 2022, 13, 838525. [Google Scholar] [CrossRef]
- Gradl, J.A. Quantifying Condensation on Shell Eggs and Its Effect on Salmonella Enteritidis Penetration into Egg Contents. Master’s Thesis, Auburn University, Auburn, AL, USA, 2016. Available online: https://auetd.auburn.edu/handle/10415/5261 (accessed on 30 September 2025).
- Yi, G.; Liu, W.; Li, J.; Zheng, J.; Qu, L.; Xu, G.; Yang, N. Genetic analysis for dynamic changes of egg weight in 2 chicken lines. Poult. Sci. 2014, 93, 2963–2969. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C. A study on egg production and quality according to the age of four Italian chicken dual-purpose purebred hens reared outdoors. Animals 2023, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Ipek, A.; Sozcu, A. Comparison of hatching egg characteristics, embryo development, yolk absorption, hatch window, and hatchability of Pekin Duck eggs of different weights. Poult. Sci. 2017, 96, 3593–3599. [Google Scholar] [CrossRef]
- Shanawany, M.M. Inter-relationship between egg weight, parental age and embryonic development. Br. Poult. Sci. 1984, 25, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Narushin, V.G.; Romanov, M.N. Modelling Growth of Chick Embryo. In Proceedings of the 9th European Poultry Conference, Plenary Papers and Contributed Papers, Glasgow, UK, 7–12 August 1994; Great Britain, Ministry of Agriculture, Fisheries and Food; World’s Poultry Science Association, UK Branch: Andover, UK, 1994; Volume 1, pp. 411–412. Available online: https://kar.kent.ac.uk/46310/ (accessed on 30 September 2025).
- Idahor, K.O. Poultry bird’s egg: An egg inside egg whose biological, nutritional and cultural value gives and sustains life. Int. J. Res. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Chekh, O.O.; Bondarenko, Y.V.; Bordunova, O. Influence of pre-incubation storage conditions and calibration of eggs on differential mortality of embryos of different sex. Bull. Sumy Natl. Agrar. Univ. Ser. Livest. 2025, 1, 105–110. [Google Scholar] [CrossRef]
- Kushner, K.F.; Moiseeva, I.G.; Tolokonnikova, E.V. Genetic polymorphism of chicken egg protein in connection with embryonal and postembryonal chicken viability. In Focus on Feeding Mankind, Proceedings and Abstracts from the XV World’s Poultry Science Congress and Exposition; U.S.A. Branch, World’s Poultry Science Association: Washington, DC, USA, 1974; Abstract W-5; pp. 497–499. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19750115133 (accessed on 30 September 2025).
- Prokudina, N.O.; Ogurtsova, N.S.; Katerynych, O.O.; Bondarenko, Y.V.; Artemenko, O.B. Features of embryohistogenesis of chickens of the new meat-and-egg population. Poult. Farm. 2007, 59, 108–124. [Google Scholar]
- Bondarenko, Y.V.; Prokudina, N.O.; Artemenko, O.B.; Ogurtsova, N.S.; Baidevlyatova, O.M.; Ruda, S.V.; Katerynych, O.O. Morphofunctional features of embryonic development and early ontogenesis of a new mini-meat population of domestically bred chickens. Sci. Messin. LNU Vet. Med. Biotech. Ser. Agric. Sci. 2007, 9, 129–134. [Google Scholar]
- Nangsuay, A.; Ruangpanit, Y.; Meijerhof, R.; Attamangkune, S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci. 2011, 90, 2648–2655. [Google Scholar] [CrossRef]
- Enting, H.; Boersma, W.J.A.; Cornelissen, J.B.W.J.; van Winden, S.C.L.; Verstegen, M.W.A.; van der Aar, P.J. The effect of low-density broiler breeder diets on performance and immune status of their offspring. Poult. Sci. 2007, 86, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Çağlayan, T.; Garip, M.; Kırıkçı, K.; Günlü, A. Effect of egg weight on chick weight, egg weight loss and hatchability in rock partridges (A. graeca). Ital. J. Anim. Sci. 2009, 8, 567–574. [Google Scholar] [CrossRef]
- Fathi, M.; Abou-Emera, O.; Al-Homidan, I.; Galal, A.; Rayan, G. Effect of genotype and egg weight on hatchability properties and embryonic mortality pattern of native chicken populations. Poult. Sci. 2022, 101, 102129. [Google Scholar] [CrossRef] [PubMed]
- Narushin, V.G.; Romanov, M.N.; Sakhatsky, N.I. Modelling growth of chick embryo: Correction for egg weight [Modelowanie wzrostu zarodka kurzego z poprawką na masę jaja]. Zesz. Nauk. Przegląd Hod. [Anim. Prod. Rev. Appl. Sci. Rep.] 1997, 31, 55–57. Available online: https://www.researchgate.net/publication/355808171 (accessed on 30 September 2025).
- Narushin, V.G.; Romanov, M.N.; Salamon, A.; Kent, J.P. Egg Quality Index: A more accurate alternative to the Haugh unit to describe the internal quality of goose eggs. Food Biosci. 2023, 55, 102968. [Google Scholar] [CrossRef]
- Baydevlyatova, O.N.; Ogurtsova, N.S.; Shomina, N.V.; Tereshchenko, A.V. Morphological indicators of egg quality in a new chicken subpopulation of the meat-egg type of productivity. Poult. Farm. 2009, 64, 109–115. Available online: http://avianua.com/archiv/ptahivnictvo/64/15.pdf (accessed on 30 September 2025).
- Bondarenko, Y.V.; Tkachik, T.E.; Zakharchenko, O.P.; Ruda, S.V.; Katerinich, O.O.; Shekhovtsov, S.S.; Kutnyuk, P.I. Morphological quality traits of eggs of subpopulations of Birky meat-egg type chickens. Poult. Farm. 2007, 59, 29–36. [Google Scholar]
- Tixier-Boichard, M.; Joffrin, C.; Gourichon, D.; Bordas, A. Improvement of Yolk Percentage by Crossbreeding Between a Commercial Brown-egg Layer and a Local Breed, the Fayoumi. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Minas Gerais, Brazil, 13–18 August 2006; Instituto Prociência: Minas Gerais, Brazil, 2006. Report no. 32-09. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20063170246 (accessed on 30 September 2025).
- Narushin, V.G.; Selina, M.V.; Romanov, M.N. Development of Non-destructive Technologies and Mathematical Methods for Assessing the Egg Quality. In Molecular Genetic Technologies for Analysis of Gene Expression Related to Animal Productivity and Disease Resistance, Materials of the 2nd International Scientific and Practical Conference; Sel’skokhozyaistvennye Tekhnologii: Moscow, Russia, 2020; pp. 151–164. [Google Scholar] [CrossRef]
- Kushner, H.F.; Moiseeva, I.G.; Tolokonnikova, E.V. Genetic and poultry selection genetic parameters of egg quality traits. Proceeding of the Abstracts of Scientific Communications of the XIV World’s Poultry Congress (Section I. Genetics and Reproduction, Section II. Physiology and Nutrition), Madrid, Spain, 6–12 September 1970; Ministerio de Agricultura: Madrid, Spain, 1970; pp. 94–101. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19720100924 (accessed on 30 September 2025).
- Moiseeva, I.G.; Tolokonnikova, E.V. The correlation of various indices of internal egg quality with each other and with productivity of hens. Trans. All-Union Poult. Res. Inst. 1966, 30, 22–30. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19670102999 (accessed on 30 September 2025).
- Moiseeva, I.G.; Tolokonnikova, E.V. Use of the principles of population genetics in selecting fowls for egg quality. Zhivotnovodstvo 1974, 5, 34–37. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19740110600 (accessed on 30 September 2025).
- Moiseyeva, I.G.; Tolokonnikova, E.V. Correlation of some quality traits of hens’ eggs and the level of hens’ productivity with chick hatchability. In Papers on Genetics and Immunogenetics of Animals, Eggs and Chicks Hatchability; Elsevier: Amsterdam, The Netherlands, 1968; Volume 2, p. 54. Available online: https://scholar.google.com/citations?view_op=view_citation&citation_for_view=tFr9MGIAAAAJ:9yKSN-GCB0IC (accessed on 30 September 2025).
- Ostryakova, O.E.; Gadyuchko, O.T.; Bondarenko, Y.V.; Podstreshny, O.P.; Katerynych, O.O. Using genetic methods in predicting selection changes based on oomorphological indicators in ducks. Poult. Farm. 2010, 66, 232–240. [Google Scholar]
- Ostryakova, O.E.; Podstreshny, O.P.; Gadyuchko, O.T.; Bondarenko, Y.V. The use of genetic markers in the selection of ducks based on oomorphological indicators. Mod. Poult. Farm. 2011, 5–6, 33–39. [Google Scholar]
- Liu, Z.; Sun, C.; Yan, Y.; Li, G.; Wu, G.; Liu, A.; Yang, N. Genome-wide association analysis of age-dependent egg weights in chickens. Front. Genet. 2018, 9, 128. [Google Scholar] [CrossRef]
- Lordelo, M.; Cid, J.; Cordovil, C.M.; Alves, S.P.; Bessa, R.J.; Carolino, I. A comparison between the quality of eggs from indigenous chicken breeds and that from commercial layers. Poult. Sci. 2020, 99, 1768–1776. [Google Scholar] [CrossRef]
- Zita, L.; Tůmová, E.; Štolc, L. Effects of genotype, age and their interaction on egg quality in brown-egg laying hens. Acta Vet. Brno 2009, 78, 85–91. [Google Scholar] [CrossRef]
- Romanov, M.N. Qualitative and Quantitative Egg Characteristics in Laying Hens of Different Genotype. In Egg and Egg Products Quality, Proceedings of the 6th European Symposium on Quality of Eggs and Eggs Products, Zaragoza, Spain, 25–29 September 1995; World’s Poultry Science Association, Spanish Branch; Tres Cantos: Madrid, Spain, 1995; pp. 203–206. Available online: https://kar.kent.ac.uk/46404/ (accessed on 30 September 2025).
- Larkina, T.A.; Barkova, O.Y.; Peglivanyan, G.K.; Mitrofanova, O.V.; Dementieva, N.V.; Stanishevskaya, O.I.; Vakhrameev, A.B.; Makarova, A.V.; Shcherbakov, Y.S.; Pozovnikova, M.V.; et al. Evolutionary subdivision of domestic chickens: Implications for local breeds as assessed by phenotype and genotype in comparison to commercial and fancy breeds. Agriculture 2021, 11, 914. [Google Scholar] [CrossRef]
- Grela, E.R.; Knaga, S.; Winiarska-Mieczan, A.; Zięba, G. Effects of dietary alfalfa protein concentrate supplementation on performance, egg quality, and fatty acid composition of raw, freeze-dried, and hard-boiled eggs from Polbar laying hens. Poult. Sci. 2020, 99, 2256–2265. [Google Scholar] [CrossRef]
- Jia, W.; Slominski, B.A.; Guenter, W.; Humphreys, A.; Jones, O. The effect of enzyme supplementation on egg production parameters and omega-3 fatty acid deposition in laying hens fed flaxseed and canola seed. Poult. Sci. 2008, 87, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.; Scheideler, S.E. Long-term effects of feeding flaxseed-based diets. 1. Egg production parameters, components, and eggshell quality in two strains of laying hens. Poult. Sci. 2001, 80, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Katerynych, O.O.; Ryabokon, Y.O.; Bondarenko, Y.V.; Nalyvaiko, V.P.; Bochko, A.M.; Lunina, L.A. Breeding, Growing and Keeping Birkivski Meat and Egg Chickens (Breeding Recommendations); n.p.: Birky, Ukraine, 2005. [Google Scholar]
- Katerynych, O.O.; Pankova, S.M.; Tereshchenko, O.V.; Ruda, S.V.; Havilei, O.V.; Riabinina, O.V.; Muzyka, N.M.; Ionov, I.A. Rearing, Maintenance and Feeding of Egg and Meat-Egg Hens: Scientific and Practical Guide; Poultry Research Institute, DDSP NAAS: Birky, Ukraine, 2017. [Google Scholar]
- Samiullah, S.; Roberts, J.R.; Chousalkar, K.K. Effect of production system and flock age on egg quality and total bacterial load in commercial laying hens. J. Appl. Poult. Res. 2014, 23, 59–70. [Google Scholar] [CrossRef]
- Dang, D.X.; Li, C.J.; Cui, Y.; Zhou, H.; Lou, Y.; Li, D. Egg quality, hatchability, gosling quality, and amino acid profile in albumen and newly-hatched goslings’ serum as affected by egg storage. Poult. Sci. 2023, 102, 102367. [Google Scholar] [CrossRef]
- Narushin, V.G.; Romanov, M.N.; Salamon, A.; Kent, J.P. An innovative non-destructive technology for controlling the storage period of chicken eggs using egg parameters. Food Bioprocess Technol. 2024, 17, 2770–2781. [Google Scholar] [CrossRef]
- Shomina, N.V.; Tkachenko, S.M.; Tagirov, M.T.; Tereshchenko, O.V. Monitoring the quality of hatching eggs during storage. Eff. Poult. Farm. 2009, 11, 29–33. [Google Scholar]
- Shalev, B.A.; Pasternak, H. Increment of egg weight with hen age in various commercial avian species. Br. Poult. Sci. 1993, 34, 915–924. [Google Scholar] [CrossRef]
- Biesiada-Drzazga, B.; Banaszewska, D.; Kaim-Mirowski, S. Analysis of selected external and internal characteristics of the eggs of Hy-Line Brown hens in relation to their age. Anim. Sci. Genet. 2022, 18, 45–56. [Google Scholar]
- Tůmová, E.; Uhlířová, L.; Tůma, R.; Chodová, D.; Máchal, L. Age related changes in laying pattern and egg weight of different laying hen genotypes. Anim. Reprod. Sci. 2017, 183, 21–26. [Google Scholar] [CrossRef]
- Rakonjac, S.; Bogosavljevic-Boskovic, S.; Škrbić, Z.; Peric, L.; Doskovic, V.; Petrovic, M.; Petricevic, V. The effect of the rearing system, genotype and laying hens age on the egg weight and share of main parts of eggs. Acta Agric. Serb. 2017, 22, 185–192. [Google Scholar] [CrossRef]
- Hussein, S.M.; Harms, R.H.; Janky, D.M. Research note: Effect of age on the yolk to albumen ratio in chicken eggs. Poult. Sci. 1993, 72, 594–597. [Google Scholar] [CrossRef]
- Wolc, A.; Arango, J.; Settar, P.; O’Sullivan, N.P.; Olori, V.E.; White, I.M.S.; Hill, W.G.; Dekkers, J.C.M. Genetic parameters of egg defects and egg quality in layer chickens. Poult. Sci. 2012, 91, 1292–1298. [Google Scholar] [CrossRef]
- Savegnago, R.P.; Caetano, S.L.; Ramos, S.B.; Nascimento, G.B.; Schmidt, G.S.; Ledur, M.C.; Munari, D.P. Estimates of genetic parameters, and cluster and principal components analyses of breeding values related to egg production traits in a White Leghorn population. Poult. Sci. 2011, 90, 2174–2188. [Google Scholar] [CrossRef]
- Yi, G.; Shen, M.; Yuan, J.; Sun, C.; Duan, Z.; Qu, L.; Dou, T.; Ma, M.; Lu, J.; Guo, J.; et al. Genome-wide association study dissects genetic architecture underlying longitudinal egg weights in chickens. BMC Genom. 2015, 16, 746. [Google Scholar] [CrossRef]
- Goto, T.; Tsudzuki, M. Genetic mapping of quantitative trait loci for egg production and egg quality traits in chickens: A review. J. Poult. Sci. 2017, 54, 1–12. [Google Scholar] [CrossRef]
- Francesch, A.; Estany, J.; Alfonso, L.; Iglesias, M. Genetic parameters for egg number, egg weight, and eggshell color in three Catalan poultry breeds. Poult. Sci. 1997, 76, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, I.G. The effect of inbreeding on the quality of fowl eggs. Genetika 1970, 6, 99–107. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19700104311 (accessed on 30 September 2025).
- Moiseeva, I.G.; Tolokonnikova, E.V. Effect of crossbreeding on egg quality in the progeny. Ptitsevodstvo 1973, 3, 28–30. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19730106499 (accessed on 30 September 2025).
- Ren, J.; Gao, Z.; Lu, Y.; Li, M.; Hong, J.; Wu, J.; Wu, D.; Deng, W.; Xi, D.; Chong, Y. Application of GWAS and mGWAS in livestock and poultry breeding. Animals 2024, 14, 2382. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, J.S.; Dang, C.G.; Sudrajad, P.; Kim, H.C.; Yeon, S.H.; Kang, H.S.; Lee, S.-H. Stories and challenges of genome wide association studies in livestock—A review. Asian-Australas. J. Anim. Sci. 2015, 28, 1371–1379. [Google Scholar] [CrossRef]
- Romanov, M.N.; Kochish, I.I.; Myasnikova, O.V.; Nikonov, I.N.; Surai, P.F.; Selina, M.V. Modern Biotechnology and Optimization of Intestinal Microbiota in Poultry Industry. In Genetics, Breeding, Biotechnology: Integration of Science and Practice in Animal Husbandry, Materials of the International Scientific and Practical Conference; VNIIGRZh: Pushkin, Russia, 2021; pp. 138–141. Available online: https://elibrary.ru/item.asp?id=47925130 (accessed on 30 September 2025).
- Underwood, G.; Andrews, D.; Phung, T. Advances in genetic selection and breeder practice improve commercial layer hen welfare. Anim. Prod. Sci. 2021, 61, 856–866. [Google Scholar] [CrossRef]
- Tkachyk, T.E.; Tereshchenko, O.V.; Katerynych, O.O. The use of DNA typing in the breeding and selection process in poultry farming. In Methodology of Scientific Research on Issues of Breeding, Genetics and Biotechnology in Animal Husbandry, Materials of the Scientific and Practical Conference; Ahrarna nauka: Kyiv, Ukraine, 2010; pp. 118–119. [Google Scholar]
- Bondarenko, Y.V.; Sakhatsky, M.I. The use of autosexing in poultry farming. In Genetics and Selection in Ukraine at the Turn of the Millennium; Logos: Kyiv, Ukraine, 2001; Volume 4, pp. 314–336. [Google Scholar]
- Volkova, N.A.; German, N.Y.; Larionova, P.V.; Vetokh, A.N.; Romanov, M.N.; Zinovieva, N.A. Identification of SNPs and candidate genes associated with abdominal fat deposition in quails (Coturnix japonica). Agric. Biol. 2023, 58, 1079–1087. [Google Scholar] [CrossRef]
- Semyenova, S.K.; Moiseev, I.G.; Vasil’ev, V.A.; Filenko, A.L.; Nikiforov, A.A.; Sevast’yanova, A.A.; Ryskov, A.P. Genetic polymorphism of Russian, European, and Asian chicken breeds as revealed with DNA and protein markers. Russ. J. Genet. 2002, 38, 1109–1112. [Google Scholar] [CrossRef]
- Abasht, B.; Dekkers, J.C.; Lamont, S.J. Review of quantitative trait loci identified in the chicken. Poult. Sci. 2006, 85, 2079–2096. [Google Scholar] [CrossRef]
- Emara, M.G.; Kim, H. Genetic markers and their application in poultry breeding. Poult. Sci. 2003, 82, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Loywyck, V.; Bed’Hom, B.; Pitel, F.; Verrier, É.; Bijma, P. Evolution of the polymorphism at molecular markers in QTL and non-QTL regions in selected chicken lines. Genet. Sel. Evol. 2008, 40, 639–661. [Google Scholar] [CrossRef] [PubMed]
- Kutnyuk, P.I.; Gadyuchko, O.T.; Bondarenko, Y.V. Locus Om as Body Weight Marker in Process of Frequent-dependent Selection of Turkeys. In The Poultry Industry Towards the 21st Century, Proceedings of the 10th European Poultry Conference, Jerusalem, Israel, 21–26 June 1998; World’s Poultry Science Association: Jerusalem, Israel, 1998; p. 67. Available online: https://scholar.google.com/citations?view_op=view_citation&citation_for_view=c1PnDYEAAAAJ:mlAyqtXpCwEC (accessed on 30 September 2025).
- Jahuey-Martínez, F.J.; Martínez-Quintana, J.A.; Rodríguez-Almeida, F.A.; Parra-Bracamonte, G.M. Exploration and enrichment analysis of the QTLome for important traits in livestock species. Genes 2024, 15, 1513. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Tuberosa, R. The crop QTLome comes of age. Curr. Opin. Biotechnol. 2015, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Plemyashov, K.V.; Smaragdov, M.G.; Romanov, M.N. Molecular Genetic Polymorphism in Animal Populations and Its Application in Intensive Breeding of Dairy Cattle—A Review. In Molecular Genetic Technologies for Analysis of Gene Expression Related to Animal Productivity and Disease Resistance, Materials of the 3rd International Scientific and Practical Conference; Sel’skokhozyaistvennye tekhnologii: Moscow, Russia, 2021; pp. 368–378. [Google Scholar] [CrossRef]
- Chu, J.; Ma, Y.; Song, H.; Zhao, Q.; Wei, X.; Yan, Y.; Fan, S.; Zhou, B.; Li, S.; Mou, C. The genomic characteristics affect phenotypic diversity from the perspective of genetic improvement of economic traits. iScience 2023, 26, 106426. [Google Scholar] [CrossRef]
- Moiseyeva, I.G.; Volokhovich, V.A. Quantitative trait variability in the domestic fowl/Variation of qualitative traits of chicken exterior. In Selection and Technological Processes in Poultry Industry; Știința: Kishinev, USSR, 1987; pp. 70–74. [Google Scholar]
- Brito, A.C.; Oliveira, S.A.S.; Oliveira, E.J. Genome-wide association study for resistance to cassava root rot. J. Agric. Sci. 2017, 155, 1424–1441. [Google Scholar] [CrossRef]
- Li, C.Q.; Dong, N.; Fu, Y.Z.; Sun, R.R.; Wang, Q.L. Marker detection and elite allele mining for yield traits in Upland cotton (Gossypium hirsutum L.) by association mapping. J. Agric. Sci. 2017, 155, 613–628. [Google Scholar] [CrossRef]
- Banestani, E.S.; Esmailizadeh, A.; Momen, M.; Ayatollahi Mehrgardi, A.A.; Mokhtari, M. Genome-wide association study identifies significant SNP and related genes associated with body size in Yorkshire pigs using latent variable modelling. J. Agric. Sci. 2023, 161, 599–605. [Google Scholar] [CrossRef]
- dos Santos, J.C.G.; de Araújo Neto, F.R.; Fernandez, G.S.; de Abreu Santos, D.J.; Cunha, F.P.; Aspilcueta-Borquis, R.R.; Tonhati, H. Comparison of single-step methods for genomic prediction of age at first calving in dairy buffaloes. J. Agric. Sci. 2024, 162, 377–383. [Google Scholar] [CrossRef]
- Nisa, F.U.; Asif, M.; Nisa, Q.U.; Naqvi, R.Z.; Rehman, M.S.U.; Mukhtar, Z. Copy number variation profiling in the genome of crossbred dairy cattle from Pakistan. J. Agric. Sci. 2025, 163, 585–596. [Google Scholar] [CrossRef]
- Sun, C.; Qu, L.; Yi, G.; Yuan, J.; Duan, Z.; Shen, M.; Qu, L.; Xu, G.; Wang, K.; Yang, N. Genome-wide association study revealed a promising region and candidate genes for eggshell quality in an F2 resource population. BMC Genom. 2015, 16, 565. [Google Scholar] [CrossRef]
- Liu, W.; Li, D.; Liu, J.; Chen, S.; Qu, L.; Zheng, J.; Xu, G.; Yang, N. A Genome-wide SNP scan reveals novel loci for egg production and quality traits in White Leghorn and brown-egg dwarf layers. PLoS ONE 2011, 6, e28600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, F.; Liu, L.; Zheng, C.W.; Wang, D.H.; Hou, Z.C.; Ning, Z.H. Integrating transcriptome and genome re-sequencing data to identify key genes and mutations affecting chicken eggshell qualities. PLoS ONE 2015, 10, e0125890. [Google Scholar] [CrossRef] [PubMed]
- Schreiweis, M.A.; Hester, P.Y.; Settar, P.; Moody, D.E. Identification of quantitative trait loci associated with egg quality, egg production, and body weight in an F2 resource population of chickens. Anim. Genet. 2006, 37, 106–112. [Google Scholar] [CrossRef]
- Abdelmanova, A.S.; Dotsev, A.V.; Romanov, M.N.; Stanishevskaya, O.I.; Gladyr, E.A.; Rodionov, A.N.; Vetokh, A.N.; Volkova, N.A.; Fedorova, E.S.; Gusev, I.V.; et al. Unveiling comparative genomic trajectories of selection and key candidate genes in egg-type Russian White and meat-type White Cornish chickens. Biology 2021, 10, 876. [Google Scholar] [CrossRef]
- Dzhagaev, A.Y.; Volkova, N.A.; Zinovieva, N.A. Search for genes associated with the age at first egg in laying hens (Gallus gallus L.). Agric. Biol. 2024, 59, 658–665. [Google Scholar] [CrossRef]
- Volkova, N.A.; Romanov, M.N.; Dzhagaev, A.Y.; Larionova, P.V.; Volkova, L.A.; Abdelmanova, A.S.; Vetokh, A.N.; Griffin, D.K.; Zinovieva, N.A. Genome-wide association studies and candidate genes for egg production traits in layers from an F2 crossbred population produced using two divergently selected chicken breeds, Russian White and Cornish White. Genes 2025, 16, 583. [Google Scholar] [CrossRef]
- Volkova, N.A.; Romanov, M.N.; Vetokh, A.N.; Larionova, P.V.; Volkova, L.A.; Abdelmanova, A.S.; Sermyagin, A.A.; Griffin, D.K.; Zinovieva, N.A. Genome-wide association study reveals the genetic architecture of growth and meat production traits in a chicken F2 resource population. Genes 2024, 15, 1246. [Google Scholar] [CrossRef]
- Volkova, N.A.; Romanov, M.N.; German, N.Y.; Larionova, P.V.; Vetokh, A.N.; Volkova, L.A.; Sermyagin, A.A.; Shakhin, A.V.; Griffin, D.K.; Sölkner, J.; et al. Genome-wide association studies in Japanese quails of the F2 resource population elucidate molecular markers and candidate genes for body weight parameters. Int. J. Mol. Sci. 2025, 26, 8243. [Google Scholar] [CrossRef]
- Dementeva, N.V.; Romanov, M.N.; Kudinov, A.A.; Mitrofanova, O.V.; Stanishevskaya, O.I.; Terletsky, V.P.; Fedorova, E.S.; Nikitkina, E.V.; Plemyashov, K.V. Studying the structure of a gene pool population of the Russian White chicken breed by genome-wide SNP scan. Agric. Biol. 2017, 52, 1166–1174. [Google Scholar] [CrossRef]
- Moiseeva, I.G. The lipid and cholesterin contents of hen’s eggs of the Russian white breed in relation to productiveness. Tr. Akad. Nauk SSSR Inst. Genet. 1965, 33, 119–128. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19660103382 (accessed on 30 September 2025). [PubMed]
- Moiseeva, I.G. Content of lipids and cholesterol in eggs of Russian White chickens. Collect. Works Young Sci. All-Union Res. Tech. Poult. Inst. 1966, 8, 225–235. Available online: https://scholar.google.com/citations?view_op=view_citation&citation_for_view=tFr9MGIAAAAJ:5ugPr518TE4C (accessed on 30 September 2025).
- Romanov, M.N.; Shakhin, A.V.; Abdelmanova, A.S.; Volkova, N.A.; Efimov, D.N.; Fisinin, V.I.; Korshunova, L.G.; Anshakov, D.V.; Dotsev, A.V.; Griffin, D.K.; et al. Dissecting selective signatures and candidate genes in grandparent lines subject to high selection pressure for broiler production and in a local Russian chicken breed of Ushanka. Genes 2024, 15, 524. [Google Scholar] [CrossRef]
- Bondarenko, Y.V.; Sergheyeva, V.D.; Kuranova, E.N.; Krasnozhon, S.A.; Romanov, M.N. Autosexing maternal form of meat-type chickens. Poult. Farm. 1987, 40, 6–11. Available online: https://kar.kent.ac.uk/46286/ (accessed on 30 September 2025).
- Melnyk, V.O.; Tereshchenko, O.V.; Zhukorskyi, O.M.; Ivko, I.I.; Katerynych, O.O.; Khvostyk, V.P.; Bratyshko, N.I.; Nalyvaiko, L.I.; Artemenko, O.B.; Melnyk, O.V.; et al. Resource-Saving Environmentally Friendly Technologies for Growing, Keeping and Feeding Poultry; n.p.: Kyiv, Ukraine, 2015. [Google Scholar]
- Tereshchenko, A.V.; Artemenko, A.B.; Pudov, V.Y. A hidden source of increasing the production of broiler chickens. Exclus. Agro 2007, 4, 64–65. [Google Scholar]
- Kochish, I.I.; Romanov, M.N.; Myasnikova, O.V.; Smolensky, V.I.; Martynov, V.V.; Nikonov, I.N.; Selina, M.V.; Kolesnikova, R.R.; Bernikova, K.E.; Motin, M.S. Practical Recommendations for the Use of Feed Additives to Improve the Productivity and Stress Resistance of Egg Poultry; Sel’skokhozyaistvennye Tekhnologii: Moscow, Russia, 2019. [Google Scholar]
- Gorobets, A.I.; Katerynych, O.O.; Bratyshko, N.I.; Bondarenko, Y.V.; Klymenko, T.E. Birkivski Meat and Egg Chickens (Feeding Recommendations); Poultry Research Institute, UAAN, SE DG Borky: Birky, Ukraine, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 30 September 2025).
- R Core Team. R-4; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://cran.r-project.org/src/base/R-4/ (accessed on 30 September 2025).
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Purcell, S. PLINK 1.9.; Center for Human Genetic Research: Boston, MA, USA; Massachusetts General Hospital: Boston, MA, USA; Broad Institute of Harvard & MIT: Cambridge, MA, USA, 2017; Available online: https://zzz.bwh.harvard.edu/plink/index.shtml (accessed on 30 September 2025).
- DataCamp. Principal Component Analysis in R Tutorial. Tutorials, R Programming; DataCamp, Inc.: New York, NY, USA, 2023; Available online: https://www.datacamp.com/tutorial/pca-analysis-r (accessed on 30 September 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- GitHub. ggplot2. Tidyverse; GitHub, Inc.: San Francisco, CA, USA, 2023; Available online: https://github.com/tidyverse/ggplot2 (accessed on 30 September 2025).
- Carbonetto, P.; Stephens, M. Scalable variational inference for Bayesian variable selection in regression, and its accuracy in genetic association studies. Bayesian Anal. 2012, 7, 73–108. [Google Scholar] [CrossRef]
- Utts, J. Simple Linear Regression Model. Statistics 110 and 201—Fall 2017; Department of Statistics University of California: Irvine, CA, USA, 2017; Available online: https://ics.uci.edu/~jutts/110/Lecture7Compact.pdf (accessed on 30 September 2025).
- Guo, W. Chapter 2 Multiple Regression I (Part 1); Department of Mathematical Sciences, New Jersey Institute of Technology: Newark, NJ, USA, 2012; Available online: https://web.njit.edu/~wguo/Math644_2012/Math644_Chapter%202_part1.pdf (accessed on 30 September 2025).
- Turner, S.D. qqman: An R package for visualizing GWAS results using Q-Q and Manhattan plots. J. Open Source Softw. 2018, 3, 731. [Google Scholar] [CrossRef]
- GitHub. qqman; GitHub, Inc.: San Francisco, CA, USA, 2024; Available online: https://github.com/qqman (accessed on 30 September 2025).
- NCBI. Gallus gallus Genome Assembly GRCg6a; Genome; National Library of Medicine: Bethesda, MD, USA, 2018. Available online: https://ncbi.nlm.nih.gov/datasets/genome/GCF_000002315.6/ (accessed on 30 September 2025).
- NCBI. Gallus gallus (Chickens); Genome; National Library of Medicine: Bethesda, MD, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/?taxon=9031 (accessed on 30 September 2025).
- Kinsella, R.J.; Kähäri, A.; Haider, S.; Zamora, J.; Proctor, G.; Spudich, G.; Almeida-King, J.; Staines, D.; Derwent, P.; Kerhornou, A.; et al. Ensembl BioMarts: A hub for data retrieval across taxonomic space. Database 2011, 2011, bar030. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Bello, S.F.; Lawal, R.A.; Adeola, A.C.; Nie, Q. The study of selection signature and its applications on identification of candidate genes using whole genome sequencing data in chicken—A review. Poult. Sci. 2023, 102, 102657. [Google Scholar] [CrossRef]
- Moiseyeva, I.G.; Bannikova, L.V.; Altukhov, Y.P. State of poultry breeding in Russia: Genetic monitoring. Int. Agron. J. 1993, 5–6, 66–69. [Google Scholar]
- Gilyazova, I.; Korytina, G.; Kochetova, O.; Savelieva, O.; Mikhaylova, E.; Vershinina, Z.; Chumakova, A.; Markelov, V.; Abdeeva, G.; Karunas, A.; et al. Advances in genomics and postgenomics in poultry science: Current achievements and future directions. Int. J. Mol. Sci. 2025, 26, 8285. [Google Scholar] [CrossRef]
- Bondarenko, Y.V.; Podstreshny, A.P. Genetic Monitoring of Chicken Populations. In Proceedings of the Abstracts of the 2nd International Conference on Molecular Genetic Markers of Animals, Kiev, Ukraine, 15–17 May 1996; Agrarna Nauka: Kiev, Ukraine, 1996; pp. 47–48. [Google Scholar]
- Romanov, M.N.; Sazanov, A.A.; Moiseyeva, I.G.; Smirnov, A.F. Poultry. In Genome Mapping and Genomics in Animals, Volume 3: Genome Mapping and Genomics in Domestic Animals; Cockett, N.E., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 75–141. [Google Scholar] [CrossRef]
- Qu, L.; Shen, M.M.; Dou, T.C.; Ma, M.; Lu, J.; Wang, X.G.; Guo, J.; Hu, Y.P.; Li, Y.F.; Wang, K.H. Genome-wide association studies for mottled eggs in chickens using a high-density single-nucleotide polymorphism array. Animal 2021, 15, 100051. [Google Scholar] [CrossRef] [PubMed]
- Dementieva, N.V.; Kudinov, A.A.; Pozovnikova, M.V.; Nikitkina, E.V.; Pleshanov, N.V.; Silyukova, Y.L.; Krutikova, A.A.; Plemyashov, K.V. Genome-wide association studies of cryostability of semen in roosters. Pol. J. Vet. Sci. 2020, 23, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Shen, L.; Zhou, J.; Cao, Z.; Luan, P.; Li, Y.; Xiao, F.; Guo, H.; Li, H.; Zhang, H. Genome-wide association studies for growth traits in broilers. BMC Genom. Data 2022, 23, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Dunn, I.C.; Wilson, P.W.; Pertinez, S.P.; Fulton, J.E.; Arango, J.; Andersson, B.; Schmutz, M.; Wolc, A. Genome wide association analysis of cuticle deposition in laying hens. Poult. Sci. 2023, 102, 102990. [Google Scholar] [CrossRef]
- Haqani, M.I.; Nomura, S.; Nakano, M.; Goto, T.; Nagano, A.J.; Takenouchi, A.; Nakamura, Y.; Ishikawa, A.; Tsudzuki, M. Quantitative trait loci for growth-related traits in Japanese quail (Coturnix japonica) using restriction-site associated DNA sequencing. Mol. Genet. Genom. 2021, 296, 1147–1159. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Jiang, X.; Wu, Q.; Lin, R.; Chen, H.; Zhang, M.; Zeng, T.; Tian, Y.; Xu, E.; et al. Genome-wide association study identified candidate genes for egg production traits in the Longyan Shan-ma duck. Poult. Sci. 2024, 103, 104032. [Google Scholar] [CrossRef]
- Zhao, X.; Nie, C.; Zhang, J.; Li, X.; Zhu, T.; Guan, Z.; Chen, Y.; Wang, L.; Lv, X.Z.; Yang, W.; et al. Identification of candidate genomic regions for chicken egg number traits based on genome-wide association study. BMC Genom. 2021, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, W.; Zhao, J.; Tian, J.; Yang, L.; Wang, H.; Chen, S.; Ma, R.; Gu, Y.; Wei, D.; et al. Metabolomics and transcriptomics reveal age-dependent development of meat quality traits in Jingyuan chicken. Animals 2025, 15, 1938. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.A.; Argañaraz, M.E.; Barrera, A.D.; Rodler, D.; Mutto, A.Á.; Sinowatz, F. Biology and biotechnology of follicle development. Sci. World J. 2012, 2012, 938138. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Amevor, F.K.; Feng, Q.; Kang, X.; Song, W.; Zhu, Q.; Wang, Y.; Li, D.; Zhao, X. Sexual maturity promotes yolk precursor synthesis and follicle development in hens via liver-blood-ovary signal axis. Animals 2020, 10, 2348. [Google Scholar] [CrossRef]
- Kaspers, B. An egg a day—The physiology of egg formation. Lohmann Inf. 2016, 50, 12–17. Available online: https://lohmann-breeders.com/media/2020/08/VOL-50-Kaspers-An-egg-day.pdf (accessed on 30 September 2025).
- Kuksis, A. Yolk lipids. Biochim. Biophys. Acta Lipids Lipid Metab. 1992, 1124, 205–222. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Wang, N.; Lu, S.; Dong, J.; Qi, Z.; Zhou, J.; Wang, Q. Evaluation of changes in egg yolk lipids during storage based on lipidomics through UPLC-MS/MS. Food Chem. 2023, 398, 133931. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.C.; Huang, X.; Ahn, D.U. Egg yolk lipids: Separation, characterization, and utilization. Food Sci. Biotechnol. 2022, 31, 1243–1256. [Google Scholar] [CrossRef]
- Ji, Q.; Chang, P.; Dou, Y.; Zhao, Y.; Chen, X. Egg Yolk fat deposition is regulated by diacylglycerol and ceramide enriched by adipocytokine signaling pathway in laying hens. Animals 2023, 13, 607. [Google Scholar] [CrossRef]
- Yan, X.; Liu, H.; Hu, J.; Han, X.; Qi, J.; Ouyang, Q.; Hu, B.; He, H.; Li, L.; Wang, J.; et al. Transcriptomic analyses of the HPG axis-related tissues reveals potential candidate genes and regulatory pathways associated with egg production in ducks. BMC Genom. 2022, 23, 281. [Google Scholar] [CrossRef]
- Du, X.; Xu, X.; Liu, Y.; Wang, Z.; Qiu, H.; Zhao, A.; Lu, L. Cell heterogeneity analysis revealed the key role of fibroblasts in the magnum regression of ducks. Animals 2024, 14, 1072. [Google Scholar] [CrossRef]
- Yang, K.T.; Lin, C.Y.; Liou, J.S.; Fan, Y.H.; Chiou, S.H.; Huang, C.W.; Wu, C.P.; Lin, E.C.; Chen, C.F.; Lee, Y.P.; et al. Differentially expressed transcripts in shell glands from low and high egg production strains of chickens using cDNA microarrays. Anim. Reprod. Sci. 2007, 101, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Zhong, C.; Jiang, X.; Wu, G.; Li, G.; Yan, Y.; Yang, N.; Sun, C. Genetic patterns and genome-wide association analysis of eggshell quality traits of egg-type chicken across an extended laying period. Poult. Sci. 2024, 103, 103458. [Google Scholar] [CrossRef] [PubMed]
- Narushin, V.G.; Romanov, M.N.; Griffin, D.K. Non-destructive evaluation of the volumes of egg shell and interior: Theoretical approach. J. Food Eng. 2021, 300, 110536. [Google Scholar] [CrossRef]
- Narushin, V.G.; Griffin, A.W.; Romanov, M.N.; Griffin, D.K. Measurement of the neutral axis in avian eggshells reveals which species conform to the golden ratio. Ann. N. Y. Acad. Sci. 2022, 1517, 143–153. [Google Scholar] [CrossRef]
- Narushin, V.G.; Romanov, M.N.; Griffin, D.K. Egg-inspired engineering in the design of thin-walled shelled vessels: A theoretical approach for shell strength. Front. Bioeng. Biotechnol. 2022, 10, 995817. [Google Scholar] [CrossRef] [PubMed]
- Ketta, M.; Tůmová, E. Relationship between eggshell thickness and other eggshell measurements in eggs from litter and cages. Ital. J. Anim. Sci. 2017, 17, 234–239. [Google Scholar] [CrossRef]
- Almeida, O.A.C.; Moreira, G.C.M.; Rezende, F.M.; Boschiero, C.; de Oliveira Peixoto, J.; Ibelli, A.M.G.; Ledur, M.C.; de Novais, F.J.; Coutinho, L.L. Identification of selection signatures involved in performance traits in a paternal broiler line. BMC Genom. 2019, 20, 449. [Google Scholar] [CrossRef]
- Boschiero, C.; Moreira, G.C.M.; Gheyas, A.A.; Godoy, T.F.; Gasparin, G.; Mariani, P.D.S.C.; Paduan, M.; Cesar, A.S.M.; Ledur, M.C.; Coutinho, L.L. Genome-wide characterization of genetic variants and putative regions under selection in meat and egg-type chicken lines. BMC Genom. 2018, 19, 83. [Google Scholar] [CrossRef]
- Malila, Y.; Uengwetwanit, T.; Thanatsang, K.V.; Arayamethakorn, S.; Srimarut, Y.; Petracci, M.; Soglia, F.; Rungrassamee, W.; Visessanguan, W. Insights into transcriptome profiles associated with wooden breast myopathy in broilers slaughtered at the age of 6 or 7 weeks. Front. Physiol. 2021, 12, 691194. [Google Scholar] [CrossRef] [PubMed]
- Kanlisi, R.A.; Amuzu-Aweh, E.N.; Naazie, A.; Otsyina, H.R.; Kelly, T.R.; Gallardo, R.A.; Lamont, S.J.; Zhou, H.; Dekkers, J.; Kayang, B.B. Genetic architecture of body weight, carcass, and internal organs traits of Ghanaian local chickens. Front. Genet. 2024, 15, 1297034. [Google Scholar] [CrossRef]
- Dadousis, C.; Somavilla, A.; Ilska, J.J.; Johnsson, M.; Batista, L.; Mellanby, R.J.; Headon, D.; Gottardo, P.; Whalen, A.; Wilson, D.; et al. A genome-wide association analysis for body weight at 35 days measured on 137,343 broiler chickens. Genet. Sel. Evol. 2021, 53, 70. [Google Scholar] [CrossRef]
- Bani Saadat, H.; Vaez Torshizi, R.; Masoudi, A.A.; Ehsani, A.; Shahinfar, S. Identification of genes affecting growth traits in broiler chickens using linear regression and machine learning methods. Iran. J. Anim. Sci. 2024, 55, 583–602. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Zhao, F.; Ren, H.; Xu, L.; Lu, J.; Zhang, S.; Zhang, X.; Wei, C.; Lu, G.; et al. Genome-wide association studies for growth and meat production traits in sheep. PLoS ONE 2013, 8, e66569. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Deng, T.; Xie, R.; Han, J.; Du, L.; Zhao, F.; Wang, L. Genome-wide scan for run of homozygosity and identification of corresponding candidate genes in sheep populations. Acta Vet. Zoot. Sin. 2019, 50, 1554–1566. [Google Scholar] [CrossRef]
- Yu, H.; Yu, S.; Guo, J.; Cheng, G.; Mei, C.; Zan, L. Genome-wide association study reveals novel loci associated with body conformation traits in Qinchuan cattle. Animals 2023, 13, 3628. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; An, Y.; Tian, C.; Wang, L.; Liu, Z.; Qi, D. Identification of genes related to growth and amino acid metabolism from the transcriptome profile of the liver of growing laying hens. Poult Sci. 2024, 103, 103181. [Google Scholar] [CrossRef]
- Twumasi, G.; Wang, H.; Xi, Y.; Qi, J.; Li, L.; Bai, L.; Liu, H. Genome-wide association studies reveal candidate genes associated with pigmentation patterns of single feathers of Tianfu Nonghua ducks. Animals 2024, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z.; Chen, G.; Luo, Q.; Nie, Q.; Zhang, X.; Luo, W. Characterization of chicken skin yellowness and exploration of genes involved in skin yellowness deposition in chicken. Front. Physiol. 2021, 12, 585089. [Google Scholar] [CrossRef] [PubMed]
- Podisi, B.K.; Knott, S.A.; Dunn, I.C.; Law, A.S.; Burt, D.W.; Hocking, P.M. Overlap of quantitative trait loci for early growth rate, and for body weight and age at onset of sexual maturity in chickens. Reproduction 2011, 141, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Vakhrameev, A.B.; Narushin, V.G.; Larkina, T.A.; Barkova, O.Y.; Peglivanyan, G.K.; Dysin, A.P.; Dementieva, N.V.; Makarova, A.V.; Shcherbakov, Y.S.; Pozovnikova, M.V.; et al. Selection-driven chicken phenome and phenomenon of pectoral angle variation across different chicken phenotypes. Livest. Sci. 2022, 264, 105067. [Google Scholar] [CrossRef]
- Vakhrameev, A.B.; Narushin, V.G.; Larkina, T.A.; Barkova, O.Y.; Peglivanyan, G.K.; Dysin, A.P.; Dementieva, N.V.; Shcherbakov, Y.S.; Pozovnikova, M.V.; Griffin, D.K.; et al. Pectoral angle: A glance at a traditional phenotypic trait in chickens from a new perspective. J. Agric. Sci. 2023, 161, 606–615. [Google Scholar] [CrossRef]
| Age, Weeks | Indicator 1 | Weight, g | ||||
|---|---|---|---|---|---|---|
| Egg | Yolk | Egg White | Thick Albumen | Shell | ||
| 18–28 | M ± m | 43.00 ± 0.36 | 12.00 ± 0.20 | 24.90 ± 0.38 | 15.30 ± 0.26 | 6.10± 0.11 |
| Min...Max | 33.5...57.8 | 9.9...15.3 | 21.5...34.9 | 9.7...20.5 | 4.7...8.4 | |
| CV, % | 8.2 | 11.2 | 10.6 | 16.3 | 13.3 | |
| 29–41 | M ± m | 50.80 ± 0.54 * | 15.60 ± 0.17 * | 28.70 ± 0.30 * | 16.80 ± 0.25 * | 6.50 ± 0.08 * |
| Min...Max | 36.6...63.7 | 12.5...19.2 | 26.0...35.6 | 10.7...23.5 | 5.1...8.1 | |
| CV, % | 9.3 | 7.7 | 7.3 | 12.6 | 8.2 | |
| 42–52 | M ± m | 57.80 ± 0.53 * | 18.70 ± 0.21 * | 31.70 ± 0.33 * | 18.10 ± 0.27 * | 7.40 ± 0.12 * |
| Min...Max | 51.4...70.0 | 15.4...20.6 | 24.2...36.5 | 14.9...23.6 | 5.9...9.8 | |
| CV, % | 6.7 | 7.9 | 7.6 | 10.5 | 12.1 | |
| Traits | Effects 1 | R2 | |||||
|---|---|---|---|---|---|---|---|
| Age | Parent Group | Hatch | |||||
| F | p-Value | F | p-Value | F | p-Value | ||
| Egg weight | 237.9 | 0.000 *** | 1.66 | 0.119 | 2.02 | 0.077 t | 0.717 |
| Yolk weight | 245.5 | 0.000 *** | 1.16 | 0.330 | 1.92 | 0.109 | 0.762 |
| Egg white weight | 94.5 | 0.000 *** | 2.07 | 0.058 t | 2.23 | 0.067 t | 0.584 |
| Thick albumen weight | 40.3 | 0.000 *** | 2.19 | 0.046 * | 1.58 | 0.181 | 0.469 |
| Eggshell weight | 51.4 | 0.000 *** | 0.49 | 0.814 | 0.91 | 0.458 | 0.492 |
| Traits | LS Means-Based Trait Estimates | ||
|---|---|---|---|
| Age, Weeks | |||
| 18–28 | 29–41 | 42–52 | |
| Egg weight, g | 44.05 ± 0.69 | 52.00 ± 0.70 | 57.74 ± 0.78 |
| Yolk weight, g | 12.21 ± 0.30 | 15.97 ± 0.30 | 18.64 ± 0.33 |
| Egg white weight, g | 25.65 ± 0.45 | 29.43 ± 0.44 | 31.45 ± 0.49 |
| Thick albumen weight, g | 15.28 ± 0.34 | 16.90 ± 0.34 | 18.27 ± 0.37 |
| Eggshell weight, g | 6.03 ± 0.13 | 6.69 ± 0.13 | 7.35 ± 0.14 |
| Trait | No. of SNPs | Chromosomes |
|---|---|---|
| Thick albumen weight (18–28 weeks) | 85 | GGA1–GGA5, GGA7–GGA9, GGA11, GGA12, GGA14–GGA18, GGA20, GGA25, GGA27 |
| Thick albumen weight (29–41 weeks) | 2 | GGA2, GGA8 |
| Yolk weight (18–28 weeks) | 33 | GGA1, GGA5, GGA6, GGA7, GGA9–GGA11, GGA17, GGA20, GGA21 |
| Shell weight (18–28 weeks) | 4 | GGA1, GGA3, GGA17 |
| Chromosomes 1 | Genes | SNPs 2 | SNP Position | β | R2 | p-Value | Trait 3 |
|---|---|---|---|---|---|---|---|
| GGA1 | SYTL5 | GGaluGA038925 | 114,366,975 | 2.158 | 0.2862 | 7.91 × 10−8 | YW1 |
| GGaluGA038927 | 114,379,202 | 5.183 | 0.3453 | 3.05 × 10−7 | TAW1 | ||
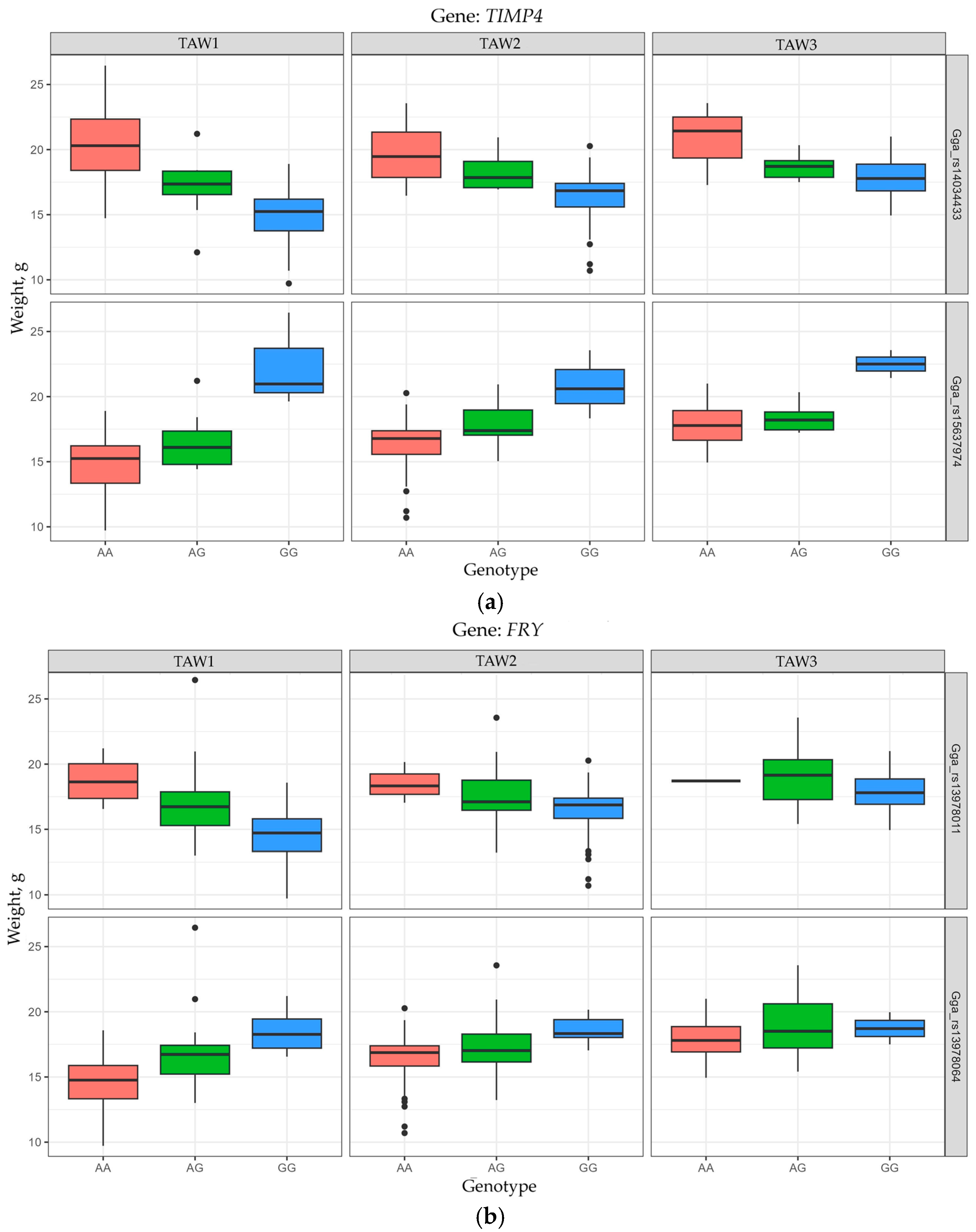
| FRY | Gga_rs13978011 | 175,956,011 | 2.310 | 0.2570 | 3.98 × 10−7 | TAW1 | |
| Gga_rs13978064 | 175,971,650 | 2.137 | 0.2584 | 3.13 × 10−7 | TAW1 | ||
| GABRG3 | Gga_rs13939653 | 132,426,482 | 2.667 | 0.3725 | 3.98 × 10−8 | TAW1 | |
| Gga_rs15424427 | 132,514,348 | 6.371 | 0.2772 | 6.67 × 10−8 | TAW1 | ||
| Gga_rs13939653 | 132,426,482 | 5.064 | 0.2163 | 5.52 × 10−7 | YW1 | ||
| GGA6 | VCL | Gga_rs16546266 | 16,277,262 | −1.609 | 0.2653 | 2.41 × 10−7 | YW1 |
| Gga_rs14576710 | 16,283,091 | −1.608 | 0.2646 | 2.15 × 10−7 | YW1 | ||
| GGA10 | ALDH1A3 | Gga_rs14952507 | 17,878,734 | 1.754 | 0.2295 | 5.60 × 10−7 | YW1 |
| Gga_rs14952510 | 17,878,899 | 1.754 | 0.2295 | 5.60 × 10−7 | YW1 | ||
| GGaluGA072046 | 17,898,445 | 1.784 | 0.2450 | 1.48 × 10−7 | YW1 | ||
| Gga_rs10730304 | 17,910,258 | 1.747 | 0.2343 | 3.35 × 10−7 | YW1 | ||
| GGA11 | HYDIN | Gga_rs15601378 | 1,592,394 | 1.819 | 0.2504 | 9.87 × 10−7 | YW1 |
| Gga_rs14018273 | 1,614,369 | 1.846 | 0.2901 | 9.43 × 10−7 | YW1 | ||
| GGaluGA074476 | 1,626,527 | 1.929 | 0.2836 | 1.85 × 10−7 | YW1 | ||
| GGA12 | TIMP4 | Gga_rs14034433 | 5,165,421 | 2.628 | 0.2611 | 2.67 × 10−7 | TAW1 |
| Gga_rs15637974 | 5,176,426 | 2.664 | 0.2802 | 8.12 × 10−8 | TAW1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, N.A.; Romanov, M.N.; Larionova, P.V.; Dzhagaev, A.Y.; Volkova, L.A.; Sermyagin, A.A.; Griffin, D.K.; Zinovieva, N.A. Genome-Wide Egg Hunt: Unhiding Candidate Genes for Egg Component Traits in Layers of an F2 Resource Population. Animals 2025, 15, 3391. https://doi.org/10.3390/ani15233391
Volkova NA, Romanov MN, Larionova PV, Dzhagaev AY, Volkova LA, Sermyagin AA, Griffin DK, Zinovieva NA. Genome-Wide Egg Hunt: Unhiding Candidate Genes for Egg Component Traits in Layers of an F2 Resource Population. Animals. 2025; 15(23):3391. https://doi.org/10.3390/ani15233391
Chicago/Turabian StyleVolkova, Natalia A., Michael N. Romanov, Polina V. Larionova, Alan Yu. Dzhagaev, Ludmila A. Volkova, Alexander A. Sermyagin, Darren K. Griffin, and Natalia A. Zinovieva. 2025. "Genome-Wide Egg Hunt: Unhiding Candidate Genes for Egg Component Traits in Layers of an F2 Resource Population" Animals 15, no. 23: 3391. https://doi.org/10.3390/ani15233391
APA StyleVolkova, N. A., Romanov, M. N., Larionova, P. V., Dzhagaev, A. Y., Volkova, L. A., Sermyagin, A. A., Griffin, D. K., & Zinovieva, N. A. (2025). Genome-Wide Egg Hunt: Unhiding Candidate Genes for Egg Component Traits in Layers of an F2 Resource Population. Animals, 15(23), 3391. https://doi.org/10.3390/ani15233391










