Do Hatchery-Reared Southern Pygmy Perch (Nannoperca australis) Develop Effective Survival Behaviour in a Soft-Release Site?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Source of Fish
2.2. Laboratory Housing
2.3. Behavioural Testing
2.4. Predator Tests
2.5. Novel Food Test
2.6. Statistical Analysis
3. Results
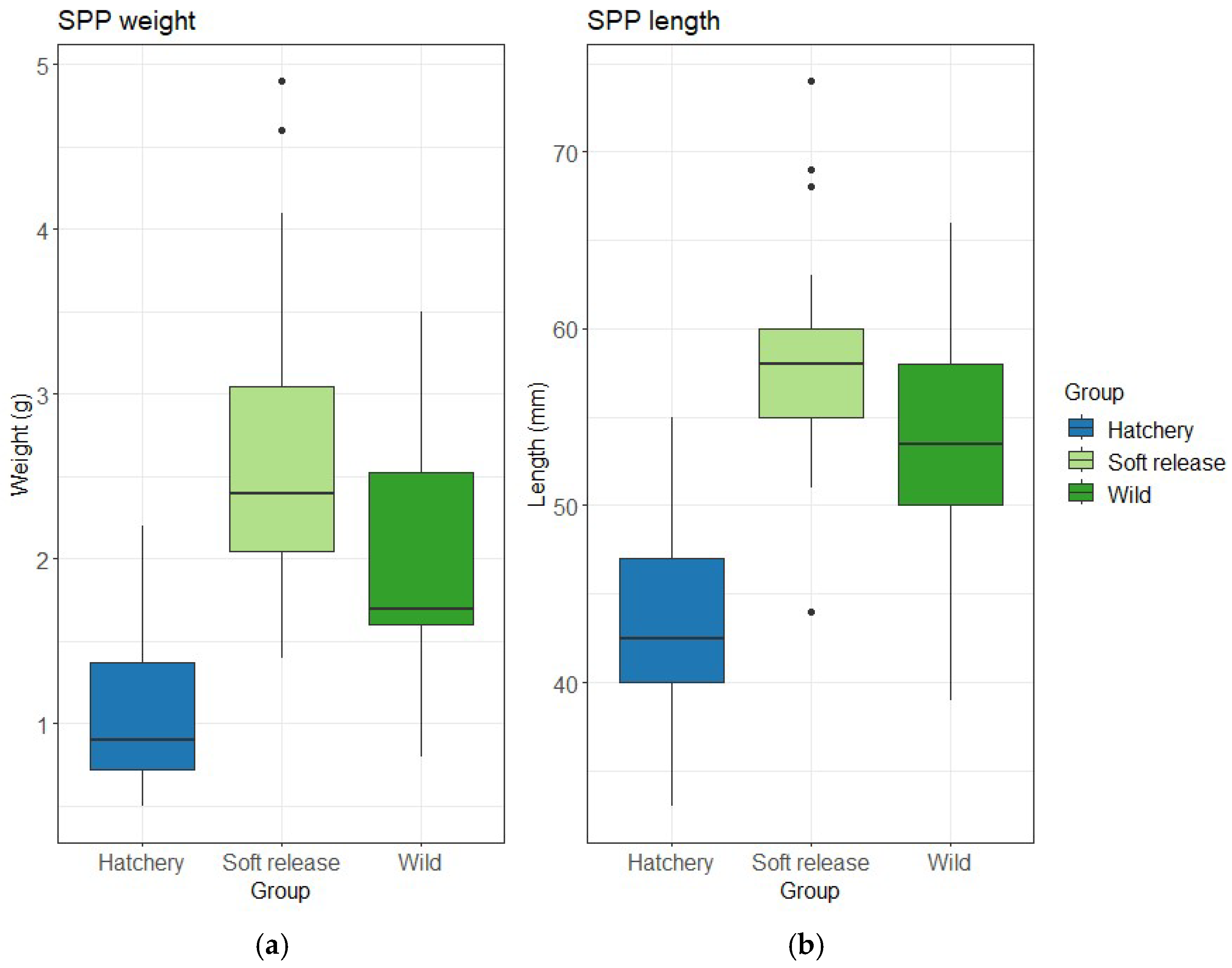
3.1. Fish Size
3.2. Behaviour
3.3. Predator Tests
3.4. Novel Food Test
3.5. Developmental Context: Juvenile Baseline Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lintermans, M. Review of on-ground recovery actions for threatened freshwater fish in Australia. Mar. Freshw. Res. 2013, 64, 775–791. [Google Scholar] [CrossRef]
- Koehn, J.D.; Raymond, S.M.; Stuart, I.; Todd, C.R.; Balcombe, S.R.; Zampatti, B.P.; Bamford, H.; Ingram, B.A.; Bice, C.M.; Burndred, K.; et al. A compendium of ecological knowledge for restoration of freshwater fishes in Australia. Mar. Freshw. Res. 2020, 71, 1391. [Google Scholar] [CrossRef]
- Lake, P.S.; Bond, N.R. Australian futures: Freshwater ecosystems and human water usage. Futures 2007, 39, 288–305. [Google Scholar] [CrossRef]
- Todd, C.R.; Koehn, J.D.; Pearce, L.; Dodd, L.; Humphries, P.; Morrongiello, J.R. Forgotten fishes: What is the future for small threatened freshwater fish? Population risk assessment for southern pygmy perch, Nannoperca australis. Aquat. Conserv. 2017, 27, 1290–1300. [Google Scholar] [CrossRef]
- Philippart, J.C. Is captive breeding an effective solution for the preservation of endemic species? Biol. Conserv. 1995, 72, 281–295. [Google Scholar] [CrossRef]
- Rytwinski, T.; Kelly, L.A.; Donaldson, L.A.; Taylor, J.J.; Smith, A.; Drake, D.A.R.; Martel, A.L.; Geist, J.; Morris, T.J.; George, A.L.; et al. What evidence exists for evaluating the effectiveness of conservation-oriented captive breeding and release programs for imperilled freshwater fishes and mussels? Can. J. Fish. Aquat. Sci. 2021, 78, 1332–1346. [Google Scholar] [CrossRef]
- Näslund, J. Reared to become wild-like: Addressing behavioral and cognitive deficits in cultured aquatic animals destined for stocking into natural environments—A critical review. Bull. Mar. Sci. 2021, 97, 489–538. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Sperry, J.H.; DeGregorio, B.A. Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biol. Conserv. 2019, 236, 324–331. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Ballew, N.G.; Kjelvik, M.K. Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 2014, 71, 927–944. [Google Scholar] [CrossRef]
- Hutchison, M.; Butcher, A.; Norris, A.; Kirkwood, J.; Chilcott, K. Review of Domestication Effects on Stocked Fishes, Strategies to Improve Post Stocking Survival of Fishes and Their Potential Application to Threatened Fish Species Recovery Programs in the Murray–Darling Basin; Murray-Darling Basin Authority: Canberra, Australia, 2012. [Google Scholar]
- Johnsson, J.I.; Brockmark, S.; Näslund, J. Environmental effects on behavioural development consequences for fitness of captive-reared fishes in the wild. J. Fish Biol. 2014, 85, 1946–1971. [Google Scholar] [CrossRef]
- Carrera-García, E.; Rochard, E.; Acolas, M.-L. Effects of rearing practice on post-release young-of-the-year behavior: Acipenser sturio early life in freshwater. Endanger. Species Res. 2017, 34, 269–281. [Google Scholar] [CrossRef]
- Brown, C.; Day, R.L. The future of stock enhancements: Lessons for hatchery practice from conservation biology. Fish Fish. 2002, 3, 79–94. [Google Scholar] [CrossRef]
- Salvanes, A.G.V. Are antipredator behaviours of hatchery Salmo salar juveniles similar to wild juveniles? J. Fish Biol. 2017, 90, 1785–1796. [Google Scholar] [CrossRef]
- Jackson, C.D.; Brown, G.E. Differences in antipredator behaviour between wild and hatchery-reared juvenile Atlantic salmon (Salmo salar) under seminatural conditions. Can. J. Fish. Aquat. Sci. 2011, 68, 2157–2166. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 2007, 318, 100–103. [Google Scholar] [CrossRef]
- Baerwald, M.R.; Kwan, N.; Pien, C.; Auringer, G.; Carson, E.W.; Cocherell, D.E.; Ellison, L.; Fangue, N.A.; Finger, A.J.; Gille, D.A.; et al. Captive-reared Delta Smelt (Hypomesus transpacificus) exhibit high survival in natural conditions using in situ enclosures. PLoS ONE 2023, 18, e0286027. [Google Scholar] [CrossRef]
- Sneddon, L.U. The bold and the shy: Individual differences in rainbow trout. J. Fish Biol. 2003, 62, 971–975. [Google Scholar] [CrossRef]
- Sales, E.; Rogers, L.; Freire, R.; Luiz, O.; Kopf, R.K. Bold-shy personality traits of globally invasive, native and hatchery-reared fish. R. Soc. Open Sci. 2023, 10, 231035. [Google Scholar] [CrossRef]
- Lintermans, M. Southern pygmy perch, Nannoperca australis. In A Guide to the Freshwater Fishes of South-Eastern Australia; CSIRO Publishing: Clayton, Australia, 2013; pp. 165–167. [Google Scholar]
- Pearce, L.; Bice, C.; Whiterod, N.; Raadik, T. Nannoperca australis, Southern Pygmy Perch. In The IUCN Red List of Threatened Species 2019; e.T123358579A123382811; International Union for Conservation of Nature and Natural Resources: Fontainebleau, France, 2019. [Google Scholar]
- Buckley, S.J.; Brauer, C.; Lamin, C.; Rose, P.; Vornicu, D.E.; Beheregaray, L.B. A community-driven captive-breeding and reintroduction program maintains genetic diversity in a threatened freshwater fish. Conserv. Sci. Pract. 2024, 6, e13054. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Stuart, I.G.; Sharpe, C. The Native Fish Recovery Plan: Gunbower and Lower Loddon. Report Prepared for the North Central Catchment Management Authority. Available online: https://www.nccma.vic.gov.au/media/documents/native_fish_recovery_plan_gunbower_and_lower_loddon_2014.pdf (accessed on 16 September 2025).
- Raymond, S.M.; Ryall, J.; Fanson, B.; Day, S.; Koehn, J.D.; Todd, C.R.; Kitchingman, A.; Loeun, K.; Iscaro, B.; Hogan, L.; et al. Farm dams: A valuable interim step in small-bodied threatened fish conservation. Austral Ecol. 2024, 49, e70001. [Google Scholar] [CrossRef]
- Freire, R.; Michie, M.; Rogers, L.; Shamsi, S. Age-related changes in survival behaviour in parasite-free hatchery-reared Rainbow trout (Oncorhynchus mykiss). Animals 2024, 14, 1315. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 17 July 2024).
- Nash, R.D.; Valencia, A.H.; Geffen, A.J. The origin of Fulton’s condition factor—Setting the record straight. Fisheries 2006, 31, 236–238. [Google Scholar]
- Fonken, D.R.; Conner, M.M.; Walsworth, T.E.; Thompson, P.D. Benefits of stocking fewer but larger individuals with implications for native fish recovery. Can. J. Fish. Aquat. Sci. 2023, 80, 439–450. [Google Scholar] [CrossRef]
- Hyvärinen, P.; Vehanen, T. Effect of brown trout body size on post-stocking survival and pike predation. Ecol. Freshw. Fish 2004, 13, 77–84. [Google Scholar] [CrossRef]
- Humphries, P. Life history, food and habitat of southern pygmy perch, Nannoperca australis, in the Macquarie River, Tasmania. Mar. Freshw. Res. 1995, 46, 1159–1169. [Google Scholar] [CrossRef]
- Warfe, D.M.; Barmuta, L.A. Habitat structural complexity mediates foraging success in freshwater fish. Oecologia 2004, 141, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Domenici, P. The visually mediated escape response in fish: Predicting prey responsiveness and the locomotor behaviour of predators and prey. Mar. Freshwr. Behav. Physiol. 2002, 35, 87–110. [Google Scholar] [CrossRef]
- Meager, J.J.; Rodewald, P.; Domenici, P.; Fernö, A.; Järvi, T.; Skjaeraasen, J.E.; Sverdrup, G.K. Behavioural responses of hatchery-reared and wild cod Gadus morhua to mechano-acoustic predator signals. J. Fish Biol. 2011, 78, 1437–1450. [Google Scholar] [CrossRef]
- Brown, C. Familiarity with the test environment improves escape responses in the crimson spotted rainbowfish, Melanotaenia duboulayi. Anim. Cogn. 2001, 4, 109–113. [Google Scholar] [CrossRef]
- Berejikian, B.A. The effects of hatchery and wild ancestry and experience on the relative ability of steelhead trout fry (Oncorhynchus mykiss) to avoid a benthic predator. Can. J. Fish. Aquat. Sci. 1995, 52, 2476–2482. [Google Scholar] [CrossRef]
- Oulton, L.J.; Haviland, V.; Brown, C. Predator Recognition in Rainbowfish, Melanotaenia duboulayi, Embryos. PLoS ONE 2013, 8, e76061. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Davidson, T.; Laland, K. Environmental enrichment and prior experience of live prey improve foraging behaviour in hatchery-reared Atlantic salmon. J. Fish Biol. 2003, 63, 187–196. [Google Scholar] [CrossRef]
- Ballew, N.G.; Mittelbach, G.G.; Scribner, K.T.; Doorn, G.S.V.; Alice, A.W. Fitness Consequences of Boldness in Juvenile and Adult Largemouth Bass. Am. Nat. 2017, 189, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish.2 Environ. Biol. Fish. 2015, 98, 825–832. [Google Scholar] [CrossRef]


| Behaviour/Test | Global Test (χ2/F, df, p) | Significant Pairwise Differences (Dunn or GLM) |
|---|---|---|
| Size—Weight | KW χ2 = 46.465, df = 2, p < 0.001 | Soft release > Hatchery (p < 0.001); Wild > Hatchery (p < 0.001); Soft release > Wild (p = 0.036) |
| Size—Length | KW χ2 = 42.721, df = 2, p < 0.001 | Soft release > Hatchery (p < 0.001); Wild > Hatchery (p < 0.001); Soft release vs. Wild ns (p = 0.062) |
| Emergence latency | KW χ2 = 6.132, df = 2, p = 0.105 | None |
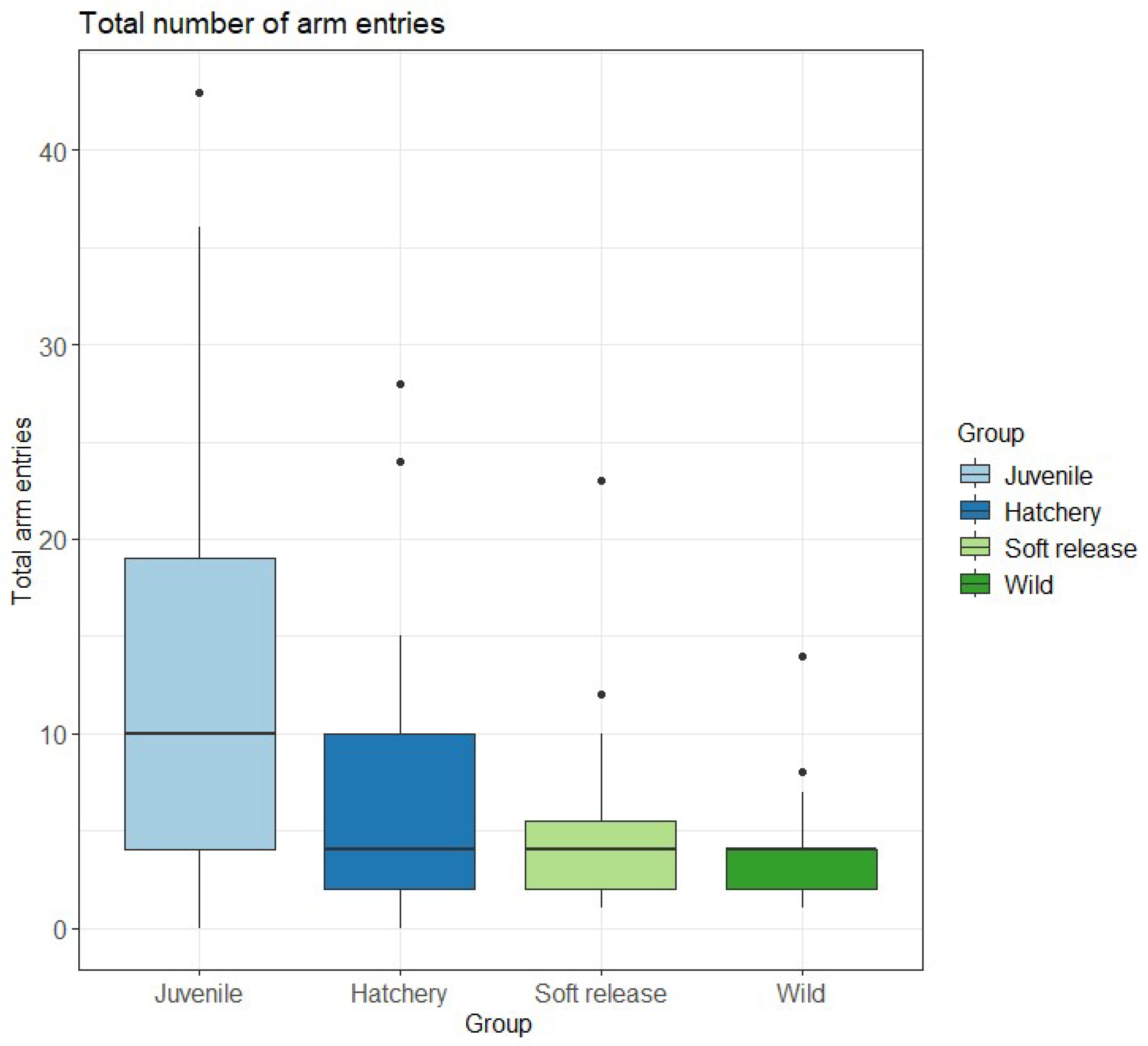
| Exploration (arms) | KW χ2 = 12.281, df = 3, p = 0.007 | Juvenile > Soft release (p = 0.029); Juvenile > Wild (p = 0.010) |
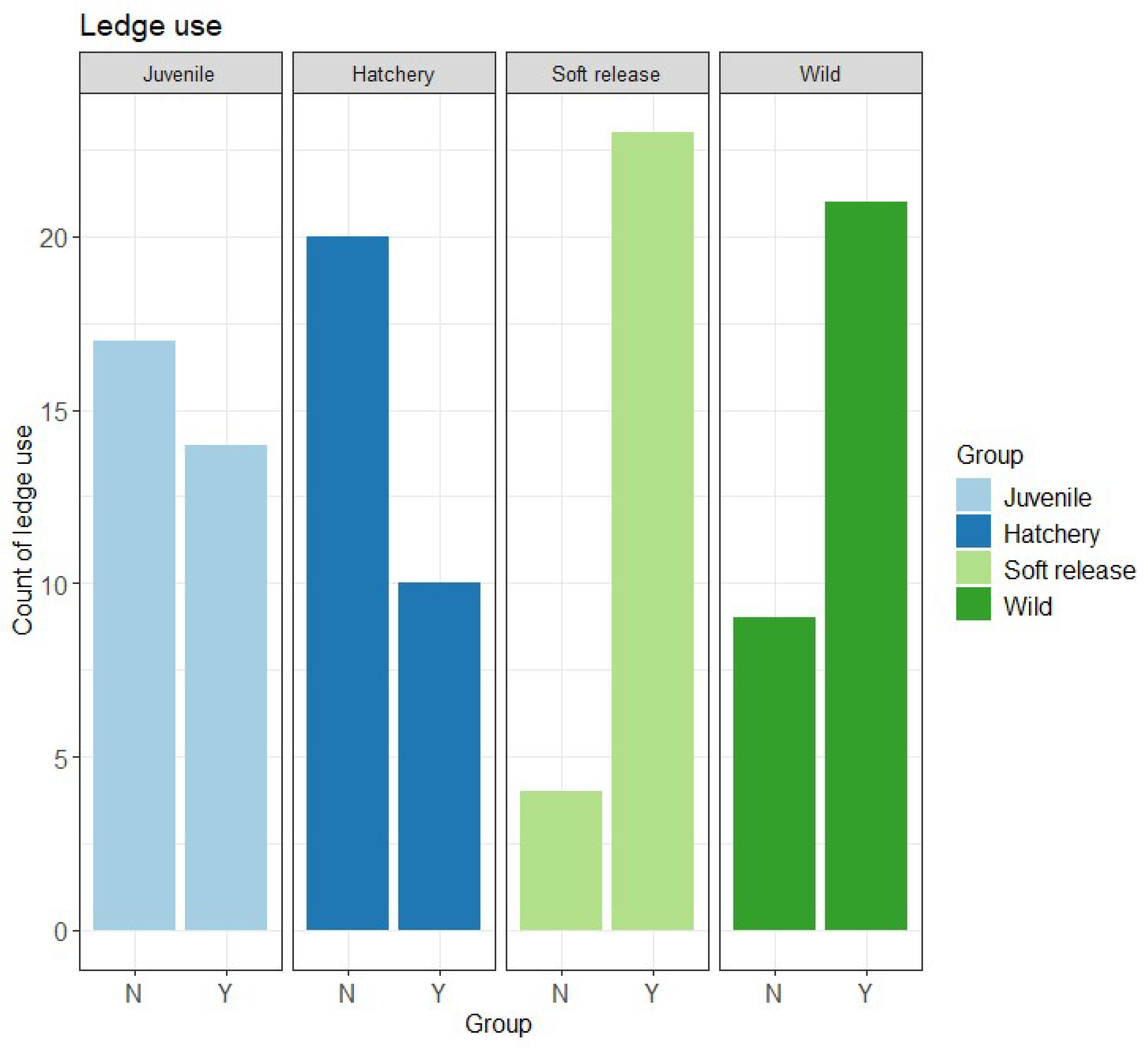
| Ledge use (binary) | GLM F3,114 = 20.647, p = 0.0001 | Wild and Soft release > Hatchery and Juvenile |
| Avian avoid (likelihood) | GLM F3,114 = 3.786, p = 0.285 | None |
| Fish avoid (likelihood) | GLM F3,114 = 2.101, p = 0.551 | None |
| Avian freeze (likelihood) | GLM F3,114 = 7.381, p = 0.060 | None |
| Fish freeze (likelihood) | GLM F3,114 = 1.079, p = 0.782 | None |
| Latency—Avian avoid | KW χ2 = 6.693, df = 3, p = 0.082 | None |
| Latency—Fish avoid | KW χ2 = 11.454, df = 3, p = 0.010 | Wild < Juvenile (p = 0.011) |
| Latency—Avian freeze | KW χ2 = 8.992, df = 3, p = 0.029 | Soft release > Hatchery (p = 0.025) |
| Latency—Fish freeze | KW χ2 = 13.832, df = 3, p = 0.003 | Soft release > Juvenile (p = 0.026) |
| Novel food (inspect) | GLM F3,114 = 6.748, p = 0.080 | None |
| Novel food (latency) | KW χ2 = 1.685, df = 3, p = 0.640 | None |
| Group | Empty | Small Rocks | Large Rocks | Plants |
|---|---|---|---|---|
| Juvenile | 6.8% | 36.8% | 27.9% | 28.6% |
| Hatchery | 4.7% | 30.7% | 22.7% | 41.9% |
| Soft-Release | 4.8% | 33.3% | 28.5% | 33.3% |
| Wild | 7.8% | 36.6% | 21.7% | 33.9% |
| Total | 6.1% | 34.4% | 25.1% | 34.4% |
| KW χ2 (df = 3) | 1.01 | 0.38 | 1.807 | 1.134 |
| p | 0.799 | 0.944 | 0.613 | 0.769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, J.; Rose, P.; Price, A.; Freire, R. Do Hatchery-Reared Southern Pygmy Perch (Nannoperca australis) Develop Effective Survival Behaviour in a Soft-Release Site? Animals 2025, 15, 2754. https://doi.org/10.3390/ani15182754
King J, Rose P, Price A, Freire R. Do Hatchery-Reared Southern Pygmy Perch (Nannoperca australis) Develop Effective Survival Behaviour in a Soft-Release Site? Animals. 2025; 15(18):2754. https://doi.org/10.3390/ani15182754
Chicago/Turabian StyleKing, James, Peter Rose, Amina Price, and Rafael Freire. 2025. "Do Hatchery-Reared Southern Pygmy Perch (Nannoperca australis) Develop Effective Survival Behaviour in a Soft-Release Site?" Animals 15, no. 18: 2754. https://doi.org/10.3390/ani15182754
APA StyleKing, J., Rose, P., Price, A., & Freire, R. (2025). Do Hatchery-Reared Southern Pygmy Perch (Nannoperca australis) Develop Effective Survival Behaviour in a Soft-Release Site? Animals, 15(18), 2754. https://doi.org/10.3390/ani15182754







