A New Species of Boulenophrys (Anura, Megophryidae) from Northern Jiangxi, China †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
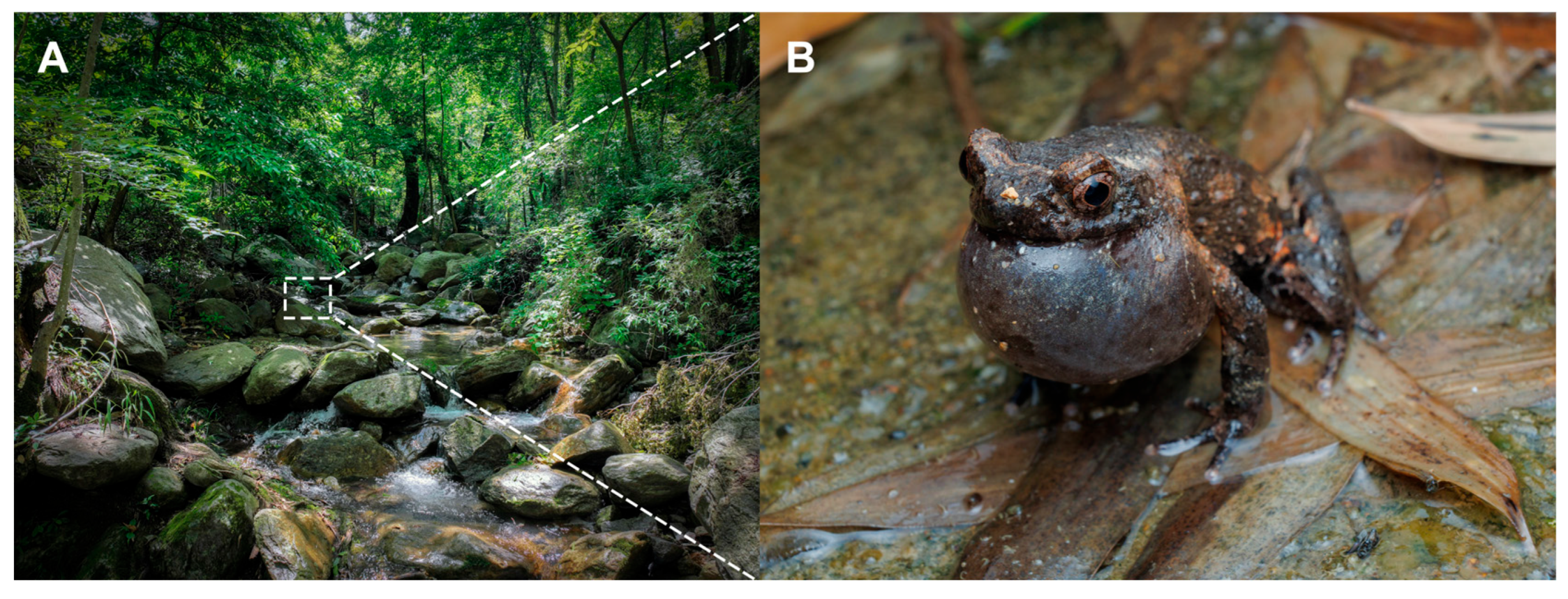
2.1. Sampling
2.2. Morphological Analyses
2.3. DNA Sequencing and Molecular Analyses
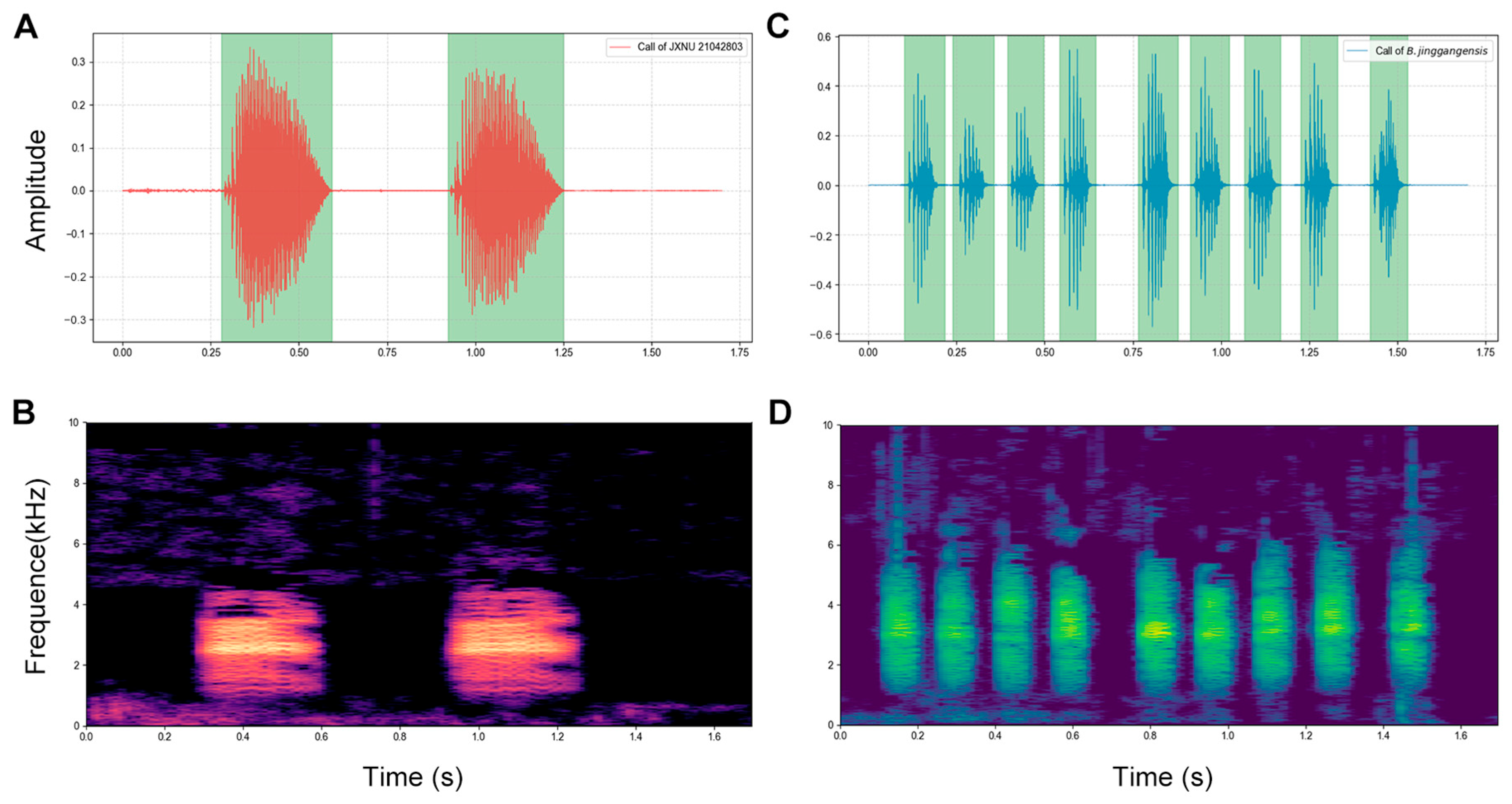
2.4. Acoustic Analyses
3. Results
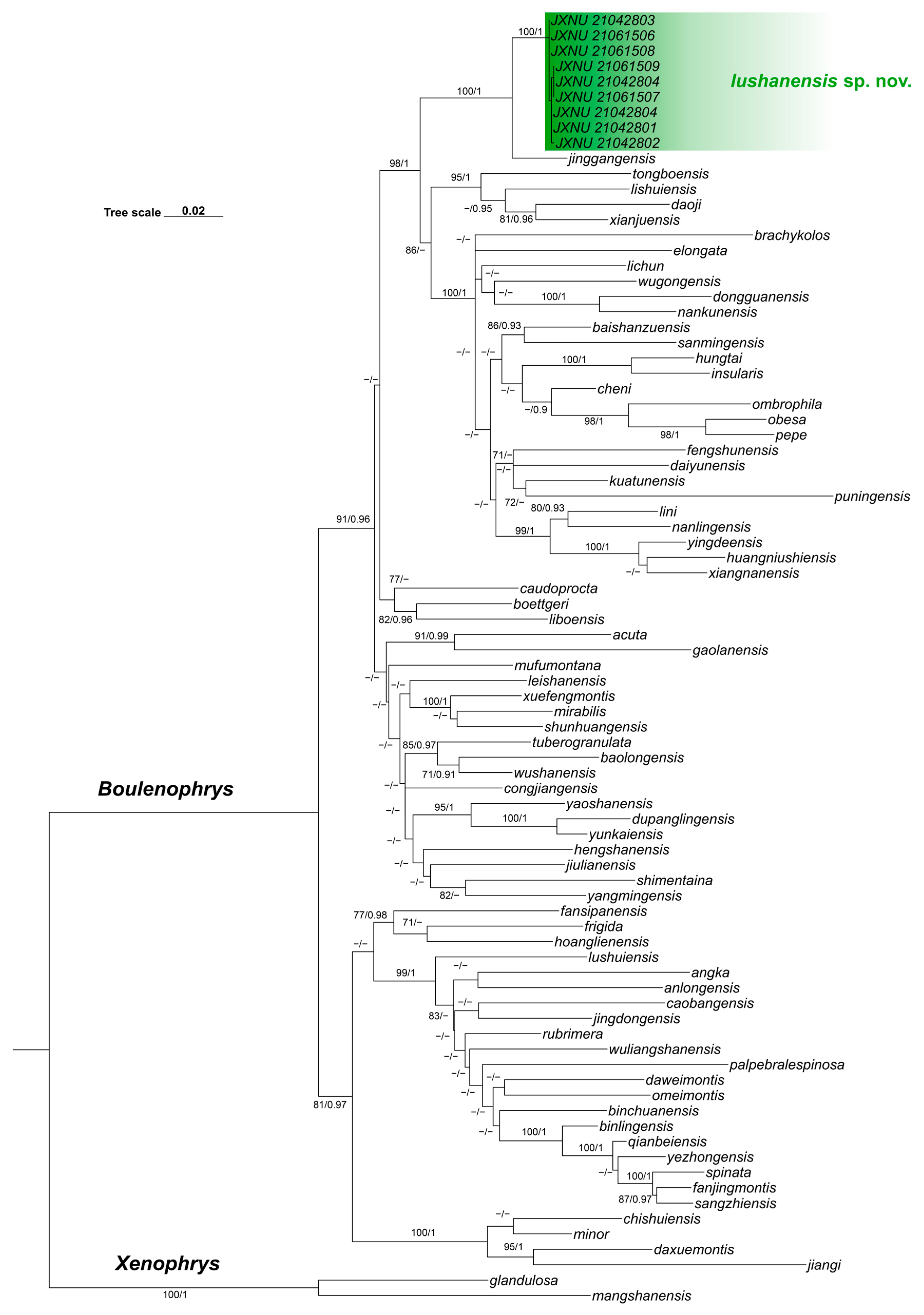
3.1. Phylogenetic Analyses and Genetic Divergence
3.2. Taxonomic Account
| JXNU 21042801 | JXNU 21042802 | JXNU 21042803 | JXNU 21042804 | JXNU 21061505 | JXNU 21061506 | JXNU 21061507 | JXNU 21061508 | JXNU 21061509 | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Male | Female | Female | Female | Male | Male | Male |
| SVL | 50.2 | 44.7 | 43 | 50.3 | 46 | 46.3 | 41.1 | 40.5 | 42.7 |
| HL | 16.8 | 14.7 | 13.7 | 16.5 | 15.3 | 15.2 | 14 | 13.7 | 14.3 |
| HW | 16.7 | 14.4 | 14.2 | 16.9 | 15.6 | 15.8 | 14.4 | 14.7 | 14.6 |
| SL | 4.7 | 4.6 | 4.5 | 4.7 | 4.6 | 4.8 | 4.3 | 4.5 | 4.5 |
| IND | 4.2 | 3.6 | 3.6 | 3.9 | 3.8 | 3.9 | 3.7 | 3.8 | 3.6 |
| IOD | 4 | 3.5 | 3.8 | 4.1 | 3.5 | 3.6 | 3.7 | 3.5 | 3.4 |
| ED | 5.3 | 4.9 | 4.9 | 5.3 | 5 | 4.9 | 4.6 | 4.3 | 5.1 |
| TD | 3.4 | 3.3 | 2 | 2.9 | 2.6 | 2.6 | 1.7 | 2.2 | 2.6 |
| TED | 2.2 | 1.5 | 1.9 | 2.1 | 2 | 2.3 | 2 | 1.8 | 1.7 |
| HNL | 10.9 | 9.6 | 9.9 | 10.7 | 9.9 | 10.1 | 9.5 | 9.2 | 9.2 |
| FAL | 11.9 | 10.7 | 9.9 | 11.3 | 11.4 | 10.5 | 10.2 | 10.2 | 10.4 |
| TL | 21 | 18.7 | 18.2 | 20.2 | 20.3 | 19.5 | 18 | 18 | 17 |
| FTL | 19.8 | 17.8 | 17.7 | 18.8 | 18.4 | 17.6 | 16.8 | 16.9 | 16.6 |
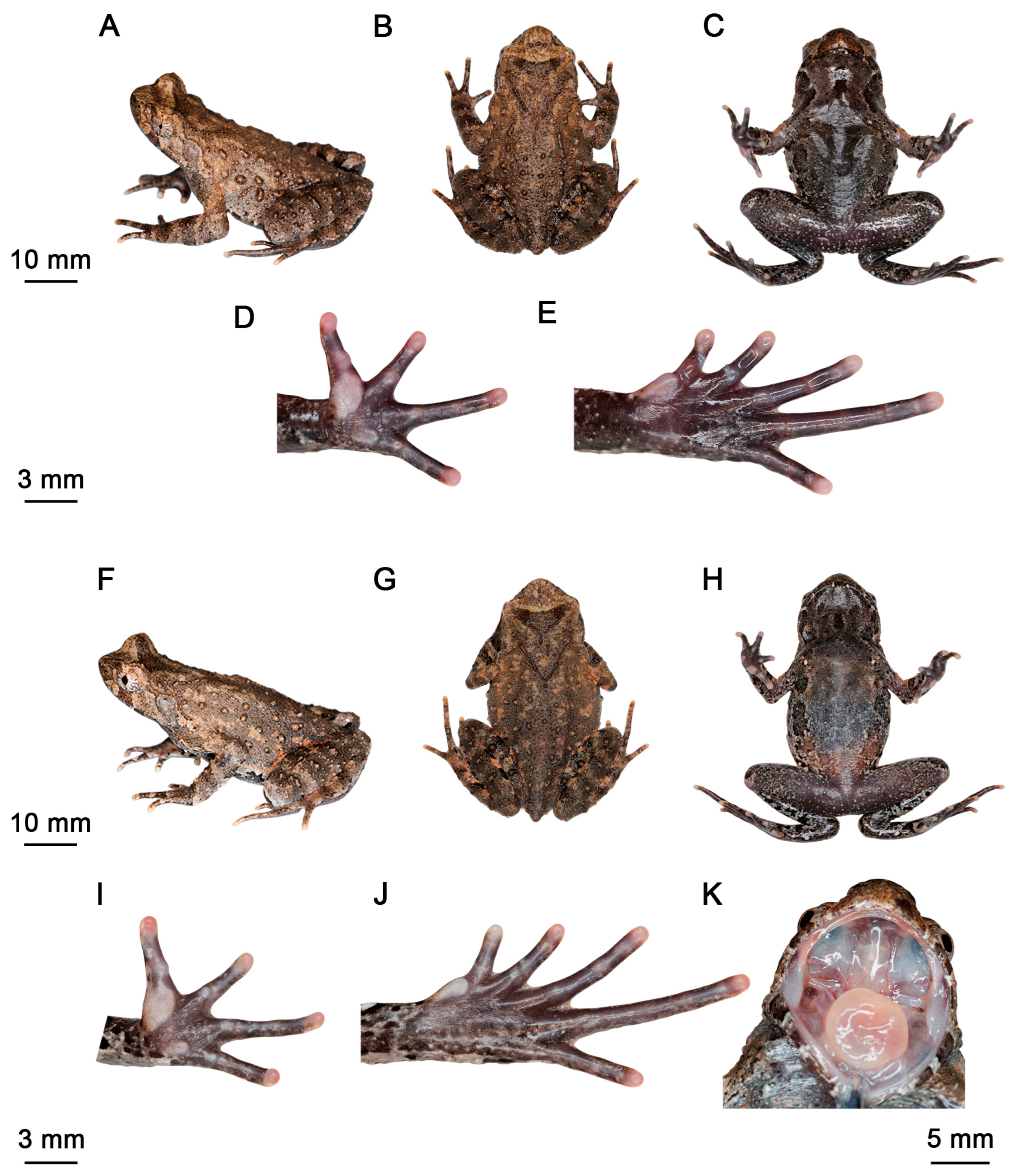
3.3. Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AmphibiaWeb: Information on Amphibian Biology and Conservation, University of California, Berkeley, California. Available online: http://amphibiaweb.org/ (accessed on 17 October 2025).
- AmphibiaChina: The Database of Chinese Amphibians. Kunming Institute of Zoology (CAS), Kunming, Yunnan, China. Available online: http://www.amphibiachina.org/ (accessed on 17 October 2025).
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Available online: https://amphibiansoftheworld.amnh.org (accessed on 17 October 2025).
- Chen, J.M.; Zhou, W.W.; Poyarkov, N.J.; Stuart, B.L.; Brown, R.M.; Lathrop, A.; Wang, Y.Y.; Yuan, Z.Y.; Jiang, K.; Hou, M.; et al. A novel multilocus phylogenetic estimation reveals unrecognized diversity in Asian horned toads, genus Megophrys sensu lato (Anura: Megophryidae). Mol. Phylogenetics Evol. 2017, 106, 28–43. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Chen, G.L.; Zhu, T.Q.; Zeng, Z.C.; Lyu, Z.T.; Wang, J.; Messenger, K.; Greenberg, A.J.; Guo, Z.; Yang, Z.H.; et al. Prevalence of cryptic species in morphologically uniform taxa—Fast speciation and evolutionary radiation in Asian frogs. Mol. Phylogenetics Evol. 2018, 127, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Mahony, S.; Foley, N.M.; Biju, S.D.; Teeling, E.C. Evolutionary History of the Asian Horned Frogs (Megophryinae): Integrative Approaches to Timetree Dating in the Absence of a Fossil Record. Mol. Biol. Evol. 2017, 34, 744–771. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.T.; Qi, S.; Wang, J.; Zhang, S.Y.; Zhao, J.; Zeng, Z.C.; Wan, H.; Yang, J.H.; Mo, Y.M.; Wang, Y.Y. Generic classification of Asian horned toads (Anura: Megophryidae: Megophryinae) and monograph of Chinese species. Zool. Res. 2023, 44, 380–450. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, Y.H.; Zhou, W.W.; Chen, H.M.; Zhang, B.L.; Chen, J.M.; Xu, W.; Rao, D.Q.; Zhao, H.; Yan, F.; et al. Hidden hotspots of amphibian biodiversity in China. Proc. Natl. Acad. Sci. USA 2024, 121, e2320674121. [Google Scholar] [CrossRef]
- Pope, C.H. Notes on Amphibians from Fukien, Hainan, and Other Parts of China. Bull. Am. Mus. Nat. Hist. 1931, 61, 397–611. [Google Scholar]
- Boulenger, G.A. On a collection of reptiles and batrachians made by Mr. J. D. La Touche in N.W. Fokien, China. Proc. Zool. Soc. Lond. 1899, 1899, 159–172. [Google Scholar]
- Yang, D.D.; Gu, Y.L.; Liu, S.; Xiong, J.L.; Wang, L.; Hu, S.C. Survey and Evaluation of Amphibian Resources in Lushan Nature Reserve of Jiangxi Province. Sichuan J. Zool. 2007, 26, 362–364. [Google Scholar]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica, Amphibia, Volume 2, Anura; Science Press: Beijing, China, 2009; p. 957. [Google Scholar]
- Fei, L.; Ye, C.Y. Amphibians of China; Chengdu Institute of Biology Chinese Academy of Sciences: Chengdu, China; Science Press: Beijing, China, 2016; Volume 1. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcot, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- McFee, B.; Raffel, C.; Liang, D.; Ellis, D.P.W.; McVicar, M.; Battenberg, E.; Nieto, O. librosa: Audio and music signal analysis in python. In Proceedings of the 14th Python in Science Conference, Austin, TX, USA, 6–12 July 2015. [Google Scholar]
- Tapley, B.; Cutajar, T.; Nguyen, L.T.; Portway, C.; Mahony, S.; Nguyen, C.T.; Harding, L.; Luong, H.V.; Rowley, J.J.L. A new potentially endangered species of Megophrys (Amphibia: Megophryidae) from Mount Ky Quan San, north west Vietnam. J. Nat. Hist. 2021, 54, 2543–2575. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, Z.; Zhou, J.; Zhou, J.; Wang, W. The Evolutionary Process and Mechanism of Cultural Landscapes: An Integrated Perspective of Landscape Ecology and Evolutionary Economic Geography. Land 2022, 11, 2062. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhang, H.; Yan, P.; Xue, H.; Wu, X. Vicariance and Its Impact on the Molecular Ecology of a Chinese Ranid Frog Species Complex (Odorrana schmackeri, Ranidae). PLoS ONE 2015, 10, e0138757. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, Z.T.; Liu, Z.Y.; Liao, C.K.; Zeng, Z.C.; Zhao, J.; Li, Y.L.; Wang, Y.Y. Description of six new species of the subgenus Panophrys within the genus Megophrys (Anura, Megophryidae) from southeastern China based on molecular and morphological data. Zookeys 2019, 851, 113–164. [Google Scholar] [CrossRef]
- Qi, S.; Lyu, Z.T.; Wang, J.; Mo, Y.M.; Zeng, Z.C.; Zeng, Y.J.; Dai, K.Y.; Li, Y.Q.; Grismer, L.L.; Wang, Y.Y. Three new species of the genus Boulenophrys (Anura, Megophryidae) from southern China. Zootaxa 2021, 5072, 401–438. [Google Scholar] [CrossRef]
- Song, H.; Wang, H.; Qi, S.; Wang, N.; Wang, Y. A New Species of the Genus Boulenophrys (Anura: Megophryidae: Megophryinae) from Zhuhai, Guangdong, China. Asian Herpetol. Res. 2024, 15, 251–264. [Google Scholar] [CrossRef]
- Mayr, E. Populations, Species, and Evolution: An Abridgment of Animal Species and Evolution; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1970; p. 279. [Google Scholar]
- Qian, T.Y.; Hu, K.; Mo, X.Y.; Gao, Z.W.; Zhang, N.; Yang, D.D. A new species of Boulenophrys from central Hunan Province, China (Anura: Megophryidae). Vertebr. Zool. 2023, 73, 915–930. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.Z.; Wei, G.; Xu, N.; Cheng, Y.L.; Wang, B.; Wu, J. A New Species of the Asian Toad Genus Megophrys sensu lato (Anura: Megophryidae) from Guizhou Province, China. Asian Herpetol. Res. 2020, 11, 1–18. [Google Scholar]
- Luo, T.; Wang, Y.; Wang, S.; Lu, X.; Wang, W.; Deng, H.; Zhou, J. A species of the genus Panophrys (Anura, Megophryidae) from southeastern Guizhou Province, China. Zookeys 2021, 1047, 27–60. [Google Scholar] [CrossRef]
- Li, Y.L.; Jin, M.J.; Zhao, J.; Liu, Z.Y.; Wang, Y.Y.; Pang, H. Description of two new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Heishiding Nature Reserve, Fengkai, Guangdong, China, based on molecular and morphological data. Zootaxa 2014, 3795, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Suwannapoom, C.; Poyarkov, N.A., Jr.; Pawangkhanant, P.; Xu, K.; Jin, J.Q.; Murphy, R.W.; Che, J. A new species of the genus Xenophrys Anura Megophryidae from northern Thailand. Zool. Res. 2019, 40, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.D.; Lyu, Z.T.; Wang, J.; Li, Y.L.; Liu, Z.Y.; Chen, H.H.; Rao, D.Q.; Jin, Z.F.; Zhang, C.Y.; et al. Review of the genus Brachytarsophrys (Anura: Megophryidae), with revalidation of Brachytarsophrys platyparietus and description of a new species from China. Zool. Res. 2020, 41, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.Y.; Fei, L.; Xie, F. A new species of Megophryidae—Megophyrys baolongensis from China (Amphibia, Anura). Acta Herpetol. Sin. 2007, 11, 38–41. [Google Scholar]
- Nguyen, T.Q.; Pham, C.T.; Nguyen, T.T.; Luong, A.M.; Ziegler, T. A new species of Megophrys (Amphibia: Anura: Megophryidae) from Vietnam. Zootaxa 2020, 4722, 401–422. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, D.D.; Mo, X.Y.; Li, H.H.; Chen, D. The Fauna of Hunan: Amphibia; Hunan Science and Technology Press: Changsha, China, 2014. [Google Scholar]
- Wang, Y.Y.; Zhao, J.; Yang, J.H.; Zhou, Z.; Chen, G.L.; Liu, Y. Morphology, molecular genetics, and bioacoustics support two new sympatric Xenophrys toads (Amphibia: Anura: Megophryidae) in southeast China. PLoS ONE 2014, 9, e93075. [Google Scholar] [CrossRef]
- Xu, N.; Li, S.Z.; Liu, J.; Wei, G.; Wang, B. A new species of the horned toad Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 943, 119–144. [Google Scholar]
- Lyu, Z.T.; Zeng, Z.C.; Wang, J.; Liu, Z.Y.; Huang, Y.Q.; Li, W.Z.; Wang, Y.Y. Four new species of Panophrys (Anura, Megophryidae) from eastern China, with discussion on the recognition of Panophrys as a distinct genus. Zootaxa 2021, 4927, 9–40. [Google Scholar] [CrossRef]
- Zeng, Z.C.; Wang, J.; Chen, H.H.; Xiao, W.W.; Zhan, B.B.; Li, Y.H.; Lin, S.S. A New Species of the Genus Boulenophrys (Anura, Megophryidae) from Eastern Guangdong, China. Asian Herpetol. Res. 2024, 15, 12–21. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, L.; Ran, H.; Shen, Z.X. Megophrys binlingensis fanjingmontis: A New Subspecies of Megophryidae from Guizhou, China. Chin. J. Zool. 2012, 47, 135–138. [Google Scholar]
- Tapley, B.; Cutajar, T.; Nguyen, L.T.; Nguyen, C.T.; Harding, L.; Portway, C.; Van Luong, H.; Rowley, J.J. A new locality and elevation extension for Megophrys rubrimera (Tapley et al., 2017) in Bat Xat Nature Reserve, Lao Cai Province, northern Vietnam. Herpetol. Notes 2018, 11, 865–868. [Google Scholar]
- Wang, J.; Zeng, Z.C.; Lyu, Z.T.; Qi, S.; Liu, Z.Y.; Chen, H.H.; Lu, Y.H.; Xiao, H.W.; Lin, C.R.; Chen, K.; et al. Description of three new Boulenophrys species from eastern Guangdong, China, emphasizing the urgency of ecological conservation in this region (Anura, Megophryidae). Zootaxa 2022, 5099, 91–119. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.; Mahony, S.; Nguyen, C.T.; Dau, V.Q.; Luong, A.M.; Le, D.T.; Nguyen, T.T.; Nguyen, T.Q.; Portway, C.; et al. Two new and potentially highly threatened Megophrys Horned frogs (Amphibia: Megophryidae) from Indochina’s highest mountains. Zootaxa 2018, 4508, 301–333. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.Y.; Lyu, Z.T.; Zeng, Z.C.; Wang, Y.Y. A new species of the genus Xenophrys (Amphibia: Anura: Megophryidae) from an offshore island in Guangdong Province, southeastern China. Zootaxa 2017, 4324, 541–556. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, T.D.; Zhao, J.; Sung, Y.H.; Yang, J.H.; Pang, H.; Zhang, Z. Description of a new species of the genus Xenophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Jinggang, China, based on molecular and morphological data. Zootaxa 2012, 3546, 53–67. [Google Scholar] [CrossRef]
- Li, S.Z.; Xu, N.; Liu, J.; Jiang, J.P.; Wei, G.; Wang, B. A New Species of the Asian Toad Genus Megophrys sensu lato (Amphibia: Anura: Megophryidae) from Guizhou Province, China. Asian Herpetol. Res. 2018, 9, 224–239E. [Google Scholar]
- Lin, S.S.; Chen, H.H.; Li, Y.H.; Peng, Z.N.; Zeng, Z.C.; Wang, J. A new Boulenophrys species (Anura, Megophryidae) from the coastal hills of eastern Fujian Province, China. Zookeys 2024, 1216, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.C.; Li, D.H.; Zhu, W.B.; Wang, J.; Jiang, J.P.; Ye, C.Y.; Fei, L.; Wang, B. Description of a new toad of Megophrys Kuhl & Van Hasselt, 1822 (Amphibia: Anura: Megophryidae) from western Yunnan Province, China. Zootaxa 2021, 4942, 351–381. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Li, G.; Xiao, N.; Li, J.Q.; Pan, T.; Wang, H.; Zhang, B.W.; Zhou, J. A New Species of the Genus Xenophrys (Amphibia: Anura: Megophryidae) from Libo County, Guizhou, China. Asian Herpetol. Res. 2017, 8, 75–85. [Google Scholar]
- Wang, L.; Deng, X.J.; Liu, Y.; Wu, Q.Q.; Liu, Z. A new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Hunan, China. Zootaxa 2019, 4695, 301–330. [Google Scholar] [CrossRef]
- Lyu, Z.T.; Li, Y.Q.; Zeng, Z.C.; Zhao, J.; Liu, Z.Y.; Guo, G.X.; Wang, Y.Y. Four new species of Asian horned toads (Anura, Megophryidae, Megophrys) from southern China. ZooKeys 2020, 942, 105–140. [Google Scholar] [CrossRef] [PubMed]
- Messenger, K.R.; Dahn, H.A.; Liang, Y.; Xie, P.; Wang, Y.; Lu, C. A new species of the genus Megophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Wuyi, China. Zootaxa 2019, 4554, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, S.S.; Gan, J.S.; Chen, H.H.; Yu, L.M.; Pan, Z.; Xiao, J.J.; Zeng, Z.C. A new species of the genus Boulenophrys from South China (Anura, Megophryidae). Zootaxa 2024, 5514, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Su, H.J.; Shi, S.C.; Wu, Y.Q.; Li, G.R.; Yao, X.G.; Wang, B.; Li, S.Z. Description of a new horned toad of Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 974, 131–159. [Google Scholar]
- Tapley, B.; Cutajar, T.; Mahony, S.; Nguyen, C.T.; Dau, V.Q.; Nguyen, T.T.; Luong, H.V.; Rowley, J.J.L. The Vietnamese population of Megophrys kuatunensis (Amphibia: Megophryidae) represents a new species of Asian horned frog from Vietnam and southern China. Zootaxa 2017, 4344, 465–492. [Google Scholar] [CrossRef]
- Jiang, J.P.; Ye, C.Y.; Fei, L. A New Horn Toad Megophrys sangzhiensis from Hunan, China (Amphibia, Anura). Zool. Res. 2008, 29, 219–222. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Gu, X.M.; Sun, A.Q. A new species of Xenophrys in China (Amphibia: Pelobatidae). Acta Zootaxonomica Sin. 2000, 25, 462–466. [Google Scholar]
- Mo, X.Y.; Shen, Y.H.; Li, H.H.; Wu, X.S. A new species of Megophrys (Amphibia: Anura: Megophryidae) from the northwestern Hunan Province, China. Curr. Zool. 2010, 56, 432–436. [Google Scholar] [CrossRef]
- Fei, L. Atlas of Amphibians in China; Field Edition; Henan Science and Technology Press: Zhengzhou, China, 2020; p. 432. [Google Scholar]
- Wang, B.; Wu, Y.Q.; Peng, J.W.; Shi, S.C.; Lu, N.N.; Wu, J. A new Megophrys Kuhl & Van Hasselt (Amphibia, Megophryidae) from southeastern China. Zookeys 2020, 904, 35–62. [Google Scholar]
- Xiao, B.; Xi, J.; Shi, S.; Li, H.; Zhu, L.; Maimaiti, A.; Xu, Y.; Liao, S.; Wang, B.; Mo, X. A New Species of the Genus Boulenophrys (Anura, Megophryidae) from Southern Hunan Province, Central China. Animals 2025, 15, 440. [Google Scholar] [CrossRef]
- Kuang, D.Y.; Wei, W.F.; Luo, Y.S.; Pei, K.W.; Cao, Y.Y.; Zhang, M.; Huang, T.F.; Pu, L.; Shi, S. A new species of Boulenophrys (Megophryidae) from Mt. Hengshan, Hunan Province, China, with re-description of B. hengshanensis. Animals 2025, 15, 2745. [Google Scholar] [CrossRef]
- Li, S.; Shi, S.; Liu, J.; Zhao, J.; Gao, S.; Wang, B. A new species of the genus Boulenophrys (Anura, Megophryidae) from Hubei, China. Zoosystematics Evol. 2025, 101, 1213–1226. [Google Scholar] [CrossRef]
- Liu, J.; Feng, C.B.; Shen, T.; Li, S.Z.; Cheng, Y.L.; Wei, G.; Wang, B.; Su, H.J. A new species of the genus Boulenophrys (Anura, Megophryidae) from Guizhou, China. Herpetozoa 2025, 38, 117–136. [Google Scholar] [CrossRef]
- Wang, H.T.; Wu, X.G.; Song, H.M.; Huang, Z.; Zhuo, X.H.; Wang, Y.Y. A new species of the genus Boulenophrys (Anura: Megophryidae: Megophryinae) from Jiulianshan National Nature Reserve, southern Jiangxi, China. Zootaxa 2025, 5691, 376–398. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Chen, J.; Huang, H.; Gao, X.; Chen, B.; Liu, N. A new horned toad of Boulenophrys (Anura: Megophryidae) discovered in Hubei Province, China. ZooKeys 2025, 1235, 305–320. [Google Scholar] [CrossRef]

| Species | SVL in Males (in mm) | SVL in Females (in mm) | Horn-Like Tubercle at Upper Eyelid: Larger (T); Smaller (F) | Vomerine Teeth: Present (T); Absent (F) | Tongue: Notched(T); Not Notched (F) | Heels Over- Lapping: (T) Just Meeting (N); Not Meeting (F) | Lateral Fringes on Toes: Wide (T); Narrow (N); Absent (F) | Webs on Toes: One-Fourth (T); Rudimentary(N); Lacking (F) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | B. lushanensis sp. nov. | 40.5–44.7 | 46.0–50.2 | F | T | F | F | N | N |
| 2 | B. acuta | 27.1–33.0 | 28.1–33.6 | T | F | F | F | N | N |
| 3 | B. angka | 31.2–32.1 | 37.5–39.2 | F | F | F | T/N | F | N |
| 4 | B. anlongensis | 40.0–45.5 | 48.9–51.2 | F | F | F | T | N | N |
| 5 | B. baishanzuensis | 28.4–32.4 | / | F | F | F | T | N | F |
| 6 | B. baolongensis | 42.0–45.0 | / | F | F | T | N | F | F |
| 7 | B. binchuanensis | 32.0–36.0 | 40.2–42.5 | F | F | T/F | N | T | N |
| 8 | B. binlingensis | 45.1–51.0 | / | F | F | T | T | / | N |
| 9 | B. boettgeri | 34.5–37.8 | 39.7–46.8 | F | F | T | N | T | N |
| 10 | B. brachykolos | 33.7–39.3 | 33.9–45.9 | F | F | F | F | F | N |
| 11 | B. caobangensis | 34.9–38.9 | / | F | F | F | / | F | N |
| 12 | B. caudoprocta | 81.3 | / | T | T | F | N | / | N |
| 13 | B. congjiangensis | 28.6–33.4 | 38.4–40.2 | F | F | F | T | N | N |
| 14 | B. changyangensis | 39.4–43.3 | / | F | F | F | T | F | F |
| 15 | B. cheni | 26.2–29.5 | 31.8–34.1 | F | F | T | T | T | N |
| 16 | B. chishuiensis | 43.4–44.1 | 44.8–49.8 | F | F | F | T | F | N |
| 17 | B. daiyunensis | 27.6–28.7 | 33.7–35.6 | F | T | F | T/N | N | N |
| 18 | B. daoji | 32.6–33.6 | 37.5–41.4 | F | F | F | F | N | N |
| B. dalaolingensis | 49.9–56.2 | 50.3–60.0 | F | F | T | T | T | T | |
| 19 | B. fanjingmontis | 58.2–63.6 | 62.8–72.2 | F | T | T | N | T | N |
| 20 | B. dongguanensis | 30.2–39.3 | / | F | T | F | F | F | N |
| 21 | B. dupanglingensis | 37.8–40.2 | 41.8–45.9 | F | F | F | T | F | N |
| 22 | B. elongata | 28.2–28.5 | 35.1–37.6 | F | T | F | N | N | F |
| 23 | B. hoanglienensis | 37.4–47.6 | 59.6 | F | T | T | / | F | N/F |
| B. huangnniushienensis | 37.9–40.7 | / | F | F | F | T/N | F | F | |
| 24 | B. fansipanensis | 30.9–44.3 | 41.7–42.5 | F | T | T/F | / | F | F |
| 25 | B. fengshunensis | 34.3–39.4 | 42.5–44.9 | F | T | F | F | F | N |
| 26 | B. frigida | 30.3–31.8 | / | F | T | T/F | / | F | F |
| 27 | B. gaolanensis | 29.2–30.9 | 32.0–34.9 | F | F | F | F | F | F |
| B. gutu | 34.4–38.0 | 48.4 | F | F | F | F | N | N | |
| 28 | B.hengshanensis | 35.7–41.2 | 37.5–50.2 | F | F | F | F | F | F |
| 29 | B. jingdongensis | 53.0–56.5 | 63.5 | F | T | T | T | T | T |
| 30 | B. hungtai | 25.8–33.3 | / | F | F | F | F | F | F |
| 31 | B. insularis | 36.8–41.2 | 47.1 | F | T | T | F | F | N |
| 32 | B. jiangi | 34.4–39.2 | 39.5–40.4 | F | F | F | T | F | N |
| 33 | B. jiulianensis | 30.4–33.9 | 34.1–37.5 | F | T | T | T | F | N |
| 34 | B. jinggangensis | 35.1–36.7 | 38.4–41.6 | T | T | F | T | N | N |
| 35 | B. liboensis | 60.5–67.7 | 60.8–70.6 | T | T | T | T | T | N |
| 36 | B. kuatunensis | 26.2–31.4 | 26.6–37.3 | F | F | T | T/F | F/N | F |
| 37 | B. leishanensis | 30.4–38.7 | 42.3 | F | F | F | T | F | N |
| 38 | B. lichun | 33.5–37.0 | 47.1 | F | T | F | F | F | F |
| 39 | B. lushuiensis | 31.0–34.8 | / | F | F | T | / | N | N |
| 40 | B. lini | 34.1–39.7 | 37.0–39.9 | F | F | F | T | T | N |
| 41 | B. lishuiensis | 30.7–34.7 | 36.9–40.4 | F | F | F | / | F | F |
| 42 | B. minor | 34.5–41.2 | / | F | F | T | T/N | F | N |
| 43 | B. nanlingensis | 30.5–37.3 | / | F | T | T | T | N | N |
| 44 | B. mirabilis | 55.8–61.4 | 68.5–74.8 | T | F | F | T | N | N |
| 45 | B. mufumontana | 30.1–30.8 | 36.3 | F | F | F | T | N | N |
| 46 | B. nankunensis | 29.9–34.9 | 39.4–41.9 | F | T | F | F | F | N |
| 47 | B. omeimontis | 56.0–59.5 | 68.0–72.5 | F | T | T | T | N | N |
| 48 | B. daxuemontis | 41.2–46.2 | 51.8–58.6 | F | F | F | T | N | N |
| 49 | B. obesa | 35.6 | 37.5–41.2 | F | F | F | F | F | N |
| 50 | B. ombrophila | 27.4–34.5 | 32.8–35.0 | F | F | F | F | F | F |
| 51 | B. qianbeiensis | 49.3–58.2 | / | F | T | T | T | T | T |
| 52 | B. palpebralespinosa | 36.2–38.0 | / | T | T | F | T | T | T |
| 53 | B. pepe | 35.3–36.4 | / | F | F | T | F | F | F |
| 54 | B. puningensis | 31.7–34.6 | 37.8–38.3 | F | T | F | F | F | N |
| 55 | B. sangzhiensis | 54.7 | / | F | T | T | T | N | N |
| 56 | B. rubrimera | 26.6–30.8 | / | F | T | T/F | / | N | F |
| 57 | B. sanmingensis | 27.0–29.5 | 29.5 | F | F | T | T | T | N |
| 58 | B. shuichengensis | 102.0–118.3 | 99.8–115.6 | T | F | T | / | T | T |
| 59 | B. shimentaina | 28.0–30.6 | / | F | T | F | T | N | N |
| 60 | B. spinata | 47.2–54.4 | 54.0–55.0 | F | F | T | T | T | T |
| 61 | B. shunhuangensis | 30.3–33.7 | 37.6 | F | F | F | T | F | F |
| 62 | B. tongboensis | 26.5–31.5 | / | F | T | T | T | F | F |
| 63 | B. daweimontis | 34.0–37.0 | 40.0–46.0 | F | T | / | / | F | F |
| 64 | B. tuberogranulatus | 33.2–39.0 | 50.5 | F | F | F | / | F | N |
| 65 | B. wugongensis | 31.0–34.1 | 38.5–42.8 | F | F | F | F | F | N |
| 66 | B. wuliangshanensis | 27.3–31.6 | 41.3 | F | F | T/F | T | F | F |
| 67 | B. wushanensis | 30.4–35.5 | 38.4 | F | F | F | F | F/T | T |
| 68 | B. xiangnanensis | 38.6–42.0 | 44.4 | F | F | F | N | T | N |
| 69 | B. xianjuensis | 31.0–36.3 | 41.6 | F | F | F | T | N | N |
| 70 | B. xuefengmontis | 37.0–38.3 | 45.3–48.9 | F | F | F | T/N | F | F |
| 71 | B. yaoshanensis | 32.5–42.6 | 46.6–47.4 | F | F | F | T/N | F | N |
| 72 | B. yangmingensis | 33.2–37.1 | 45.2 | F | F | F | T | N | N |
| 73 | B. yezhongensis | 41.2–46.2 | 51.8–58.6 | F | T | F | T | N | N |
| 74 | B. yingdeensis | 33.2–35.3 | 36.3–45.8 | F | T | F | T/N | F | N |
| 75 | B. yunkaiensis | 35.3–40.0 | 45.3–46.1 | F | F | F | T/N | F | N |
| Call Character | Boulenophrys lushanensis sp. nov. | Boulenophrys jinggangensis | ||
|---|---|---|---|---|
| Unvouchered | JXNU21042803 | JXNU21042802 | Unvouchered | |
| Number of call groups measured | 9 | 6 | 7 | 9 |
| Call duration (ms) | 290.6 ± 15.6 (271.3–322.0) | 301.4 ± 12.4 (286.9–322.3) | 298.2 ± 18.1 (267.6–326.6) | 107.0 ± 4.6 (100.3–113.1) |
| Intercall interval (ms) | 737.7 ± 112.6 (623.4–960.3) | 834.4 ± 114.5 (634.3–960.5) | 790.0 ± 124.3 (644.5–969.2) | 165.1 ± 27.1 (140.1–222.9) |
| Call repetition rate (calls/s) | 1.36 | 1.2 | 1.27 | 6.07 |
| Pulses/call | 33–53 | 51–59 | 41–50 | 7–16 |
| Dominant frequency (kHz) | 2.54 ± 0.03 (2.50–2.58) | 2.64 ± 0.04 (2.56–2.68) | 2.56 ± 0.06 (2.48–2.63) | 3.28 ± 0.30 (3.06–3.75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Zhou, H.; Messenger, K.R.; Amin, H.; Wang, Z.; Xu, J.; Xu, S.; Li, Y. A New Species of Boulenophrys (Anura, Megophryidae) from Northern Jiangxi, China. Animals 2025, 15, 3197. https://doi.org/10.3390/ani15213197
Shen D, Zhou H, Messenger KR, Amin H, Wang Z, Xu J, Xu S, Li Y. A New Species of Boulenophrys (Anura, Megophryidae) from Northern Jiangxi, China. Animals. 2025; 15(21):3197. https://doi.org/10.3390/ani15213197
Chicago/Turabian StyleShen, Deming, Haiying Zhou, Kevin R. Messenger, Hina Amin, Zhenyu Wang, Jun Xu, Shi Xu, and Yankuo Li. 2025. "A New Species of Boulenophrys (Anura, Megophryidae) from Northern Jiangxi, China" Animals 15, no. 21: 3197. https://doi.org/10.3390/ani15213197
APA StyleShen, D., Zhou, H., Messenger, K. R., Amin, H., Wang, Z., Xu, J., Xu, S., & Li, Y. (2025). A New Species of Boulenophrys (Anura, Megophryidae) from Northern Jiangxi, China. Animals, 15(21), 3197. https://doi.org/10.3390/ani15213197










