Simple Summary
Clostridium butyricum possesses probiotic and metabolic properties. This study investigated the effects of C. butyricum on growth performance, gut microbiota, and various physiological parameters in Hanwoo calves. C. butyricum supplementation caused changes in blood amylase and acid–base parameters, suggesting improved metabolic stability and buffering capacity. Microbial analysis showed normal microbial diversity, increased abundance of beneficial microbes, and reduced levels of potential pathogens. Conclusively, C. butyricum may help establish a favorable intestinal environment in neonatal calves, supporting early gut health and disease prevention.
Abstract
A healthy gut microbiota in calves is necessary for optimal performance and development. Considering that probiotics have been shown to improve gut microbiota, the aim of this study was to investigate the effects of Clostridium butyricum on growth performance, blood parameters, and gut microbiota in Hanwoo calves. In total, 92 calves from two farms were randomly assigned to four groups: a control and three treatment groups that received increasing doses of C. butyricum (CB1, CB2, and CB3) during the first 5 days after birth. Independent experiments were conducted at each farm with different measurements, where body weight was monitored and blood, rumen, and fecal samples were collected to assess physiological responses and microbial profiles. Notably, significant differences were observed in blood amylase and acid–base parameters, suggesting that C. butyricum supplementation may enhance metabolic stability and buffering capacity. Microbial profiling revealed preserved alpha diversity and compositional shifts in both the rumen and fecal microbiota. Particularly, there was an increase in the relative abundances of Prevotella and Muribaculaceae and a decrease in the abundances of the pathogenic genera Escherichia and Shigella in calves fed C. butyricum-supplemented diets. These changes, along with a trend toward a reduced frequency and severity of diarrhea, suggest that C. butyricum supplementation may support gut health and promote stable early growth in neonatal calves.
1. Introduction
Optimal feeding management is essential to reduce neonatal calf mortality and ensure healthy growth by minimizing pathogen exposure, promoting rumen development, and supporting the proliferation of beneficial microbiota [1,2,3]. Traditionally, antibiotics have been widely administered to prevent and treat diseases such as diarrhea in calves [4,5,6], and their use has also contributed to improved feed efficiency and growth performance [7,8,9]. Recently, the use of antimicrobial feed additives has been restricted/prohibited in several countries owing to concerns regarding antibiotic residues and environmental pollution [10,11]. Consequently, the identification of suitable antibiotic alternatives has attracted increasing interest. Probiotics, defined as live microorganisms such as lactic acid bacteria, yeasts, and butyrate-producing bacteria, have been investigated as promising alternatives to conventional antibiotics. Research findings indicate that probiotic supplementation in animal diets can support gut health and potentially enhance disease resistance [12,13,14].
Among the probiotic species, Clostridium butyricum has been recognized as a promising candidate. C. butyricum is a gram-positive, endophytic, anaerobic bacterium that produces short-chain fatty acids (SCFAs), particularly butyric acid. Unlike lactic acid bacteria, which require protective coatings to survive gastric conditions, C. butyricum naturally forms spores—highly resistant structures that enable the bacterium to withstand gastric acid, bile salts, digestive enzymes, and antibiotics, thereby maintaining its viability throughout the gastrointestinal tract [15,16]. As an anaerobe, C. butyricum primarily acts in the distal gut where oxygen levels are low, facilitating the growth of beneficial microbiota and suppressing harmful pathogens. Its fermentation metabolites, including SCFAs such as acetate, propionate, and butyrate, help maintain an acidic intestinal environment, which inhibits the colonization of pathogenic bacteria, supports the growth of beneficial microbes, and stimulates intestinal peristalsis, thereby promoting regular bowel movement [13,15,17]. Moreover, some studies have reported that butyrate downregulates the expression of various virulence-associated genes [18].
Several studies have investigated the effects of C. butyricum supplementation under practical conditions of livestock. Previous studies on the growth and gut health benefits of probiotics have mainly focused on monogastric animals [19,20,21,22,23,24]. In ruminants, C. butyricum supplementation has been reported to promote growth [25,26,27] and modulate the structure and composition of the gut microbiota [26,27]. Recent research has shown that C. butyricum supplementation during heat stress enhances rumen fermentation and alleviates the adverse physiological impacts of thermal stress [25]. Therefore, C. butyricum has the potential to improve the ruminal microbial environment, thereby contributing to improved productivity. However, most studies have been conducted in monogastric animals and adult ruminants, and there is limited information on the effects of C. butyricum supplementation in Hanwoo calves. Considering that calves have a relatively underdeveloped gastrointestinal microbiome, we hypothesized that the early introduction of beneficial microorganisms can improve their growth and overall health.
Therefore, the aim of this study was to investigate the effects of C. butyricum supplementation on growth performance, blood parameters, fecal microbiota composition, and disease incidence in Hanwoo calves, with a focus on promoting healthy early growth. Specifically, we designed experiments I and II to evaluate the effects of C. butyricum supplementation on growth performance and various health-related parameters in Hanwoo calves.
2. Materials and Methods
The current study was conducted from February to June 2024. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Kangwon National University (Chuncheon, Republic of Korea) (protocol number: KW-250529-2), following the recommendations of the guidelines for animal research. Newborn Hanwoo calves (n = 92) were used, and experiments I (n = 52) and II (n = 40) were independently conducted on separate farms (hereafter referred to as Farm A and Farm B, respectively). Experiment I focused on body weight and rumen microbiota, whereas experiment II focused on blood parameters, fecal microbiota, and diarrhea frequency. Both experiments were designed to ensure an adequate sample size and uniform age distribution to minimize confounding factors unrelated to C. butyricum supplementation. Additionally, conducting multiple assessment protocols simultaneously can be stressful for newborn calves. Despite being conducted at different locations, both farms maintained the same feeding management practices and conditions to ensure consistency throughout the experiment.
2.1. Experiment I
2.1.1. Experimental Design and Diets
Experiment I was performed to evaluate the effects of C. butyricum supplementation on body weight and ruminal microbiota in Hanwoo calves. The feeding trial was performed from March to August 2024 at Farm A using 52 newborn male calves. Newborn Hanwoo calves with an average body weight of 29.5 ± 4.2 kg (mean ± standard deviation) were randomly assigned to four treatment groups at birth (13 calves per group), with no significant difference in initial body weight among groups (p = 0.259): control (no supplementation; CON) and three C. butyricum supplementation groups receiving 108 (CB1), 109 (CB2), and 1010 (CB3) colony-forming units (CFU). For each supplemented group, a 2 g solid tablet containing the assigned concentration of C. butyricum with a corn starch-based excipient was dissolved in approximately 10 mL of water and administered orally for five consecutive days immediately after birth. Notably, the supplementation period was designed to target the early life stage when calves experience high microbial colonization to adapt to the external environment [28,29,30]. Our aim was to evaluate whether supplementation during this stage influences subsequent rumen fermentation and growth performance. Calves in the control group received an identical 2 g tablet containing only the excipient, administered at the same time and frequency as the treatment groups. All calves were housed with their dams in a communal rearing pen and were weaned at 3 months of age.
All the calves were fed 1.31 kg/day of calf starter in accordance with the Korean Feeding Standard for Hanwoo Steers [31]. They received food twice daily (at 08:00 and 17:00 h) and had ad libitum access to water. The ingredients and nutritional composition of the diets are listed in Table 1. The chemical composition of the experimental diets was analyzed using the methods recommended by the Association of Official Analytical Chemists [32]. Crude protein was determined using the Kjeldahl method, and crude fat by ether extraction. Crude fiber was analyzed using the Weende method, and ash content was determined by combustion at 550 °C. Moisture content was measured by oven drying at 105 °C. Neutral detergent fiber and acid detergent fiber were measured using the procedure described by Van Soest et al. [33].

Table 1.
Chemical composition of experimental diets for Hanwoo calves (DM basis).
2.1.2. Body Weight and Average Daily Gain
Body weight was measured at birth and at 3 months of age. Additionally, the average daily gain (ADG) was calculated by subtracting birth weight from body weight at three months of age and dividing it by the total number of days in the experimental period.
2.1.3. Rumen Fluid Sampling
Rumen fluid was collected at weaning (3 months of age) using an appropriate catheter, with three calves randomly selected from each group, to evaluate the effects of C. butyricum supplementation on rumen microbiota composition. Approximately 100 mL of rumen fluid was aseptically collected in a sterilized and immediately filtered through four layers of cheesecloth to remove the feed particles. Thereafter, the filtrate was stored at −80 °C until further analysis.
2.1.4. 16S rRNA Gene Sequencing
Genomic DNA was extracted from the bacterial samples using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Thereafter, the quantity and quality of the extracted DNA were assessed using a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). For 16S rRNA gene sequencing, the V3–V4 region of the bacterial 16S rRNA gene was amplified using MiSeq sequencing technology (Illumina, San Diego, CA, USA). A sequencing library was prepared according to the Illumina 16S Metagenomic Sequencing Library Preparation Protocol. All polymerase chain reactions (PCRs) for library preparation were conducted using 2X KAPA HiFi HotStart Ready Mix (Roche, Mannheim, Germany). The first PCR (1st PCR) was performed using universal primers with the following Illumina adapter overhang sequences: forward primer: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′; reverse primer: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′. The PCR conditions were as follows: initial denaturation at 95 °C for 30 s, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 min. Amplified PCR products were purified using AMPure beads (Beckman Coulter, Brea, CA, USA). A second PCR (2nd PCR) was conducted to incorporate the Illumina index sequences using Nextera XT Indexed Primers (Illumina). The cycling conditions were identical to those of the first PCR, except for the number of cycles, which was reduced to 10. The final PCR products were purified using the AMPure beads. Purified libraries were quantified via qPCR according to the qPCR Quantification Protocol Guide (KAPA Library Quantification Kit for Illumina sequencing; Roche, Mannheim, Germany). Size distribution of the final libraries was assessed using a TapeStation D1000 ScreenTape (Agilent Technologies, Waldbronn, Germany). Sequencing was performed on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) with a 2 × 300 bp paired-end sequencing strategy.
2.1.5. Experiment I: Microbial Diversity and Functional Profiling
Briefly, the 16S amplicon sequence reads were analyzed using QIIME2 version 2024.10.1 (http://qiime.org/ accessed on 1 October 2024). The adapter and primer sequences were trimmed using the CutAdapt software version 4.9, and the chimeric sequences were filtered out. Denoising and merging were conducted using the plugin DADA2 to create an amplicon sequence variant (ASV) feature table. Taxonomic classifiers were manually constructed using the naïve Bayes classifier with the SILVA version 138 database. Thereafter, taxonomic assignment of ASVs was performed using the plugin q2-feature-classifier with 99% bacterial identity and representative sequences. Following the first taxonomic classification, an extra filtration step was used to obtain bacterial sequence data. Unassigned ASVs, chloroplasts, mitochondria, and non-bacterial taxa were excluded from the taxonomic filtration.
Additionally, alpha and beta diversities of the rumen and fecal microbiota were analyzed using QIIME 2 based on the ASV biological observation matrix (BIOM). Alpha diversity metrics included species richness (Chao1, ACE, observed ASVs, observed genera, and observed species) as well as Fisher’s, Shannon’s, and Simpson’s indices. Beta diversity was visualized using principal coordinate analysis (PCoA) based on the Bray–Curtis dissimilarity matrix. Hierarchical clustering was visualized using a dendrogram. All visualizations were generated using QIIME 2 artifacts (.qzv) and viewed using the QIIME 2 View web application (https://view.qiime2.org/, accessed on 10 September 2025). Diversity analyses were also conducted using MicrobiomeAnalyst based on the BIOM file. The same alpha diversity metrics were calculated, and PCoA and dendrograms were used to compare the microbial compositions. A Venn diagram was created using Venny software version 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/, accessed on 17 July 2024) to compare shared and exclusive ASVs among the groups. Functional genetic profiling was performed using PICRUSt2 version 2.5.176. Moreover, the relative abundance of the MetaCyc pathway was used to infer functional differences among the treatment groups.
2.2. Experiment II
2.2.1. Experimental Design and Diets
Experiment II was conducted to investigate the effects of C. butyricum supplementation on blood parameters, fecal scores, diarrhea frequency, and microbial diversity and functional profiles in Hanwoo calves. Feeding trial was performed from March to August 2024 at Farm B using 40 newborn male calves. Newborn Hanwoo calves were randomly selected and assigned to the experimental treatment groups at birth. All calves were then randomly allocated to four treatment groups (10 calves per group): a control group (no supplementation; CON), and three C. butyricum supplementation groups receiving 108 (CB1), 109 (CB2), and 1010 CFU (CB3). For each supplement group, a 2 g solid tablet containing the assigned concentration of C. butyricum was dissolved in approximately 10 mL of water and administered orally for five consecutive days immediately following birth. To prevent cross-contamination among treatments, the calves were kept in pens (three to four animals per pen) equipped with individual feed bins. All calves were nursed in the same pen as their dams and weaned at 3 months of age. Feeding management, diet formulation, and chemical analyses were performed as described in Experiment I. The nutritional composition of the calf starter is presented in Table 1.
2.2.2. Blood Collection and Laboratory Analysis
Blood samples were collected from the jugular vein of 40 newborn calves at 7 d of age and immediately divided into heparin tubes containing EDTA (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) and serum tubes (Greiner BioOne, Kremsmünster, Austria). Thereafter, the collected blood samples were transported on ice to the laboratory, where hematological analysis and serum separation were performed via centrifugation (2000× g, 15 min, 4 °C).
Hematological parameters were measured using an automated hemocytometer (IDEXX Laboratories, Westbrook, ME, USA). The erythrogram included red blood cell count, hemoglobin concentration, hematocrit, mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and reticulocyte count. Leukograms were assessed by determining the total white blood cell (WBC), neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts, along with platelet counts. Blood biochemical parameters, including glucose, total cholesterol, triglycerides, amylase, non-esterified fatty acids (NEFAs), albumin, globulin, albumin/globulin ratio (A/G), total protein, total bilirubin, blood urea nitrogen (BUN), creatinine, gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), calcium, phosphorus, and magnesium, were analyzed using an automated dry biochemical analyzer (Boule Medical AB, Spanga, Sweden).
Blood gas parameters, including pH, partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), bicarbonate (HCO3−), total carbon dioxide (TCO2), base excess (BE), and oxygen saturation (sO2), were analyzed using an automated blood gas analyzer (model, manufacturer, country) according to the manufacturer’s protocol. For quality control, calibration and internal standardization were performed before each set of measurements using certified reference materials provided by the manufacturer. All analyses were completed within 30 min of plasma separation to prevent gas diffusion and ensure data accuracy.
2.2.3. Fecal Score and Diarrhea Frequency
Fecal scores and diarrhea frequency were recorded for each calf by trained evaluators to monitor the intestinal health and assess the effects of C. butyricum supplementation. Fecal scores were evaluated weekly before afternoon feeding using the four-point fecal scoring system described by Larson et al. [34]. The scoring system classified the fecal scores as follows: 1 = firm and well-formed, 2 = soft but maintained shape, 3 = loose and unformed, and 4 = watery diarrhea. Diarrhea was defined as a fecal score of 3 or higher, and the number of diarrhea days per calf was recorded throughout the experimental period. The total duration of diarrhea was calculated as the cumulative number of days for which each calf exhibited a fecal score of three or higher.
2.2.4. Fecal Sampling, Microbial Diversity, and Functional Profiling
Fecal samples were collected from calves at 7 d of age, with three calves randomly selected from each group, to assess the effect of C. butyricum supplementation on gut microbiota. Approximately 10 g of feces were aseptically obtained from the rectum using sterile gloves, placed in sterile bags, transported on ice, and stored at −80 °C until analysis. Genomic DNA was extracted using a QIAamp PowerFecal Pro DNA Kit (Qiagen, Hilden, Germany). DNA amplification, sequencing, and downstream analyses were conducted using protocols identical to those described for rumen fluid in Experiment I, except for the sample type.
2.3. Statistical Analysis
Statistical analyses were performed using the R software (version 4.3.1; R Core Team, Vienna, Austria). Considering that the two experiments were conducted independently in different herds, each addressing distinct outcome variables, the datasets were analyzed separately. For each body weight and blood metabolite parameter, data were assessed for normality using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. When both the normality and homogeneity of variance assumptions were satisfied, a Type II analysis of variance (ANOVA) was conducted, and multiple comparisons were performed using Tukey’s honest significant difference (HSD) test. If the normality assumption was met but the variances were heterogeneous, Welch’s ANOVA was used. For non-normally distributed data, the Kruskal–Wallis test was applied, followed by Dunn’s test for multiple comparisons, with Bonferroni correction. In addition, estimated marginal means were used to perform planned contrasts between the control group and the average of the groups supplemented with C. butyricum. Subsequently, polynomial contrasts were applied to test for linear and quadratic effects of supplementation. For nonparametric data, aligned rank transform models were applied to enable factorial contrasts, and robust standard errors were used when appropriate. Descriptive statistics were expressed as group means, standard errors of the mean (SEMs), and sample sizes. Statistical significance was set at p < 0.05, and tendencies were considered at 0.05 ≤ p ≤ 0.10.
3. Results and Discussions
3.1. Body Weight and Average Daily Gain
Table 2 shows the effects of C. butyricum on the body weight, ADG, and FCR of Hanwoo calves. Birth and weaning weights were not significantly affected by C. butyricum supplementation (birth weight, p = 0.259; weaning weight, p = 0.896). Although ADG showed an increasing trend with increasing levels of C. butyricum supplementation, ADG was not significantly different (p = 0.835) among the groups, with values ranging from 0.86 to 0.91 kg/day.

Table 2.
Effects of C. butyricum on body weight in Hanwoo calves.
Research findings indicate that the effects of C. butyricum supplementation on growth performance vary among different livestock species and management conditions. For example, Li et al. [26] found that C. butyricum supplementation significantly increased DMI and body weight in cows, whereas Zhang et al. [27] found no significant changes in the growth indicators in goats. These differences suggest that the effects of C. butyricum supplementation may be affected by various factors, including species, current health status, feeding environment, and composition of gut microbes.
In the present study, C. butyricum supplementation did not considerably affect growth performance parameters, including birth weight, weaning weight, and ADG, in Hanwoo calves. This may reflect the limited direct impact of probiotics on growth, although it is also possible that factors such as dosage, trial duration, and sample size may have influenced the statistical outcomes. Therefore, future studies should adopt longer feeding trials, include a range of dosage levels, and ensure adequate sample sizes to determine the effect of C. butyricum on the growth performance of calves.
3.2. Blood Biochemical Parameters
Table 3 shows the effects of C. butyricum supplementation on blood metabolite levels. Blood amylase was markedly lower in the CB1 and CB2 groups compared with the CON group (overall, p < 0.01), whereas the CB3 group showed intermediate values. Triglyceride concentrations also exhibited a quadratic response (p = 0.036), being higher in the CB1 and CB2 groups but declining in the CB3 group. Other blood metabolites, including cholesterol, NEFA, total protein, albumin, and liver enzymes (AST, GGT, ALP), did not differ significantly among groups (p > 0.05).

Table 3.
Effects of C. butyricum on blood metabolites in Hanwoo calves.
Amylase is a digestive enzyme primarily secreted by the pancreas and salivary glands and is present at high concentrations in digestive fluids, with small amounts detected in the blood and urine [35,36,37]. In ruminant species, salivary secretion of amylase is negligible, distinguishing them from non-ruminant animals [38,39,40]. Circulating amylase levels are commonly utilized as biomarkers for pancreatic and gastrointestinal functions and have been employed in the clinical evaluation of pathological conditions such as pancreatitis and gastroenteritis.
Recently, there has been growing recognition that indicators such as serum amylase should be interpreted within the framework of host–microbiota interactions, particularly along the gut–pancreas axis. This concept has led to increasing research interest on the bidirectional physiological mechanisms underlying this axis. Among various microbial metabolites, VFAs have been reported to influence pancreatic function [12,41]. Particularly, sodium butyrate modulates pancreatic function by suppressing nuclear factor-kappa B (NF-κB) activity and inhibiting histone deacetylase, thereby regulating inflammation and fibrosis [41,42].
In the present study, the observed decrease in serum amylase concentration following C. butyricum supplementation suggests that changes in the intestinal environment indirectly influence pancreatic function via the gut–pancreas axis. However, ruminants possess a complex microbial ecosystem within the rumen and distinct microbial communities along the intestinal tract, resulting in a multilayered microbiota structure. Because of this complexity, the physiological link between microbial populations and digestive organs, including the pancreas, is highly intricate and not well understood in ruminants. Furthermore, as this study did not directly assess pancreatic responses or the expression of genes related to pancreatic function, definitive conclusions regarding the underlying mechanisms of the observed phenomenon cannot be drawn.
However, previous studies have reported that metabolites, such as VFAs, can improve intestinal homeostasis, modulate immune and metabolic functions, and potentially affect extraintestinal organs such as the pancreas [23,24,25]. Our findings may provide foundational evidence for future investigations into microbiota–organ interactions mediated by the gut–pancreas axis in ruminants. However, further studies are required to elucidate the precise biological mechanisms underlying this process.
Table 4 shows the hematological parameters of calves fed diets supplemented with different C. butyricum doses. No significant differences were observed among the groups for most parameters. In contrast, the white blood cell count tended to differ (overall, p = 0.078; linear, p = 0.072) and showed a significant quadratic trend (p = 0.014), increasing in the CON group, decreasing in the CB1 and CB2 groups, and then increasing in the CB3 group. Neutrophil counts were significantly higher in the CON group than in the CB1 and CB2 groups (overall, p = 0.033; quadratic response [p = 0.006]), with the CB3 group showing intermediate values.

Table 4.
Effects of C. butyricum on hematological parameters in Hanwoo calves.
Notably, differences in WBC and neutrophil counts were observed among the treatment groups. Although the mechanisms of C. butyricum in immune regulation and inflammation were not directly investigated in the present study, previous studies have reported that C. butyricum exhibits anti-inflammatory and immunomodulatory effects by regulating cytokine production and immune cell differentiation [43,44]. Specifically, C. butyricum enhances the host immune system by upregulating pro-inflammatory cytokines such as IL-8, IL-6, and TNF-α, and also exerts beneficial effects through the production of anti-inflammatory cytokines such as IL-10 [45,46]. Additionally, some studies have shown that C. butyricum may suppress the NF-κB signaling pathway, thereby regulating the transcription of inflammatory genes and inhibiting excessive inflammatory responses and immune cell activation [42,47]. Similarly, Zhang et al. [21] reported that dietary supplementation with C. butyricum upregulated TNF-α and IL-4 concentrations in the jejunal mucosa of broiler chickens compared with those in the control group. Additionally, studies in piglets have shown that 0.4% C. butyricum supplementation increases the relative mRNA expression of TLR2 and IL-10 in the ileum. Although these findings were obtained from monogastric animals, they suggest that C. butyricum supplementation may modulate immune responses by balancing pro-inflammatory and anti-inflammatory signaling pathways, contributing to improved intestinal immune regulation. However, a limitation of this study is that specific immune-related markers, such as cytokine levels, were not analyzed. Future studies should incorporate comprehensive assessments of inflammation-related markers and hematological parameters to elucidate the immunomodulatory effects of C. butyricum.
Furthermore, the effects of C. butyricum supplementation on blood acid–base parameters are presented in Table 5. No significant differences were observed in pH levels among the groups (overall, p > 0.05). However, there was a significant increase in TCO2, HCO3, and base excess and a decrease in anion gap in the CB3 group compared with those in the CON group (overall, p < 0.01). Additionally, these parameters showed clear linear responses across the supplementation levels (linear, p < 0.01).

Table 5.
Effects of C. butyricum on blood acid base parameters in Hanwoo calves.
TCO2, HCO3−, and pH are critical indicators of systemic acid–base balance. Among these, HCO3−—which constitutes the major component of TCO2—functions as a primary physiological buffer and is widely recognized as a key marker for assessing metabolic acid–base disorders, including metabolic acidosis and alkalosis. The bicarbonate buffering system not only maintains systemic acid–base homeostasis but also plays a vital role in regulating the ruminal environment of gastrointestinal regions with active microbial fermentation [48,49,50]. In the rumen, the accumulation of VFAs during fermentation predisposes the environment to acidification; however, HCO3− secreted via saliva contributes to the neutralization of this acidity, thereby stabilizing ruminal pH. Consequently, HCO3− and VFAs in the rumen are essential for sustaining microbial homeostasis and acid–base equilibrium. Moreover, localized buffering activity in the rumen may have systemic implications, potentially influencing circulating HCO3− concentrations [51,52,53].
In the present study, C. butyricum supplementation significantly increased both serum HCO3− concentration and blood pH. Although C. butyricum was not directly investigated in previous ruminal acidosis models, the existing literature has demonstrated that co-administration of probiotics and sodium bicarbonate ameliorates acid–base disturbances and elevates blood pH under experimentally induced acidosis [14]. Collectively, these findings imply that probiotics may contribute to mitigating acidic ruminal conditions and restoration of systemic pH by modulating SCFA production and enhancing ruminal buffering capacity.
Therefore, these findings suggest that C. butyricum supplementation may influence the profile of ruminal fermentation metabolites, thereby enhancing the activity of the bicarbonate buffering system and promoting ruminal pH stability, which may contribute to the maintenance of systemic acid–base homeostasis.
3.3. Diarrhea Frequency and Severity
Table 6 shows the effects of C. butyricum supplementation on the frequency and severity of diarrhea in Hanwoo calves. Although overall group differences were not significant (p > 0.05), both parameters showed a tendency to decrease linearly with increasing supplementation levels (frequency, p = 0.051; severity, p = 0.052). The highest frequency and severity were observed in the CON group (5.90 and 2.00, respectively), whereas the lowest values were recorded in the CB3 group (3.10 and 1.10, respectively).

Table 6.
Effects of C. butyricum on diarrhea frequency and severity in Hanwoo calves.
Although the reductions in diarrhea frequency and severity were not statistically significant, the numerical trends suggest that C. butyricum supplementation may play a beneficial role in mitigating gastrointestinal disturbances in young calves. These observations are consistent with previous findings showing that butyrate-producing probiotics can enhance gut barrier integrity, modulate local immune responses, and suppress pathogenic bacteria, thereby reducing diarrhea incidence and severity [19,54]. Additionally, the observed dose-dependent decline, particularly in the CB3 group, supports the hypothesis that higher levels of C. butyricum may effectively stabilize the gastrointestinal environment during the early growth phase. Moreover, the lack of statistical significance may be attributed to sample size limitations or variability in individual responses; however, these findings warrant further investigation under controlled conditions in larger cohorts.
3.4. Rumen and Fecal Alpha Diversity and Microbiota
In this study, we investigated the alpha diversities of the rumen and fecal microbiota of calves fed C. butyricum-supplemented diets (Table 7). No significant differences were observed among the treatment groups for any of the indices evaluated, including the ASVs, Chao1, Shannon, and Gini–Simpson indices, in both the rumen and feces (p > 0.05). In the rumen, the Shannon index ranged from 5.84 to 6.70, and the Gini–Simpson index remained below 0.05 across all groups. In fecal samples, the Shannon index was consistently above 5, and the Gini–Simpson index ranged from 0.059 to 0.121, suggesting a more even microbial community than that in the rumen. Overall, these values indicate that the ecological stability of microbial diversity and evenness was preserved despite probiotic supplementation.

Table 7.
Effects of C. butyricum on alpha diversity of microbiota in Hanwoo calves.
Additionally, the composition of rumen microbiota is summarized in Figure 1 and Tables S1 and S2. At the phylum level, Firmicutes and Bacteroidetes were predominant across all groups, accounting for approximately 40–51% and 41–55% of the total sequences, respectively. Other phyla, including Actinobacteria, Fibrobacterota, Cyanobacteria, and Spirochaetota, were detected at lower relative abundances (<5%), with no significant differences among the groups (p > 0.05). Additionally, similarities and differences in community structure among groups were determined and visualized using principal coordinate analysis (PCoA) based on beta diversity metrics (Supplementary Figure S1).
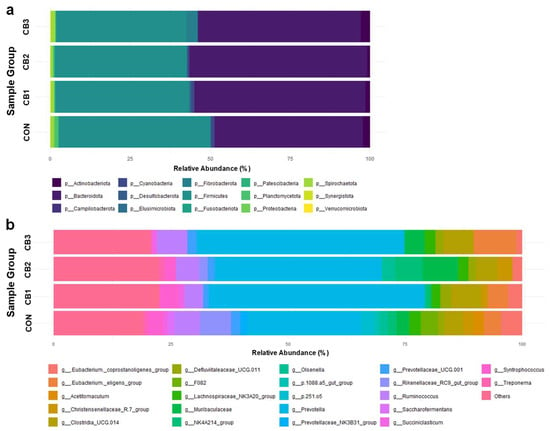
Figure 1.
Effects of C. butyricum on the rumen microbiota composition in Hanwoo calves. CON = control group (no supplementation, n = 3); CB1 = C. butyricum 108 CFU (n = 3); CB2 = C. butyricum 109 CFU (n = 3); CB3 = C. butyricum 1010 CFU (n = 3). The relative abundances of (a) group average of phyla and (b) the group average of genera are visualized. Genera with a relative abundance in the bottom 20% and phyla with a relative abundance below 1% were grouped under “Others”.
At the genus level, Prevotella was the most dominant taxon, with a relative abundance ranging from 24.13% in the CON group to 45.93% in the CB2 group. Although these differences were not statistically significant (p = 0.238), higher abundances were observed in all C. butyricum-supplemented groups than in the CON group. Similarly, Muribaculaceae abundance increased from 2.95% in the CON group to 7.52% in the CB3 group, showing a near-significant trend (p = 0.056). In contrast, the relative abundance of the Rikenellaceae RC9 gut and F082 decreased notably in the supplementation groups.
Prevotella plays a central role in carbohydrate and hydrogen metabolism in the rumen of ruminants. Members of this genus possess a wide range of enzymes that are capable of degrading various polysaccharides, thereby contributing to VFA production [55,56]. Additionally, some species have been reported to be proteolytic bacteria that produce cysteine proteases and collagen-degrading enzymes [55,57]. Although the functional importance of Prevotella has been well demonstrated in previous studies, the present study did not include metabolomic analyses, such as VFA profiling, to directly assess its metabolic activity or its contribution to fermentation. In future studies, metabolomic approaches should be integrated to quantitatively evaluate the effects of C. butyricum on rumen fermentation parameters and clarify its relationship with microbial composition.
In the present study, an increasing trend in the relative abundances of not only Prevotella but also Muribaculaceae was observed in the C. butyricum-supplemented groups. Muribaculaceae, primarily belonging to the phylum Bacteroidetes, is a group of commensal bacteria that produces enzymes specialized in degrading dietary polysaccharides and fibers [58,59]. Their abundance has been shown to vary in response to the dietary forage-to-concentrate ratio, specific feed ingredients, and probiotic supplementation [60,61] and they may interact with fermentation conditions and rumen microbial community structure in response to environmental changes [62,63].
Previous studies have reported that dietary supplementation with C. butyricum increases the relative abundance of genera such as Prevotella, Ruminococcaceae, and Megasphaera and promotes the production of VFAs [23,27]. Moreover, appropriate levels of C. butyricum supplementation can facilitate probiotic colonization of the gastrointestinal tract, enhance gut barrier function, and protect against pathogenic bacterial invasion [24]. In the present study, all groups were fed an identical total mixed ration and maintained under standardized housing conditions to minimize the effects of external factors. Under these controlled conditions, the observed increase in the relative abundances of Prevotella and Muribaculaceae in the C. butyricum-supplemented groups suggests that this strain may indirectly promote the growth of these microbes by improving the gut microenvironment, reducing the abundance of competing bacteria, and altering the availability of substrates.
Furthermore, the composition of fecal microbiota is summarized in Figure 2 and Tables S3 and S4. At the phylum level, Firmicutes was the most dominant taxon across all groups, accounting for approximately 63–71% of the total sequences, followed by Proteobacteria (4.8–18.2%), Bacteroidetes (10.6–31.9%), and Actinobacteria (5.6–11.8%). Other phyla, such as Cyanobacteria and Verrucomicrobiota, were present in lower proportions (<5%), with no significant differences among the treatment groups (p > 0.05). Moreover, similarities and differences in community structure among groups were determined and visualized using PCoA based on beta diversity metrics (Supplementary Figure S2).
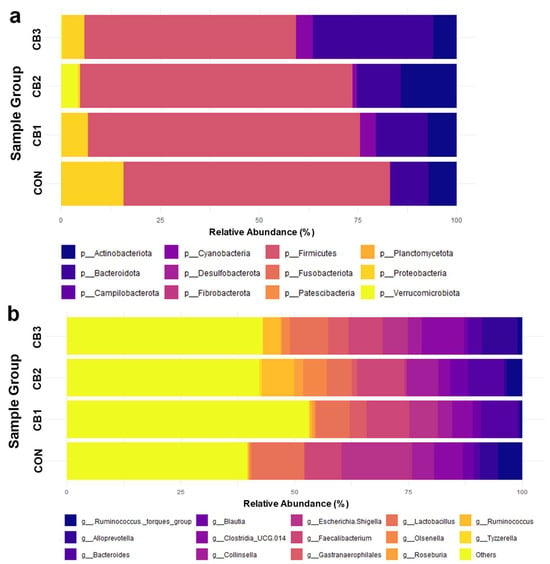
Figure 2.
Effects of C. butyricum on fecal microbiota composition in Hanwoo calves. CON = control group (no supplementation, n = 3); CB1 = C. butyricum 108 CFU (n = 3); CB2 = C. butyricum 109 CFU (n = 3); CB3 = C. butyricum 1010 CFU (n = 3). The relative abundances of (a) the group average of phyla and (b) the group average of genera are visualized. Genera with a relative abundance in the bottom 20% and phyla with a relative abundance below 1% were grouped under “Others”.
Similarly, no significant differences (p > 0.05) were observed in the relative abundances of most genera among the treatment groups. However, several genera showed trends in response to C. butyricum supplementation. Escherichia–Shigella showed a decreasing trend, with the highest abundance observed in the control group (13.78%) and progressively lower values in the treatment groups, reaching 2.31% in CB3. In contrast, increasing trends were observed for genera such as Bacteroides, Faecalibacterium, and Gastranaerophilales in the C. butyricum-supplemented group. Although Tyzzerella showed a significant increase in CB3, its overall abundance was low, suggesting that its biological relevance is limited.
The reduced abundance of E. coli in the C. butyricum-supplemented groups suggests that the probiotic suppresses potentially pathogenic bacteria. The Escherichia–Shigella genera includes several strains known for their pathogenicity, such as enterotoxigenic E. coli (ETEC), which is a major cause of diarrhea and gastrointestinal disturbances in animals [64,65]. These pathogens can cause diarrhea, dehydration, and impaired nutrient absorption, ultimately leading to reduced growth and increased mortality in neonatal calves. Therefore, the observed decrease in E. coli abundance following C. butyricum supplementation may be associated with its benefits in reducing the frequency and severity of calf diarrhea.
4. Conclusions
In this study, we investigated the effects of C. butyricum supplementation on growth performance, blood parameters, and gut microbiota composition in Hanwoo calves. Although no significant differences were observed in growth performance, calves in the C. butyricum-supplemented groups exhibited a tendency toward increased weight gain compared with those in the control group. Physiological responses were also detected in certain blood parameters and acid–base indicators, particularly in the C. butyricum-supplemented groups, suggesting improved buffering capacity and enhanced metabolic stability. Microbial analysis showed normal alpha diversity and notable shifts in both rumen and fecal microbiota compositions in response to C. butyricum supplementation. Specifically, the relative abundances of beneficial genera, such as Prevotella and Muribaculaceae, tended to increase, whereas potentially pathogenic Escherichia and Shigella showed a decreasing trend. Overall, these findings suggest that C. butyricum supplementation may modulate the ruminal environment and microbial composition to support gut health and immune balance in calves. Additionally, the observed trend toward reduced diarrhea frequency and severity indicates the potential benefits of promoting intestinal stability and supporting early growth in neonatal calves. Among the treatment groups, the highest dosage (1010 CFU, CB3) appeared to induce the most pronounced physiological and microbial changes. However, this study did not include a dose–response analysis or follow-up trials to determine the optimal dosage, highlighting the need for further investigation. Moreover, this study was limited to the early growth phase and did not include direct profiling of rumen fermentation metabolites. Additionally, the number of sampling points was restricted, preventing repeated measurements over time. To fully understand the long-term benefits and microbial mechanisms of C. butyricum supplementation, future studies should include extended growth monitoring, repeated sampling designs, and integrative analyses such as metabolomic profiling and microbial co-occurrence network analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15192785/s1, Figure S1: Principal coordinate analysis (PCoA) of ruminal microbiota in Hanwoo calves supplemented with C. butyricum; Figure S2: Principal coordinate analysis (PCoA) of fecal microbiota in Hanwoo calves supplemented with C. butyricum; Table S1: Effects of C. butyricum on the rumen microbiota composition (phylum level) in Hanwoo calves; Table S2: Effects of C. butyricum on the rumen microbiota composition (genus level) in Hanwoo calves; Table S3: Effects of C. butyricum on the fecal microbiota composition (phylum level) in Hanwoo calves; Table S4: Effects of C. butyricum on the rumen microbiota composition (genus level) in Hanwoo calves.
Author Contributions
Conceptualization, B.K.P., S.K.K., S.A.K., I.G.J., G.H.J., S.J.H., K.D.B., and E.J.B.; methodology, M.J.K., Y.L.K., and S.H.L.; formal analysis, M.J.K., Y.L.K., and S.H.L.; investigation, M.J.K., Y.L.K., and S.H.L.; resources, S.K.K., S.A.K., I.G.J., G.H.J., S.J.H., K.D.B., E.J.B., and B.K.P.; data curation, M.J.K., Y.L.K., J.S.S., and S.H.L.; writing—original draft preparation, M.J.K.; writing—review and editing, M.J.K., Y.L.K., S.H.L., J.S.S., and B.K.P.; visualization, M.J.K., Y.L.K., and S.H.L.; supervision, B.K.P.; project administration, B.K.P.; funding acquisition, S.K.K., S.A.K., I.G.J., G.H.J., S.J.H., K.D.B., E.J.B., and B.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from Natural Good Things Inc., Republic of Korea (grant number: 202400460001).
Institutional Review Board Statement
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Kangwon National University (Chuncheon, Republic of Korea) (protocol number: KW-250529-2, approval date: 13 March 2024), following the recommendations of the guidelines for animal research.
Informed Consent Statement
Written informed consent was obtained from the farm owners.
Data Availability Statement
The data supporting this study are not publicly available due to restrictions imposed by the funding agency and contractual/ethical obligations with participating farms. Limited, de-identified data may be shared upon reasonable request for non-commercial academic purposes only, subject to prior approval by the corresponding author and the funding institution.
Conflicts of Interest
Authors Sang Kook Kim, Soo An Kim, In Gi Jo, and Gyung Hyun Jo are employees of Natural Good Things Inc. Seong Jeong Han, Ki Deuk Bae and Eu Jin Ban are employees of Natural Pure Korea Inc. The other authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| SCFA | Short chain fatty acid |
| ADG | Average daily gain |
| FCR | Feed conversion ratio |
| DMI | Dry matter intake |
| MCV | Mean cell volume |
| MCHC | Mean corpuscular hemoglobin concentration |
| WBC | White blood cell |
| NEFA | Non-esterified fatty acids |
| BUN | Blood urea nitrogen |
| GGT | Gamma-glutamyl transferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
References
- Govil, K.; Yadav, D.S.; Patil, A.K.; Nayak, S.; Baghel, R.P.S.; Yadav, P.K.; Malapure, C.D.; Thakur, D. Feeding Management for Early Rumen Development in Calves. J. Entomol. Zool. Stud. 2017, 5, 1132–1139. [Google Scholar]
- Diao, Q.; Zhang, R.; Fu, T. Review of Strategies to Promote Rumen Development in Calves. Animals 2019, 9, 490. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Hu, M.; Hou, J.; Yang, L.; Wang, X.; Du, W.; Liu, J.; Xu, Q. Colonization and Development of the Gut Microbiome in Calves. J. Anim. Sci. Biotechnol. 2023, 14, 46. [Google Scholar] [CrossRef]
- Sawant, A.A.; Sordillo, L.M.; Jayarao, B.M. A Survey on Antibiotic Usage in Dairy Herds in Pennsylvania. J. Dairy Sci. 2005, 88, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Busani, L.; Graziani, C.; Binkin, N.; Franco, A.; Di Egidio, A.; Battisti, A. Survey of the Knowledge, Attitudes and Practice of Italian Beef and Dairy Cattle Veterinarians Concerning the Use of Antibiotics. Vet. Rec. 2004, 155, 733–738. [Google Scholar] [PubMed]
- Zwald, A.G.; Ruegg, P.L.; Kaneene, J.B.; Warnick, L.D.; Wells, S.J.; Fossler, C.; Halbert, L.W. Management Practices and Reported Antimicrobial Usage on Conventional and Organic Dairy Farms. J. Dairy Sci. 2004, 87, 191–201. [Google Scholar] [CrossRef]
- De Souza, K.A.; Cooke, R.F.; Schubach, K.M.; Brandão, A.P.; Schumaher, T.F.; Prado, I.N.; Marques, R.S.; Bohnert, D.W. Performance, Health and Physiological Responses of Newly Weaned Feedlot Cattle Supplemented with Feed-Grade Antibiotics or Alternative Feed Ingredients. Animal 2018, 12, 2521–2528. [Google Scholar] [CrossRef]
- Sarker, M.S.K.; Ko, S.Y.; Lee, S.M.; Kim, G.M.; Choi, J.K.; Yang, C.J. Effect of Different Feed Additives on Growth Performance and Blood Profiles of Korean Hanwoo Calves. Asian-Australas. J. Anim. Sci. 2009, 23, 52–60. [Google Scholar] [CrossRef]
- Berge, A.C.B.; Lindeque, P.; Moore, D.A.; Sischo, W.M. A Clinical Trial Evaluating Prophylactic and Therapeutic Antibiotic Use on Health and Performance of Preweaned Calves. J. Dairy Sci. 2005, 88, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Cars, O.; Mölstad, S.; Melander, A. Variation in Antibiotic Use in the European Union. Lancet 2001, 357, 1851–1853. [Google Scholar] [CrossRef]
- Morley, P.S.; Apley, M.D.; Besser, T.E.; Burney, D.P.; Fedorka-Cray, P.J.; Papich, M.G.; Traub-Dargatz, J.L.; Weese, J.S. Antimicrobial Drug Use in Veterinary Medicine. J. Vet. Intern. Med. 2005, 19, 617–629. [Google Scholar] [CrossRef]
- Pan, Y.; Li, J.; Fan, Z.; Chen, Y.; Huang, X.; Wu, D. New Insights into Chronic Pancreatitis: Potential Mechanisms Related to Probiotics. Microorganisms 2024, 12, 1760. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium Butyricum: From Beneficial to a New Emerging Pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Dagnaw Fenta, M.; Gebremariam, A.A.; Mebratu, A.S. Effectiveness of Probiotic and Combinations of Probiotic with Prebiotics and Probiotic with Rumenotorics in Experimentally Induced Ruminal Acidosis Sheep. Vet. Med. 2023, 14, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-Producing Human Gut Symbiont, Clostridium Butyricum, and Its Role in Health and Disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef] [PubMed]
- Tang, X. Probiotic Roles of Clostridium Butyricum in Piglets: Considering Aspects of Intestinal Barrier Function. Animals 2024, 14, 1069. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R. The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Hautefort, I.; Thompson, A.; Hinton, J.C.; Van Immerseel, F. Butyrate Specifically Down-Regulates Salmonella Pathogenicity Island 1 Gene Expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Zheng, J.; Li, W.; Jiang, X.; Zhao, X.; Li, J.; Che, L.; Lin, Y.; Xu, S. Effects of Dietary Clostridium Butyricum Supplementation on Growth Performance, Intestinal Development, and Immune Response of Weaned Piglets Challenged with Lipopolysaccharide. J. Anim. Sci. Biotechnol. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Long, L.; Jin, X.; Li, Y.; Wu, Q.; Chen, X.; Geng, Z.; Zhang, C. Effects of Clostridium Butyricum on Growth Performance, Meat Quality, and Intestinal Health of Broilers. Front. Vet. Sci. 2023, 10, 1107798. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of Dietary Supplementation of Probiotic, Clostridium Butyricum, on Growth Performance, Immune Response, Intestinal Barrier Function, and Digestive Enzyme Activity in Broiler Chickens Challenged with Escherichia Coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, B.; Wang, L.; Sun, Q.; Deng, W.; Wei, F.; Ma, H.; Fu, C.; Wang, G.; Li, S. Effects of Clostridium Butyricum on Growth Performance, Gut Microbiota and Intestinal Barrier Function of Broilers. Front. Microbiol. 2021, 12, 777456. [Google Scholar] [CrossRef]
- Liang, J.; Kou, S.; Chen, C.; Raza, S.H.A.; Wang, S.; Ma, X.; Zhang, W.-J.; Nie, C. Effects of Clostridium Butyricum on Growth Performance, Metabonomics and Intestinal Microbial Differences of Weaned Piglets. BMC Microbiol. 2021, 21, 85. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Wan, X.; Li, K.; Jian, F.; Li, W.; Jiang, R.; Han, R.; Li, H.; Kang, X. Dietary Supplementation with Clostridium Butyricum Improves Growth Performance of Broilers by Regulating Intestinal Microbiota and Mucosal Epithelial Cells. Anim. Nutr. 2021, 7, 1105–1114. [Google Scholar] [CrossRef]
- Cai, L.; Hartanto, R.; Zhang, J.; Qi, D. Clostridium Butyricum Improves Rumen Fermentation and Growth Performance of Heat-Stressed Goats In Vitro and In Vivo. Animals 2021, 11, 3261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Lv, J.; Dou, X.; Zhang, Y. Effects of Dietary Supplementation with Clostridium Butyricum on the Amelioration of Growth Performance, Rumen Fermentation, and Rumen Microbiota of Holstein Heifers. Front. Nutr. 2021, 8, 763700. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Q.; Wang, J.; Yu, Y.; Zhang, Y.; Sun, Y. Effects of Dietary Supplementation with Clostridium Butyricum on Growth Performance, Apparent Digestibility, Blood Metabolites, Ruminal Fermentation and Bacterial Communities of Fattening Goats. Front. Nutr. 2022, 9, 888191. [Google Scholar] [CrossRef] [PubMed]
- Guzman, C.E.; Wood, J.L.; Egidi, E.; White-Monsant, A.C.; Semenec, L.; Grommen, S.V.H.; Hill-Yardin, E.L.; De Groef, B.; Franks, A.E. A Pioneer Calf Foetus Microbiome. Sci. Rep. 2020, 10, 17712. [Google Scholar] [CrossRef]
- Elolimy, A.; Alharthi, A.; Zeineldin, M.; Parys, C.; Helmbrecht, A.; Loor, J.J. Supply of Methionine during Late-Pregnancy Alters Fecal Microbiota and Metabolome in Neonatal Dairy Calves without Changes in Daily Feed Intake. Front. Microbiol. 2019, 10, 2159. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Guan, L.L. Regulation of Rumen Development in Neonatal Ruminants Through Microbial Metagenomes and Host Transcriptomes. Genome Biol. 2019, 20, 172. [Google Scholar] [CrossRef]
- National Institute of Animal Science. Korean Feeding Standard for Hanwoo; National Institute of Animal Science: Wanju, Republic of Korea, 2017.
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Larson, L.L.; Owen, F.G.; Albright, J.L.; Appleman, R.D.; Lamb, R.C.; Muller, L.D. Guidelines toward More Uniformity in Measuring and Reporting Calf Experimental Data. J. Dairy Sci. 1977, 60, 989–991. [Google Scholar] [CrossRef]
- Matull, W.R.; Pereira, S.P.; O’donohue, J.W. Biochemical Markers of Acute Pancreatitis. J. Clin. Pathol. 2006, 59, 340–344. [Google Scholar] [CrossRef]
- Sitrin, M.D. Digestion and Absorption of Carbohydrates and Proteins. In The Gastrointestinal System: Gastrointestinal, Nutritional and Hepatobiliary Physiology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 137–158. [Google Scholar]
- Whitcomb, D.C.; Lowe, M.E. Human Pancreatic Digestive Enzymes. Dig. Dis. Sci. 2007, 52, 1–17. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on Ruminant Saliva. 1. The Composition and Output of Sheep’s Saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef]
- Vargas-Rodriguez, C.F.; Engstrom, M.; Azem, E.; Bradford, B.J. Effects of Dietary Amylase and Sucrose on Productivity of Cows Fed Low-Starch Diets. J. Dairy Sci. 2014, 97, 4464–4470. [Google Scholar] [CrossRef]
- Boehlke, C.; Zierau, O.; Hannig, C. Salivary Amylase–The Enzyme of Unspecialized Euryphagous Animals. Arch. Oral Biol. 2015, 60, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Kanika, G.; Khan, S.; Jena, G. Sodium Butyrate Ameliorates L-arginine-induced Pancreatitis and Associated Fibrosis in Wistar Rat: Role of Inflammation and Nitrosative Stress. J. Biochem. Mol. Toxicol. 2015, 29, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Lee, E.-J.; Lee, J.-C.; Kim, W.-K.; Kim, H.-S. Anti-Inflammatory Effects of Short Chain Fatty Acids in IFN-γ-Stimulated RAW 264.7 Murine Macrophage Cells: Involvement of NF-ΚB and ERK Signaling Pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Sasaki, M.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T.; Kusunoki, M. Preventive Efficacy of Butyrate Enemas and Oral Administration of Clostridium Butyricum M588 in Dextran Sodium Sulfate-Induced Colitis in Rats. J. Gastroenterol. 2000, 35, 341–346. [Google Scholar] [CrossRef]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T. A Single Strain of Clostridium Butyricum Induces Intestinal IL-10-Producing Macrophages to Suppress Acute Experimental Colitis in Mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef]
- Hua, M.-C.; Lin, T.-Y.; Lai, M.-W.; Kong, M.-S.; Chang, H.-J.; Chen, C.-C. Probiotic Bio-Three Induces Th1 and Anti-Inflammatory Effects in PBMC and Dendritic Cells. World J. Gastroenterol. WJG 2010, 16, 3529. [Google Scholar] [CrossRef]
- Gao, Q.; Qi, L.; Wu, T.; Wang, J. Clostridium Butyricum Activates TLR2-Mediated MyD88-Independent Signaling Pathway in HT-29 Cells. Mol. Cell. Biochem. 2012, 361, 31–37. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive Effect of Short-Chain Fatty Acids on Production of Proinflammatory Mediators by Neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Mao, J.; Wang, L. Rumen Acidosis in Ruminants: A Review of the Effects of High-Concentrate Diets and the Potential Modulatory Role of Rumen Foam. Front. Vet. Sci. 2025, 12, 1595615. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lopez, E.; Petri, R.M.; Ricci, S.; Rivera-Chacon, R.; Sener-Aydemir, A.; Sharma, S.; Reisinger, N.; Zebeli, Q. Dynamic Changes in Salivation, Salivary Composition, and Rumen Fermentation Associated with Duration of High-Grain Feeding in Cows. J. Dairy Sci. 2021, 104, 4875–4892. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.B.; Aslam, M.; Lema, M.; Shin, I.S.; Le Ruyet, P.; Hogue, J.F.; Buchanan, D.S.; Miller, T.P.; Adams, G.D. Sodium Bicarbonate or Multielement Buffer via Diet or Rumen: Effects on Performance and Acid-Base Status of Lactating Cows. J. Dairy Sci. 1992, 75, 2409–2420. [Google Scholar] [CrossRef]
- Hassan, W.; Selim, H.M.; Abdelaal, A.M.; Abdallah, A. Evaluation of the Efficacy of Different Dietary Rumen Buffers on Prevention of Ruminal Acidosis in Goats. Slov. Veter. Res. 2023, 60, 17–27. [Google Scholar]
- Gianesella, M.; Morgante, M.; Cannizzo, C.; Stefani, A.; Dalvit, P.; Messina, V.; Giudice, E. Subacute Ruminal Acidosis and Evaluation of Blood Gas Analysis in Dairy Cow. Vet. Med. Int. 2010, 2010, 392371. [Google Scholar] [CrossRef]
- Apper-Bossard, E.; Faverdin, P.; Meschy, F.; Peyraud, J.-L. Effects of Dietary Cation-Anion Difference on Ruminal Metabolism and Blood Acid-Base Regulation in Dairy Cows Receiving 2 Contrasting Levels of Concentrate in Diets. J. Dairy Sci. 2010, 93, 4196–4210. [Google Scholar] [CrossRef]
- Han, Y.; Tang, C.; Li, Y.; Yu, Y.; Zhan, T.; Zhao, Q.; Zhang, J. Effects of Dietary Supplementation with Clostridium Butyricum on Growth Performance, Serum Immunity, Intestinal Morphology, and Microbiota as an Antibiotic Alternative in Weaned Piglets. Animals 2020, 10, 2287. [Google Scholar] [CrossRef]
- Potempa, M.; Potempa, J.; Kantyka, T.; Nguyen, K.-A.; Wawrzonek, K.; Manandhar, S.P.; Popadiak, K.; Riesbeck, K.; Eick, S.; Blom, A.M. Interpain A, A Cysteine Proteinase from Prevotella Intermedia, Inhibits Complement by Degrading Complement Factor C3. PLoS Pathog. 2009, 5, e1000316. [Google Scholar] [CrossRef]
- Wu, Q.-C.; Wang, W.-K.; Zhang, F.; Li, W.-J.; Wang, Y.-L.; Lv, L.-K.; Yang, H.-J. Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs. Microorganisms 2022, 10, 1105. [Google Scholar] [CrossRef]
- Doust, R.H.; Mobarez, A.M. Collagenase Activity in Prevotella Bivius Isolated from Patients with Premature Rupture of Membranes. Med. J. Islam. Repub. Iran 2004, 18, 61–66. [Google Scholar]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B. Sequence and Cultivation Study of Muribaculaceae Reveals Novel Species, Host Preference, and Functional Potential of This yet Undescribed Family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Ding, Y.; Soh, M.; Low, A.; Seedorf, H. Muribaculum gordoncarteri sp. Nov., an Anaerobic Bacterium from the Faeces of C57BL/6J Mice. Int. J. Syst. Evol. Microbiol. 2020, 70, 4725–4729. [Google Scholar] [CrossRef]
- Mao, J.; Wang, L.; Wang, Z.; Xue, B.; Peng, Q.; Hu, R.; Xiao, J. High Concentrate Diets Altered the Structure and Function of Rumen Microbiome in Goats. Front. Microbiol. 2024, 15, 1416883. [Google Scholar] [CrossRef]
- Cheng, L.; Kun, K.; Gang, T.; Mohammad, A.-M.; Bai, X.U.E.; Gicheha, M.G. Effects of Yeast and Yeast Cell Wall Polysaccharides Supplementation on Beef Cattle Growth Performance, Rumen Microbial Populations and Lipopolysaccharides Production. J. Integr. Agric. 2020, 19, 810–819. [Google Scholar] [CrossRef]
- Isaacson, R.; Kim, H.B. The Intestinal Microbiome of the Pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Ma, Q.; Liu, Y.; Khan, M.Z.; Wu, B.; Chen, W.; Liu, X.; Wang, C.; Li, Y. Exploring the Effect of Gastrointestinal Prevotella on Growth Performance Traits in Livestock Animals. Animals 2024, 14, 1965. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, M.; Zhou, X.; Li, B.; Zhang, J. Epidemic Characterization and Molecular Genotyping of Shigella Flexneri Isolated from Calves with Diarrhea in Northwest China. Antimicrob. Resist. Infect. Control 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Hamidian, M.; Rezadehbashi, M.; Doyle, M.; Salmanzadeh-Ahrabi, S.; Derakhshan, F.; Reza Zali, M. Prevalence and Antimicrobial Resistance of Diarrheagenic Escherichia Coli and Shigella Species Associated with Acute Diarrhea in Tehran, Iran. Can. J. Infect. Dis. Med. Microbiol. 2009, 20, e56–e62. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).