Simple Summary
In this study, a new species of the genus Pseudocalotes is described from Yingjiang County, Dehong Dai, and Jingpo Autonomous Prefecture, Yunnan Province, China, based on four female specimens. Phylogenetic analyses based on NADH dehydrogenase subunit 2 (ND2) and NADH dehydrogenase subunit 4 (ND4) indicated that the new taxon is different from its congeners. Morphologically, the new species can be diagnosed from the other species by a combination of 18 characters. The recognition of this new species brings the number of described Pseudocalotes species to 22.
Abstract
In this study, a new species of the genus Pseudocalotes is described from Yingjiang County, Dehong Dai and Jingpo Autonomous Prefecture, Yunnan Province, China, based on four female specimens. It can be distinguished from its congeners by the following combination of characters: (1) interoculabials 3 or 4; (2) canthals 5–7; (3) cicrcumorbitals 8–11; (4) 1 scale between rostral and nasal; (5) interparietal 1; (6) superciliaries 4–6; (7) supralabials 6–7, the 1st in contact with the nasal; (8) infralabials 6–8; (9) transverse gular fold and antehumeral fold present; (10) 2–3 enlarged scales between eye and ear; (11) nuchal crest single, consists of 3–5 erected spines; (12) dorsal crest row single, discontinuous and low, located between two keeled, parallel and enlarged scale rows; (13) enlarged postrictals absent; (14) scales around midbody 53–62, dorsal body scales heterogenous in size and shape; (15) midventrals smaller than dorsals; (16) subdigital scales on the 4th finger 20–26, and on the 4th toe 24–29; (17) dorsal background coloration light taupe with four irregular brown patches along the middle of dorsal; (18) inner lips wathet, tongue aurantiacus, throat bluish black. The population from Yingjiang County was nested within a highly supported lineage, formed a sister taxon with P. kakhienensis (SH 97/UFB 100) and according to the p-distance, the new species differed from its congeners by 14.5% to 35.2% for NADH dehydrogenase subunit 2 (ND2) and 15.5% to 25.0% for NADH dehydrogenase subunit 4 (ND4).
1. Introduction
The agamid lizards of the genus Pseudocalotes Fitzinger, 1843, which type species is P. tympanistriga (Gray, 1831), represents a mountainous, arboreal group that are widely distributed in South to South East Asia, from southern China, Laos, Myanmar, Vietnam, Thailand, Cambodia to the Malay Peninsula and major landmasses of the Sunda Shelf [1,2,3,4,5,6,7,8]. Currently, it contains at least 21 recognized species, of which 4 are found in China: P. brevipes (Werner, 1904), P. kakhienensis (Anderson, 1879), P. kingdonwardi (Smith, 1935), and P. microlepis (Boulenger, 1888) [8,9].
Yingjiang County lies in southwest Yunnan Province, geographically at the southeastern-corner of the Tibetan Plateau and at the southern-tip of the Hengduan Mountains, which is the most affected by the southwest tropical monsoon in China. Within the territory, numerous mountains, wide valleys, and rivers intersect with each other, forming several climatic zones and resulting in the regional divergences of species and high biodiversity. However, compared with other areas with high biodiversity, this region has not been surveyed in details for herpetological diversity. So, the species diversity in this region might still be underestimated.
During a herpetological survey in Yingjiang County, Dehong Dai and Jingpo Autonomous Prefecture, Yunnan Province, China (Figure 1) in November 2023, we collected four lizard specimens. Although they could be assigned to Pseudocalotes by having distinctively small scales of central gular region; dorsal scales heterogenous in size and shape; smooth scales of the lateral head; absent post-orbital and post-occipital spines; elongated and tall nuchal crest scales; the middle suborbital scale row significantly enlarged; and feeble and low dorsal crest scales, but they cannot be identified as any known species morphologically [3,4,7]. Furthermore, molecular analyses supported by these specimens comprise a separate evolution lineage. Thus, we described the specimens from Yunnan Province, China as a new species.
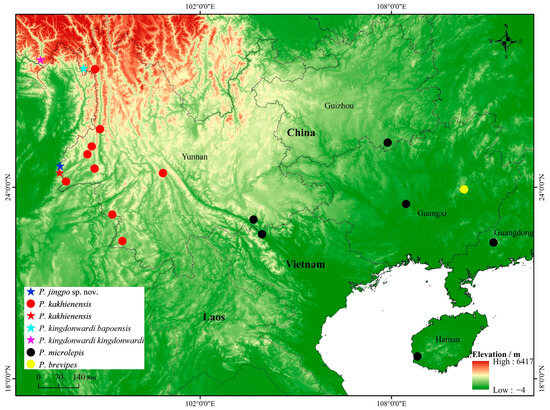
Figure 1.
Known distribution of species of the genus Pseudocalotes in China: Pseudocalotes jingpo sp. nov. (dark blue pentacle), P. kakhienensis (red pentacle and red circle), P. kingdonwardi kingdonwardi (pink pentacle), P. kingdonwardi bapoensis (wathet pentacle), P. microlepis (black circle), and P. brevipes (yellow circle). Pentacles represent the type locality, and circles represent the known localities.
2. Material and Methods
2.1. Sampling
Three adult and one subadult lizard specimens were collected from Yingjiang County, Yunnan Province, China. Sex was determined by dissection of the specimens, and determining if ovaries were present. The specimens were humanely euthanized using a lethal injection of 0.7% tricaine methanesulfonate (MS222) solution. Fresh liver tissue was extracted and immediately preserved in 95% ethanol. The specimens were fixed in 10% formaldehyde, then transferred to 75% ethanol for permanent preservation, and deposited at the Qinghai University Museum. Sampling procedures involving live lizards were conducted in accordance with the Wild Animals Protection Law of China.
2.2. Molecular Phylogenetic Analyses
Genomic DNA were extracted from preserved liver tissues using QIAamp DNA Mini Kit (QIAGEN, Changsheng Biotechnology Co., Ltd., Changchun, China). Fragments of the NADH dehydrogenase subunit 2 (ND2) were obtained by polymerase chain reaction (PCR) using the primers (5′-CCACCAAACAACTACACCTA-3′)/Jap_1559R (5′-GGATTAATGCCCTCTGGATT-3′) in ND2 [7] and “ND4” (5′-CACCTATGACTACCAAAAGCTCATGTAGAAGC-3′)/“LEU” (5′-CATTACTTTTACTTGGATTTGCACCA-3′) in ND4 [6]. PCR products were sequenced by Shanghai Map Biotech Co., Ltd., Shanghai, China. The raw sequences were assembled using SeqMan in the DNASTAR software package [10], and compared by MEGA X software [11]. The maximum likelihood (ML) was used IQ-TREE 1.6.12 [12] to construct the phylogenetic tree. Ultrafast Bootstrap Approximation (UFB) node support was assessed by using 5000 ultrafast bootstrap replicates and the UFB (%) ≥ 95 was considered significantly supported [13]. In addition, the single branch tests were conducted by SH-like approximate likelihood ratio test (SH-aLRT) by 1000 replicates and the nodal support (SH, %) ≥80 was also considered supported well [14]. Uncorrected pairwise distances (p-distance) among closely related congeners were calculated in MEGA X software [11]. The newly generated sequences were uploaded to GenBank.
To explore the phylogenetic relationships of the specimens from Yingjiang County, we used concatenated ND2 and ND4 sequences of 15 recognized species of the genus Pseudocalotes, and the homologous sequences of 3 recognized genera of Draconinae, including Bronchocela, Dendragama, and Gonocephalus in analysis. We also used the homologous sequences of Pogona vitticeps (Amphibolurinae) and Calotes versicolor (Draconinae) as outgroups (Table 1) [5,6,7,15,16,17,18,19,20,21]. All sequences, except the newly generated sequences, were obtained from the National Center for Biotechnology Information (NCBI).

Table 1.
GenBank accession numbers, localities, and voucher information for all specimens used in this study.
2.3. Morphological Examination
Morphological characters were obtained from the newly collected specimens and many key references [1,2,3,4,5,6,7,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Measurements and scale counts were performed following Zhao et al., 1999, Harvey et al., 2014, Grismer et al., 2016 [4,5,34] and were developed using digital calipers to the nearest 0.1 mm.
Abbreviations for measurements are as follows: total length (TL); snout to vent length (SVL); tail length (TAL); head length from the tip of the snout to posterior axis of the jaw, where in front of the first scale of the nuchal crest (HL); head width at its widest point (HW); maximum head depth taken at the rear axis of the jaw (HD); forelimb length (FLL); and hindlimb length (HLL). Scalation features are as follows: interoculabials: the number of scales between supralabials and suboculars; postrostrals: the numbers of scales in contact with the rostral scale, excluding the supralabials; canthals: the number of enlarged scales between the postnasal and the anteriormost superciliary; circumorbitals: the number of enlarged scales extending from the anterior margin of the orbit and grading into larger scales that terminate at the posterior margin of the orbit, forming a semicircular arc; superciliaries: the number of flat, elongate, imbricating scales immediately above the orbit; supralabials: the number of scales extending from the rostral to just beyond the rictus; infralabials: the number of scales extending from the mental to the scales positioned beneath the last supralabial; postmentals: the number of scales in contact with the mental, excluding the infralabials; chin-shields: the number of enlarged scales between the mental that extend posteriorly along the margin of the mandible medial to the infralabials; gular scales: the number of scales between the postmentals and the prebrachial margin; nuchal crest: the number of elongated, lanceolate, vertebral scales on the nape of the neck; scales around midbody; Finger IV subdigital lamellae count: the number of subdigital lamellae scale from the base to the tip of Finger IV, excluding the claw; Toe IV subdigital lamellae count: the number of subdigital lamellae scales from the base to the tip of Toe IV, excluding the claw.
Other morphological characters considered important for taxonomic identification in the genus Pseudocalotes were recorded according to Harvey et al., 2014 and Grismer et al., 2016 [4,5]: the number of scales between the rostral and nasal; the number of supralabials in contact with the nasal; the number of enlarged scales between the eye and the ear; the number of enlarged posttemporals, supratympanics, and postrictals; the presence or absence of an interparietal scale and parietal eye; the presence or absence of transverse gular and antehumeral folds; the condition of preaxial lamellae of the third toe; the dewlap color pattern; and the presence or absence of a gular spot.
3. Results
3.1. Phylogenetic Relationship
All specimens (QHU2024001–QHU2024004) were successfully sequenced. The newly generated sequences were deposited in GenBank (Accession numbers: PP228248–PP228251 in ND2, and PP356075–PP356078 in ND4), corresponding voucher and collection number: QHU2024001, LFR2023033; QHU2024002, LFR2023040–QHU2024004, LFR2023042). The topology obtained by Maximum Likelihood analysis is shown in Figure 2, based on 1498 bp concatenated ND2 and ND4 gene sequences, indicates that the relationships of the genus is strongly supported: ((Gonocephalus, Bronchocela), Pseudocalotes). The genus Pseudocalotes is divided into two unrelated clades with low support: clade 1 undoubtedly includes the vast majority of species in the genus Pseudocalotes; but clade 2, which contains P. Dioidema, P. guttalineatus, P. cybelidermus, P. baliomus, P. rhammanotus and P. tympanistriga, is more closely related to the genus Dendragama. This result differs from the results obtained by Harvey et al., 2017 [6].
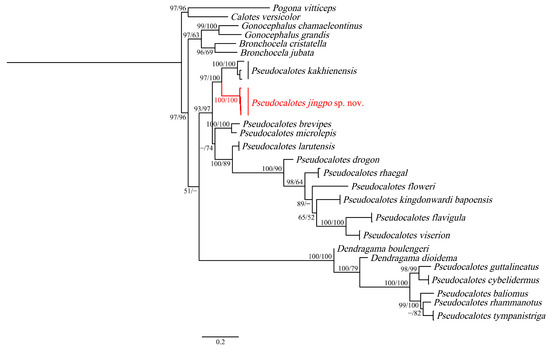
Figure 2.
The phylogenetic relationship trees of Pseudocalotes based on both Maximum Likelihood (ML) of two mitochondrial fragments (ND2 and ND4). The values on the corresponding branches indicate SH/UFB, while the values under 50% are omitted. Tips for the new species in this study are shown in red.
Meanwhile, our analyses clearly assigned the phylogenetic position of the newly collected specimens to the genus Pseudocalotes. Four specimens of the new species from Yingjiang County, Yunnan Province were nested within clade 1, and formed a sister taxon with P. kakhienensis (SH 97/UFB 100). According to the p-distance, the new species differed from its congeners by ranges from 14.5% (vs. P. kakhienensis) to 35.2% (vs. P. tympanistriga) in ND2 (Table 2) and from 15.5% (vs. P. kakhienensis) to 25.0% (vs. P. tympanistriga) in ND4 (Table 3). Moreover, morphological data also support the recognition of the specimens from Yingjiang County as distinct from all other described species of Pseudocalotes. Thus, we described the unnamed specimens as a new species.

Table 2.
Uncorrected p-distances (%) among the Pseudocalotes species based on the partial mitochondria ND2 gene.

Table 3.
Uncorrected p-distances (%) among the Pseudocalotes species based on the partial mitochondria ND4 gene.
3.2. Taxonomic Account
Squamata; Iguania; Agamidae; Draconinae; Pseudocalotes; Pseudocalotes jingpo sp. nov. XU, GONG, HOU, WENG, LIU, DENG, Hu, and PENG http://zoobank.org/urn:lsid:zoobank.org:act:FBF1A402-DF7F-44BC-878C-50C5548AD32B (accessed on 30 January 2024) Figure 3, Figure 4 and Figure 5.
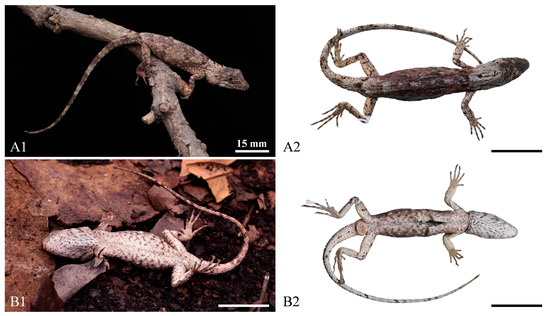
Figure 3.
Dorsal view (A1,A2) and ventral view (B1,B2) of the holotype of Pseudocalotes jingpo sp. nov. (QHU2024002) in life (A1,B1) and in preservative (A2,B2). Scale bars: (A1–B1,A2–B2) = 15 mm.
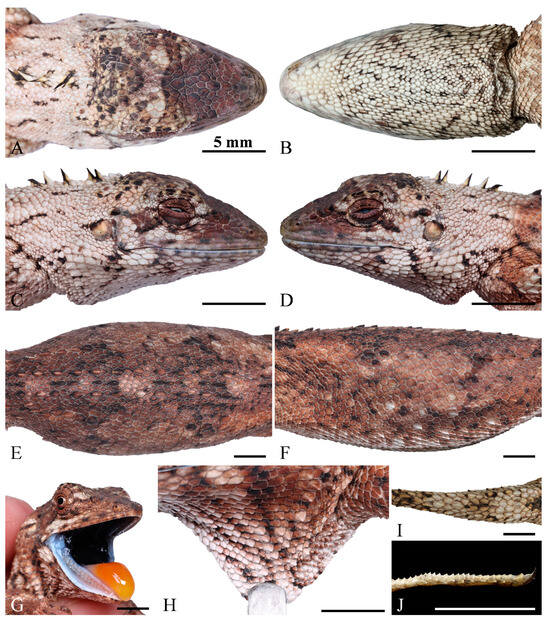
Figure 4.
Dorsal (A), ventral (B), right (C), left (D) views of the head, dorsal (E), and lateral (F) view of body, close-up view of the oral cavity (G), dewlap (H), the base of dorsal tail (I) and the lateral view of preaxial scales on the third toe (J) of the holotype (QHU2024002) of Pseudocalotes jingpo sp. nov. Scale bars: (A–J) = 5 mm.
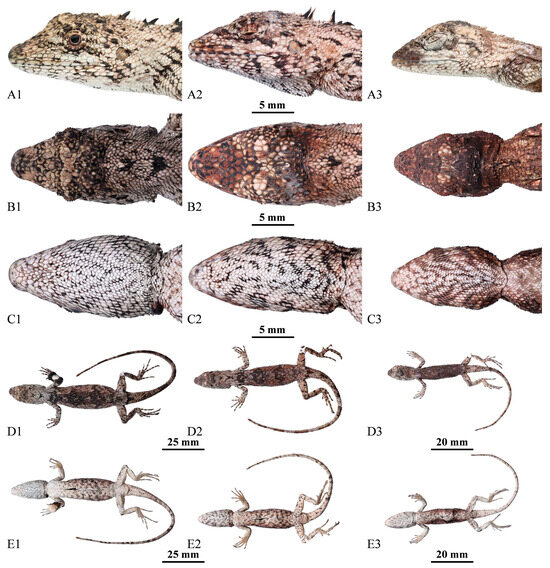
Figure 5.
Lateral (A1–A3), dorsal (B1–B3), ventral (C1–C3) views of the head, general view of dorsal (D1–D3) and ventral (E1–E3) of the paratypes of Pseudocalotes jingpo sp. nov. (A1–E1): QHU2024001, adult female; (A2–E2): QHU2024003, adult female; (A3–E3): QHU2024004, subadult female. Scale bars are shown in the figure.
3.3. Diagnosis
Pseudocalotes jingpo sp. nov. can be distinguished from its congeners in the genus Pseudocalotes by the following combination of characters: (1) interoculabials 3 or 4; (2) canthals 5–7; (3) cicrcumorbitals 8–11; (4) 1 scale between the rostral and nasal; (5) interparietal 1; (6) superciliaries 4–6; (7) supralabials 6–7, the 1st in contact with the nasal; (8) infralabials 6–8; (9) transverse gular fold and antehumeral fold present; (10) 2–3 enlarged scales between the eye and ear; (11) nuchal crest single, consists of 3–5 erected spines; (12) dorsal crest row single, discontinuous and low, located between two keeled, parallel and enlarged scale rows; (13) enlarged postrictals absent; (14) scales around the midbody 53–62, dorsal body scales heterogenous in size and shape; (15) midventrals smaller than the dorsals; (16) subdigital scales on the 4th finger 20–26, and on the 4th toe 24–29; (17) dorsal background coloration light taupe with four irregular brown patches along the middle of dorsal; (18) inner lips wathet, tongue aurantiacus, throat bluish black.
3.4. Description of Holotype
Adult female, SVL 57.5 mm and TAL 85.3 mm, tail complete, TAL/SVL ratio 1.48; TAL/TL ratio 0.60; head relatively large, subtriangular in lateral and dorsal view, HL 15.1 mm, HW 8.9 mm, HD 8.9 mm, HW/HL ratio 0.59; FLL 12.7 mm, HLL 18.2 mm; finger IV > III > II > V > I and Toe IV > III > V > II > I in relative length; nostril lateral, round, piercing in the middle of the nasal, almost invisible in dorsal view.
Dorsal head scales keeled and slightly imbricated; rostral sloped anteriorly, visible dorsally, subhexagonal, about 2.5 times as wide as tall, in contact with postrostrals and the first supralabials; postrostrals 6, small, postrostral series separated the nasal and rostral; nasal subhexagonal, width almost equal to the height; canthus rostralis sharp, canthals 5/6, keeled; in prefrontal region, 6 enlarged, heavily keeled scales forming a Y-shaped series; scales of frontal region keeled, smaller than medial supraoculars; interparietal scale small, hexagonal and keeled, about twice as long as wide, surrounded by swollen and keeled scales; a small parietal eye in the center of interparietal; circumorbitals 11/12, keeled and relatively enlarged; supraciliaries 6/5, elongate except for last one; temporal scales of varying sizes and shapes, enlarged posttemporals 1/1; tympanum naked; supratympanic 1, swollen, keeled and slightly enlarged; enlarged scales between eye and ear 2/2, separated by one small scales; interoculabials displaying 4 complete rows between supralabials and suboculars; supralabials 7/7, smooth, the 1st supralabial in contact with the nasal; infralabials 7/8, smooth; postmentals 4, the outer 2 relatively enlarged, contacting infralabials and forming the first of chin-shields; chin-shields 5/4, separated from infralabials by one anteriorly and two posteriorly rows of small scales; gular scales small and smooth, slightly thickened, with the tip directed posteromedially, 53 at midline; gular pouch well developed, the transverse gular fold present.
The nuchal crest consists of 5 elongated lanceolate scales, arranged in 3 parts (2 + 1 + 2), each of the parts are separated by 2 or 3 smaller scales, the 3rd nuchal crest the largest; dorsal crest on body weakly and composing of one row of keeled scales in normal size, extending to the base of the tail, located between 2 parallels, keeled, and enlarged scale rows; dorsal crest and 2 enlarged, discontinuous scale rows, separated from one another by small scales; the scales of the dorsal crest separated from the nuchal crest by a gap of 6 small dorsals; the antehumeral fold present; scales around midbody 57; dorsal scales are heterogenous in size and shape; not arranged in regular rows; the smaller scales feebly keeled and larger scales moderately keeled on flank of body; keels on scales of the flanks are obliquely downward; ventrals heavily keeled; and no sharp transition from scales on flanks.
Slender limbs, covered with irregular, keeled scales; the scales on outer surfaces of the limbs strongly keeled, and weakly keeled on the inner surface; hind limbs longer and slightly stronger than forelimbs; palmar and pedal subdigital lamellae bicarinate with spinose mucrons, except the base of fingers and toes; 23 lamellae beneath Finger IV, 24 lamellae beneath Toe IV; subdigital lamellae of Toe III modified, preaxial keels moderately protruded and pointed, and postaxial keels gradually weakened; tail laterally compressed, slightly swollen at base, covered with strongly keeled scales, and the vertebral scales on tail partial enlarged.
Dentition: Premaxillary teeth 3, pleurodont, the middle one is the largest, and the smaller on both sides (Figure 6). Maxillary teeth 14/14, clearly divided into two distinct groups. The anterior part consists of two pleurodont teeth, approximately canine shaped, tip slightly curved backwards, the first one smaller while the second one is significantly enlarged (as well as the largest tooth). The posterior part consists of 12 acrodont tricuspid teeth, with the anterior 7 small and the posterior 5 enlarged. Among them, the 11th–13th is significantly enlarged, and the 10th and 14th are only slightly enlarged. Mandibular teeth 16, with a composition similar to that of the maxillary teeth. The anterior part consists of three thin and sharp pleurodont teeth, the first one small, while the second and thirs enlarged. The posterior part consists of 13 acrodont tricuspid teeth, with the anterior 8 small and the posterior 5 enlarged. Among them, the 13th–15th is significantly enlarged, and the 12th and 16th only slightly enlarged. The upper and lower teeth are alternating, i.e., maxillary teeth fit into a gap in the dentary and vice versa.
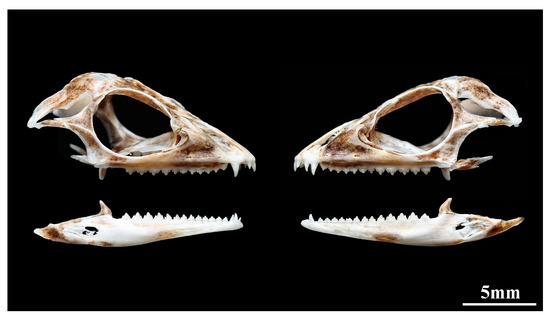
Figure 6.
Lateral view of the skull of Pseudocalotes jingpo sp. nov. (Paratype, QHU2024003). Skeletal specimen was made by Junkang Hu. Scale bar = 5 mm.
Coloration in life: In the light phase, the background coloration of the dorsal head, body, limbs, and tail is light taupe, scattered with dark brown irregular spots; three deep brown bands on on the dorsal surface of the head; multiple black brown short lines around the eyes form the radial stripe pattern; four diffused, irregular, brownish patches between limb insertions, not extending to ventral edge of flanks, the final patches incomplete and thin, over the pelvic region; nine bands on tail; small brown spots or irregular black brown rings scattered on the limbs, a light gray, irregular patch on each elbow and knee. Ventral surface of head ivory, gular region whitish and bearing brownish, oblique lines; gular spot absent; Venter ivory with light brown irregular oblique lines on both sides, lines crossing each other, extend inward, and are separated in the middle of the venter. Inner lips wathet, tongue aurantiacus, remaining oral cavity mostly bluish black.
In the dark phase, the overall color pattern is similar to the light phase. The difference is that the background coloration of the dorsal head, body, limbs, and tail is tan, scattered with blackish brown and fawn irregular spots, the irregular spots of lighter and darker coloration gives the anterior part of flank almost a reticulated appearance; as the color deepens, the reticulated pattern gradually blurs out; an oblique, white band below the eyes, extends to the venter surface of head; the dark patches on the dorsal body extend slightly to the flank, blend with the background color and almost invisible; a white, irregular patch on each elbow and knee. The brownish background color extends towards the venter, venter head and body light brown, gradually becoming lighter towards the middle; the brownish lines on the ventral surface of head and body further deepen.
3.5. Variation
Paratypes closely approximates the holotype in overall body coloration and pattern, except the postrostrals 5–6 (n = 4), cicrcumorbitals 8–12 (n = 4), canthals 5–7 (n = 4), supralabials 5–7 (n = 4), infralabials 6–8 (n = 4), enlarged scales between eye and ear 1–3 (n = 4), enlarged posttemporals 1–2 (n = 4), nuchal crest 3–5 (n = 4), postmentals 3–4 (n = 4), gulars 42–53 (n = 4), scales around midbody 53–62 (n = 4), Finger IV subdigital lamellae 20–26 (n = 4), Toe IV subdigital lamellae 24–29 (n = 4). Measurements and scalation features of the type series (n = 4) are presented in Table 4.

Table 4.
Main morphological characters of the type series of Pseudocalotes jingpo sp. nov.
3.6. Comparisons
Pseudocalotes jingpo sp. nov. can be differentiated from all other species of Pseudocalotes by having the combination of following characters: interoculabials; canthals; cicrcumorbitals; visible parietal eye; superciliaries; supralabials; infralabials; the presence of a transverse gular fold and antehumeral fold; the number of enlarged scales between eye and ear, number of enlarged posttemporals; the number of nuchal crest and dorsal crest rows; dorsal body scales heterogenous in size and shape; light taupe dorsal background coloration with four irregular brown patches along the middle of dorsal and numerous other characteristics (Table 4).
Pseudocalotes jingpo sp. nov. is most similar to its sister species P. kakhienensis. However, the new species can be distinguished from P. kakhienensis by having the 1st supralabial in contact with nasal (vs. 2 supralabials in contact with nasal); nuchal crest single, consists of 3–5 erected spines (vs. 7–13); transverse gular fold present (vs. absent); interoculabials 3–4 (vs. 2); the smaller dorsal scales feebly keeled and larger scales moderately keeled on flank of body; keels on scales of the flanks are obliquely downward (vs. the smaller dorsal scales moderately keeled and larger scales feebly keeled on flank of body, keels on those of the upper flanks are oriented obliquely upward, horizontal on the mid flanks and obliquely downward on the lower flanks); dorsal crest row discontinuous, located between two parallel and enlarged scale rows (vs. dorsal crest rows continuous, scale rows adjacent dorsal crest not enlarged); SVL up to 69.3 mm in female (vs. SVL up to 117 mm in female); inner lips wathet; tongue aurantiacus; the remaining oral cavity mostly bluish black (vs. inner lips aurantiacus, tongue flesh, remaining oral cavity mostly black); and the markedly different dorsal color patterns. For more detailed information and visual comparisons, please refer to Table 5 and Figure 7.

Table 5.
Morphological characters of Pseudocalotes obtained from specimens examined in this study and literature.
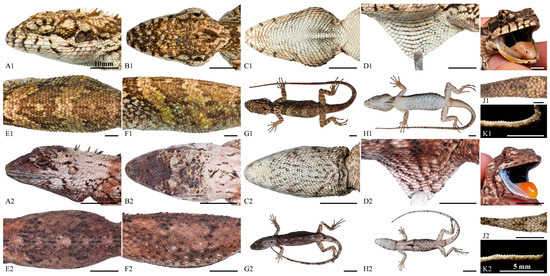
Figure 7.
Comparisons of lateral (A), dorsal (B), ventral (C) views of the head, close-up view of the dewlap (D), dorsal I, and lateral (F) view of body, general view of dorsal (G) and ventral (H), close-up view of the oral cavity (I), the base of dorsal tail (J) and the lateral view of preaxial scales on the third toe (K) among P. kakhienensis (LFR2024001; topotype; adult female) (A1–K1) and P. jingpo sp. nov. (QHU2024002; holotype; adult female) (A2–K2). Scale bars: (A1–K1,A2–J2) = 10 mm; (K2) = 5 mm.
Pseudocalotes jingpo sp. nov. differs from P. microlepis by having smooth gulars (vs. keeled); antehumeral fold present (vs. absent); enlarged supratympanics 1 (vs. absent); nuchal crest single, consists of 3–5 erected spines (vs. nuchal crest 8–10, with no gaps); scales around midbody 53–62, dorsal crest row discontinuous, located between two parallel and enlarged scale rows (vs. dorsal crest rows continuous, scale rows adjacent dorsal crest not enlarged); dorsal scales are heterogenous in size and shape (vs. scales around midbody 62–74, dorsal scales small and weakly keeled); ventrals smaller than dorsals (vs. ventrals almost equal to dorsals); Finger IV lamellae 20–26, and Toe IV lamellae 24–29 (vs. Finger IV lamellae 18–19, and Toe IV lamellae 21–25); and tail comparatively short, TAL/SVL ratio 1.32–1.48 in female (vs. tail long, TAL/SVL ratio 2.01–2.29 in female).
Pseudocalotes jingpo sp. nov. differs from P. brevipes by having smooth gulars (vs. keeled); a single nuchal crest consists of 3–5 erected spines (vs. 7–13); scales around midbody 53–62 (vs. 66–76); Finger IV lamellae 20–26 (vs. Finger IV lamellae 16–21); dorsal crest row discontinuous, located between two parallel and enlarged scale rows (vs. dorsal crest rows continuous, scale rows adjacent dorsal crest not enlarged); dorsal scales in irregular rows (vs. dorsal scales in regular rows); and the vertebral scales on tail not enlarged (vs. the vertebral scales on tail strongly keeled and enlarged).
Pseudocalotes jingpo sp. nov. differs from P. kingdonwardi by having postrostrals 5–6 (vs. 3–4); the presence of a transverse gular fold (vs. absent); nuchal crest single, consists of 3–5 erected spines (vs. 8–11); scales around midbody 53–62 (vs. 42–54); gulars 42–53 (vs. 36); dorsal crest row discontinuous, located between two parallel and enlarged scale rows (vs. dorsal crest rows continuous, scale rows adjacent dorsal crest not enlarged); and SVL up to 69.3 mm, TAL/SVL ratio 1.32–1.48 in female (vs. SVL up to 103.1mm, TAL/SVL ratio 2.02 in female).
With two other congeners that have irregular dorsal scale rows, Pseudocalotes jingpo sp. nov. differs from P. flavigula (Smith, 1924) by having scales around midbody 53–62 (vs. 38–44); interparietal present (vs. absent); dorsal crest row discontinuous, located between two parallel and enlarged scale rows (vs. dorsal crest rows continuous, scale rows adjacent dorsal crest not enlarged); and from P. poilani (Bourret, 1939) by having supralabials 5–7, infralabials 6–8 (vs. supralabials 8–9, infralabials 9–10), nuchal crest single, consists of 3–5 erected spines (vs. 7–9), and Finger IV lamellae 20–26 (vs. 18).
Pseudocalotes jingpo sp. nov. can be easily distinguished from P. tympanistriga (Gray, 1831), P. andamanensis (Boulenger, 1891), P. floweri (Boulenger, 1912), P. saravacensis Inger and Stuebing, 1994, P. larutensis Hallermann and Mcguire, 2001, P. dringi Hallermann and Böhme, 2000, P. khaonanensis Chan-ard, Cota, Makchai and Laoteow, 2008, P. ziegleri Hallermann, Truong, Orlov and Ananjeva, 2010, P. cybelidermus Harvey, Hamidy, Kurniawan, Shaney and Smith, 2014, P. guttalineatus Harvey, Hamidy, Kurniawan, Shaney and Smith, 2014, P. rhammanotus Harvey, Hamidy, Kurniawan, Shaney and Smith, 2014, P. drogon Grismer, Quah, Wood, Anuar, Muin, Davis, Murdoch, Grismer, Cota and Cobos, 2016, P. rhaegal Grismer, Quah, Wood, Anuar, Muin, Davis, Murdoch, Grismer, Cota and Cobos, 2016, P. viserion Grismer, Quah, Wood, Anuar, Muin, Davis, Murdoch, Grismer, Cota and Cobos, 2016, and P. baliomus Harvey, Shaney, Hamidy, Kurniawan and Smith, 2017 by having dorsals forming irregular rows and being heterogenous in size and shape (vs. dorsals forming regular rows). Moreover, the new species can be distinguished from P. tympanistriga, P. rhammanotus, P. floweri, P. dringi, P. khaonanensis, P. saravacensis, P. drogon, and P. viserion by having scales around midbody 53–62 (vs. 46–40 in P. tympanistriga, 51 in P. rhammanotus, 44 in P. floweri, 48–52 in P. dringi, 72–75 in P. khaonanensis, 68 in P. saravacensis, 51 in P. drogon, and 35–38 in P. viserion), from P. andamanensis, P. larutensis, P. cybelidermus, P. guttalineatus and P. rhaegal by having transverse gular fold present (vs. the transverse gular fold absent), from P. ziegleri and P. baliomus by having antehumeral fold present (vs. antehumeral fold absent).
3.7. Etymology
The specific epithet, Pseudocalotes jingpo sp. nov., is a Latinized noun in apposition, and is invariable, based on the distribution of this new species being similar to one of the minorities in China, the Jingpo Ethnic Group, which inhabit the mountain regions in southwest China, and the type locality Dehong Dai and Jingpo Autonomous Prefecture is also the concentrated area of the Jingpo Ethnic Group in China. We suggest Jǐng Pō Nǐ Shù Xī (景颇拟树蜥) as a Chinese common name and Jingpo False Garden Lizard as an English common name.
3.8. Distribution and Habitat
At present, this new species is only found at the type locality of Yingjiang County, Dehong Dai and Jingpo Autonomous Prefecture, Yunnan Province, China. We found the lizards on a clear day in the bushes by the roadside, with an air temperature of about 20 °C. The habitat environment was well-preserved tropical montane rainforest, at the elevation of approximately 1000 m.
4. Discussion
The subfamily Draconinae represent a remarkable radiation of reptiles distributed throughout Asia and Oceania, with more than 273 valid species recognized. However, despite the high diversity of this vast group, the intergeneric similarities and the morphological diversity of intrageneric species led to the unclear classification relationships among many genera. Morever, since many species in this group are still poorly understood, making it difficult to obtain samples, this results in the lack of phylogenetic research and difficult to proceed with the revisionary work.
The teeth of Draconinae are characterized by acrodont tricuspid dentition, and the dentition is also considered one of the important morphological characters for solving the intergeneric and interspecific relationships [38]. However, the dentition of the vast majority of species in the subfamily Draconinae is still unknown, and there is very little research on this aspect. In this study, we provided relevant data on the dentition of the new species. However, due to the lack of data, we are unable to determine the true characteristics of the dentition of different genera of Draconinae, and even unable to compare the differences among other species of the genus Pseudocalotes. Therefore, in the future, we need to strengthen international cooperation and conduct a large number of sample comparisons to help us further solve the classification relationships between different groups of the Draconinae.
The agamid lizards of genus Pseudocalotes has long been considered as an enigmatic assemblage of species. Although the genus has a broad distribution in southeast Asia, most of species restricted to montane refugia and have well concealment, so that it is hard to detect them in the wild, and many species were described based on a few or even a single type specimen (e.g., P. rhammanotus and P. drogon), and several species (e.g., P. poilani and P. saravacensis) do not have any sequence data accessioned [1,2,3,4]. Therefore, although some previous studies have reviewed and revised classification for the genus to some extent, but due to the lack of broader genetic and morphological sampling, we are still not clear enough about the classification status, and evolution history within this genus [1,3,4,5,7,20,21].
In this study, we combined the morphological and molecular phylogenetical analysis of the specimens in the genus Pseudocalotes. The ML tree that was generated from the dataset inferred the phylogenetic relationships: ((Gonocephalus, Bronchocela), Pseudocalotes). This result differs from the results obtained by Harvey et al., 2017 [6]. The genus Pseudocalotes was strongly suggested as a sister taxon to the genus Gonocephalus and Bronchocela (SH 97/UFB 96). The genus Pseudocalotes is a paraphyletic clade, the genus Dendragama inserted into it. Within genus Pseudocalotes is divided into two unrelated clades with low support: the clade 1 undoubtedly includes the vast majority of species in the genus Pseudocalotes; but the clade 2, which contains P. Dioidema, P. guttalineatus, P. cybelidermus, P. baliomus, P. rhammanotus and P. tympanistriga, is more closely related to the genus Dendragama. The phylogenetic relationships between the genus Gonocephalus, Bronchocela, Pseudocalotes), and Dendragama remained essentially unresolved. Meanwhile, our analyses clearly assigned the phylogenetic position of the newly collected specimens to the genus Pseudocalotes, four specimens of the new species from Yingjiang County, Yunnan Province were nested within the clade 1, formed a sister taxon with P. kakhienensis (SH 97/UFB 100).
Based on molecular evidence, Pseudocalotes jingpo sp. nov. is closely related to P. kakhienensis, but still possess a considerable level of genetic divergence from 14.5% to 16.3% in ND2 and 15.5% to 15.8% in ND4 to each other. Interestingly, the distribution pattern among two species significantly overlaps, and the type locality is adjacent to each other (Figure 1), but they can be distinguished easily by significant morphological features, so that we can be certain that it is a newly discovered lineage.
The description of Pseudocalotes jingpo sp. nov. brings the total number of Pseudocalotes species to 22. Yingjiang County, which is the type locality of the new species, is one of the areas with the most biodiverse in China. In recent years, with the progress of field surveys, many new species have been discovered here. Therefore, additional surveys may help the understanding of the biodiversity along the southwest China. Currently, the new species known only from the locality investigated, but the Yingjiang County is close to the borders of Myanmar, and this species may also occur in the adjacent area.
In China, the genus Pseudocalotes is mainly distributed in south of China mainland and Hainan Island. However, due to the bright body coloration pattern is typically in male, species of genus Pseudocalotes were often captured and traded as pets. In order to protect these beautiful species, this genus should be considered to include in the local protected animal lists to prohibit the pet trading.
5. Conclusions
We described a new species of the genus Pseudocalotes, Pseudocalotes jingpo sp. nov., based on four female specimen collected from Yingjiang County, Dehong Dai and Jingpo Autonomous Prefecture, Yunnan Province, China. Since Yingjiang County is close to the borders of Myanmar, this species may also occur in the adjacent area. However, due to their cryptic lifestyle, the discovery of the new species is largely accidental, which makes it difficult for us to make accurate judgments on the distribution and population status of this species. Further research is needed to elucidate the true distribution range and ecological niche of the new species.
Author Contributions
Conceptualization, Y.X., M.H. and L.P.; Data curation, Y.G., Y.X., J.D. and L.P.; Funding acquisition, L.P.; Methodology, Y.X., Y.G., J.H., M.H., S.L. and L.P.; Resources, Y.X. and L.P.; Software, Y.X., S.W., Y.G. and L.P.; Supervision, L.P., S.L. and M.H.; Visualization, S.W. and L.P.; Writing—original draft, Y.X.; Writing—review and editing, Y.X., Y.G., J.H., M.H., S.L., J.D. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [32301325], the Project of Qinghai Science & Technology Department [2024-ZJ-965], and the Open Project of State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University [2023-ZZ-08].
Institutional Review Board Statement
All sampling and procedures involving live lizards were performed in accordance with the Wild Animals Protection Law of the People’s Republic of China. Approved by the Institutional Ethics Committee of Qinghai University (protocol code SL-2023028 and date of approval 15 March 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. ZooBank Code: urn:lsid:zoobank.org:act:FBF1A402-DF7F-44BC-878C-50C5548AD32B; urn:lsid:zoobank.org:pub:EECB346B-B738-42CA-A06C-386CC9253B1F.
Acknowledgments
We would like to thank our workmates for their help and advice. We are also thankful to the editors and reviewers for their valuable and helpful comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hallermann, J.; Böhme, W. A review of the genus Pseudocalotes (Squamata: Agamidae), with description of a new species from West Malaysia. Amphib. Reptil. 2000, 21, 193–210. [Google Scholar] [CrossRef]
- Hallermann, J.; Truong, N.O.; Orlov, N.; Ananjeva, N. A new species of the genus Pseudocalotes (Squamata: Agamidae) from Vietnam. Russ. J. Herpetol. 2010, 17, 31–40. [Google Scholar]
- Mahony, S. Systematic and taxomonic revaluation of four little known Asian agamid species, Calotes kingdonwardi Smith, 1935, Japalura kaulbacki Smith, 1937, Salea kakhienensis Anderson, 1879 and the monotypic genus Mictopholis Smith, 1935 (Reptilia: Agamidae). Zootaxa 2010, 2514, 1–23. [Google Scholar] [CrossRef]
- Harvey, M.B.; Hamidy, A.; Kurniawan, N.; Shaney, K.; Smith, E.N. Three new species of Pseudocalotes (Squamata: Agamidae) from southern Sumatra, Indonesia. Zootaxa 2014, 3841, 211–238. [Google Scholar] [CrossRef]
- Grismer, L.L.; Quah, E.S.H.; Wood, P.L., Jr.; Anuar, S.; Muin, A.; Davis, H.R.; Murdoch, M.L.; Grismer, J.L.; Cota, M.; Cobos, A.J. Dragons in the mist: Three new species of Pseudocalotes Fitzinger (Squamata: Agamidae) from the sky island archipelago of Peninsular Malaysia. Zootaxa 2016, 4136, 461–490. [Google Scholar] [CrossRef]
- Harvey, M.B.; Shaney, K.; Hamidy, A.; Kurniawan, N.; Smith, E.N. A new species of Pseudocalotes (Squamata: Agamidae) from the Bukit Barisan Range of Sumatra with an Estimation of its phylogeny. Zootaxa 2017, 4276, 215–232. [Google Scholar] [CrossRef]
- Wang, K.; Che, J.; Lin, S.M.; Deepak, V.; Aniruddha, D.; Jiang, K.; Jin, J.Q.; Chen, H.M.; Siler, C.D. Multilocus phylogeny and revised classification for mountain dragons of the genus Japalura s.l. (Reptilia: Agamidae: Draconinae) from Asia. Zool. J. Linn. Soc. 2019, 185, 246–267. [Google Scholar] [CrossRef]
- Uetz, P. The Reptile Database. Available online: http://www.reptile-database.org, (accessed on 9 January 2024).
- Cai, B.; Wang, Y.Z.; Chen, Y.Y.; Li, J.T. A revised taxonomy of Chinese reptiles. Biodivers. Sci. 2015, 23, 365–382. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.V.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Haeseler, A.V.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate Maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Macey, J.R.; Schulte, J.A.; Larson, A.; Ananjeva, N.B.; Wang, Y.; Pethiyagoda, R.; Rastegar-Pouyani, N.; Papenfuss, T.J. Evaluating trans-tethys migration: An example using acrodont lizard phylogenetics. Syst. Biol. 2000, 49, 233–256. [Google Scholar] [CrossRef]
- Amer, S.A.M.; Kumazawaa, Y. Mitochondrial genome of Pogona vitticepes (Reptilia; Agamidae): Control region duplication and the origin of Australasian agamids. Gene 2005, 346, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.M.; Kumazawaa, Y. The Mitochondrial Genome of the Lizard Calotes versicolor and a Novel Gene Inversion in South Asian Draconine Agamids. Mol. Biol. Evol. 2007, 24, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Shaney, K.J.; Wostl, E.; Hamidy, A.; Kurniawan, N.; Harvey, M.B.; Smith, E.N. Conservation challenges regarding species status assessments in biogeographically complex regions: Examples from overexploited reptiles of Indonesia. Oryx 2017, 51, 627–638. [Google Scholar] [CrossRef]
- Welton, L.J.; Siler, C.D.; Grismer, L.L.; Diesmos, A.C.; Sites, J.W.; Brown, R.M. Archipelago-wide survey of Philippine forest dragons (Agamidae: Gonocephalus): Multilocus phylogeny uncovers unprecedented levels of genetic diversity in a biodiversity hotspot. Biol. J. Linn. Soc. 2017, 120, 410–426. [Google Scholar] [CrossRef]
- Yu, X.L.; Du, Y.; Fang, M.C.; Li, H.; Lin, L.H. The Mitochondrial Genome of Pseudocalotes microlepis (Squamata: Agamidae) and its Phylogenetic Position in Agamids. Asian Herpetol. Res. 2018, 9, 24–34. [Google Scholar]
- Shaney, K.J.; Maldonado, J.; Smart, U.; Thammachoti, P.; Fujita, M.; Hamidy, A.; Kurniawan, N.; Harvey, M.B.; Smith, E.N. Phylogeography of montane dragons could shed light on the history of forests and diversification processes on Sumatra. Mol. Phylogenetics Evol. 2020, 149, 106840. [Google Scholar] [CrossRef]
- Anderson, J. Anatomical and Zoological Researches: Comprising an Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1866 and 1875; and a Monograph of the Two Cetacean Genera Platanista and Orcella; Bernard Quaritch: London, UK, 1879. [Google Scholar]
- Boulenger, G.A. Catalogue of the Lizards in the British Museum (Natural History) I. Geckonidae, Eublepharidae, Uroplatidae, Pygopodidae, Agamidae; British Museum (Natural History): London, UK, 1879. [Google Scholar]
- Boulenger, G.A. An account of the reptiles and batrachians obtained in Tenasserim by M. L. Fea, of the Genova Civic Museum. Ann. Mus. Civ. Stor. Nat. Genova 1887, 2, 474–486. [Google Scholar]
- Boulenger, G.A. On new or little known Indian and Malayan reptiles and batrachians. Ann. Mag. Nat. Hist. 1891, 6, 288–292. [Google Scholar] [CrossRef][Green Version]
- Werner, F. Beschreibung neuer Reptilien aus den Gattungen Acanthosaura, Calotes, Gastropholis und Typhlops. Zoolgischer Anz. 1904, 27, 461–464. [Google Scholar]
- Annandale, N. Description of a new species of lizard of the genus Salea from Assam. Rec. Indian Mus. 1908, 2, 37–38. [Google Scholar] [CrossRef]
- Boulenger, G.A. A Vertebrate Fauna of the Malay Peninsula from the Isthmus of Kra to Singapore Including the Adjacent Islands. Reptilia and Amphibia; Taylor & Francis: London, UK, 1912. [Google Scholar]
- Smith, M.A. Two new lizards and a new tree frog from the Malay Peninsula. J. Fed. Malay States Mus. 1924, 11, 183–186. [Google Scholar]
- Smith, M.A. The Fauna of British India, Including Ceylon and Burma. Reptiles and Amphibia, Vol. II. Sauria; Taylor and Francis: London, UK, 1935. [Google Scholar]
- Bourret, R. Notes herpétologiques sur l’Indochine française. XVIII. Reptiles et batraciens reçus au Laboratoire des Sciences Naturelle de l’Université au cours de l’année 1939. Descriptions de quatre espéces et d’une variété nouvelles. Bull. Génér. l’Instr. Pub. Hanoi 1939, 19, 5–39. [Google Scholar]
- Yang, D.T.; Su, C.Y.; Li, S.M. New species and new subspecies of amphibians and reptiles from Gaoligong Shan, Yunnan. Acta Zootaxon. Sin. 1979, 4, 185–188. [Google Scholar]
- Inger, R.F.; Stuebing, R.B. First record of the lizard genus Pseudocalotes (Lacertidae: Agamidae) in Borneo, with description of a new species. Raffles Bull. Zool. 1994, 42, 961–965. [Google Scholar]
- Zhao, E.M.; Zhao, K.T.; Zhou, K.Y. Fauna Sinica, Reptilia, Vol. 2: Squamata, Lacertilia; Science Press: Beijing, China, 1999. [Google Scholar]
- Hallermann, J.; McGuire, J.A. A new species of Pseudocalotes (Squamata: Agamidae) from Bukit Larut, West Malaysia. Herpetologica 2001, 57, 255–265. [Google Scholar]
- Chan-ard, T.; Cota, M.; Makchai, S.; Laoteow, S. A new species of the genus Pseudocalotes (Squamata: Agamidae) from peninsular Thailand. Thail. Nat. Hist. Mus. J. 2008, 3, 25–31. [Google Scholar]
- Harikrishnan, S.; Vasudevan, K. Rediscovery of Calotes andamanensis Boulenger, 1891, and assessment of its generic allocation (Squamata: Sauria: Agamidae). Herpetozoa 2013, 26, 3–13. [Google Scholar]
- Smith, K.; Schaal, S.F.K.; Sun, W.; Li, C.T. Acrodont iguanians (Squamata) from the middle Eocene of the Huadian basin of Jilin Province, China, with a critique of the taxon “Tinosaurus “. Vertebr. Palasiat. 2011, 49, 69–84. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).