Bacterial Agents and Antimicrobial-Resistance Patterns in Canine Otitis Externa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Handling
2.2. Criteria for the Selection of Dogs with Bacterial Otitis Externa
2.3. Microbiological Analysis
2.4. Kirby–Bauer Disc Diffusion Assay
2.5. Statistical Analyses
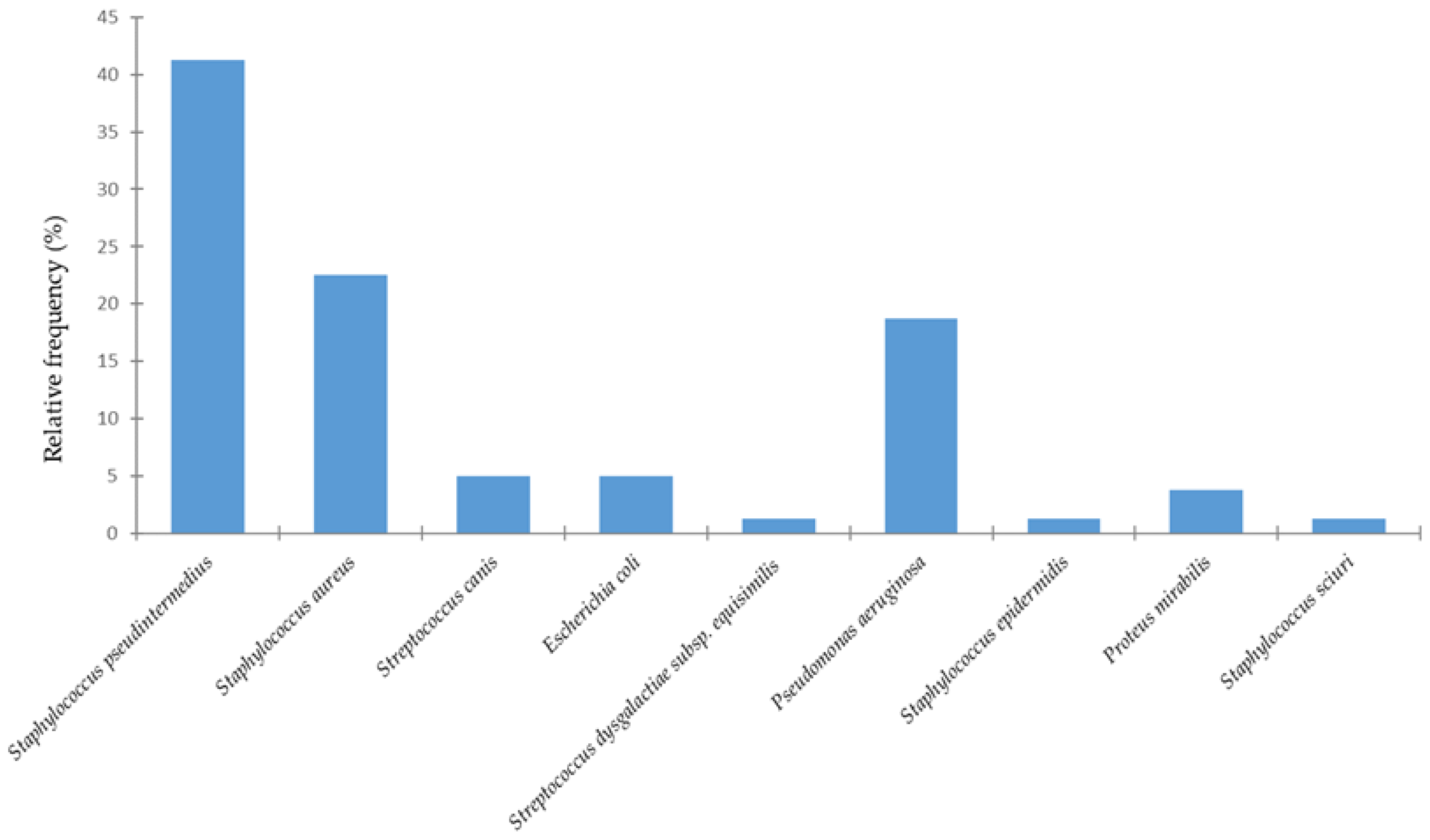
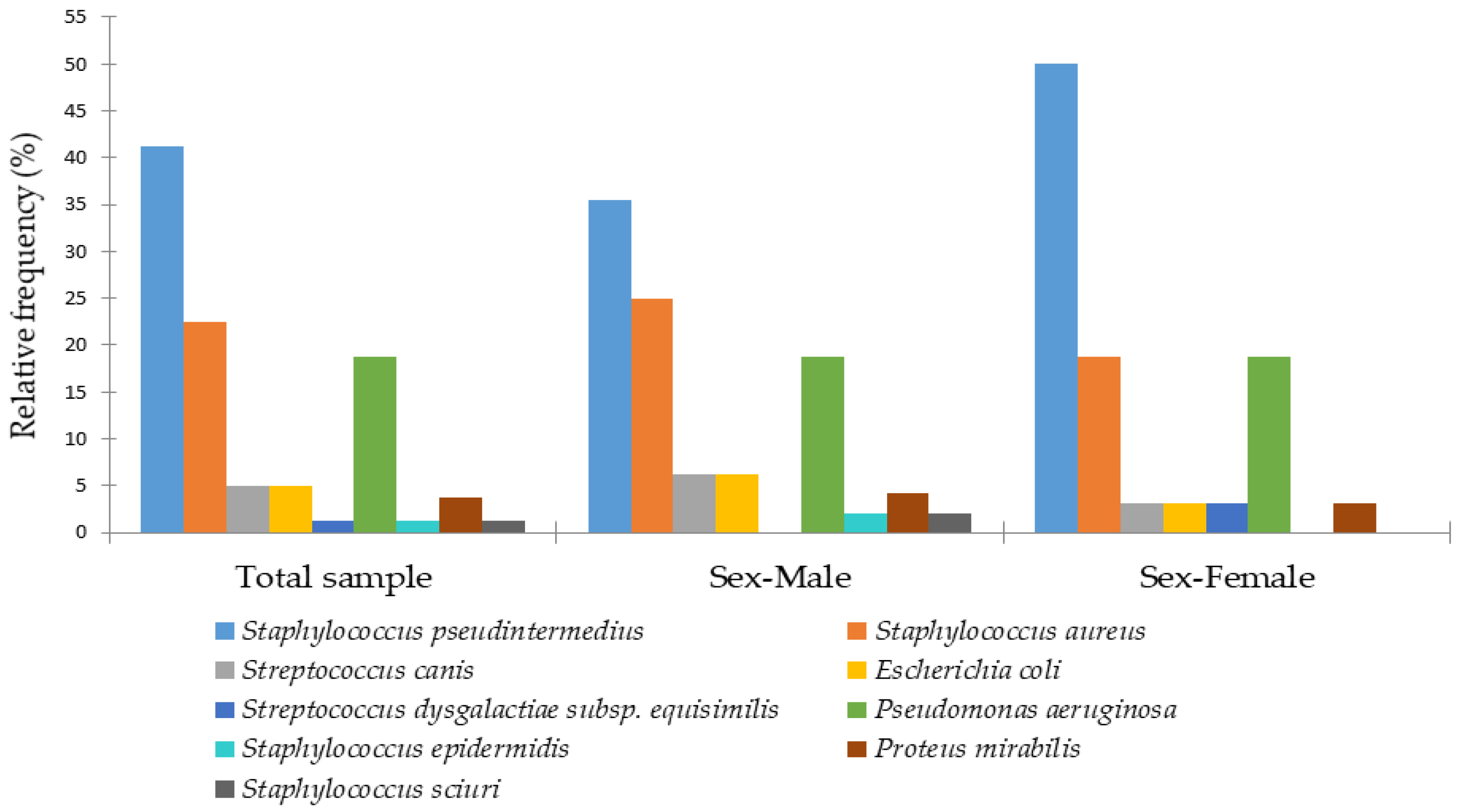
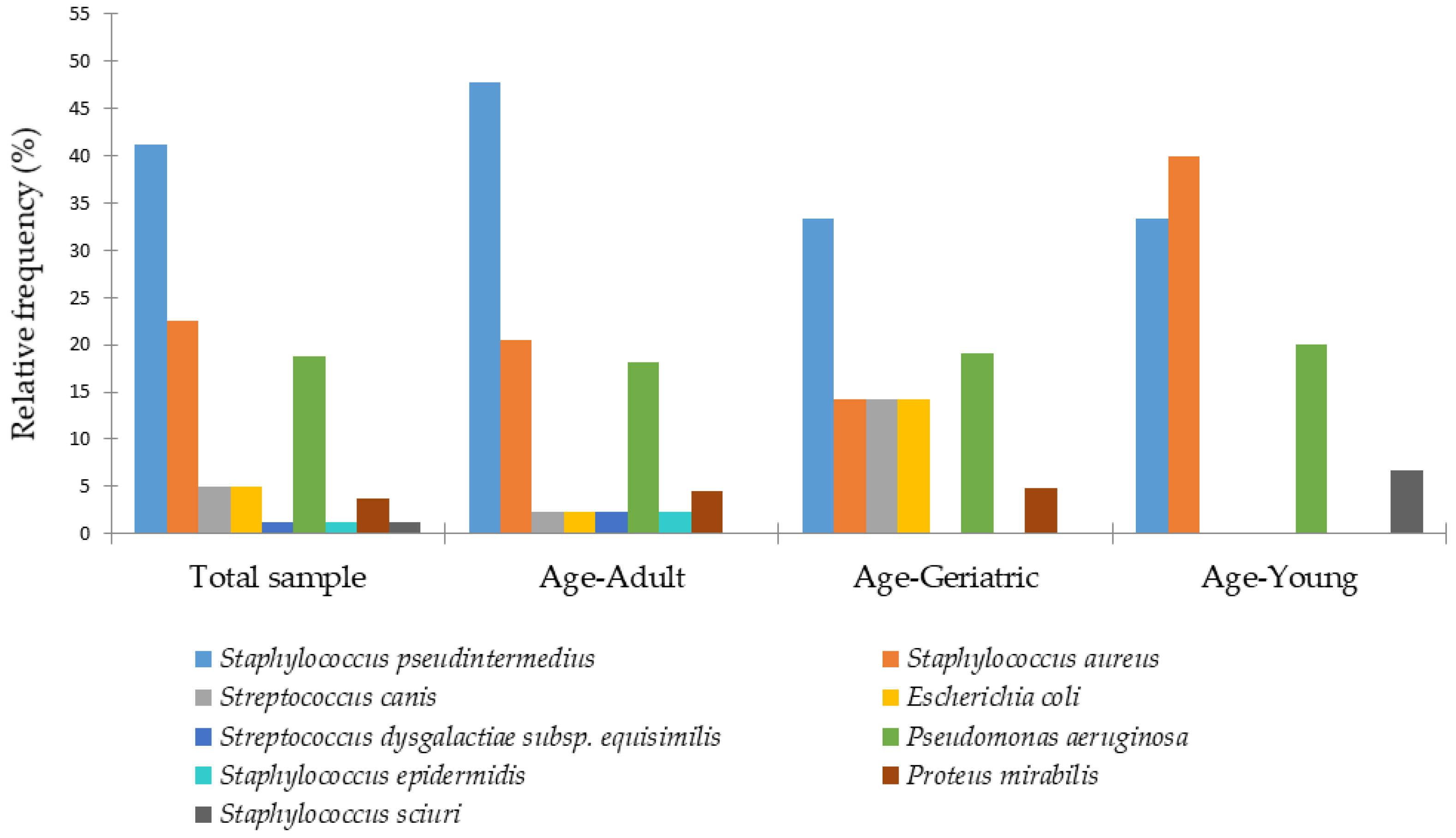
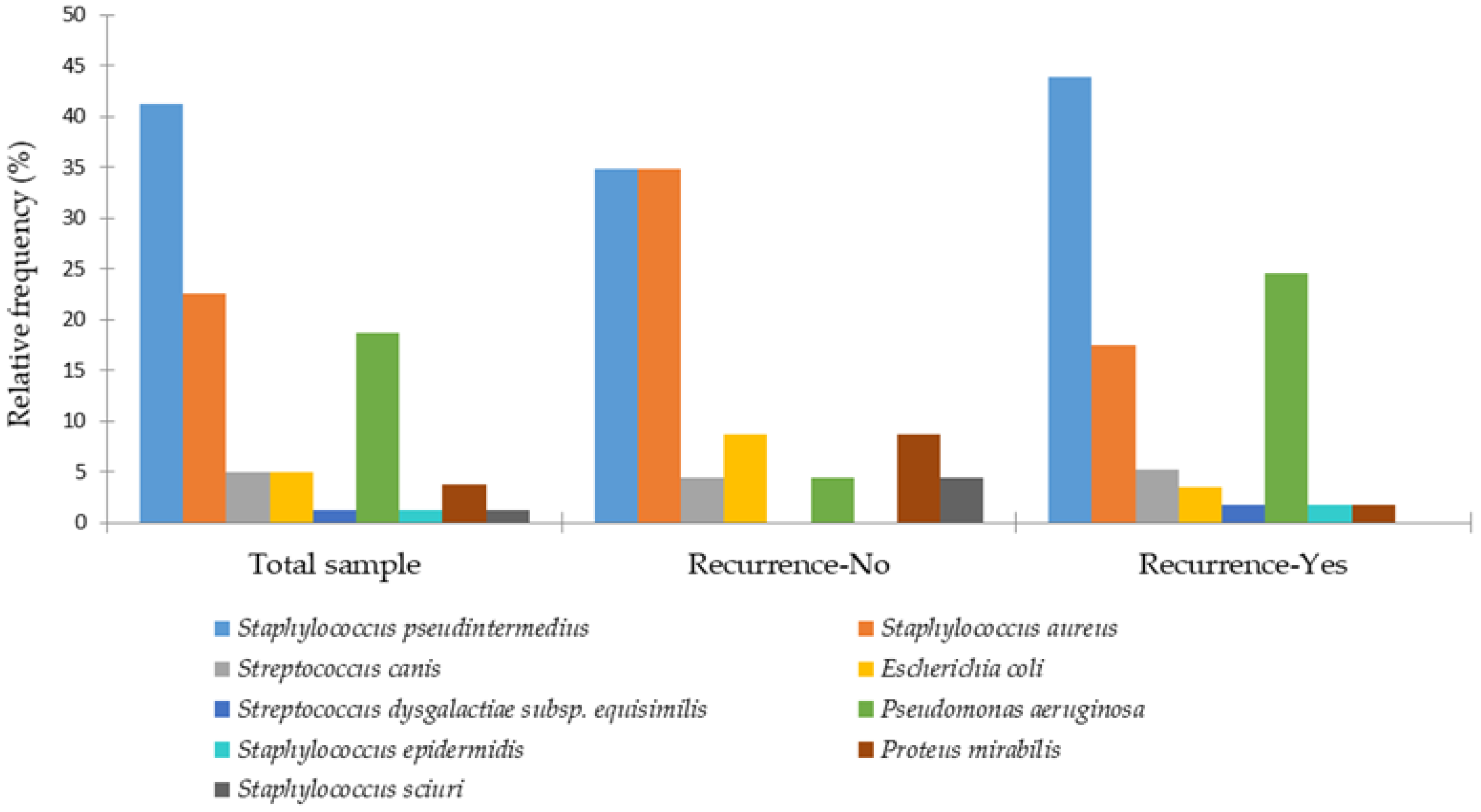
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carwardine, D.; Burton, N.J.; Knowles, T.G.; Barthelemy, N.; Parsons, K.J. Outcomes, Complications and Risk Factors Following Fluoroscopically Guided Transcondylar Screw Placement for Humeral Intracondylar Fissure. J. Small Anim. Pract. 2021, 62, 895–902. [Google Scholar] [CrossRef]
- Engdahl, K.S.; Brodbelt, D.C.; Cameron, C.; Church, D.B.; Hedhammar, Å.; O’Neill, D.G. Demography and Disorders of English Cocker Spaniels under Primary Veterinary Care in the UK. Canine Med. Genet. 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T. Managing Recurrent Otitis Externa in Dogs: What Have We Learned and What Can We Do Better? J. Am. Vet. Med. Assoc. 2023, 261, S10–S22. [Google Scholar] [CrossRef] [PubMed]
- Hemida, M.B.M.; Vuori, K.A.; Borgström, N.C.; Moore, R.; Rosendahl, S.; Anturaniemi, J.; Estrela-Lima, A.; Hielm-Björkman, A. Early Life Programming by Diet Can Play a Role in Risk Reduction of Otitis in Dogs. Front. Vet. Sci. 2023, 10, 1186131. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and Predisposing Factors for Canine Otitis Externa in the UK—A Primary Veterinary Care Epidemiological View. Canine Med. Genet. 2021, 8, 7. [Google Scholar] [CrossRef]
- Rosales, R.S.; Ramírez, A.S.; Moya-Gil, E.; de la Fuente, S.N.; Suárez-Pérez, A.; Poveda, J.B. Microbiological Survey and Evaluation of Antimicrobial Susceptibility Patterns of Microorganisms Obtained from Suspect Cases of Canine Otitis Externa in Gran Canaria, Spain. Animals 2024, 14, 742. [Google Scholar] [CrossRef]
- Kiss, G.; Radványi, S.; Szigeti, G. New Combination for the Therapy of Canine Otitis Externa: I Microbiology of Otitis Externa. J. Small Anim. Pract. 1997, 38, 51–56. [Google Scholar] [CrossRef]
- Huang, H.P.; Little, C.J.L.; McNeil, P.E. Histological Changes in the External Ear Canal of Dogs with Otitis Externa. Vet. Dermatol. 2009, 20, 422–428. [Google Scholar] [CrossRef]
- Ortiz, M.D.L.Á.V.; Rubio Arias, P.G. Comparación Del Tratamiento de Fitoterapia vs Tratamiento Convencional de Otitis Bacteriana “Cocos” En Perros. Anatomía Digit. 2024, 7, 21–40. [Google Scholar] [CrossRef]
- Malayeri, H.Z.; Jamshidi, S.; Salehi, T.Z. Identification and Antimicrobial Susceptibility Patterns of Bacteria Causing Otitis Externa in Dogs. Vet. Res. Commun. 2010, 34, 435–444. [Google Scholar] [CrossRef]
- Park, S.; Bae, S.; Kim, J.; Oh, T. Identification and Antimicrobial Susceptibility of Bacteria Isolated from Dogs with Chronic Otitis Externa. J. Vet. Clin. 2017, 34, 23. [Google Scholar] [CrossRef]
- Bugden, D.L. Identification and Antibiotic Susceptibility of Bacterial Isolates from Dogs with Otitis Externa in Australia. Aust. Vet. J. 2013, 91, 43–46. [Google Scholar] [CrossRef]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Leblond, A.; Madec, J.Y.; Haenni, M.; Gay, E. Antimicrobial Resistance Patterns of Bacteria Isolated from Dogs with Otitis. Epidemiol. Infect. 2019, 147, e121. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Maboni, G.; Battisti, R.; da Costa, L.; Selva, H.L.; Levitzki, E.D.; Gressler, L.T. High Rates of Multidrug Resistance in Bacteria Associated with Small Animal Otitis: A Study of Cumulative Microbiological Culture and Antimicrobial Susceptibility. Microb. Pathog. 2022, 165, 105399. [Google Scholar] [CrossRef] [PubMed]
- Garcias, B.; Batalla, M.; Vidal, A.; Durán, I.; Darwich, L. Trends in Antimicrobial Resistance of Canine Otitis Pathogens in the Iberian Peninsula (2010–2021). Antibiotics 2025, 14, 328. [Google Scholar] [CrossRef]
- Lilenbaum, W.; Veras, M.; Blum, E.; Souza, G.N. Antimicrobial Susceptibility of Staphylococci Isolated from Otitis Externa in Dogs. Lett. Appl. Microbiol. 2000, 31, 42–45. [Google Scholar] [CrossRef]
- Kwon, J.; Yang, M.-H.; Ko, H.-J.; Kim, S.-G.; Park, C.; Park, S.-C. Antimicrobial Resistance and Virulence Factors of Proteus Mirabilis Isolated from Dog with Chronic Otitis Externa. Pathogens 2022, 11, 1215. [Google Scholar] [CrossRef]
- Hur, B.A.; Hardefeldt, L.Y.; Verspoor, K.M.; Baldwin, T.; Gilkerson, J.R. Describing the Antimicrobial Usage Patterns of Companion Animal Veterinary Practices; Free Text Analysis of More than 4.4 Million Consultation Records. PLoS ONE 2020, 15, e0230049. [Google Scholar] [CrossRef]
- Seixas, R.; Monteiro, V.; Carneiro, C.; Vilela, C.L.; Oliveira, M. First Report of a Linezolid-Resistant MRSA (Methicillin Resistant Staphylococcus aureus) Isolated from a Dog with a Severe Bilateral Otitis in Portugal. Rev. Vet. 2011, 22, 81–84. [Google Scholar] [CrossRef]
- Couto, N.; Belas, A.; Pomba, C. Clonality of Methicillin-Resistant Staphylococcus aureus and Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Healthy and Sick Companion Animals and Humans in Portugal. Poster session. Clin. Microbiol. Infect. 2012, 18, 337–338. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0.; EUCAST: Växjö, Sweden, 2025. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 35th ed.; CLSI Supplement M100: Wayne, PA, USA, 2025. [Google Scholar]
- Kasai, T.; Fukui, Y.; Aoki, K.; Ishii, Y.; Tateda, K. Changes in the Ear Canal Microbiota of Dogs with Otitis Externa. J. Appl. Microbiol. 2021, 130, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.P.; Huang, H.M. Effects of Ear Type, Sex, Age, Body Weight, and Climate on Temperatures in the External Acoustic Meatus of Dogs. Am. J. Vet. Res. 1999, 60, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Prošić, I.; Milčić-Matić, N.; Milić, N.; Radalj, A.; Aksentijević, K.; Ilić, M.; Nišavić, J.; Radojičić, M.; Gajdov, V.; Krnjaić, D. Molecular Prevalence of MecA and MecC Genes in Coagulase-Positive Staphylococci Isolated from Dogs with Dermatitis and Otitis in Belgrade, Serbia: A One Year Study. Acta Vet. Beogr. 2024, 74, 117–132. [Google Scholar] [CrossRef]
- Dziva, F.; Wint, C.; Auguste, T.; Heeraman, C.; Dacon, C.; Yu, P.; Koma, L.M. First Identification of Methicillin-Resistant Staphylococcus pseudintermedius Strains among Coagulase-Positive Staphylococci Isolated from Dogs with Otitis Externa in Trinidad, West Indies. Infect. Ecol. Epidemiol. 2015, 5, 29170. [Google Scholar] [CrossRef]
- Zur, G.; Gurevich, B.; Elad, D. Prior Antimicrobial Use as a Risk Factor for Resistance in Selected Staphylococcus pseudintermedius Isolates from the Skin and Ears of Dogs. Vet. Dermatol. 2016, 27, 468. [Google Scholar] [CrossRef]
- Perry, L.R.; MacLennan, B.; Korven, R.; Rawlings, T.A. Epidemiological Study of Dogs with Otitis Externa in Cape Breton, Nova Scotia. Can. Vet. J. 2017, 58, 168–174. [Google Scholar]
- Guimarães, L.; Teixeira, I.M.; da Silva, I.T.; Antunes, M.; Pesset, C.; Fonseca, C.; Santos, A.L.; Côrtes, M.F.; Penna, B. Epidemiologic Case Investigation on the Zoonotic Transmission of Methicillin-Resistant Staphylococcus pseudintermedius among Dogs and Their Owners. J. Infect. Public Health 2023, 16, 183–189. [Google Scholar] [CrossRef]
- Dégi, J.; Morariu, S.; Simiz, F.; Herman, V.; Beteg, F.; Dégi, D.M. Future Challenge: Assessing the Antibiotic Susceptibility Patterns of Staphylococcus Species Isolated from Canine Otitis Externa Cases in Western Romania. Antibiotics 2024, 13, 1162. [Google Scholar] [CrossRef]
- Lynch, S.A.; Helbig, K.J. The Complex Diseases of Staphylococcus pseudintermedius in Canines: Where to Next? Vet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef]
- Dégi, J.; Imre, K.; Cătană, N.; Morar, A.; Sala, C.; Herman, V. Frequency of Isolation and Antibiotic Resistance of Staphylococcal Flora from External Otitis of Dogs. Vet. Rec. 2013, 173, 42. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Tamakan, H.; Gocmen, H. Genetic Characterization of Methicillin Resistant Staphylococcus pseudintermedius in Dogs and Cats in Cyprus: Comparison of MRSP and MRSA Results. Pak. J. Zool. 2022, 54, 1511–1519. [Google Scholar] [CrossRef]
- Vingopoulou, E.I.; Delis, G.A.; Batzias, G.C.; Kaltsogianni, F.; Koutinas, A.; Kristo, I.; Pournaras, S.; Saridomichelakis, M.N.; Siarkou, V.I. Prevalence and Mechanisms of Resistance to Fluoroquinolones in Pseudomonas aeruginosa and Escherichia coli Isolates Recovered from Dogs Suffering from Otitis in Greece. Vet. Microbiol. 2018, 213, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Penna, B.; Thomé, S.; Martins, R.; Martins, G.; Lilenbaum, W. In Vitro Antimicrobial Resistance of Pseudomonas aeruginosa Isolated from Canine Otitis Externa in Rio de Janeiro, Brazil. Braz. J. Microbiol. 2011, 42, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.V.; Moreira, J.M.A.R.; de Godoy Menezes, I.; Dutra, V.; do Bom Parto Ferreira de Almeida, A. Antibiotic Resistance Profiles and Activity of Clove Essential Oil (Syzygium aromaticum) against Pseudomonas aeruginosa Isolated of Canine Otitis. Vet. World 2022, 15, 2499–2505. [Google Scholar] [CrossRef]
- de Sousa, T.; Machado, S.; Caniça, M.; Ramos, M.J.N.; Santos, D.; Ribeiro, M.; Hébraud, M.; Igrejas, G.; Alves, O.; Costa, E.; et al. Pseudomonas aeruginosa: One Health Approach to Deciphering Hidden Relationships in Northern Portugal. J. Appl. Microbiol. 2025, 136, lxaf037. [Google Scholar] [CrossRef]
- Secker, B.; Shaw, S.; Atterbury, R.J. Pseudomonas spp. in Canine Otitis Externa. Microorganisms 2023, 11, 2650. [Google Scholar] [CrossRef]
- Leonard, C.; Thiry, D.; Taminiau, B.; Daube, G.; Fontaine, J. External Ear Canal Evaluation in Dogs with Chronic Suppurative Otitis Externa: Comparison of Direct Cytology, Bacterial Culture and 16S Amplicon Profiling. Vet. Sci. 2022, 9, 366. [Google Scholar] [CrossRef]

| Predictor | Estimates | 95% Confidence Interval | SE | Z | p | Rate Ratio (Exp(Estimate)) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Intercept | 1.96 | 1.46 | 2.45 | 0.25 | 7.69 | <0.001 | 7.07 |
| Sex | |||||||
| Female–Male | −0.46 | −0.91 | −0.01 | 0.23 | −1.99 | 0.046 | 0.63 |
| Recurrence | |||||||
| Yes–No | 0.97 | 0.48 | 1.46 | 0.25 | 3.87 | <0.001 | 2.64 |
| Habitat | |||||||
| Outdoor–Mixed | −0.69 | −1.22 | −0.17 | 0.27 | −2.59 | 0.009 | 0.50 |
| Indoor–Mixed | −0.90 | −1.47 | −0.34 | 0.29 | −3.15 | 0.002 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, S.; Calouro, R.; de Sousa, T.; Dapkevicius, M.d.L.E.; Mesquita, J.R.; Coelho, A.C.; Poeta, P. Bacterial Agents and Antimicrobial-Resistance Patterns in Canine Otitis Externa. Animals 2025, 15, 3317. https://doi.org/10.3390/ani15223317
Saraiva S, Calouro R, de Sousa T, Dapkevicius MdLE, Mesquita JR, Coelho AC, Poeta P. Bacterial Agents and Antimicrobial-Resistance Patterns in Canine Otitis Externa. Animals. 2025; 15(22):3317. https://doi.org/10.3390/ani15223317
Chicago/Turabian StyleSaraiva, Sónia, Rita Calouro, Telma de Sousa, Maria de Lurdes Enes Dapkevicius, João R. Mesquita, Ana C. Coelho, and Patrícia Poeta. 2025. "Bacterial Agents and Antimicrobial-Resistance Patterns in Canine Otitis Externa" Animals 15, no. 22: 3317. https://doi.org/10.3390/ani15223317
APA StyleSaraiva, S., Calouro, R., de Sousa, T., Dapkevicius, M. d. L. E., Mesquita, J. R., Coelho, A. C., & Poeta, P. (2025). Bacterial Agents and Antimicrobial-Resistance Patterns in Canine Otitis Externa. Animals, 15(22), 3317. https://doi.org/10.3390/ani15223317









