Optimizing Sperm Cryopreservation from Four Endangered Korean Amphibian Species: Species-Specific Effects of Cryoprotectants and Cooling Regimes on Membrane-Integrity Viability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sperm Collection
2.3. Cryoprotectant Preparation
2.4. Sperm Cryopreservation
2.5. Sperm Thawing and Viability Assessment
2.6. Statistical Analysis
3. Results
3.1. Post-Thaw Membrane-Integrity Viability Across Species and Treatments
3.2. Effects of Species, Cryoprotectant, and Cooling Conditions on Post-Thaw Membrane-Integrity Viability
3.3. Inter-Specific Variation and Optimal Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.B.; Falso, P.; Gallipeau, S.; Stice, M. The cause of global amphibian declines: A developmental endocrinologist’s perspective. J. Exp. Biol. 2010, 213, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Yaman, B.; Van Huynh, A. Public attention towards declining global amphibian species. Biol. Conserv. 2024, 290, 110472. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.N.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Cordeiro, I.F.; Lemes, C.G.d.C.; Sanchez, A.B.; da Silva, A.K.; de Paula, C.H.; de Matos, R.C.; Ribeiro, D.F.; de Matos, J.P.; Garcia, C.C.M.; Beirão, M.; et al. Amphibian tolerance to arsenic: Microbiome-mediated insights. Sci. Rep. 2024, 14, 10193. [Google Scholar] [CrossRef]
- Varga, J.F.A.; Bui-Marinos, M.P.; Katzenback, B.A. Frog Skin Innate Immune Defences: Sensing and Surviving Pathogens. Front. Immunol. 2019, 9, 3128. [Google Scholar] [CrossRef]
- Park, J.-K.; Do, Y. Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters. Animals 2023, 13, 3162. [Google Scholar] [CrossRef]
- Lo Parrino, E.; Ficetola, G.F.; Devin, M.; Manenti, R.; Falaschi, M. Integrating adult occurrence and reproduction data to identify conservation measures for amphibians. Conserv. Biol. 2025, 39, e14343. [Google Scholar] [CrossRef]
- Silla, A.J.; Calatayud, N.E.; Trudeau, V.L. Amphibian reproductive technologies: Approaches and welfare considerations. Conserv. Physiol. 2021, 9, coab011. [Google Scholar] [CrossRef]
- Browne, R.K.; Luo, Q.; Wang, P.; Mansour, N.; Kaurova, S.A.; Gakhova, E.N.; Shishova, N.V.; Uteshev, V.K.; Kramarova, L.I.; Venu, G.; et al. The Sixth Mass Extinction and Amphibian Species Sustainability Through Reproduction and Advanced Biotechnologies, Biobanking of Germplasm and Somatic Cells, and Conservation Breeding Programs (RBCs). Animals 2024, 14, 3395. [Google Scholar] [CrossRef]
- Burger, I.J.; Chen, L.-D.; Lampert, S.S.; Kouba, C.K.; Barber, D.; Smith, D.; Cobos, C.; Kouba, A.J. Applying sperm collection and cryopreservation protocols developed in a model amphibian to three threatened anuran species targeted for biobanking management. Biol. Conserv. 2023, 277, 109850. [Google Scholar] [CrossRef]
- Anastas, Z.M.; Byrne, P.G.; O’Brien, J.K.; Hobbs, R.J.; Upton, R.; Silla, A.J. The Increasing Role of Short-Term Sperm Storage and Cryopreservation in Conserving Threatened Amphibian Species. Animals 2023, 13, 2094. [Google Scholar] [CrossRef]
- Taheri-Khas, Z.; Gharzi, A.; Vaissi, S.; Heshmatzad, P.; Kalhori, Z. Advanced sperm preservation techniques in yellow spotted mountain newts Neurergus derjugini enhance genetic management and conservation efforts. Sci. Rep. 2025, 15, 9334. [Google Scholar] [CrossRef] [PubMed]
- Péricard, L.; Le Mével, S.; Marquis, O.; Locatelli, Y.; Coen, L. Intracytoplasmic Sperm Injection Using 20-Year-Old Cryopreserved Sperm Results in Normal, Viable, and Reproductive Offspring in Xenopus laevis: A Major Pioneering Achievement for Amphibian Conservation. Animals 2025, 15, 1941. [Google Scholar] [CrossRef]
- Pizzutto, C.S.; Colbachini, H.; Jorge-Neto, P.N. One Conservation: The Integrated View of Biodiversity Conservation. Anim. Reprod. 2021, 18, e20210024. [Google Scholar] [CrossRef]
- Arregui, L.; Koch, J.C.; Tiersch, T.R. Transitioning from a research protocol to a scalable applied pathway for Xenopus laevis sperm cryopreservation at a national stock center: The effect of cryoprotectants. J. Exp. Zool. Part B Mol. Dev. Evol. 2024, 342, 291–300. [Google Scholar] [CrossRef]
- Pearl, E.; Morrow, S.; Noble, A.; Lerebours, A.; Horb, M.; Guille, M. An optimized method for cryogenic storage of Xenopus sperm to maximise the effectiveness of research using genetically altered frogs. Theriogenology 2017, 92, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Upton, R.; Calatayud, N.E.; Silla, A.J.; Daly, J.; McFadden, M.S.; O’Brien, J.K. Cryopreservation Cooling Rate Impacts Post-thaw Sperm Motility and Survival in Litoria booroolongensis. Animals 2023, 13, 3014. [Google Scholar] [CrossRef] [PubMed]
- Mansour, N.; Lahnsteiner, F.; Patzner, R.A. Optimization of the cryopreservation of African clawed frog (Xenopus laevis) sperm. Theriogenology 2009, 72, 1221–1228. [Google Scholar] [CrossRef]
- Poo, S.; Hinkson, K.M. Applying cryopreservation to anuran conservation biology. Conserv. Sci. Pract. 2019, 1, e91. [Google Scholar] [CrossRef]
- Burger, I.J.; Lampert, S.S.; Kouba, C.K.; Morin, D.J.; Kouba, A.J. Development of an Amphibian Sperm Biobanking Protocol for Genetic Management and Population Sustainability. Conserv. Physiol. 2022, 10, coac032. [Google Scholar] [CrossRef]
- Clulow, J.; Upton, R.; Trudeau, V.L.; Clulow, S. Amphibian Assisted Reproductive Technologies: Moving from Technology to Application. In Reproductive Sciences in Animal Conservation; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 413–463. [Google Scholar]
- Silla, A.J.; Byrne, P.G. The Role of Reproductive Technologies in Amphibian Conservation Breeding Programs. Annu. Rev. Anim. Biosci. 2019, 7, 499–519. [Google Scholar] [CrossRef]
- Moore, A.I.; Squires, E.L.; Bruemmer, J.E.; Graham, J.K. Effect of cooling rate and cryoprotectant on the cryosurvival of equine spermatozoa. J. Equine Vet. Sci. 2006, 26, 215–218. [Google Scholar] [CrossRef]
- Chen, D.M.; Lampert, S.S.; Chen, L.-D.; Burger, I.J.; Kouba, C.K.; Allen, P.J.; Songsasen, N.; Roth, T.L.; Kouba, A.J. Production of live tiger salamander offspring using cryopreserved sperm. Sci. Rep. 2025, 15, 15702. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.; Labbé, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Shishova, N.R.; Uteshev, V.K.; Kaurova, S.A.; Browne, R.K.; Gakhova, E.N. Cryopreservation of hormonally induced sperm for the conservation of threatened amphibians with Rana temporaria as a model research species. Theriogenology 2011, 75, 220–232. [Google Scholar] [CrossRef]
- Butts, I.A.E.; Litvak, M.K.; Kaspar, V.; Trippel, E.A. Cryopreservation of Atlantic cod Gadus morhua L. spermatozoa: Effects of extender composition and freezing rate on sperm motility, velocity and morphology. Cryobiology 2010, 61, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef]
- Glazar, A.I.; Mullen, S.F.; Liu, J.; Benson, J.D.; Critser, J.K.; Squires, E.L.; Graham, J.K. Osmotic tolerance limits and membrane permeability characteristics of stallion spermatozoa treated with cholesterol. Cryobiology 2009, 59, 201–206. [Google Scholar] [CrossRef]
- Müller, K.; Müller, P.; Pincemy, G.; Kurz, A.; Labbe, C. Characterization of Sperm Plasma Membrane Properties after Cholesterol Modification: Consequences for Cryopreservation of Rainbow Trout Spermatozoa. Biol. Reprod. 2008, 78, 390–399. [Google Scholar] [CrossRef]
- Castro, M.; Leal, K.; Pezo, F.; Contreras, M.J. Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species. Animals 2025, 15, 1808. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Badyakar, D.; Chakrabarty, J. Role of Membrane Lipid Fatty Acids in Sperm Cryopreservation. Adv. Androl. 2014, 2014, 190542. [Google Scholar] [CrossRef]
- Upton, R.; Clulow, S.; Mahony, M.J.; Clulow, J. Generation of a sexually mature individual of the Eastern dwarf tree frog, Litoria fallax, from cryopreserved testicular macerates: Proof of capacity of cryopreserved sperm derived offspring to complete development. Conserv. Physiol. 2018, 6, coy043. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.S.; Byrne, P.G.; O’Brien, J.K.; Hobbs, R.J.; Silla, A.J. Antioxidant supplementation of cryopreservation extenders improves post-thaw membrane-integrity viability in the red-crowned toadlet, Pseudophryne australis. Front. Amphib. Reptile Sci. 2025, 3, 1525965. [Google Scholar] [CrossRef]
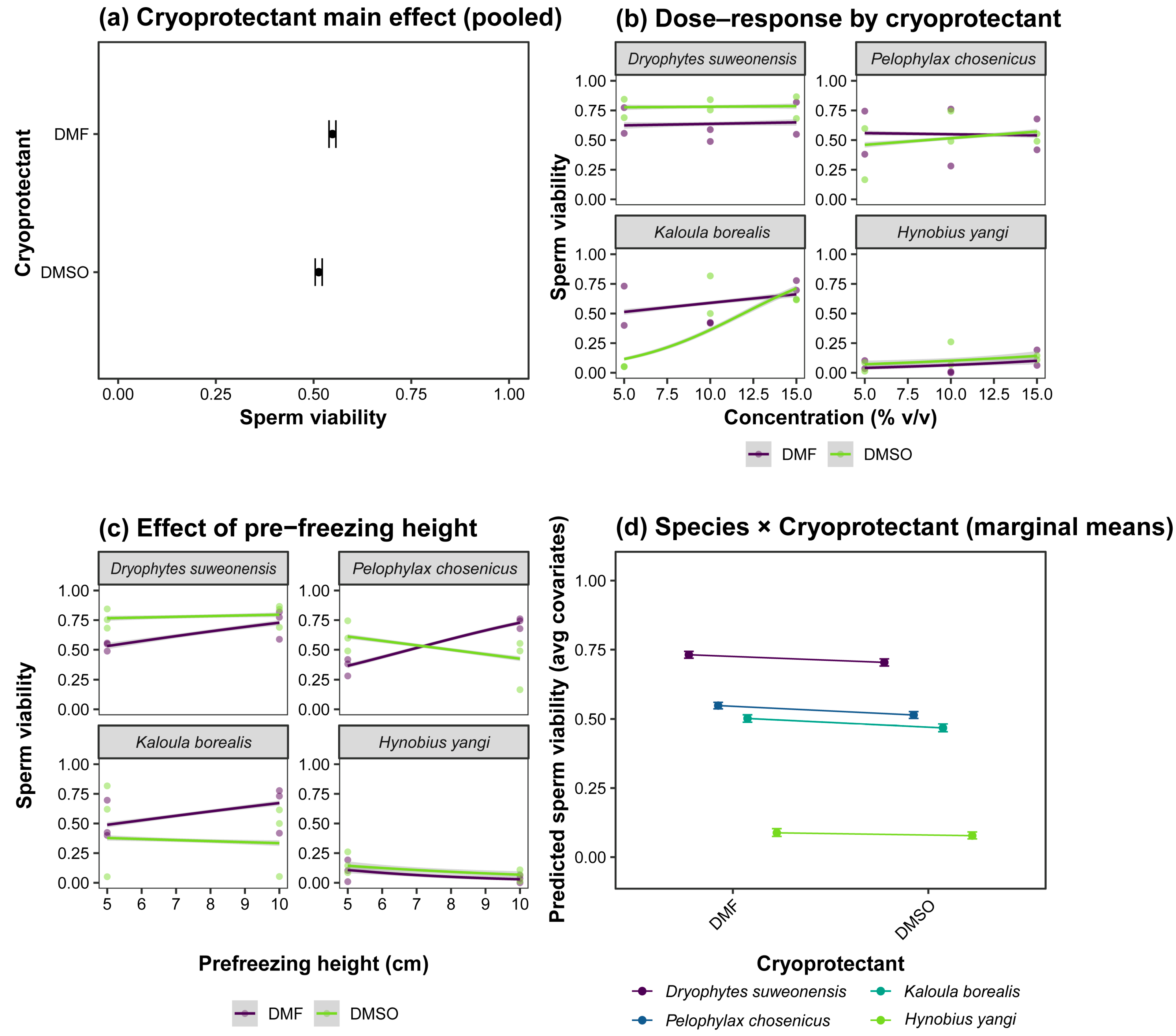
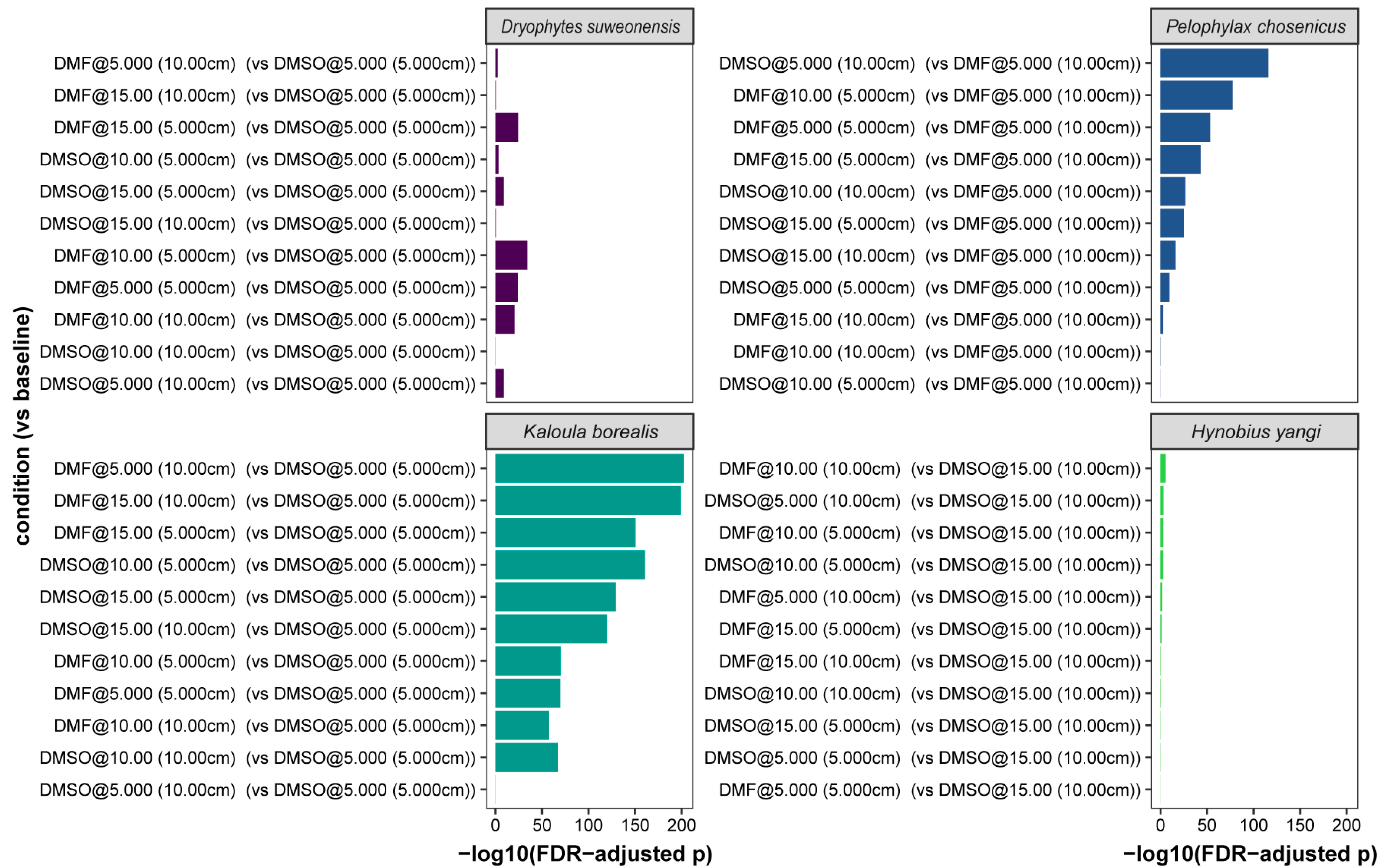
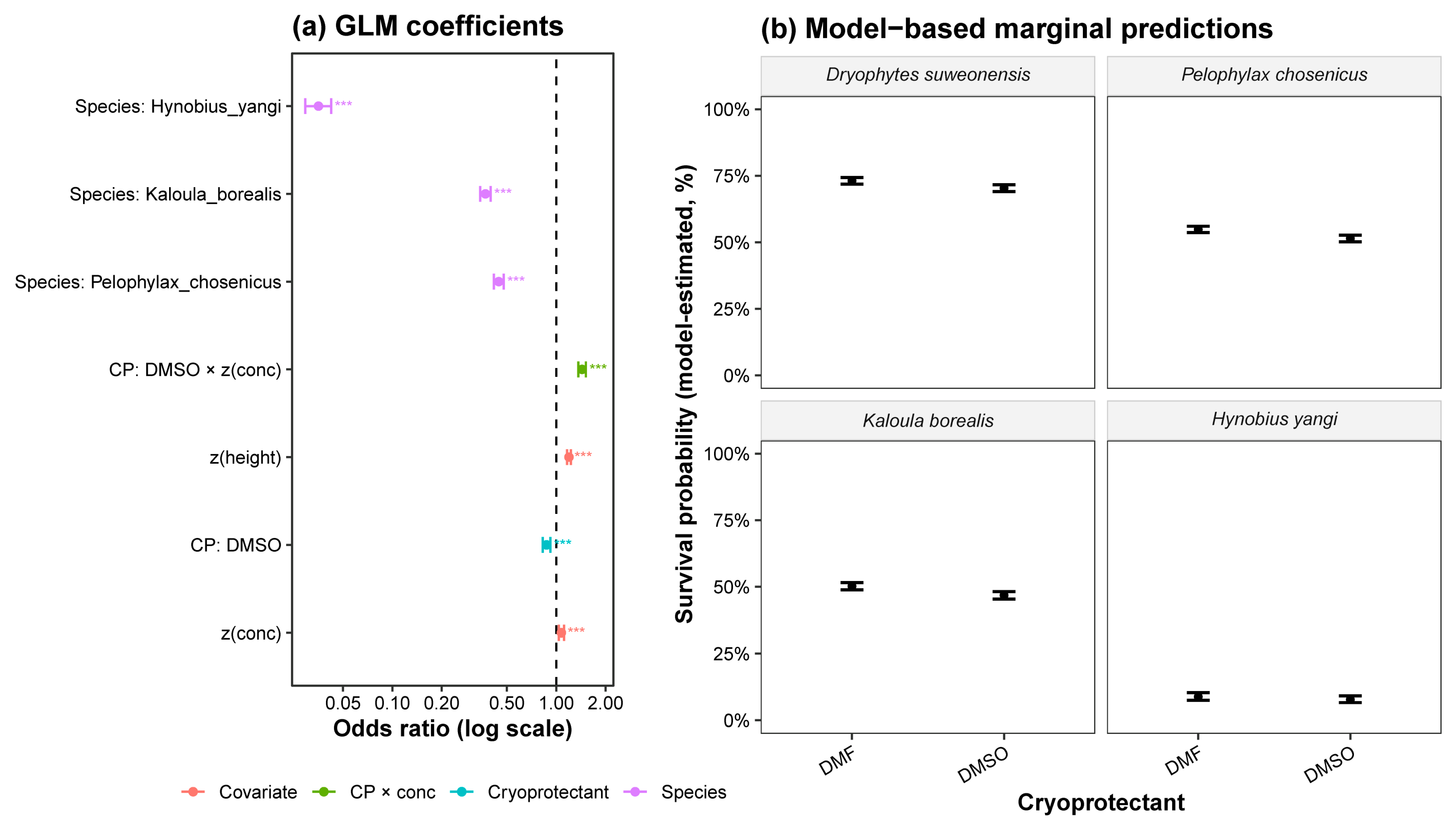
| Species | Alive/Total | Mean Viability (%) ± SE (%) | 95% CI (%) |
|---|---|---|---|
| Dryophytes suweonensis | 99/102 | 97.0% ± 1.7% | 91.7–99.0% |
| Pelophylax chosenicus | 330/343 | 96.2% ± 1.0% | 93.6–97.8% |
| Kaloula borealis | 270/282 | 95.7% ± 1.2% | 92.7–97.5% |
| Hynobius yangi | 252/263 | 95.8% ± 1.2% | 92.7–97.6% |
| Species | Alive/Total | Mean Viability (%) ± SE (%) | 95% CI (%) |
|---|---|---|---|
| Dryophytes suweonensis | 4337/6103 | 71.1% ± 0.6% | 69.9–72.2% |
| Pelophylax chosenicus | 5157/9656 | 53.4% ± 0.5% | 52.4–54.4% |
| Kaloula borealis | 3333/7030 | 47.4% ± 0.6% | 46.2–48.6% |
| Hynobius yangi | 143/1633 | 8.8% ± 0.7% | 7.5–10.2% |
| Species | Condition | vs_Baseline | Condition Mean (%, 95% CI) | Baseline Mean (%, 95% CI) | Odds Ratio [95% CI] | p-Value | FDR-Adjusted p | Significance |
|---|---|---|---|---|---|---|---|---|
| Dryophytes suweonensis | DMF@5.000 (10.00 cm) | DMSO@5.000 (5.000 cm) | 77.4 (73.6 ± 0.7) | 84.4 (81.2–87.2) | 0.63 [0.46–0.86] | 0.003 | 0.004 | ** |
| DMF@15.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 81.9 (78.4 ± 4.9) | 84.4 (81.2–87.2) | 0.83 [0.60–1.16] | 0.264 | 0.331 | ns | |
| DMSO@15.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 86.5 (83.3 ± 9.3) | 84.4 (81.2–87.2) | 1.19 [0.83–1.70] | 0.341 | 0.417 | ns | |
| DMSO@10.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 84.1 (80.8 ± 6.9) | 84.4 (81.2–87.2) | 0.98 [0.70–1.36] | 0.935 | 0.979 | ns | |
| DMF@10.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 58.8 (54.5 ± 3.0) | 84.4 (81.2–87.2) | 0.26 [0.20–0.35] | <0.001 | <0.001 | *** | |
| DMF@10.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 48.8 (44.3 ± 3.4) | 84.4 (81.2–87.2) | 0.18 [0.13–0.24] | <0.001 | <0.001 | *** | |
| DMF@15.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 54.9 (50.2 ± 9.4) | 84.4 (81.2–87.2) | 0.22 [0.17–0.30] | <0.001 | <0.001 | *** | |
| DMF@5.000 (5.000 cm) | DMSO@5.000 (5.000 cm) | 55.7 (51.2 ± 0.1) | 84.4 (81.2–87.2) | 0.23 [0.17–0.31] | <0.001 | <0.001 | *** | |
| DMSO@10.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 75.4 (71.3 ± 9.1) | 84.4 (81.2–87.2) | 0.56 [0.41–0.78] | <0.001 | <0.001 | *** | |
| DMSO@15.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 68.2 (63.9 ± 2.2) | 84.4 (81.2–87.2) | 0.40 [0.29–0.54] | <0.001 | <0.001 | *** | |
| DMSO@5.000 (10.00 cm) | DMSO@5.000 (5.000 cm) | 68.9 (65.0 ± 2.6) | 84.4 (81.2–87.2) | 0.41 [0.30–0.55] | <0.001 | <0.001 | *** | |
| Hynobius yangi | DMF@10.00 (5.000 cm) | DMSO@15.00 (10.00 cm) | 0.9 (0.2 ± 0.1) | 10.9 (7.3–76.1) | 0.08 [0.00–0.49] | <0.001 | 0.001 | ** |
| DMSO@10.00 (5.000 cm) | DMSO@15.00 (10.00 cm) | 26.1 (18.2 ± 5.9) | 10.9 (7.3–76.1) | 2.86 [1.42–5.80] | 0.002 | 0.002 | ** | |
| DMF@5.000 (10.00 cm) | DMSO@15.00 (10.00 cm) | 3.5 (1.5 ± 0.9) | 10.9 (7.3–76.1) | 0.30 [0.09–0.83] | 0.013 | 0.018 | * | |
| DMF@15.00 (5.000 cm) | DMSO@15.00 (10.00 cm) | 19.7 (13.5 ± 6.7) | 10.9 (7.3–76.1) | 1.94 [0.99–3.82] | 0.038 | 0.051 | ns | |
| DMF@15.00 (10.00 cm) | DMSO@15.00 (10.00 cm) | 6.2 (3.1 ± 2.3) | 10.9 (7.3–76.1) | 0.54 [0.19–1.39] | 0.219 | 0.283 | ns | |
| DMSO@10.00 (10.00 cm) | DMSO@15.00 (10.00 cm) | 6.8 (3.0 ± 5.1) | 10.9 (7.3–76.1) | 0.60 [0.17–1.72] | 0.366 | 0.435 | ns | |
| DMSO@15.00 (5.000 cm) | DMSO@15.00 (10.00 cm) | 14.5 (9.7 ± 1.1) | 10.9 (7.3–76.1) | 1.38 [0.68–2.78] | 0.405 | 0.469 | ns | |
| DMSO@5.000 (5.000 cm) | DMSO@15.00 (10.00 cm) | 8.5 (5.3 ± 3.4) | 10.9 (7.3–76.1) | 0.76 [0.36–1.58] | 0.49 | 0.539 | ns | |
| DMF@5.000 (5.000 cm) | DMSO@15.00 (10.00 cm) | 10.3 (6.3 ± 6.3) | 10.9 (7.3–76.1) | 0.93 [0.43–1.98] | 1 | 1 | ns | |
| DMF@10.00 (10.00 cm) | DMSO@15.00 (10.00 cm) | 0.0 (0.0 ± 0.4) | 10.9 (7.3–76.1) | 0.00 [0.00–0.22] | <0.001 | <0.001 | *** | |
| DMSO@5.000 (10.00 cm) | DMSO@15.00 (10.00 cm) | 1.4 (0.4 ± 0.8) | 10.9 (7.3–76.1) | 0.11 [0.01–0.47] | <0.001 | <0.001 | *** | |
| Kaloula borealis | DMSO@5.000 (10.00 cm) | DMSO@5.000 (5.000 cm) | 5.3 (3.8 ± 0.2) | 5.1 (3.8–4.7) | 1.04 [0.64–1.66] | 0.909 | 0.976 | ns |
| DMF@10.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 41.8 (37.2 ± 6.5) | 5.1 (3.8–4.7) | 13.37 [9.29–19.56] | <0.001 | <0.001 | *** | |
| DMF@10.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 42.4 (38.6 ± 6.4) | 5.1 (3.8–4.7) | 13.72 [9.70–19.75] | <0.001 | <0.001 | *** | |
| DMF@15.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 77.8 (74.3 ± 0.9) | 5.1 (3.8–4.7) | 64.97 [45.07–94.96] | <0.001 | <0.001 | *** | |
| DMF@15.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 69.6 (65.5 ± 3.5) | 5.1 (3.8–4.7) | 42.65 [29.68–62.15] | <0.001 | <0.001 | *** | |
| DMF@5.000 (10.00 cm) | DMSO@5.000 (5.000 cm) | 73.1 (69.9 ± 6.1) | 5.1 (3.8–4.7) | 50.49 [35.82–72.56] | <0.001 | <0.001 | *** | |
| DMF@5.000 (5.000 cm) | DMSO@5.000 (5.000 cm) | 40.0 (36.5 ± 3.5) | 5.1 (3.8–4.7) | 12.40 [8.84–17.71] | <0.001 | <0.001 | *** | |
| DMSO@10.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 50.0 (44.7 ± 5.3) | 5.1 (3.8–4.7) | 18.58 [12.73–27.57] | <0.001 | <0.001 | *** | |
| DMSO@10.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 81.6 (77.3 ± 5.5) | 5.1 (3.8–4.7) | 82.97 [54.60–128.41] | <0.001 | <0.001 | *** | |
| DMSO@15.00 (10.00 cm) | DMSO@5.000 (5.000 cm) | 61.5 (57.2 ± 5.6) | 5.1 (3.8–4.7) | 29.61 [20.76–43.08] | <0.001 | <0.001 | *** | |
| DMSO@15.00 (5.000 cm) | DMSO@5.000 (5.000 cm) | 62.0 (57.9 ± 5.9) | 5.1 (3.8–4.7) | 30.33 [21.37–43.89] | <0.001 | <0.001 | *** | |
| Pelophylax chosenicus | DMF@15.00 (10.00 cm) | DMF@5.000 (10.00 cm) | 67.8 (64.5 ± 0.9) | 74.4 (71.4–77.2) | 0.72 [0.58–0.90] | 0.003 | 0.004 | ** |
| DMF@10.00 (10.00 cm) | DMF@5.000 (10.00 cm) | 75.5 (73.1 ± 8.9) | 74.4 (71.4–77.2) | 1.10 [0.87–1.37] | 0.433 | 0.488 | ns | |
| DMSO@10.00 (5.000 cm) | DMF@5.000 (10.00 cm) | 74.4 (71.3 ± 7.4) | 74.4 (71.4–77.2) | 1.00 [0.80–1.26] | 1 | 1 | ns | |
| DMF@10.00 (5.000 cm) | DMF@5.000 (10.00 cm) | 28.1 (25.0 ± 1.5) | 74.4 (71.4–77.2) | 0.13 [0.11–0.17] | <0.001 | <0.001 | *** | |
| DMF@15.00 (5.000 cm) | DMF@5.000 (10.00 cm) | 41.8 (38.5 ± 5.1) | 74.4 (71.4–77.2) | 0.25 [0.20–0.30] | <0.001 | <0.001 | *** | |
| DMF@5.000 (5.000 cm) | DMF@5.000 (10.00 cm) | 38.0 (34.9 ± 1.3) | 74.4 (71.4–77.2) | 0.21 [0.17–0.26] | <0.001 | <0.001 | *** | |
| DMSO@10.00 (10.00 cm) | DMF@5.000 (10.00 cm) | 49.0 (45.6 ± 2.4) | 74.4 (71.4–77.2) | 0.33 [0.27–0.41] | <0.001 | <0.001 | *** | |
| DMSO@15.00 (10.00 cm) | DMF@5.000 (10.00 cm) | 55.3 (52.0 ± 8.6) | 74.4 (71.4–77.2) | 0.43 [0.35–0.52] | <0.001 | <0.001 | *** | |
| DMSO@15.00 (5.000 cm) | DMF@5.000 (10.00 cm) | 49.0 (45.4 ± 2.6) | 74.4 (71.4–77.2) | 0.33 [0.27–0.41] | <0.001 | <0.001 | *** | |
| DMSO@5.000 (10.00 cm) | DMF@5.000 (10.00 cm) | 16.6 (13.9 ± 9.6) | 74.4 (71.4–77.2) | 0.07 [0.05–0.09] | <0.001 | <0.001 | *** | |
| DMSO@5.000 (5.000 cm) | DMF@5.000 (10.00 cm) | 59.7 (56.3 ± 3.1) | 74.4 (71.4–77.2) | 0.51 [0.41–0.63] | <0.001 | <0.001 | *** |
| Species | Cryoprotectant | Predicted Integral Membrane (%) | 95% CI |
|---|---|---|---|
| Dryophytes suweonensis | DMF | 73.2% ± 0.6% [b] | 71.9–74.4% |
| DMSO | 70.4% ± 0.7% [a] | 69.1–71.7% | |
| Pelophylax chosenicus | DMF | 54.9% ± 0.6% [b] | 53.7–56.0% |
| DMSO | 51.5% ± 0.6% [a] | 50.2–52.7% | |
| Kaloula borealis | DMF | 50.2% ± 0.7% [b] | 48.9–51.5% |
| DMSO | 46.8% ± 0.7% [a] | 45.4–48.2% | |
| Hynobius yangi | DMF | 8.8% ± 0.7% [b] | 7.5–10.3% |
| DMSO | 7.8% ± 0.6% [a] | 6.6–9.1% |
| Species | Condition | Cryoprotectant | Mean ± SE (%) | 95% CI (%) | Total (Cells) | No. of Individuals | Straws Per Conditions |
|---|---|---|---|---|---|---|---|
| Dryophytes suweonensis | DMSO@15.00 (10.00 cm) | DMSO | 86.5 ± 2.3 [g] | 83.3–89.3 | 499 | 15 | 7 |
| Hynobius yangi | DMF@15.00 (5.000 cm) | DMF | 19.7 ± 5.2 [ef] | 13.5–26.7 | 135 | 5 | 5 |
| Kaloula borealis | DMSO@10.00 (5.000 cm) | DMSO | 81.6 ± 2.3 [g] | 77.3–85.5 | 345 | 15 | 5 |
| Pelophylax chosenicus | DMF@10.00 (10.00 cm) | DMF | 75.5 ± 2.6 [g] | 73.1–78.9 | 825 | 15 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Park, D.S.; Park, J.-K.; Lee, J.-E.; Moon, J.C.; Do, Y. Optimizing Sperm Cryopreservation from Four Endangered Korean Amphibian Species: Species-Specific Effects of Cryoprotectants and Cooling Regimes on Membrane-Integrity Viability. Animals 2025, 15, 3013. https://doi.org/10.3390/ani15203013
Kim J-S, Park DS, Park J-K, Lee J-E, Moon JC, Do Y. Optimizing Sperm Cryopreservation from Four Endangered Korean Amphibian Species: Species-Specific Effects of Cryoprotectants and Cooling Regimes on Membrane-Integrity Viability. Animals. 2025; 15(20):3013. https://doi.org/10.3390/ani15203013
Chicago/Turabian StyleKim, Jun-Sung, Da Som Park, Jun-Kyu Park, Ji-Eun Lee, Jeong Chan Moon, and Yuno Do. 2025. "Optimizing Sperm Cryopreservation from Four Endangered Korean Amphibian Species: Species-Specific Effects of Cryoprotectants and Cooling Regimes on Membrane-Integrity Viability" Animals 15, no. 20: 3013. https://doi.org/10.3390/ani15203013
APA StyleKim, J.-S., Park, D. S., Park, J.-K., Lee, J.-E., Moon, J. C., & Do, Y. (2025). Optimizing Sperm Cryopreservation from Four Endangered Korean Amphibian Species: Species-Specific Effects of Cryoprotectants and Cooling Regimes on Membrane-Integrity Viability. Animals, 15(20), 3013. https://doi.org/10.3390/ani15203013






