Assessment of an Ultrasound-Guided Longitudinal Approach to the Thoracic Erector Spinae Plane Block in Cat Cadavers: Description of Dye and Contrast Medium Distribution
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
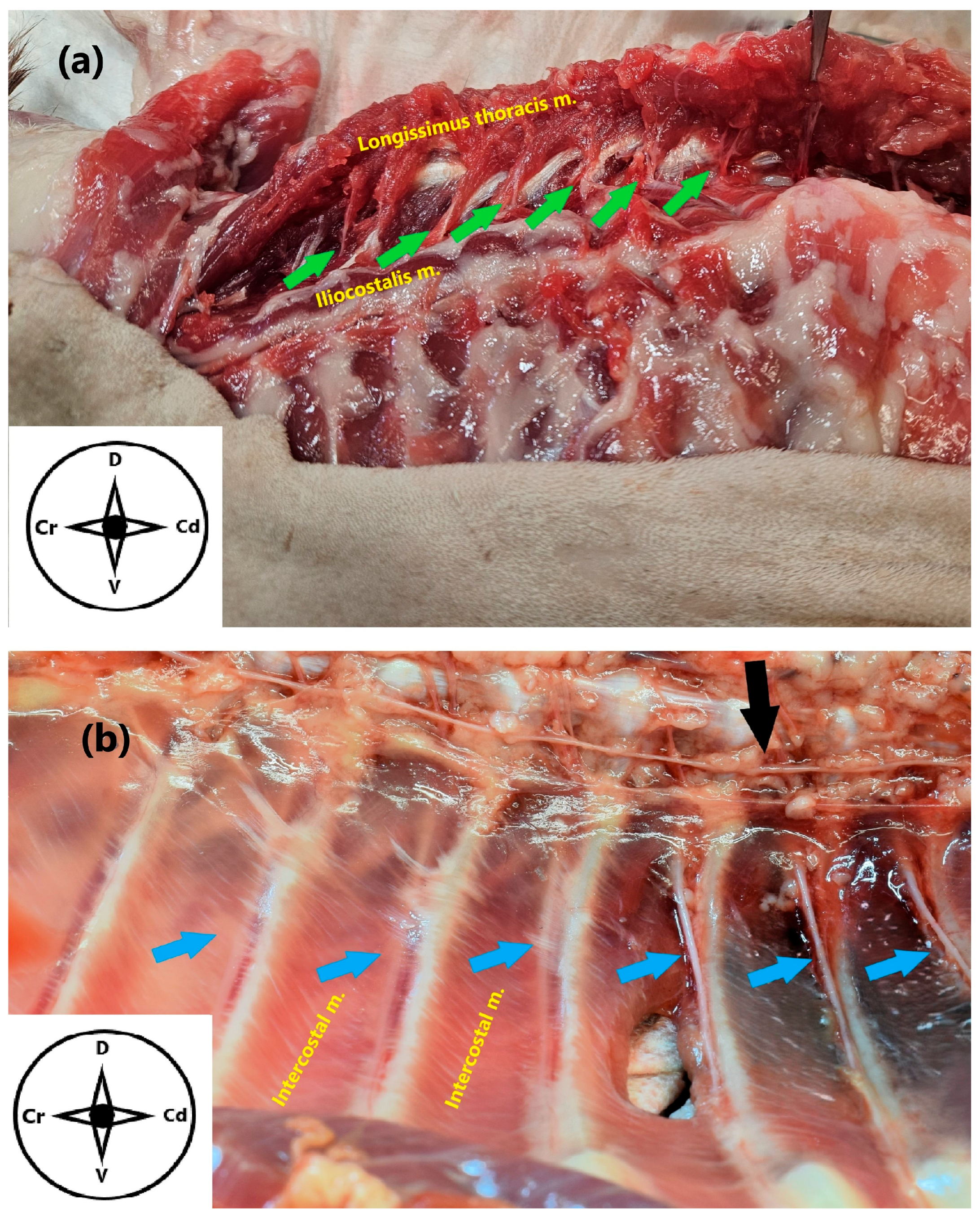
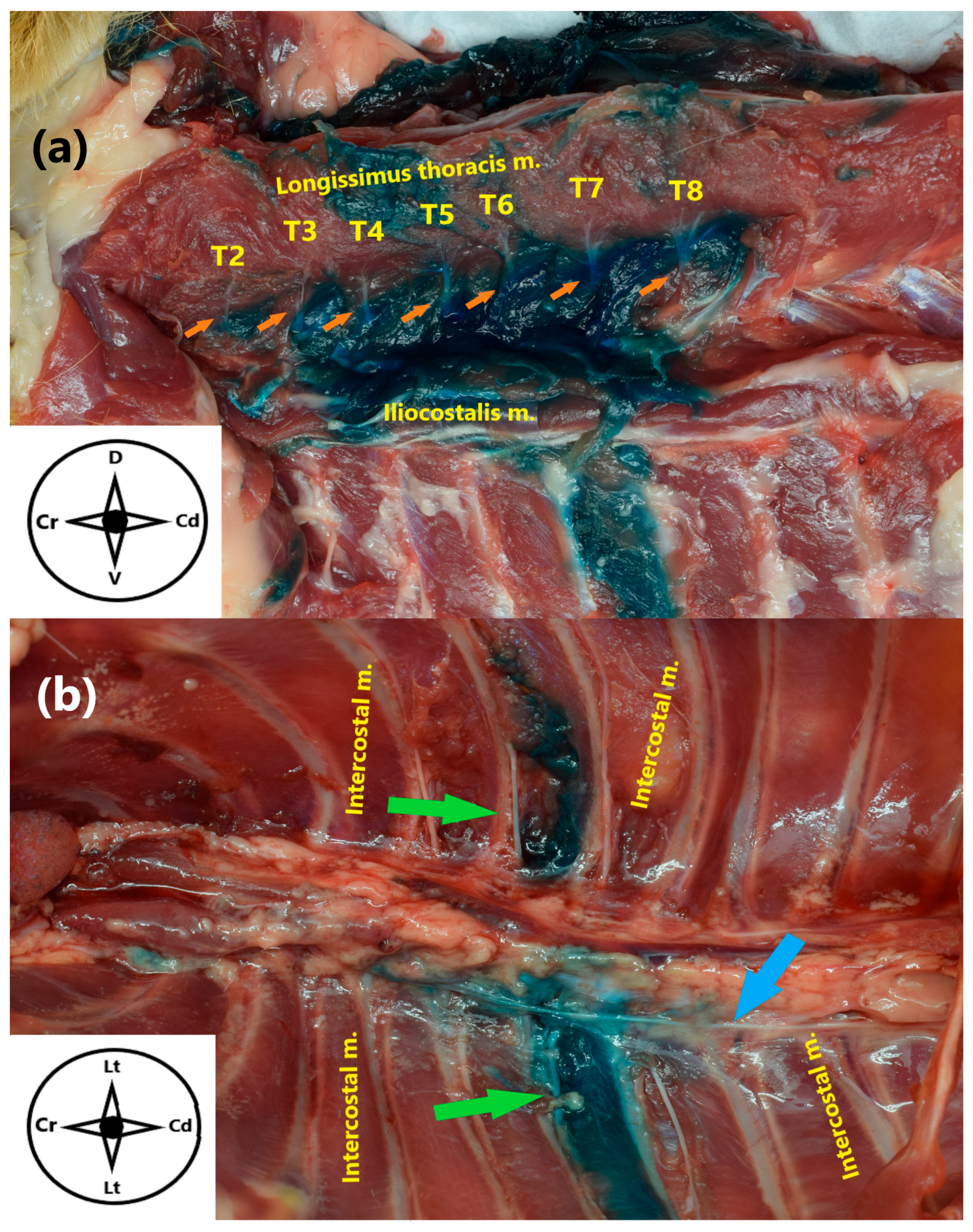
2.1. Anatomical Study
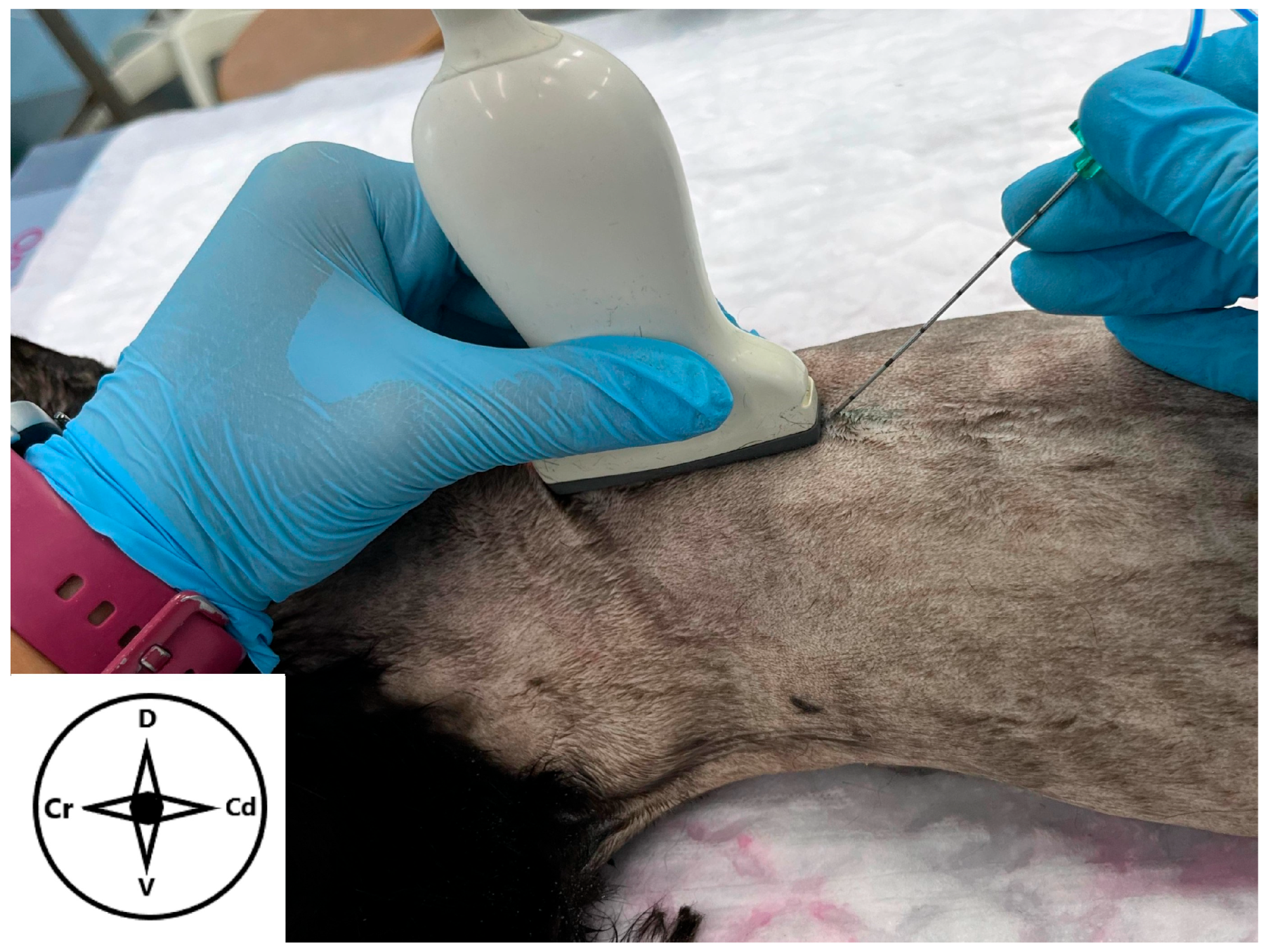
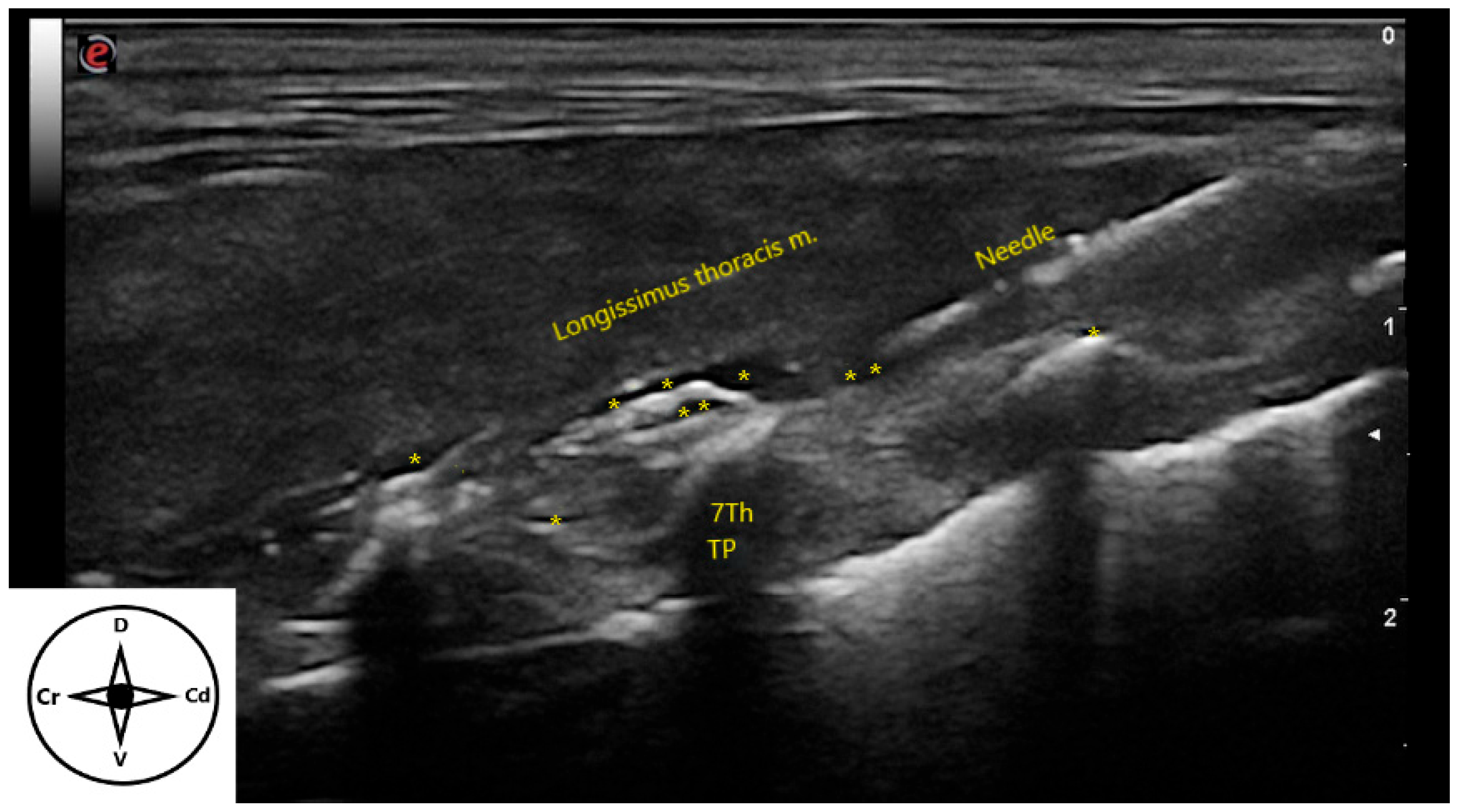
2.2. Ultrasound-Guided Technique
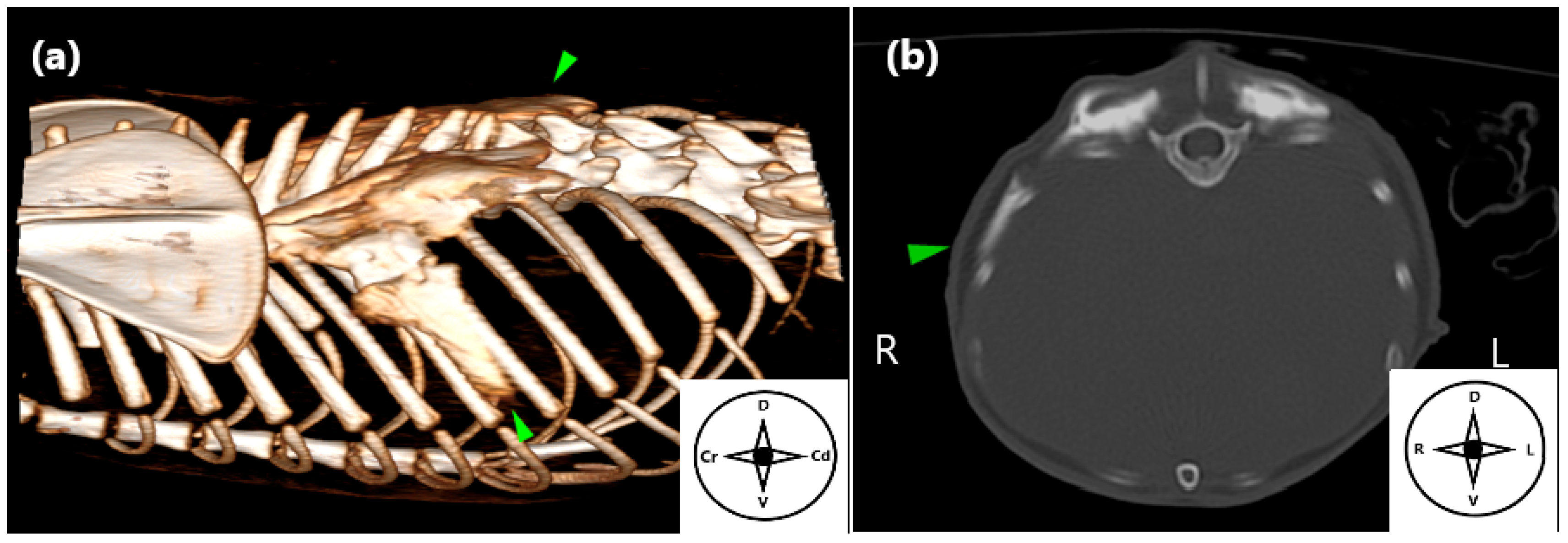
2.3. Computed Tomography (CT) Study
2.4. Spread Study
2.5. Statistical Analysis
3. Results
3.1. Anatomical Study
3.2. Ultrasound-Guided Technique
3.3. Computed Tomography (CT) Study
3.4. Spread Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESP | Erector Spinae Plane |
| DB | Dorsal Branches |
| VB | Ventral Branches |
| TP | Transverse Processes |
| MB | Methylene Blue |
| CT | Computed Tomography |
| Cr | Cranial |
| Cd | Caudal |
| D | Dorsal |
| V | Ventral |
| Lt | Lateral |
References
- Campoy, L. Development of Enhanced Recovery After Surgery (ERAS) Protocols in Veterinary Medicine through a One-Health Approach: The Role of Anesthesia and Locoregional Techniques. J. Am. Vet. Med. Assoc. 2022, 260, 1751–1759. [Google Scholar] [CrossRef]
- Redondo, J.I.; Otero, P.E.; Martínez-Taboada, F.; Doménech, L.; Hernández-Magaña, E.Z.; Viscasillas, J. Anaesthetic Mortality in Dogs: A Worldwide Analysis and Risk Assessment. Vet. Rec. 2024, 195, e3604. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, H.; Pawa, A.; Mariano, E.R. Interfascial Plane Blocks: Back to Basics. Reg. Anesth. Pain Med. 2018, 43, 341–346. [Google Scholar] [CrossRef]
- Chin, K.J.; Lirk, P.; Hollmann, M.W.; Schwarz, S.K.W. Mechanisms of Action of Fascial Plane Blocks: A Narrative Review. Reg. Anesth. Pain Med. 2021, 46, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; St James, M.; Schroeder, C.A.; Hershberger-Braker, K.L.; Teixeira, L.B.C.; Schroeder, K.M. Description of an Ultrasound-Guided Erector Spinae Plane Block and the Spread of Dye in Dog Cadavers. Vet. Anaesth. Analg. 2019, 46, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Forero, M.; Adhikary, S.D.; Lopez, H.; Tsui, C.; Chin, K.J. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg. Anesth. Pain Med. 2016, 41, 621–627. [Google Scholar] [CrossRef]
- Portela, D.A.; Castro, D.; Romano, M.; Gallastegui, A.; Garcia-Pereira, F.; Otero, P.E. Ultrasound-Guided Erector Spinae Plane Block in Canine Cadavers: Relevant Anatomy and Injectate Distribution. Vet. Anaesth. Analg. 2020, 47, 229–237. [Google Scholar] [CrossRef]
- Medina-Serra, R.; Foster, A.; Plested, M.; Sanchis, S.; Gil-Cano, F.; Viscasillas, J. Lumbar Erector Spinae Plane Block: An Anatomical and Dye Distribution Evaluation of Two Ultrasound-Guided Approaches in Canine Cadavers. Vet. Anaesth. Analg. 2021, 48, 125–133. [Google Scholar] [CrossRef]
- Cavalcanti, M.; Teixeira, J.G.; Medina-Serra, R.; Stern, A.W.; Romano, M.; Johnson, R.D.; Otero, P.E.; Portela, D.A. Erector Spinae Plane Block at the Thoracolumbar Spine: A Canine Cadaveric Study. Vet. Anaesth. Analg. 2022, 49, 656–663. [Google Scholar] [CrossRef]
- Cavalcanti, M.; Otero, P.E.; Romano, M.; Medina-Serra, R.; Chiavaccini, L.; Vettorato, E.; Maxwell, E.A.; Portela, D.A. Ultrasound-Guided Retromammillary Injections in Dogs: A Feasibility, Descriptive and Anatomical Study. Vet. Anaesth. Analg. 2024, 51, 695–701. [Google Scholar] [CrossRef]
- Kitchell, R.L. Chapter 17: Spinal Nerves. In Miller’s Anatomy of the Dog; Evans, H.E., de Lahunta, A., Eds.; Elselvier: St. Louis, MO, USA, 2012; pp. 611–657. [Google Scholar]
- Qiu, Y.; Zhang, T.-J.; Hua, Z. Erector Spinae Plane Block for Lumbar Spinal Surgery: A Systematic Review. J. Pain Res. 2020, 13, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.N.; Chauhan, S.; Bhoi, D.; Kaushal, B.; Hasija, S.; Sangdup, T.; Bisoi, A.K. Bilateral Erector Spinae Plane Block for Acute Post-Surgical Pain in Adult Cardiac Surgical Patients: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2019, 33, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.F.; Abdelghany, M.S.; Abdelraheem, T.M.; Abu Elyazed, M.M. Ultrasound-guided Erector Spinae Plane Block for Postoperative Analgesia in Pediatric Patients Undergoing Splenectomy: A Prospective Randomized Controlled Trial. Pediatr. Anesth. 2019, 29, 1201–1207. [Google Scholar] [CrossRef]
- Adhikary, S.D.; Liu, W.M.; Fuller, E.; Cruz-Eng, H.; Chin, K.J. The Effect of Erector Spinae Plane Block on Respiratory and Analgesic Outcomes in Multiple Rib Fractures: A Retrospective Cohort Study. Anaesthesia 2019, 74, 585–593. [Google Scholar] [CrossRef]
- Gürkan, Y.; Aksu, C.; Kuş, A.; Yörükoğlu, U.H. Erector Spinae Plane Block and Thoracic Paravertebral Block for Breast Surgery Compared to IV-Morphine: A Randomized Controlled Trial. J. Clin. Anesth. 2020, 59, 84–88. [Google Scholar] [CrossRef]
- Delgado, O.B.D.; Louro, L.F.; Rocchigiani, G.; Verin, R.; Humphreys, W.; Senior, M.; Campagna, I. Ultrasound-Guided Erector Spinae Plane Block in Horses: A Cadaver Study. Vet. Anaesth. Analg. 2021, 48, 577–584. [Google Scholar] [CrossRef]
- Nobre, A.V.; Gurgel, H.J.; Torres, E.C.B.; Aleixo, G.D.L.; Farias, D.J.L.D.; Souza Júnior, P.D.; Thiesen, R. Description of Ultrasound-Guided Lumbar Erector Spinae Plane (ESP) Block and Comparison of the Spread of Two Volumes of Dye in Cat Cadavers. Animals 2025, 15, 2157. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.R.; Martínez, C.P.; Santoro, F.; Herrera-Linares, M.E.; Jiménez, C.P. Perioperative Analgesic Effects of the Erector Spinae Plane Block with Bupivacaine or Bupivacaine-Dexmedetomidine in Dogs Undergoing Hemilaminectomy: A Randomized Controlled Trial. Can. Vet. J. 2024, 65, 473–480. [Google Scholar]
- Bendinelli, C.; D’Angelo, M.; Leonardi, F.; Verdier, N.; Cozzi, F.; Lombardo, R.; Portela, D.A. Erector Spinae Plane Block in Dogs Undergoing Hemilaminectomy: A Prospective Randomized Clinical Trial. Vet. Anaesth. Analg. 2024, 51, 279–287. [Google Scholar] [CrossRef]
- Chiavaccini, L.; Cavalcanti, M.; De Gasperi, D.; Portela, D.A. Clinical Efficacy of Ultrasound-Guided Bilateral Erector Spinae Plane Block for Standing Lumbar Spinous Osteotomy in a Horse. Vet. Anaesth. Analg. 2022, 49, 517–519. [Google Scholar] [CrossRef]
- Rodriguez, A.; Medina-Serra, R.; Lynch, N.; Veres-Nyeki, K. Erector Spinae Plane Block as Part of a Multimodal Analgesic Approach in an Anaesthetised Horse Undergoing Dorsal Spinous Process Ostectomy and Desmotomy. Vet. Rec. Case Rep. 2022, 10, e345. [Google Scholar] [CrossRef]
- Alza Salvatierra, D.N.; Herrera Linares, M.E.; Motta, L.; Martinez, M. Ultrasound-Guided Erector Spinae Interfascial Plane Block for Spinal Surgery in Three Cats. J. Feline Med. Surg. Open Rep. 2021, 7, 20551169211043814. [Google Scholar] [CrossRef]
- Ivanusic, J.; Konishi, Y.; Barrington, M.J. A Cadaveric Study Investigating the Mechanism of Action of Erector Spinae Blockade. Reg. Anesth. Pain Med. 2018, 43, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Linares, M.E.; Rico-Pérez, B.; Yaffy, D.; Fernández-Parra, R.; Llanos, C.; Parra-Martínez, C.; Herrera-Gutiérrez, M.E.; Sanchis-Mora, S. Cadaveric Study of the Ultrasound-Guided Erector Spinae Plane Block over the Transverse Process of the Twelfth Thoracic Vertebra in Dogs: Transversal vs Longitudinal Approach. Vet. J. 2024, 304, 106094. [Google Scholar] [CrossRef]
- Otero, P.E.; Fuensalida, S.E.; Russo, P.C.; Verdier, N.; Blanco, C.; Portela, D.A. Mechanism of Action of the Erector Spinae Plane Block: Distribution of Dye in a Porcine Model. Reg. Anesth. Pain Med. 2020, 45, 198–203. [Google Scholar] [CrossRef]
- Trujanovic, R.; Verdier, N.; Calice, I.; Knecht, C.; Otero, P.E. Axillary Ultrasound-Guided Approach for the Brachial Plexus in Pig Cadavers: A Descriptive Study. Lab. Anim. 2022, 56, 165–171. [Google Scholar] [CrossRef]
- Bogduk, N. The Lumbosacral Dorsal Rami of the Cat. J. Anat. 1976, 122, 653–662. [Google Scholar] [PubMed]
- Bailey, C.S.; Kitchell, R.L.; Haghighi, S.S.; Johnson, R.D. Cutaneous Innervation of the Thorax and Abdomen of the Dog. Am. J. Vet. Res. 1984, 45, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, K.; Sakai, H.; Tsuzuki, N.; Nagashima, M. Topographic Anatomy of the Posterior Ramus of Thoracic Spinal Nerve and Surrounding Structures. Spine 2012, 37, E817–E822. [Google Scholar] [CrossRef]
- Payne, G.L. Miller’s Anatomy of the Dog. 4th Edn. Edited by HEEvans and AdeLahunta. Elsevier, St Louis, 2013. 850 Pages. Price $153. ISBN 978 1 43770 812 7. Aust. Vet. J. 2012, 90, 504. [Google Scholar] [CrossRef]
- Yang, H.-M.; Choi, Y.J.; Kwon, H.-J.; O, J.; Cho, T.H.; Kim, S.H. Comparison of Injectate Spread and Nerve Involvement between Retrolaminar and Erector Spinae Plane Blocks in the Thoracic Region: A Cadaveric Study. Anaesthesia 2018, 73, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; Adhikary, S.D.; Forero, M. Erector Spinae Plane (ESP) Block: A New Paradigm in Regional Anesthesia and Analgesia. Curr. Anesthesiol. Rep. 2019, 9, 271–280. [Google Scholar] [CrossRef]
- Martin-Flores, M. Clinical Pharmacology and Toxicology of Local Anesthetics and Adjuncts. In Small Animal Regional Anesthesia and Analgesia; Campoy, L., Read, M.R., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 25–40. ISBN 978-0-8138-1994-5. [Google Scholar]
- Duke-Novakovski, T. Pain Management II: Local and Regional Anaesthetic Techniques. In BSAVA Manual of Canine and Feline Anaesthesia and Analgesia; Duke-Novakovski, T., de Vries, M., Seymour, C., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2016; pp. 143–158. [Google Scholar]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2022 WSAVA Guidelines for the Recognition, Assessment and Treatment of Pain. J. Small Anim. Pract. 2023, 64, 177–254. [Google Scholar] [CrossRef]
- Zannin, D.; Isaka, L.J.; Pereira, R.H.; Mencalha, R. Opioid-Free Total Intravenous Anesthesia with Bilateral Ultrasound-Guided Erector Spinae Plane Block for Perioperative Pain Control in a Dog Undergoing Dorsal Hemilaminectomy. Vet. Anaesth. Analg. 2020, 47, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Portela, D.A.; Romano, M.; Zamora, G.A.; Garcia-Pereira, F.; Pablo, L.S.; Gatson, B.J.; Johnson, A.N.; Otero, P.E. The Effect of Erector Spinae Plane Block on Perioperative Analgesic Consumption and Complications in Dogs Undergoing Hemilaminectomy Surgery: A Retrospective Cohort Study. Vet. Anaesth. Analg. 2021, 48, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, Y.; Aksu, C.; Kuş, A.; Yörükoğlu, U.H.; Kılıç, C.T. Ultrasound Guided Erector Spinae Plane Block Reduces Postoperative Opioid Consumption Following Breast Surgery: A Randomized Controlled Study. J. Clin. Anesth. 2018, 50, 65–68. [Google Scholar] [CrossRef]
- Nagaraja, P.; Ragavendran, S.; Singh, N.; Asai, O.; Bhavya, G.; Manjunath, N.; Rajesh, K. Comparison of Continuous Thoracic Epidural Analgesia with Bilateral Erector Spinae Plane Block for Perioperative Pain Management in Cardiac Surgery. Ann. Card. Anaesth. 2018, 21, 323. [Google Scholar] [CrossRef]
- Jones, M.R.; Urits, I.; Shnider, M.R.; Matyal, R. Confirmation of Erector Spinae Plane Block Analgesia for 3 Distinct Scenarios: A Case Report. A A Pract. 2019, 12, 141–144. [Google Scholar] [CrossRef]
- Tsui, B.C.H.; Fonseca, A.; Munshey, F.; McFadyen, G.; Caruso, T.J. The Erector Spinae Plane (ESP) Block: A Pooled Review of 242 Cases. J. Clin. Anesth. 2019, 53, 29–34. [Google Scholar] [CrossRef]
- Ferré, B.M.I.; Drozdzynska, M.; Vettorato, E. Ultrasound-Guided Bilateral Erector Spinae Plane Block in Dogs Undergoing Sternotomies Anaesthetised with Propofol-Dexmedetomidine Continuous Infusion. Vet. Res. Commun. 2022, 46, 1331–1337. [Google Scholar] [CrossRef]
- Bartholomew, K.J.; Ferreira, T.H. Ultrasound-Guided Erector Spinae Plane Block as Part of a Multimodal Analgesic Approach in a Dog with Acute Pancreatitis. Vet. Anaesth. Analg. 2021, 48, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Baba, L.H.K.; Elrod, S.M.; Johnson, J.A.; Clark-Price, S.C.; Hofmeister, E.H. Comparison of Ultrasound-Guided Sciatic Nerve Staining with Methylene Blue or Blue Tissue Marker. Vet. Anaesth. Analg. 2025, 52, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Onishi, E.; Toda, N.; Kameyama, Y.; Yamauchi, M. Comparison of Clinical Efficacy and Anatomical Investigation between Retrolaminar Block and Erector Spinae Plane Block. BioMed Res. Int. 2019, 2019, 2578396. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Flores, S.; Soler, M.; Gil, F.; Polo-Paredes, G.; Laredo, F.G.; Agut, A.; Belda, E. Assessment of an Ultrasound-Guided Longitudinal Approach to the Thoracic Erector Spinae Plane Block in Cat Cadavers: Description of Dye and Contrast Medium Distribution. Animals 2025, 15, 3311. https://doi.org/10.3390/ani15223311
Carrillo-Flores S, Soler M, Gil F, Polo-Paredes G, Laredo FG, Agut A, Belda E. Assessment of an Ultrasound-Guided Longitudinal Approach to the Thoracic Erector Spinae Plane Block in Cat Cadavers: Description of Dye and Contrast Medium Distribution. Animals. 2025; 15(22):3311. https://doi.org/10.3390/ani15223311
Chicago/Turabian StyleCarrillo-Flores, Sara, Marta Soler, Francisco Gil, Gonzalo Polo-Paredes, Francisco G. Laredo, Amalia Agut, and Eliseo Belda. 2025. "Assessment of an Ultrasound-Guided Longitudinal Approach to the Thoracic Erector Spinae Plane Block in Cat Cadavers: Description of Dye and Contrast Medium Distribution" Animals 15, no. 22: 3311. https://doi.org/10.3390/ani15223311
APA StyleCarrillo-Flores, S., Soler, M., Gil, F., Polo-Paredes, G., Laredo, F. G., Agut, A., & Belda, E. (2025). Assessment of an Ultrasound-Guided Longitudinal Approach to the Thoracic Erector Spinae Plane Block in Cat Cadavers: Description of Dye and Contrast Medium Distribution. Animals, 15(22), 3311. https://doi.org/10.3390/ani15223311







