Effect of Portulaca oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility, Serum Parameters, and Excreta Microbiota Metabolism in Tumbler Pigeons
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Experimental Animals and Design
2.3. Sample Collection
2.4. Sampling and Determination of Nutrient Digestibility and Metabolism
2.5. Serum Parameters
2.6. Excreta Microbial Community
2.7. Metabolites Extraction, UHPLC-MS/MS, and Metabolomic Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of P. oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility and Metabolism in Tumbler Pigeons
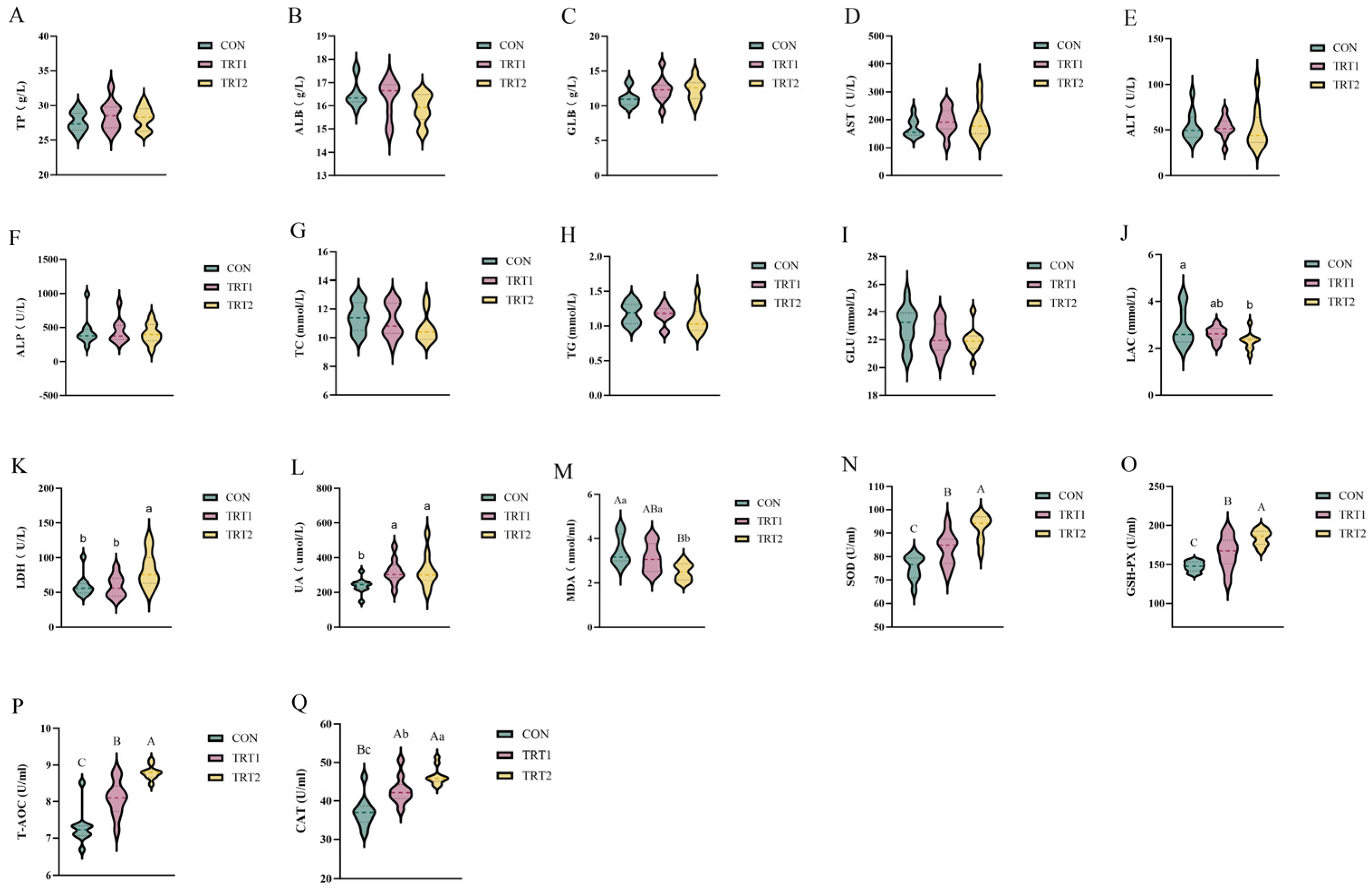
3.2. Effects of P. oleracea Addition in Health Care Sand on Serum Biochemical Parameters and Antioxidant Capacity in Tumbler Pigeons
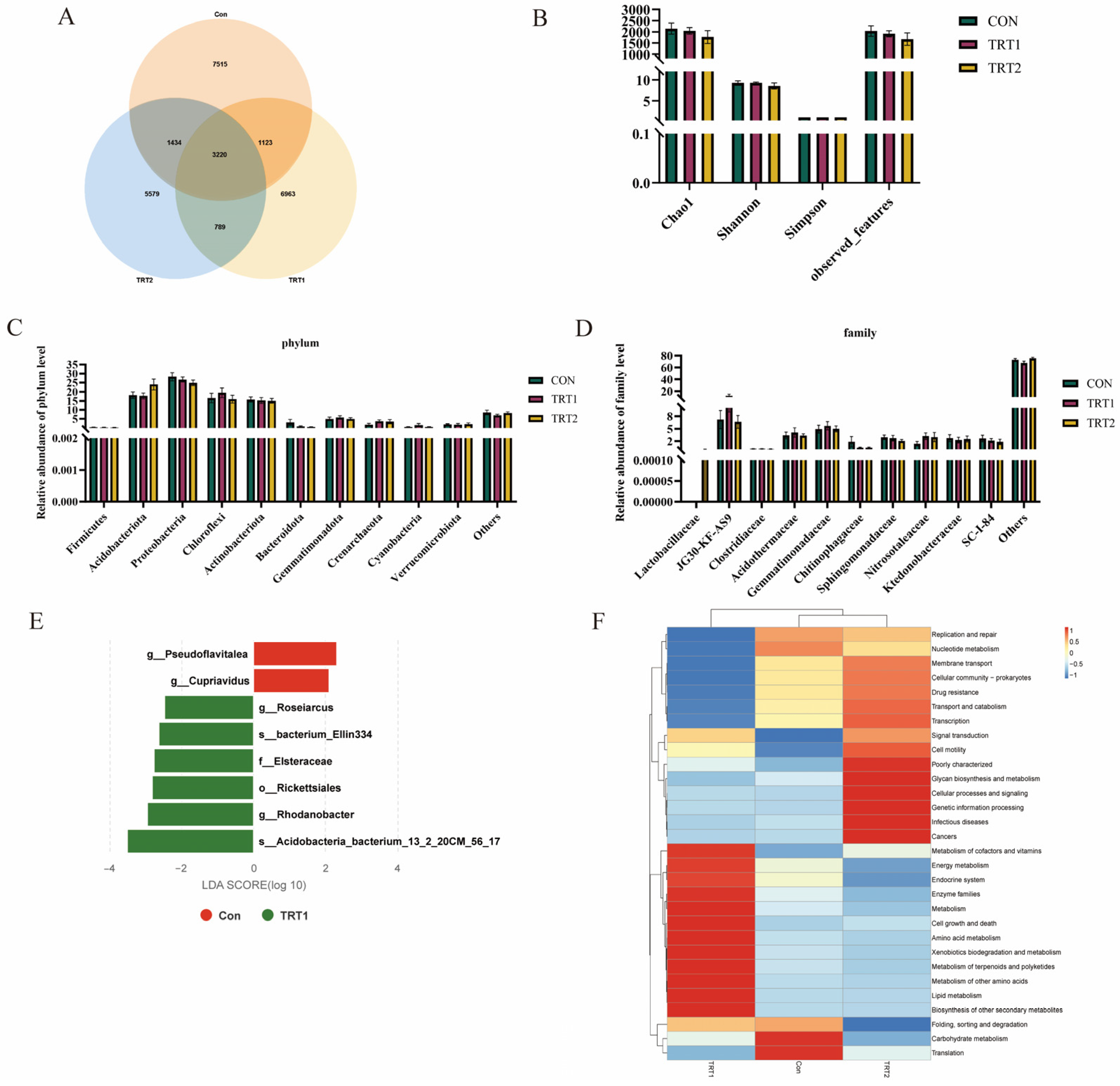
3.3. Microbial Composition and Function of Excreta
3.4. Metabolomics Analysis Results
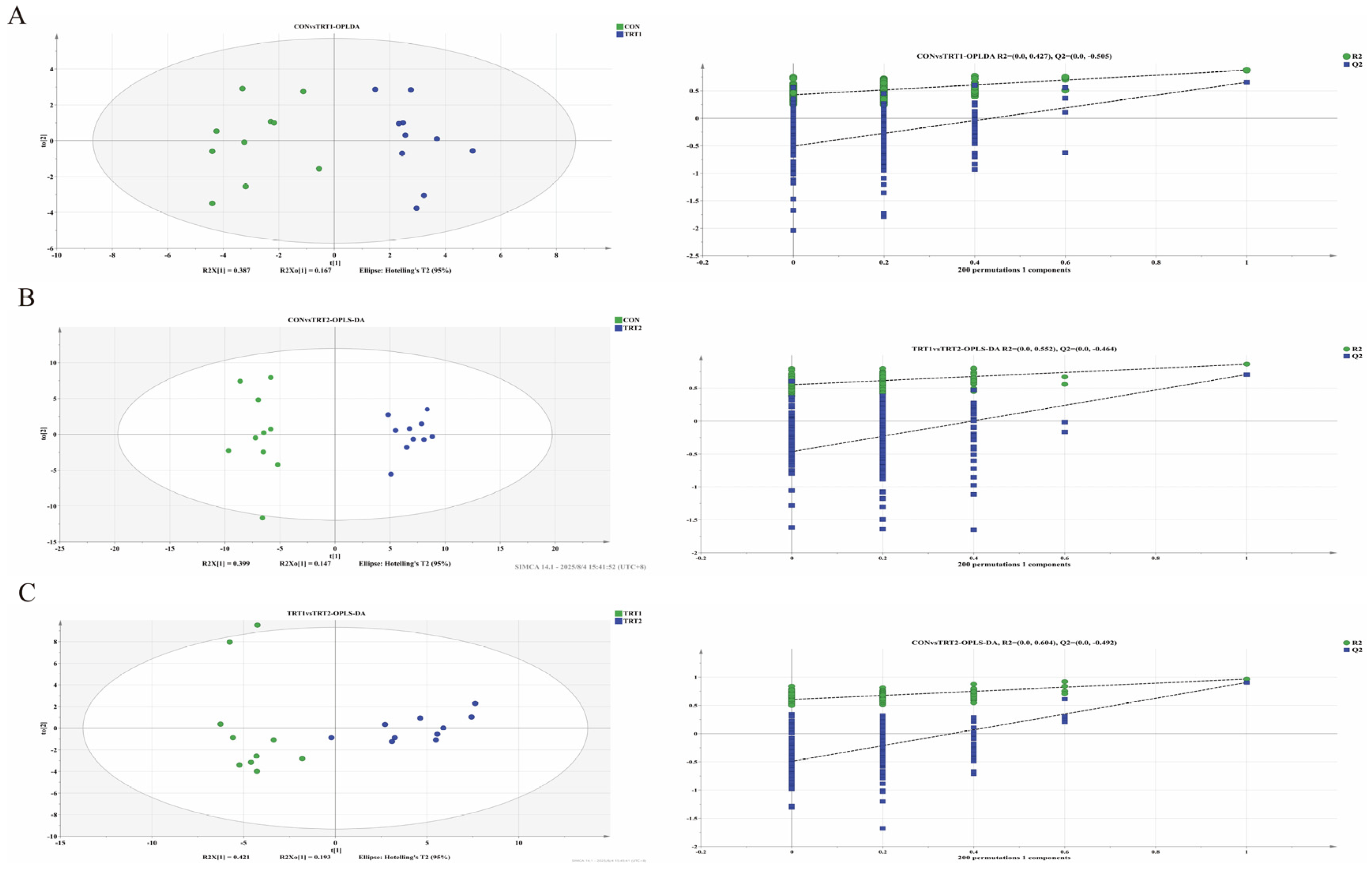
3.4.1. OPLS-DA Analysis
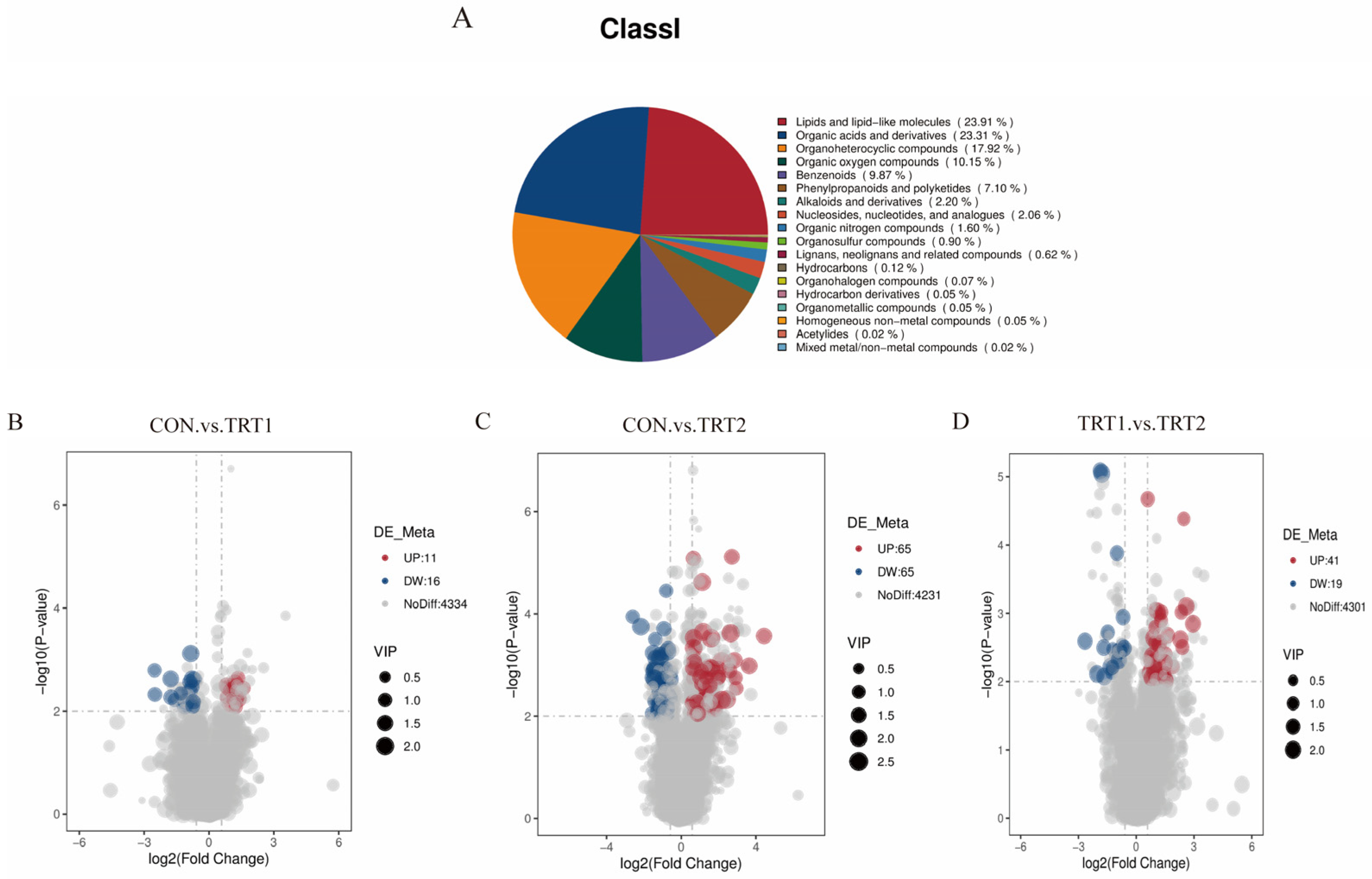
3.4.2. Metabolite Classification and Differential Metabolite Analysis
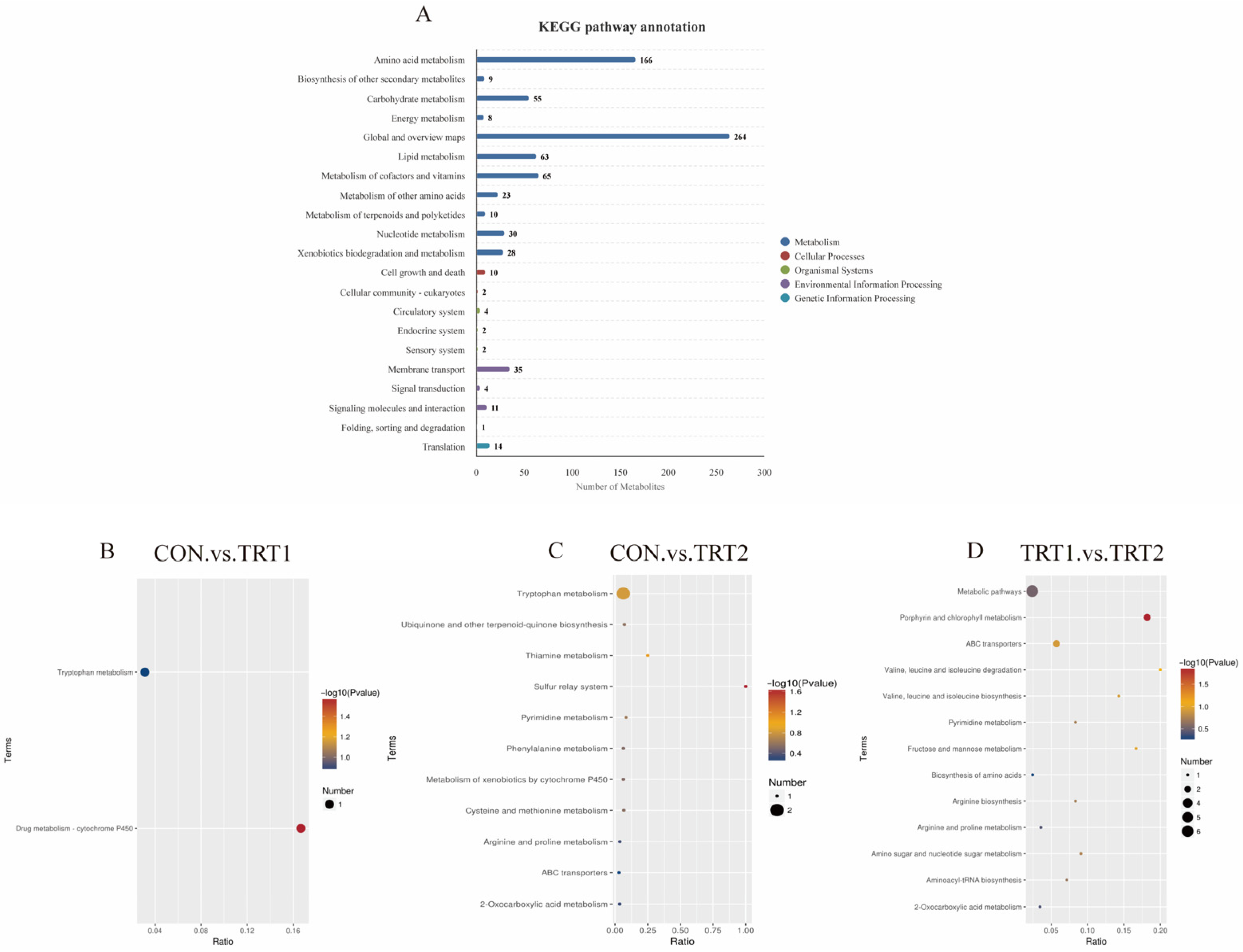
3.4.3. KEGG Classification and Enrichment Pathway Analysis of Differentially Expressed Metabolites
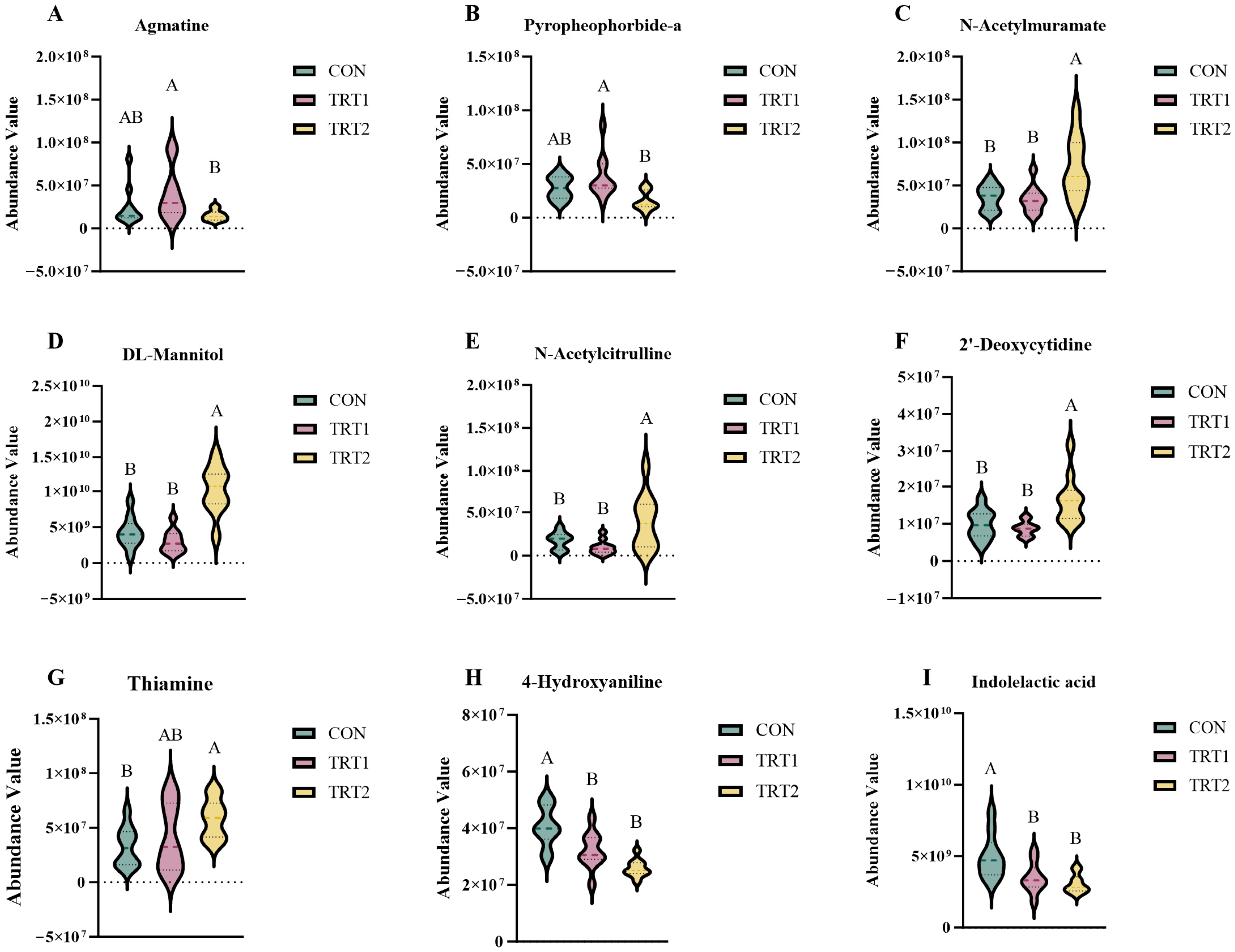
3.4.4. Analysis of Highly Significant Differential Metabolites Enriched in the KEGG Pathway
4. Discussion
4.1. Effects of P. oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility and Metabolism in Tumbler
4.2. Effects of P. oleracea Addition in Health Care Sand on Serum Biochemical Parameters and Antioxidant Capacity in Tumbler Pigeons
4.3. Effects of P. oleracea Addition in Health Care Sand on the Microbial Community Composition in Excreta of Tumbler Pigeons
4.4. Effects of P. oleracea Addition in Health Care Sand on the Metabolites in Excreta of Tumbler Pigeons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Entrikin, R.K.; Bryant, S.H. Tumbling in pigeons. Nature 1974, 252, 706–708. [Google Scholar] [CrossRef]
- STUDENS. The Genesis of Species. Nature 1871, 3, 347. [Google Scholar] [CrossRef]
- Li, X.; Guo, X.; Li, H.; Liu, J.; Lin, J.; Zheng, S.; Ke, L. Effects of different metabolizable energy levels on apparent nutrient digestibility and metabolism, blood biochemical indicators, and fecal flora diversity in racing pigeons undergoing exercise training. Front. Microbiol. 2025, 16, 1632529. [Google Scholar] [CrossRef]
- Ning, C.; Bu, W.; Meng, X.; Li, H.; Zhang, X.; Tang, Y.; Hu, F.; Wang, S.; Tan, C.; Guo, C.; et al. Study on anti-fatigue effect and mechanism of iron source combined with Angelica sinensis and Agrimonia pilosa on pigeons under exercise stress. Poult. Sci. 2025, 104, 105804. [Google Scholar] [CrossRef]
- Kastelic, M.; Pšeničnik, I.; Gračner, G.G.; Kadunc, N.Č.; Knific, R.L.; Slavec, B.; Krapež, U.; Rataj, A.V.; Rojs, O.Z.; Pulko, B.; et al. Health status and stress in different categories of racing pigeons. Animals 2021, 11, 2686. [Google Scholar] [CrossRef]
- Yu, Q.P.; Feng, D.Y.; Xia, M.H.; He, X.J.; Liu, Y.H.; Tan, H.Z.; Zou, S.G.; Ou, X.H.; Zheng, T.; Cao, Y.; et al. Effects of a traditional Chinese medicine formula supplementation on growth performance, carcass characteristics, meat quality and fatty acid profiles of finishing pigs. Livest. Sci. 2017, 202, 135–142. [Google Scholar] [CrossRef]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef]
- Gorske, S.F.; Rhodes, A.M.; Hopen, H.J. A numerical taxonomic study of Portulaca oleracea. Weed Sci. 1979, 27, 96–102. [Google Scholar] [CrossRef]
- Li, K.; Xia, T.; Jiang, Y.; Wang, N.; Lai, L.; Xu, S.; Yue, X.; Xin, H. A review on ethnopharmacology, phytochemistry, pharmacology and potential uses of Portulaca oleracea L. J. Ethnopharmacol. 2024, 319, 117211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, L.; Yan, H.; Wu, M.; Hao, X.; Liu, H. Nutritional values, bioactive compounds and health benefits of purslane (Portulaca oleracea L.): A comprehensive review. Food Sci. Hum. Wellness 2024, 13, 2480–2501. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Jalali, J.; Ghasemzadeh Rahbardar, M. Ameliorative effects of Portulaca oleracea L. (purslane) on the metabolic syndrome: A review. J. Ethnopharmacol. 2022, 299, 115672. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiu, J.; Zhang, X.; Sun, J.; Ying, X. Four alkaloids from Portulaca oleracea L. and their anti-inflammatory. Nat. Prod. Res. 2025, 39, 5742–5748. [Google Scholar] [CrossRef]
- Du, Y.K.; Liu, J.; Li, X.M.; Pan, F.F.; Wen, Z.G.; Zhang, T.C.; Yang, P.L. Flavonoids extract from Portulaca oleracea L. induce Staphylococcus aureus death by apoptosis-like pathway. Int. J. Food Prop. 2017, 20, S534–S542. [Google Scholar] [CrossRef]
- Bai, Y.; Zang, X.; Ma, J.; Xu, G. Anti-diabetic effect of Portulaca oleracea L. Polysaccharideandits mechanism in diabetic rats. Int. J. Mol. Sci. 2016, 17, 1201. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Li, T.; Cao, J.; Guan, Z.; Xu, T.; Jia, G.; Ma, G.; Zhao, R. Portulaca oleracea L. polysaccharide inhibits porcine rotavirus in vitro. Animals 2023, 13, 2306. [Google Scholar] [CrossRef] [PubMed]
- Tian, X. Protective Effect and Mechanism of Portulaca oleracea L. Dietary Fiber on Cadmium Exposure in Mice. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2022. Available online: http://202.201.224.138/vpn/3/https/NNYHGLUDN3WXTLUPMW4A/kcms2/article/abstract?v=4qE7rfs4XRG-uvdjVl5asfjEq4nyF6HcNji-xC94EF6eewLhQGpkY6Wk-t76WHR05LDpBuBlbEqwAFbVkTQCEzNEGNgE4y5QcH4B-YQJgKh8HWK_Pn6maDt2xKzOVPVloBCu5BGfh1n28AQ0dLZ9aBx9UivrDIQF_l8sLVuOALvApmSFJDhwfk5PKyayracl&uniplatform=NZKPT&language=CHS (accessed on 2 October 2025).
- GB/T 6432-1994; Method for Determination of Crude Protein in Feed. National Standardization Administration: Beijing, China, 1994.
- GB/T 6433-2006; Determination of Crude Fat in Feeds. Standards Press of China: Beijing, China, 2006.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- GB/T 6437-2002; Determination of Total Phosphorus in Feed (Spectrophotometric Method). National Standardization Administration: Beijing, China, 2002.
- Oliveira, D.R.; Lopes, A.C.A.; Pereira, R.A.; Cardoso, P.G.; Duarte, W.F. Selection of potentially probiotic Kluyveromyces lactis for the fermentation of cheese whey–based beverage. Ann Microbiol. 2019, 69, 1361–1372. [Google Scholar] [CrossRef]
- Shivamathi, C.S.; Gunaseelan, S.; Soosai, M.R.; Vignesh, N.S.; Varalakshmi, P.; Kumar, R.S.; Karthikumar, S.; Kumar, R.V.; Baskar, R.; Rigby, S.P.; et al. Process optimization and characterization of pectin derived from underexploited pineapple peel biowaste as a value-added product. Food Hydrocoll. 2022, 123, 107141. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- GB/T 6435-2014; Determination of Moisture Content in Feed. National Standardization Administration: Beijing, China, 2014.
- GB/T 6438-2007; Determination of Crude Ash Content in Feed. National Standardization Administration: Beijing, China, 2007.
- GB/T 14489.1-2008; Determination of Moisture and Volatile Matter Content in Oilseeds. People’s Republic of China and Standardization Administration of China: Beijing, China, 2008.
- Huang, X.; He, L.; Ma, J.; Li, Y.; Li, J.; Zang, C.; Hou, M.; Li, X. Ellagic acid on milk production performance, blood and milk hormones, antioxidant capacity and fecal microbial communities in lactating Yili mares. Front. Microbiol. 2025, 16, 1656100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tan, Y.; Li, H.; Huang, J.; Zhao, D.; Zhang, Z.; Yi, M.; Zhu, L.; Hui, S.; Yang, J.; et al. Fecal 16S rRNA sequencing and multi-compartment metabolomics revealed gut microbiota and metabolites interactions in APP/PS1 mice. Comput. Biol. Med. 2022, 151, 106312. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Li, H.; Liu, J.; Yang, F.; Zhao, X.; Liang, Y. 16S rRNA sequencing and metabolomics to analyze correlation between fecal flora and metabolites of squabs and parent pigeons. Animals 2025, 15, 74. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alabdali, A.Y.M.; Aldhalmi, A.K.; Reda, F.M.; Bassiony, S.S.; Selim, S.; El-Saadony, M.T.; Alagawany, M. Impacts of Purslane (Portulaca oleracea) extract supplementation on growing japanese quails’ growth, carcass traits, blood indices, nutrients digestibility and gut microbiota. Poult. Sci. 2022, 101, 102166. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Ye, F.; Tang, H.; Xiong, Y.; Wu, Y.; Wang, L.; Feng, X.; Zhang, S.; Wan, Y.; et al. Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. AMB Express 2021, 11, 31. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y.; Zhang, N.; Liu, H.; Zhang, Y.; Du, X.; He, X.; Zhao, X. Effects of Portulaca oleracea extract on growth performance, antioxidant capacity and intestinal barrier of Wenchang chickens. Feed Res. 2025, 48, 36–41. Available online: http://202.201.224.138/vpn/3/https/NNYHGLUDN3WXTLUPMW4A/kcms2/article/abstract?v=4qE7rfs4XRFxzXHObmmRJxzr98J3TIZOo7sOQuY5NrX6Hi1d-fcQ77O7QxCqe_AAhZ9bLkpvhQ0k9CuEDqLke4tQXcEhYTLS9mzbsIn8xf7BFyFNS3uVhJWIvEQEceOcZDSlHcwK6-lrR8OXSuQcvCimtX0mzn60gsMY25Fl6i1ukhTpaaG-zw&uniplatform=NZKPT&language=CHS&captchaId=67c0c2f1-a782-4f2b-8e72-0e3745c2bbae (accessed on 26 October 2025). (In Chinese).
- Ning, K.; Shi, C.; Chi, Y.Y.; Zhou, Y.F.; Zheng, W.; Duan, Y.; Tong, W.; Xie, Q.; Xiang, H. Portulaca oleracea L. polysaccharide alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal homeostasis. Int. J. Biol. Macromol. 2024, 256, 128375. [Google Scholar] [CrossRef]
- Fu, Q.; Zhou, S.; Yu, M.; Lu, Y.; He, G.; Huang, X.; Huang, Y. Portulaca oleracea Polysaccharides Modulate Intestinal Microflora in Aged Rats in Vitro. Front. Microbiol. 2022, 13, 841397. [Google Scholar] [CrossRef] [PubMed]
- Habibian, M.; Sadeghi, G.; Karimi, A. Comparative effects of powder, aqueous and methanolic extracts of purslane (Portulaca oleracea L.) on growth performance, antioxidant status, abdominal fat deposition and plasma lipids in broiler chickens. Anim. Prod. Sci. 2019, 59, 89–100. [Google Scholar] [CrossRef]
- Eidi, A.; Mortazavi, P.; Moghadam, J.Z.; Mardani, P.M. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharm. Biol. 2015, 53, 1042–1051. [Google Scholar] [CrossRef]
- Westerblad, H.; Allen, D.G.; Lännergren, J. Muscle fatigue: Lactic acid or inorganic phosphate the major cause? Physiology 2002, 17, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L.; Howlett, R.A.; Heigenhauser, G.J.F. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000, 32, 756–763. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; Wang, Z.; Li, H. The effect of the purslane polyphenols on the structure of rabbit meat myofibrillar protein under malondialdehyde-induced oxidative stress. J. Food Sci. 2023, 88, 1924–1938. [Google Scholar] [CrossRef]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Xu, L.; Gao, G.; Zhou, Z.; Wei, Z.; Sun, W.; Li, Y.; Jiang, X.; Gu, J.; Li, X.; Pi, Y. Fermented purslane (Portulaca oleracea L.) supplementation enhances growth and immune function parallel to the regulation of gut microbial butyrate production in weaned piglets. Microorganisms 2024, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Dahran, N.; Alotaibi, B.S.; Abd-Elhakim, Y.M.; Mohamed, A.A.-R.; Ibrahim, R.E.; Metwally, M.M.M.; Khamis, T.; Eskandrani, A.A.; Alosaimi, M.E.; Aly, M.Y.M.; et al. Dietary purslane (Portulaca oleracea L.) leaf powder maintains growth and intestinal health in Oreochromis niloticus under chronic water-borne cadmium exposure by strengthening the gut barriers, modulating the intestinal nutrient transporters, and relieving oxidative stress. Fish Physiol. Biochem. 2025, 51, 8. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, J.E.; Duke, J.A. Common purslane: A source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr. 1992, 11, 374–382. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Duan, Y.; Ying, Z.; Zhang, M.; Ying, X.; Yang, G. Two new homoisoflavones from Portulaca oleracea L. and their activities. Nat. Prod. Res. 2020, 36, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Wu, G.D. Influence of the Microbiota on Host Physiology—Moving beyond the Gut. J. Physiol. 2017, 595, 433–435. [Google Scholar] [CrossRef]
- Bonder, M.J.; Kurilshikov, A.; Tigchelaar, E.F.; Mujagic, Z.; Imhann, F.; Vila, A.V.; Deelen, P.; Vatanen, T.; Schirmer, M.; Smeekens, S.P.; et al. The Effect of host genetics on the gut microbiome. Nat. Genet. 2016, 48, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J. Host–microbe interactions and gut health in poultry—Focus on innate responses. Microorganisms 2019, 7, 139. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhang, S.; Zhao, Y.; Gao, D.; Xing, J.; Cao, Y.; Xu, G. Purslane (Portulaca oleracea L.) polysaccharide attenuates carbon tetrachloride-induced acute liver injury by modulating the gut microbiota in mice. Genomics 2025, 117, 110983. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Hui, M.L.; Tan, L.T.; Letchumanan, V.; He, Y.W.; Fang, C.M.; Chan, K.G.; Law, J.W.; Lee, L.H. The extremophilic actinobacteria: From microbes to medicine. Antibiotics 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Trosvik, P.; de Muinck, E.J. Ecology of bacteria in the human gastrointestinal tract—Identification of keystone and foundation taxa. Microbiome 2015, 3, 44. [Google Scholar] [CrossRef]
- Pinheiro, G.L.; Correa, R.F.; Cunha, R.S.; Cardoso, A.M.; Chaia, C.; Clementino, M.M.; Garcia, E.S.; de Souza, W.; Frasés, S. Isolation of aerobic cultivable cellulolytic bacteria from different regions of the gastrointestinal tract of giant land snail Achatina fulica. Front. Microbiol. 2015, 6, 860. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Li, Z.; Chu, T.; Sun, X.; Zhuang, S.; Hou, D.; Zhang, Z.; Sun, J.; Liu, Y.; Li, J.; Bian, Y. Polyphenols-rich Portulaca oleracea L. (Purslane) alleviates ulcerative colitis through restiring the intestinal barrier, gut microbiota and metabolites. Food Chem. 2025, 468, 142391. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Zhou, X.; Alam, M.S.; Fan, J.; Guo, Z.; Zhang, H.; Gubry-Rangin, C.; Zhongjun, J. Long-Term Adaptation of Acidophilic Archaeal Ammonia Oxidisers Following Different Soil Fertilisation Histories. Microb. Ecol. 2022, 83, 424–435. [Google Scholar] [CrossRef]
- Sikorski, J.; Baumgartner, V.; Birkhofer, K.; Boeddinghaus, R.S.; Bunk, B.; Fischer, M.; Fösel, B.U.; Friedrich, M.W.; Göker, M.; Hölzel, N.; et al. The evolution of ecological diversity in Acidobacteria. Front. Microbiol. 2022, 13, 715637. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.J.V.; de Araujo, M.R.; Junior, R.C.M.; Machado, N.M.; Joshi, R.K.; Buglio, D.d.S.; Lamas, C.B.; Direito, R.; Laurindo, L.F.; Tanaka, M.; et al. Investigating the neuroprotective and cognitive-enhancing effects of Bacopa monnieri: A systematic review focused on inflammation, oxidative stress, mitochondrial dysfunction, and apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Tanes, C.; Hu, W.; Friedman, E.; Hecht, A.; Daniel, S.; Clish, C.; Lewis, J.D.; Wu, G.D.; Bittinger, K. Distinguishing Diet- and Microbe-Derived Metabolites in the Human Gut. Microbiome 2025, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, R.; Zhang, H.; Su, Y.; Zhu, W. Swine Gut Microbiota and Its Interaction with Host Nutrient Metabolism. Anim. Nutr. 2020, 6, 410–420. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Noerman, S.; Kårlund, A.; Raita, J.; Meuronen, T.; Koistinen, V.; Landberg, R.; Hanhineva, K. Nutritional metabolomics: Recent developments and future needs. Curr. Opin. Chem. Biol. 2023, 77, 102400. [Google Scholar] [CrossRef]
- Chang, J.Y.; Kim, J.; Kosonen, R.; Kim, J.Y.; Lee, J.E. The Role of Agmatine in modulating autophagy under neuroinflammatory conditions induced by metabolic alteration in mouse brain. Exp. Neurobiol. 2025, 34, 95–107. [Google Scholar] [CrossRef]
- Cobos-Puc, L.E.; Aguayo-Morales, H. Agmatine mitigates diabetes-related memory loss in female mice by targeting i2/i3 imidazoline receptors and enhancing brain antioxidant defenses. Antioxidants 2025, 14, 837. [Google Scholar] [CrossRef]
- Bahremand, T.; Payandemehr, P.; Riazi, K.; Noorian, A.R.; Payandemehr, B.; Sharifzadeh, M.; Dehpour, A.R. Modulation of the anticonvulsant effect of swim stress by agmatine. Epilepsy Behav. 2018, 78, 142–148. [Google Scholar] [CrossRef]
- Nakano, M.; Sakamoto, T.; Itoh, Y.; Kitano, Y.; Tsukakoshi, K.; Bono, H.; Tabunoki, H. The metabolic ability of swallowtails results in the production of bioactive substances from plant components. PLoS ONE 2025, 20, e0321438. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.E.; Fish, K.; Fu, Y.-X.; Zhou, L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Pei, W.; Shen, Y.; Wang, L.; Zhu, J.; Zhang, Y.; Fan, S.; Wu, Q.; Li, L.; Zhang, Z. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021, 12, 10184–10195. [Google Scholar] [CrossRef]
- Yin, J.; Song, Y.; Hu, Y.; Wang, Y.; Zhang, B.; Wang, J.; Ji, X.; Wang, S. Dose-dependent beneficial effects of tryptophan and its derived metabolites on Akkermansia in vitro: A preliminary prospective study. Microorganisms 2021, 9, 1511. [Google Scholar] [CrossRef]
- Hu, M.; Xu, Y.; Wang, Y.; Huang, Z.; Wang, L.; Zeng, F.; Qiu, B.; Liu, Z.; Yuan, P.; Wan, Y.; et al. Gut microbial-derived N-acetylmuramic acid alleviates colorectal cancer via the AKT1 pathway. Gut 2025, 74, 1230–1245. [Google Scholar] [CrossRef]
- Fu, X.; Chen, T.S.; Ray, M.B.; Nagasawa, H.T.; Williams, W.M. p-Aminophenol-induced hepatotoxicity in hamsters: Role of glutathione. J. Biochem. Mol. Toxicol. 2004, 18, 154–161. [Google Scholar] [CrossRef]
- Bozic, I.; Lavrnja, I. Thiamine and benfotiamine: Focus on their therapeutic potential. Heliyon 2023, 9, e21839. [Google Scholar] [CrossRef] [PubMed]
- Rokita, S.E.; Yang, J.; Pande, P.; Greenberg, W.A. Quinone Methide Alkylation of Deoxycytidine. J. Org. Chem. 1997, 62, 3010–3012. [Google Scholar] [CrossRef]
- Reiterer, C.; Hu, K.; Sljivic, S.; Falkner von Sonnenburg, M.; Fleischmann, E.; Kabon, B. The effect of mannitol on oxidation-reduction potential in patients undergoing deceased donor renal transplantation—A randomized controlled trial. Acta Anaesthesiol. Scand. 2021, 65, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Jaime, S.J.; Morita, M.; Gonzales, J.U.; Moinard, C. L-Citrulline Supports Vascular and Muscular Benefits of Exercise Training in Older Adults. Exerc. Sport Sci. Rev. 2020, 48, 133–139. [Google Scholar] [CrossRef] [PubMed]
| Composition | (Content, g/kg) | Nutrient Index | Nutritional Status |
|---|---|---|---|
| Corn | 650 | DM (%) | 91.96 |
| Soybeanmeal | 200 | OM (%) | 96.18 |
| Secondary powder | 50 | ME MJ/kg | 10.87 |
| Cotton protein | 50 | CP (%) | 18.34 |
| Soybean oil | 10 | EE (%) | 2.00 |
| Limestone (0~2 mm) | 10 | CF (%) | 3.05 |
| Limestone (2~4 mm) | 10 | Ca (%) | 0.94 |
| 2% Premix | 20 | P (%) | 0.58 |
| Total | 1000 | Lys (%) | 0.86 |
| Met (%) | 0.44 |
| Composition | CON | TRT1 | TRT2 |
|---|---|---|---|
| Shell powder | 33.5 | 33.5 | 33.5 |
| Red clay | 24.8 | 24.8 | 24.8 |
| Maifan stone | 13.6 | 13.6 | 13.6 |
| Red peck soil | 8.5 | 8.5 | 8.5 |
| Zeolite powder | 11.4 | 10.65 | 10.4 |
| Sea salt | 4.2 | 4.2 | 4.2 |
| Charcoal | 3 | 3 | 3 |
| Mycotoxin adsorbent | 1 | 1 | 1 |
| Portulaca oleracea | 0 | 0.75 | 1 |
| Items | CON | TRT1 | TRT2 |
|---|---|---|---|
| DM% | 72.30 ± 2.05 Aa | 64.74 ± 1.74 Bb | 70.24 ± 4.67 ABa |
| OM% | 77.44 ± 1.40 ab | 76.01 ± 0.74 b | 79.24 ± 2.36 a |
| CP% | 41.16 ± 4.01 ABb | 37.79 ± 4.41 Bb | 48.04 ± 3.49 Aa |
| GE% | 79.06 ± 1.02 A | 76.33 ± 0.69 B | 79.32 ± 2.04 A |
| ME MJ/kg | 10.87 ± 0.14 A | 10.49 ± 0.09 B | 10.90 ± 0.28 A |
| EE% | 78.90 ± 2.37 | 80.30 ± 11.46 | 78.65 ± 4.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, J.; Li, H.; Li, X.; Zhang, P.; Guo, X.; Lin, J.; Liao, K.; Ke, L. Effect of Portulaca oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility, Serum Parameters, and Excreta Microbiota Metabolism in Tumbler Pigeons. Animals 2025, 15, 3349. https://doi.org/10.3390/ani15223349
Li H, Zhang J, Li H, Li X, Zhang P, Guo X, Lin J, Liao K, Ke L. Effect of Portulaca oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility, Serum Parameters, and Excreta Microbiota Metabolism in Tumbler Pigeons. Animals. 2025; 15(22):3349. https://doi.org/10.3390/ani15223349
Chicago/Turabian StyleLi, Hu, Jian Zhang, Haiying Li, Xiaobin Li, Ping Zhang, Xinsheng Guo, Jianwei Lin, Kunyu Liao, and Lifeng Ke. 2025. "Effect of Portulaca oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility, Serum Parameters, and Excreta Microbiota Metabolism in Tumbler Pigeons" Animals 15, no. 22: 3349. https://doi.org/10.3390/ani15223349
APA StyleLi, H., Zhang, J., Li, H., Li, X., Zhang, P., Guo, X., Lin, J., Liao, K., & Ke, L. (2025). Effect of Portulaca oleracea Addition in Health Care Sand on Apparent Nutrient Digestibility, Serum Parameters, and Excreta Microbiota Metabolism in Tumbler Pigeons. Animals, 15(22), 3349. https://doi.org/10.3390/ani15223349




