The Red-Colored Oddball—A New Ladybird Spider with Unusual Coloring from Morocco, Eresus rubrocephalus sp. nov. (Araneae: Eresidae)
Simple Summary
Abstract
1. Introduction

2. Materials and Methods
2.1. Samples
2.2. Morphological Examination
2.3. SEM
2.4. Genetic Examination
2.5. Software
3. Results
3.1. Material Examined
3.1.1. Holotype
3.1.2. Paratype
3.2. Etymology
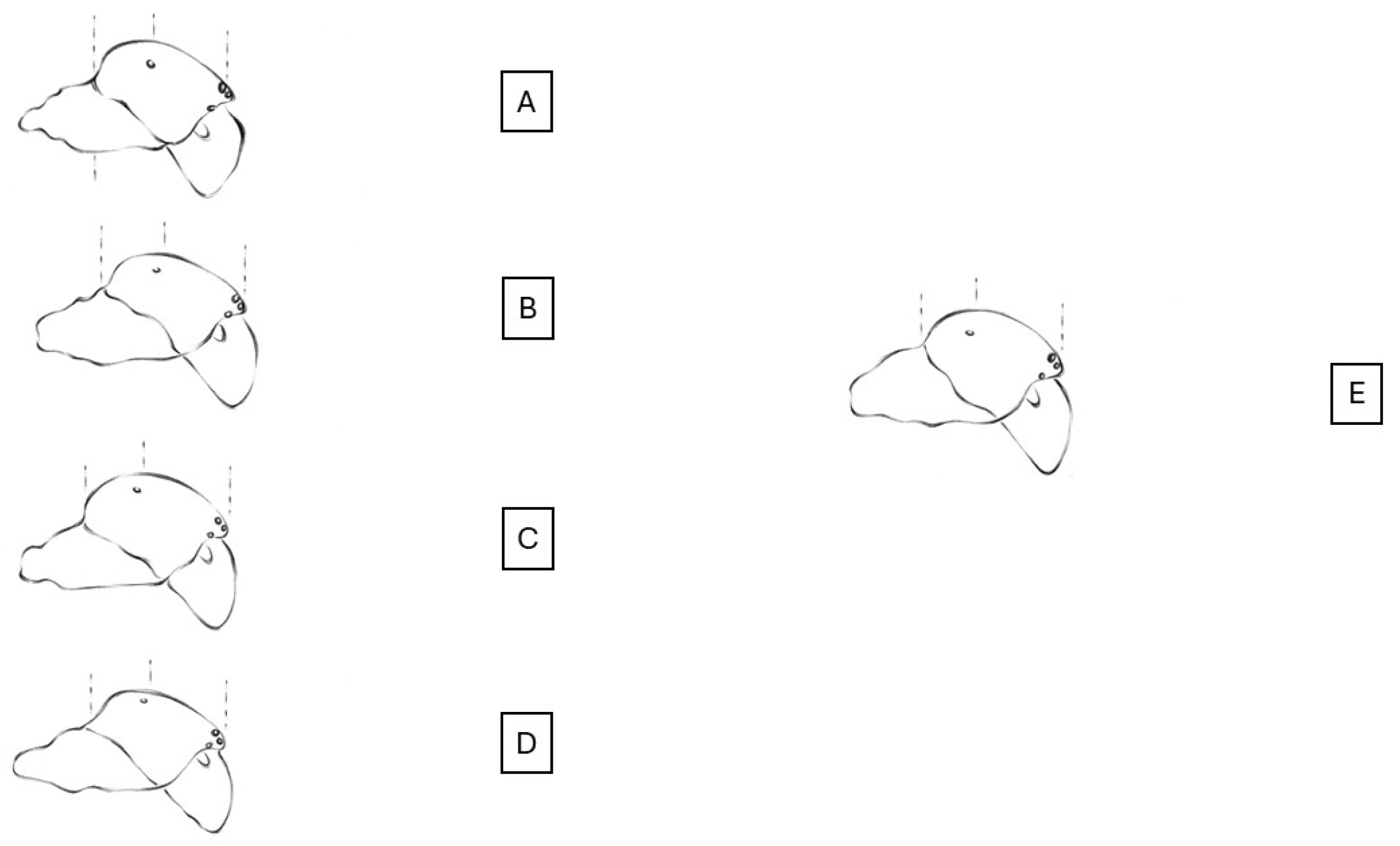
3.3. Diagnosis
3.4. Description
3.4.1. Male
3.4.2. Female
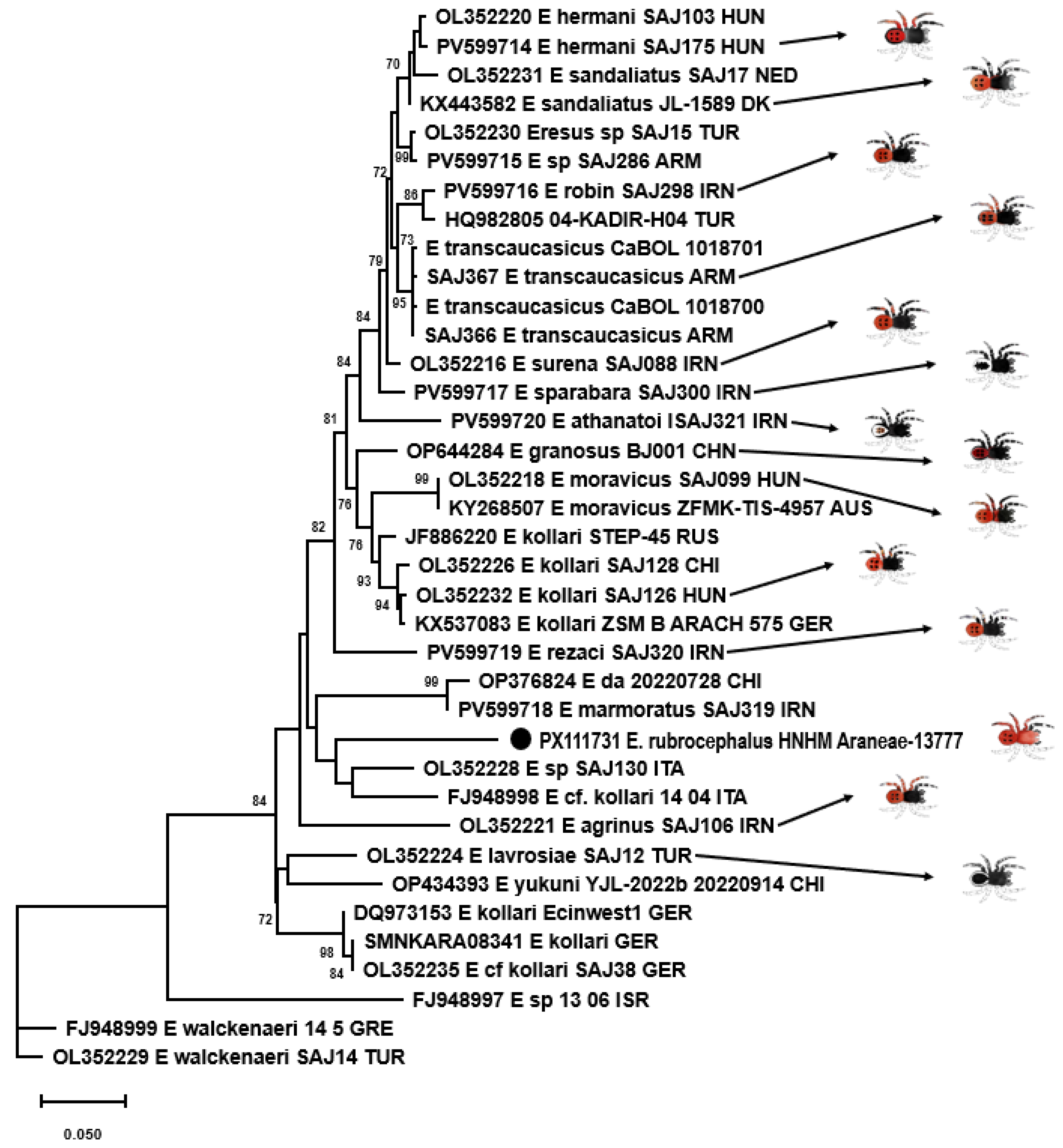
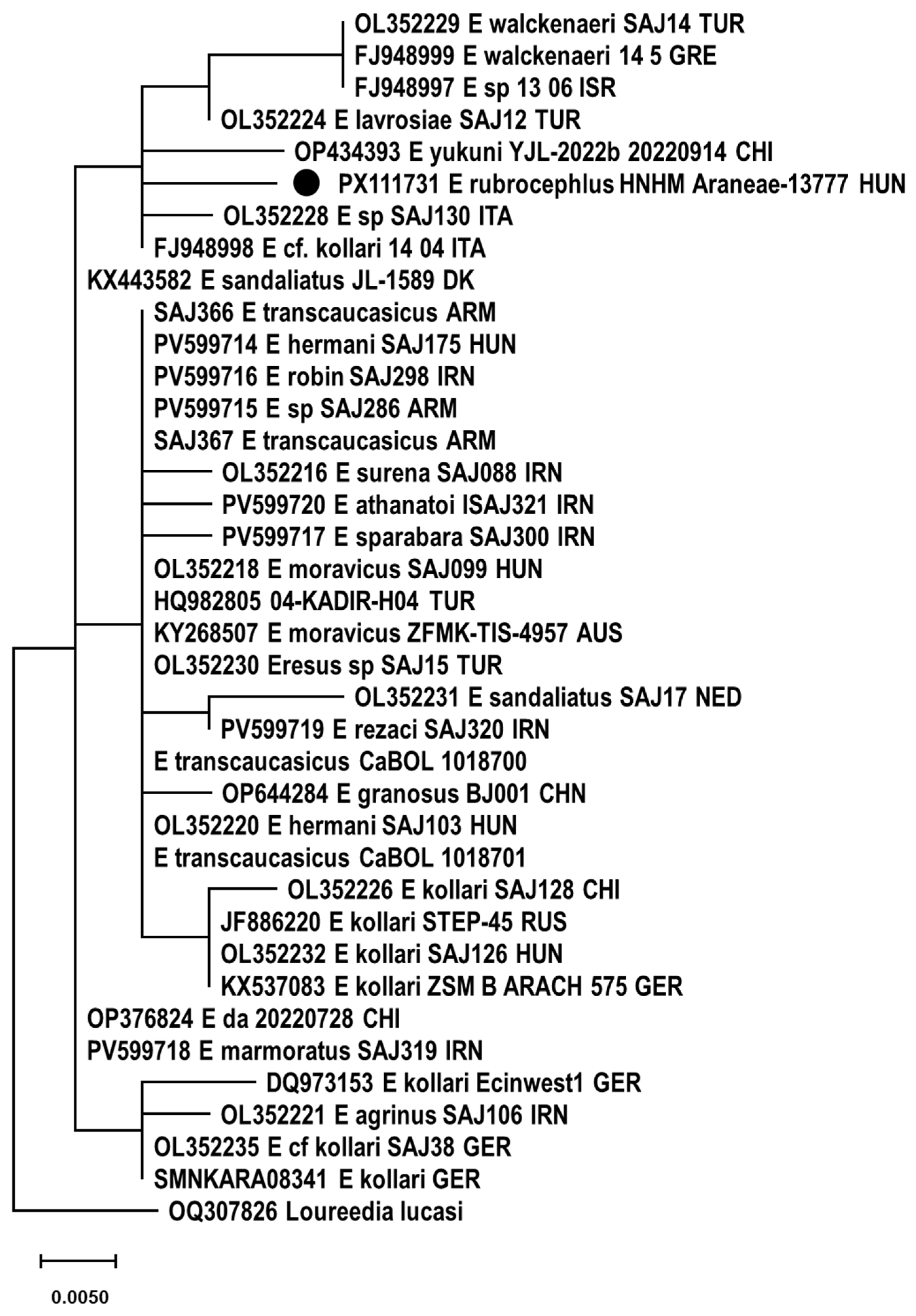
3.5. Genetic Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Lm | lamella |
| Lmg | lamellar groove |
| Sc | shoulder of the conductor |
| Tt | terminal tooth |
References
- Canard, A.; Cruveillier, M. Rectifications nomenclaturales á apporter au catalogue Mondial des araigées (1ére note). Rev. Arachnol. 2016, 3, 42–46. [Google Scholar]
- Geci, D.; Naumova, M.; Ibrahimi, H.; Grapci-Kotori, L.; Gashi, A.; Bilalli, A.; Musliu, M. Contribution to spider fauna (Arachnida: Araneae) from Bjeshket e Nemuna Mountains (Kosovo). Nat. Croat. 2023, 32, 535–547. [Google Scholar] [CrossRef]
- Thaler, K.; Knoflach, B. Zur Fantastik der Spinnen (Araneae) von Österreich: Atypidae, Haplogynae, Eresidae, Zodariidae, Mimetidae. Linz. Biol. Beitr. 2002, 34, 413–444. [Google Scholar]
- El-Hannawy, H.K. Review of spiders of genus Eresus in Egypt (Araneida: Eresidae). Serket 2004, 9, 25–35. [Google Scholar]
- Rezác, M.; Pekár, S.; Johannsen, J. Taxonomic reniew and phylogenetic analysis of central European Eresus species (Araneae: Eresidae). Zool. Scr. 2008, 37, 263–287. [Google Scholar] [CrossRef]
- Wang, J.F. Two species of spiders of the genus Eresus from China (Araneae: Eresidae). J. Hebei Norm. Univ 1994, 11–13. Available online: https://wsc.nmbe.ch/reference/7843 (accessed on 5 September 2025).
- Breitling, E.; Bauer, T.; Schafer, M.; Morano, E.; Barrientos, J.A.; Blick, T. Phantom spider 2: More notes on dubious spider species from Europe. Arachnol. Mitt. 2016, 52, 50–77. [Google Scholar] [CrossRef]
- Naumova, M.; Deltshev, C. New faunistic and taxonomic notes on the haplogyne and cribellate spiders (Araneae: Dictynidae, Dysderidae, Eresidae, Filistatidae, Sicariidae) from three Balkan countries. Acta Zool. Acad. Sci. Hung. 2021, 67, 63–76. [Google Scholar] [CrossRef]
- Yanul, V.; Terekhova, V.; Polchaninova, N. New data on the rare spider species (Arachnida, Araneae) from Kyiv region (Ukraine). Zoodiversity 2022, 56, 181–188. [Google Scholar] [CrossRef]
- Hoyas, J.; Ferrnández, M.Á. Aranas (Araneae) de la Reserva fluvial del Rio Guadyerbas, Toledo centro de Espana. Rev. Iber. Arachnol. 2023, 43, 67–74. [Google Scholar]
- Kara, C.; Demir, H.; Seyyar, O. New locality record of Eresus lavrosii Mcheidze, 1997 (Araneae: Eresidae) in Antolia. Serket 2025, 20, 435–438. [Google Scholar]
- Seropian, A.; Bulbulashvili, N.; Makharadze, G.; Kovács, G. From burrows to spotlight: First description of the female of Eresus lavrosii Mcheidte, 1997 (Araneae, Eresidae), with notes ont he natural history. Caucasiana 2025, 4, 1–14. [Google Scholar]
- Zamani, A.; Seropian, A.; Zarikian, N.; Bulbulashvili, N.; Szűts, T. Different, but still the same: Integrative taxonomy confirms a new species of Eresus Walckenaer, 1805 (Araneae, Eresidae) from the South Caucasus. ZooKeys 2025, 1249, 1–13. [Google Scholar] [CrossRef] [PubMed]
- World Spider Catalog. Version 26. Available online: https://wsc.nmbe.ch/ (accessed on 12 August 2025).
- Lecigne, S.; Moutaouakil, S.; Lips, J. Contribution to the knowledge of the spider fauna of Morocco (Arachnida: Araneae)–Second note, on new species and new records from caves and miscellaneous terrestrial ecosystems. J. Belg. Arachnol. Soc. 2025, 40, 1–184. [Google Scholar]
- Rezác, M.; Vanek, O.; Strestík, V. Eresus elhennawyi sp. n. (Araneae: Eresidae), a new velvet spider mimicking mutilid wasps from north-western Africa. Serket 2023, 19, 340–354. [Google Scholar]
- Kovács, G.; Prazsák, I.; Eichardt, J.; Vári, G.; Gyurkovics, H. A new ladybird spider from Hungary (Araneae, Eresidae). ZooKeys 2015, 494, 13–30. [Google Scholar] [CrossRef]
- Zamani, A.; Altin, C.; Szűts, T. A black sheep in Eresus (Araneae: Eresidae): Taxonomic notes on the ladybird spiders of Iran and Turkey, with a new species. Zootaxa 2020, 4851, 559–572. [Google Scholar] [CrossRef]
- Popovici, G.; Iorgu, E.I. First record of Eresus moravicus Rezác, 2008 (Araneae: Eresidae) from Romania. Arachnology 2022, 19, 31–37. [Google Scholar] [CrossRef]
- Marusik, Y.M.; Azarkina, G.N. Who is Eresus tristis Kroneberg, 1875 (Aranei: Eresidae)? Arthropoda Sel. 2020, 29, 470–474. [Google Scholar] [CrossRef]
- Al-Yacoub, G.A.A.; Al-Budeiri, A.S.M.; Zamani, A. A new species of Eresus Walckenaer, 1805 (Araneae: Eresidae) from Iraq. Arachnology 2025, 20, 31–33. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, L.Y. Chinese Spiders Illustrated; Chongqing University Press: Chongqing, China, 2017; p. 954. [Google Scholar]
- Simon, E. Aranéides nouveaux ou peu connus du midi de l’Europe (2e mémorie). Mémories Soc. R. Sci. Liége 1873, 5, 1–174. [Google Scholar]
- Breitling, R. South European spiders from the Duffey collection in Manchester Museum (Arachnida: Araneae). Arachnology 2020, 18, 333–362. [Google Scholar] [CrossRef]
- Zamani, A.; Szabó, K.; Szűts, T. Persian treasures: Integrative tanonomy reveals high species’ diversity of ladybird spiders (Araneae: Eresidae: Eresus). Zool. J. Linn. Soc. 2025, 204, 1–19. [Google Scholar] [CrossRef]
- Fehér, E.; Kaszab, E.; Bali, K.; Hoitsy, M.; Sós, E.; Bányai, K. Novel Circoviruses from Birds Share Common Evolutionary Roots with Fish Origin Circoviruses. Life 2022, 12, 368. [Google Scholar] [CrossRef]
- Larsson, A. A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Vences, M.; Miralles, A.; Brouillet, S.; Ducasse, J.; Fedosov, A.; Kharchev, V.; Kumari, S.; Patmanidis, S.; Puillandre, N.; Scherz, M.D.; et al. iTaxoTools 0.1: Kickstarting a specimen-based software toolkit for taxonomists. Megataxa 2021, 6, 77–92. [Google Scholar] [CrossRef]
- Miller, J.A.; Griswold, C.E.; Scharff, N.; Rezác, M.; Szűcs, T.; Marhabaie, M. The velvet spiders: An atlas of the Eresidae (Arachnida, Araneae). ZooKeys 2012, 195, 1–144. [Google Scholar] [CrossRef]
- Lin, Y.; Li, S.; Zhao, X.; Chen, Z.; Chen, H. Two new Eresus species (Araneae, Eresidae) from Xinjiang, China. Biodivers. Data J. 2022, 10, e94853. [Google Scholar] [CrossRef]
- Robinson, E.A.; Blagoev, G.A.; Hebert, P.D.N.; Adamowicz, S.J. Prospects for using DNA barcoding to identify spiders in species-rich genera. ZooKeys 2009, 16, 27–46. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, J.; Kovács, G.; Vincze, Z.; Keve, G.; Páll-Gergely, B.; Bagyó, R.; Fehér, E.; Bali, K.; Kaszab, E. The Red-Colored Oddball—A New Ladybird Spider with Unusual Coloring from Morocco, Eresus rubrocephalus sp. nov. (Araneae: Eresidae). Animals 2025, 15, 2707. https://doi.org/10.3390/ani15182707
Gál J, Kovács G, Vincze Z, Keve G, Páll-Gergely B, Bagyó R, Fehér E, Bali K, Kaszab E. The Red-Colored Oddball—A New Ladybird Spider with Unusual Coloring from Morocco, Eresus rubrocephalus sp. nov. (Araneae: Eresidae). Animals. 2025; 15(18):2707. https://doi.org/10.3390/ani15182707
Chicago/Turabian StyleGál, János, Gábor Kovács, Zoltán Vincze, Gergő Keve, Barna Páll-Gergely, Richárd Bagyó, Enikő Fehér, Krisztina Bali, and Eszter Kaszab. 2025. "The Red-Colored Oddball—A New Ladybird Spider with Unusual Coloring from Morocco, Eresus rubrocephalus sp. nov. (Araneae: Eresidae)" Animals 15, no. 18: 2707. https://doi.org/10.3390/ani15182707
APA StyleGál, J., Kovács, G., Vincze, Z., Keve, G., Páll-Gergely, B., Bagyó, R., Fehér, E., Bali, K., & Kaszab, E. (2025). The Red-Colored Oddball—A New Ladybird Spider with Unusual Coloring from Morocco, Eresus rubrocephalus sp. nov. (Araneae: Eresidae). Animals, 15(18), 2707. https://doi.org/10.3390/ani15182707






