Simple Summary
In chicken breeding, direct breeding selection on phenotype is challenging and expensive. Molecular-marker-assisted selection breeding remains an efficient and effective breeding method. Through association analysis, we identified VNN1 as a significant marker for chicken carcass traits. Single nucleotide polymorphisms in VNN1 can serve as molecular markers to assist in selection breeding. We propose that these markers can enhance the production efficiency of broilers.
Abstract
This study aimed to investigate the association between hepatic VNN1 expression and carcass traits in Mahuang chickens as well as to identify polymorphisms in the upstream and downstream regions of VNN1 that could potentially be associated with these carcass traits. The study revealed that VNN1 expression levels in liver correlated with various carcass traits such as dressed weight, eviscerated weight, and abdominal fat weight. A total of 39 polymorphic sites were identified, among which 23 were found to be associated with 15 different carcass traits. These polymorphic sites were organized into three distinct haplotype blocks, with BLOCK2 and BLOCK3 being associated with various eviscerated weight percentages, thigh weight, breast muscle weight, wing weight, and other traits. The study underscores the significant role of VNN1 in influencing the carcass traits of Mahuang chickens and sheds light on the genetic foundations of these traits. The findings provide valuable insights that could inform breeding strategies aimed at optimizing traits relevant to market demands and slaughtering efficiency.
1. Introduction
Chicken meat is an important source of protein in the human diet. In China, yellow-feather broilers accounted for 25.21% of the total chicken meat yield in 2023 [1]. Breeding efforts for yellow-feather broilers have traditionally focused on body weight and feather color, leading to suboptimal carcass traits. To meet the demands of segmented sales, improving carcass traits has become an important goal in the breeding of yellow-feather broilers.
Carcass traits in chickens, which encompass characteristics such as live weight (LW), dressed weight (DW), eviscerated weight (EW), eviscerated weight with giblet (EWG), breast muscle weight (BMW), thigh weight (TW), wing weight (WW), and abdominal fat weight (AFW), are of critical importance for production and are closely linked to the economic performance of the broilers. In recent years, genome-wide association studies (GWASs) have been utilized to identify the quantitative trait loci (QTLs) and genes associated with carcass traits [2]. However, QTLs and related genes associated with carcass traits often do not overlap across different breed populations [3,4,5,6], making these markers non-generalizable. Additionally, genomic selection breeding is currently expensive, whereas marker-assisted selection (MAS) remains a cost-effective and rapid breeding method [7].
Mahuang chickens are a renowned yellow-feather broiler breed for their growth performance and meat quality. There is a lack of markers for carcass traits in Mahuang chicken breeding. The liver serves as a metabolic central organ that interconnects to various tissues, including skeletal muscle and adipose tissue, thereby controlling the energy metabolism of the whole body [8]. A key gene involved in liver metabolism is VNN1, which encodes for pantetheine hydrolase (Vanin-1) and is predominantly expressed in the liver [9]. Previous research has demonstrated that VNN1 is regulated by PPARα and influences lipid and glucose metabolism in the liver [10]. Moreover, gain- and loss-of-function studies have revealed that VNN1 is activated by the synergistic interaction of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and hepatocyte nuclear factor-4α (HNF4α), which subsequently induce the expression of phosphoenolpyruvate carboxykinases (PEPCK) and glucose 6-phosphatase (G6Pase), activating hepatic gluconeogenesis [11]. In chickens, VNN1 is regulated by PPARα and is speculated to participate in liver lipid metabolism [12,13,14]. However, further studies to confirm whether VNN1 is related to carcass traits are still lacking.
In this study, the carcass traits included were statistically determined in 432 Mahuang chickens, the liver VNN1 expression levels were detected, and SNPs in the regions flanking the VNN1 gene were screened. The aim was to analyze the relationship between carcass traits and hepatic VNN1 expression, and to find SNP markers within VNN1 associated with these carcass traits.
2. Materials and Methods
2.1. Carcass Traits Data
The South China Agricultural University Institutional Animal Care and Use Committee approved all sampling and laboratory procedures adopted in this study (approval ID: SCAU#2021F074).
A total of 432 Mahuang chickens of same breeding line were the animals used in this study. After hatching, meconium avian leukosis was checked by using a double antibody sandwich enzyme-linked immunosorbent assay kit (P27 antigen), and positive individuals were eliminated. Individuals with an initial body weight of less than 42 g were eliminated. Qualified chickens were raised according to the standard feeding and management procedures by KwangFeng Industrial Co., Ltd. (Guangzhou, China). All the chickens were kept in a brooder house until 6 weeks of age and were then moved to the grower house with single-cage rearing. All of the chickens were reared in stepped cages [15] in the same pen under intensive management providing the same management regimen with ad libitum feed (Table 1) and watering until 90 days of age.

Table 1.
Nutrition during feeding.
All 90-day-old chickens were humanely slaughtered by cervical dislocation, blood drained from carotid artery, and tissues dissected. The carcass traits including LW, DW, EW, EWG, BMW, TW, WW, AFW, and their percentages (DWP, EWP, EWGP, BMWP, TWP, WWP, and AFWP) were measured and calculated as described by Yang et al. [16].
2.2. Primer Design
Primers were designed with Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome, accessed on 10 March 2022) according to the VNN1 gene sequence (ENSGALG00000013993), as shown in Table 2.

Table 2.
Details of primers used in this study.
2.3. DNA Extraction, Polymerase Chain Reaction, and DNA Sequencing
All of the blood of Mahuang chickens was collected during slaughtering. DNA was extracted from the blood using E.Z.N.A.® NRBC Blood DNA Kit (OMEGA, Norcross, GA, USA) according to the protocol manual. DNA quality was checked using NANODROP ONE (Thermo Scientific, Waltham, MA, USA), with the criterion of 1.8 < A260/A280 < 2.0, A260/A230 > 2.0, and concentration > 50 ng/μL. A total of 432 DNA samples that met these criteria were used to amplify the dsDNA of VNN1 fragments with 2× Taq MasterMix (CWBIO, Nanjing, China). PCR conditions were 94 °C for 2 min, followed by cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and a final extension of 72 °C for 5 min. Finally, Sanger sequencing of the PCR products was carried out by Guangzhou Tianyi Huiyuan Gene Technology Co., Ltd. (Guangzhou, China).
2.4. RNA Extraction, Complementary DNA (cDNA) Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
For RNA extraction, liver tissues were collected from 47 female Mahuang chickens randomly. The tissues were immediately placed into cryovials and frozen inside liquid nitrogen during slaughtering. Afterward, they were stored at −80 °C.
Total RNA was isolated from livers using RNA isolator Total RNA Extraction Reagent (Vazyme, Nanjing, China) following the manufacturer’s instructions. A total of 200 μL cDNA of approximately 800 ng RNA per sample was synthesized with MonScript™ RTIII All-in-One Mix with dsDNase (Monad, Wuhan, China). The qRT-PCR program (95 °C for 30 s, followed by cycles of 95 °C for 10 s, 60 °C for 30 s) was carried out in a Bio-Rad CFX96 system (Bio-Rad, Hercules, CA, USA) with ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) with 5 μL ChamQ SYBR qPCR Master Mix, 4.2 μL cDNA, and 8 μL primer (10 μM) used for each reaction. Chicken GAPDH was used as the internal control. The relative expression of genes was analyzed with the comparative 2−∆∆Ct method [17].
2.5. Sequencing and Genotyping
For quality control, sequence reads shorter than 700 bp were filtered out. Qualified reads were then examined using the SnapGene software suite (version 4.3.6) to assess the chromatograms. The criteria for qualified reads included the following:
Peak Heights: reads with peak heights of 500 or more for most bases.
Peak Masses: reads with peak masses of 50 or more for most bases.
Finally, the first 30 bp and the last 30 bp of each read were trimmed to remove low-quality bases commonly found at the ends of sequencing reads.
The sequence reads were aligned with the VNN1 sequence (ENSGALG00000013993) by using SnapGene software suite (version 4.3.6) for genotyping. We genotyped SNPs according to Yang et al. [16].
2.6. Statistics and Analysis
The phenotype data was estimated with the program R (version 4.1.2). The allele frequency, genotype frequency, and Hardy–Weinberg disequilibrium (HWE) were calculated with PLINK 1.9 [18,19]. The polymorphism information content (PIC) [20] was calculated with Gene-Calc (https://gene-calc.pl/, accessed on 4 November 2022). The linkage disequilibrium and haplotypes were determined and visualized with the gaston package in R (version 1.5.7) [21].
Correlation analysis between VNN1 mRNA expression and carcass traits fitted a simple linear regression model created using Graphpad prism (version 9.5). Association analysis between SNPs (or haplotypes) and carcass traits was carried out with a general linear model (GLM) combined variable using the linear command in PLINK 1.9. The GLM model used was as follows:
where Y is the phenotype value of carcass traits, μ is the population means, G is the fixed effect of the genotype (or haplotype), S is the fixed effect of sex, and e represents the random error.
Y = μ + G + S + e,
Analysis of Variance (ANOVA) was performed to estimate the phenotypic differences between genotypes (or haplotypes) in R software (version 4.2.1).
3. Results
3.1. Information on Carcass Traits
To comprehensively understand the phenotypic information of the 432 (342 female and 89 male) investigated Mahuang chickens, we counted the mean, maximum, minimum, and standard deviation of the traits LW, DW, EW, EWG, BMW, TW, WW, AFW, DWP, EWP, EWGP, BMWP, TWP, WWP, and AFWP by sex. We found significant differences in these traits in both the females and males (Table 3). We then performed Pearson correlation analyses for carcass traits in the male and female chickens. The results show that LW was significantly positive correlated to DW, EW, EWG, BMW, TW, WW, and AFW, and in addition, LW was significantly negatively correlated with WWP (Figure 1). Interestingly, we found that AFWP was negatively correlated with EWGP, BMW (BMWP), TW (TWP), and WW (WWP), and was particularly significant in the females (Figure 1). These findings provide important insights into the relationships between different carcass traits in Mahuang chickens and can inform future breeding and selection strategies for improved meat quality.

Table 3.
Descriptive statistics of carcass traits.
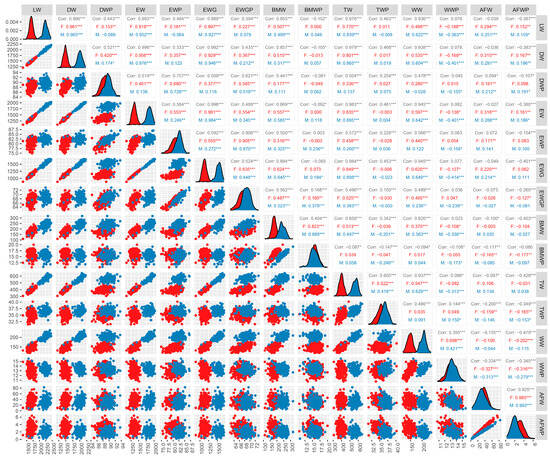
Figure 1.
Correlation between carcass traits in Mahuang chickens. Abbreviations: LW = live weight; DW = dressed weight; DWP = dressed weight percentage; EW = eviscerated weight; EWP = eviscerated weight percentage; EWG = eviscerated with giblet; EWGP = eviscerated with giblet percentage; BMW = breast muscle weight; BMWP = breast muscle weight percentage; TW = thigh weight; TWP = thigh weight percentage; WW = wing weight; WWP = wing weight percentage; AFW = abdominal fat weight; AFWP = abdominal fat weight percentage; F (red): female; M (blue): male. “***” if the p-value is <0.001; “**” if the p-value is <0.01; “*” if the p-value is <0.05.
3.2. Association between VNN1 Expression and Carcass Traits
Given the pivotal role of VNN1 in hepatic lipid metabolism, we investigated the association between hepatic VNN1 expression and carcass traits in chickens. We quantified the relative expression level of VNN1 using qPCR and performed a simple linear regression with the carcass traits. Our results demonstrated a significant association between hepatic VNN1 expression level and several carcass traits, including DW, DWP, EW, EWP, EWG, AFW, and AFWP (p < 0.05, Figure 2A–G), and a non-significant association with LW, EWGP, TW, TWP, WW, and WWP (p > 0.05, Figure S1A–H). The correlation coefficients (r2) of AFW and AFWP phenotype with VNN1 expression levels were 0.2337 and 0.1961, respectively (Figure 2F,G). These findings suggest a strong association between hepatic VNN1 expression levels and abdominal fat deposition.
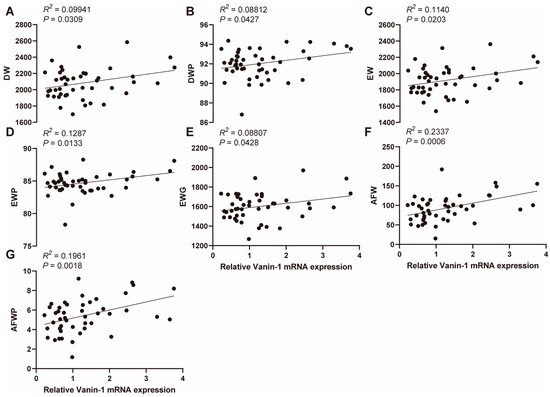
Figure 2.
Association between liver VNN1 mRNA expression and carcass traits (A–G). Association between liver VNN1 mRNA expression and DW, DWP, EW, EWP, EWG, AFW, and AFWP in 47 female Mahuang chickens. Abbreviations: DW = dressed weight; DWP = dressed weight percentage; EW = eviscerated weight; EW = eviscerated weight percentage; EWG = eviscerated with giblet; AFW = abdominal fat weight; AFWP = abdominal fat weight percentage.
3.3. Information on SNPs
To explore genetic variation in VNN1, we identified 39 polymorphic sites, including 19 upstream and 20 downstream SNPs, in the Mahuang chicken population (Figure 3). Among these SNPs, eight were newly identified (C56889810T, C56889913G, T56890010C, G56890028A, C56890154T, A56906727T, A56907254G, T56907387C), while the others can already be found in the Genetic Variation of Ensembl. We found that one SNP was a deletion mutation, and two SNPs were insertion mutations, while the others were base conversion mutations (Table 4). Table 5 shows the genotype and allelic frequencies, Hardy–Weinberg disequilibrium (HWE), and polymorphism information content (PIC) for each SNP. Our results suggest that the SNPs in the downstream region deviated from the HWE (p < 0.05), possibly due to selection during breeding. The PIC ranged from 0.054 to 0.519, indicating low or moderate levels of polymorphism, with the exception of rs734452255, which exhibited high polymorphism (PIC > 0.5).
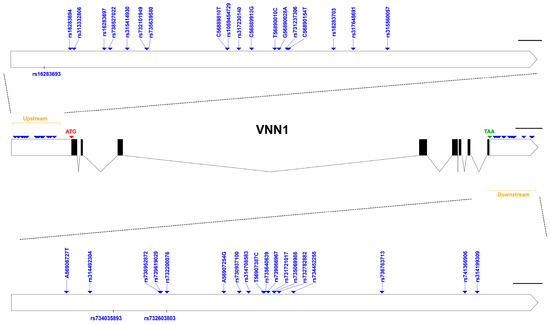
Figure 3.
Location distribution of SNPs on VNN1 gene.

Table 4.
SNPs identified in the VNN1 gene.

Table 5.
Genotype frequencies, allelic frequencies, and HWE of SNPs.
3.4. Association of SNPs with Carcass Traits and Haplotype Reconstruction
We analyzed the association between carcass traits and 39 SNPs using GLM, with a significance threshold of p < 0.05. Of these SNPs, 23 were significantly associated with carcass traits (Figure 4A, Table S1). ANOVA was performed to estimate differences in AFW between genotypes at rs736763713. Chickens with the AA genotype had significantly higher AFW compared to those with GA or GG genotypes (p < 0.05, Supplementary Materials File S1).
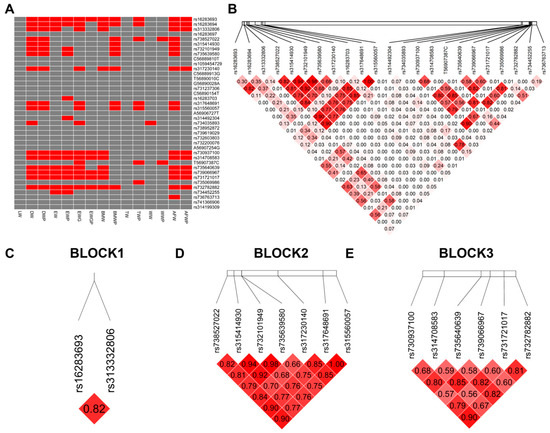
Figure 4.
LD plot shows the linkage status of 23 SNPs associated with carcass traits in the VNN1 gene. (A) Association analysis of the SNPs with carcass traits in chickens. “Red” indicates a p value less than 0.05, while “grey” indicates a p value more than 0.05. (B) The linkages between 23 SNPs. (C–E) Three blocks constructed from 23 SNPs. The color of the block indicates the LD status of SNPs; deep red means high linkages between SNPs. The number of the block shows the linkages between SNPs in r2. Abbreviations: LW = live weight; DW = dressed weight; DWP = dressed weight percentage; EW = eviscerated weight; EWP = eviscerated weight percentage; EWG = eviscerated with giblet; EWGP = eviscerated with giblet percentage; BMW = breast muscle weight; BMWP = breast muscle weight percentage; TW = thigh weight; TWP = thigh weight percentage; WW = wing weight; WWP = wing weight percentage; AFW = abdominal fat weight; AFWP = abdominal fat weight percentage.
To investigate the multi-loci association between the haplotype structures of the SNPs and carcass traits, we constructed linkage disequilibrium (LD) blocks. We defined three blocks from SNPs associated with carcass traits, with r2 measures of LD ranging from 0 to 1 (Figure 4B–E and Figure S1). Haplotypes with frequencies > 0.01 and diplotypes with frequencies > 0.005 were reserved and reconstructed based on population genotype data, which are shown in Table 6.

Table 6.
The information of haplotypes.
3.5. Association of Haplotypes with Carcass Traits
To comprehensively elucidate the relationship between SNPs and carcass traits, the LD blocks obtained from linkage disequilibrium were analyzed using the GLM with 15 carcass traits. As indicated in Table S2, BLOCK1 was not associated with any traits, while BLOCK2 was related to EW, EWP, EWGP, TW, and TWP, and BLOCK3 was associated with EW, EWP, EWG, EWGP, BMW, BMWP, TW, TWP, WW, and WWP. Multiple comparisons of phenotypic differences between the diplotypes were performed using ANOVA. No significant phenotypic differences were observed between the diplotypes of BLOCK2 and BLOCK3 (Table 7, Table 8, and Table S3). However, the H1H2 diplotype of BLOCK2 was advantageous for EW, EWP, EWGP, and TW, as shown in Table 7. In Table 8, the H2H2 diplotype demonstrated higher values of EW, EWP, EWG, BMW, TW, and WW than other diplotypes.

Table 7.
Association analysis of BLOCK2 with carcass traits in F2 generation partridge chickens.

Table 8.
Association analysis of BLOCK3 with carcass traits in F2 generation partridge chickens.
4. Discussion
Here, we present an association analysis of carcass traits and VNN1 gene in Mahuang chickens. Our analysis reveals a relationship between hepatic VNN1 expression and several carcass traits, including DW, DWP, EW, EWP, EWG, AFW, and AFWG. Additionally, we found associations between SNPs located upstream and downstream of the VNN1 gene and these carcass traits. The upstream SNPs, including rs738527022, rs315414930, rs732101949, rs735639580, rs317230140, rs317648691, and rs315560057, form a haplotype (BLOCK2) that is associated with EW, EWP, EWGP, TW, and TWP. The downstream SNPs, including rs730937100, rs314708583, rs735640639, rs739066967, rs731721017, and rs732782882, form another haplotype (BLOCK3) that is associated with EW, EWP, EWG, EWGP, BMW, BMWP, TW, TWP, WW, and WWP.
Our findings demonstrate that hepatic VNN1 expression levels are associated with various carcass traits such as DW, DWP, EW, EWP, EWG, AFW, and AFWP, and especially AFW and AFWP, with the latter showing the strongest correlation. There is a high correlation between DW and carcass performance (EW and EWG). These results suggest that VNN1 may affects carcass traits. Carcass traits have been studied by other authors and associated with gene expression and gene polymorphisms [22,23,24,25,26,27]. Identifying SNPs of VNN1 could be a potential way to find molecular markers associated with carcass traits, thereby facilitating faster genetic gain in breeding programs.
The upstream and downstream regions of genes are often rich in elements that influence transcription and post-transcription regulation, such as transcription factor binding sites, miRNA binding sites, and upstream open reading frames (uORFs) [28,29,30]. Prior research has linked polymorphisms in these regions to variations in gene expression and growth performance [31,32,33,34,35]. In our study, we screened the VNN1 flanking regions and identified 39 SNPs, with 23 linked to carcass traits and 8 reported for the first time. Notably, we constructed three haplotype blocks, with BLOCK2 and BLOCK3 showing strong associations with carcass traits. These blocks offer potent markers for breeding strategies.
Genetic variation occurs continuously throughout the genome. In chickens, different breeds are subjected to varying selection pressures, resulting in differences in genetic structure and SNP distribution. The SNPs and haplotypes associated with the carcass traits in Mahuang populations identified in this study may not be directly applicable to other breeds. However, among the 39 SNPs examined, only 8 were novel to Mahuang chickens, and these novel SNPs were not significantly associated with carcass traits. Interestingly, the SNPs found to be associated with carcass traits in Mahuang chickens have also been identified in other breeds (named by Ensembl), suggesting the potential for broader application of these SNP markers across different chicken populations.
5. Conclusions
Our research identified potential effects of hepatic VNN1 expression on carcass traits and identified SNPs and haplotype blocks on the VNN1 gene, providing promising markers for improving carcass traits in Mahuang chicken breeding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14131888/s1, Supplementary File S1: Supplementary figures and tables; Supplementary Tables: Association statistics of SNPs and carcass traits; Supplementary Materials: Gene expression data, phenotypes, and genotypes of all individuals.
Author Contributions
Conceptualization, S.Z. and Z.L.; methodology, S.Z.; software, X.F.; validation, R.W., S.Z. and X.F.; formal analysis, S.Z.; investigation, S.Z.; resources, Q.N.; data curation, Z.L.; writing—original draft preparation, S.Z.; writing—review and editing, Z.L.; visualization, S.Z.; supervision, Z.L.; project administration, Z.L.; funding acquisition, Q.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangdong Basic and Applied Basic Research Foundation (Grant No. 2022B1515120049); the National Key R & D Program of China (Grant No. 2021YFD1300100), the STI 2030 Major Projects (2023ZD04064); the Science and Technology Program of Guangzhou, China (202103000084, 202201010507); the Construction Project of Modern Agricultural Science and Technology Innovation Alliance in Guangdong Province (2021KJ128); the Science and Technology Program of Guangdong province, China (2020B1212060060); and the Project of the Seed Industry Revitalization of Department of Agriculture and Rural Affairs of Guangdong Province (2022-XPY-05-001).
Institutional Review Board Statement
The South China Agricultural University Institutional Animal Care and Use Committee approved all sampling and laboratory procedures adopted in this study (approval ID: SCAU#2021F074).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zheng, M.; Wen, J. Current Status of Broiler Breeding Industry Development. Chin. Livest. Pout. Breed. 2023, 19, 182–189. [Google Scholar]
- Pan, R.; Qi, L.; Xu, Z.; Zhang, D.; Nie, Q.; Zhang, X.; Luo, W. Weighted Single-Step GWAS Identified Candidate Genes Associated with Carcass Traits in a Chinese Yellow-Feathered Chicken Population. Poult. Sci. 2024, 103, 103341. [Google Scholar] [CrossRef]
- Huang, S.; He, Y.; Ye, S.; Wang, J.; Yuan, X.; Zhang, H.; Li, J.; Zhang, X.; Zhang, Z. Genome-Wide Association Study on Chicken Carcass Traits Using Sequence Data Imputed from SNP Array. J. Appl. Genet. 2018, 59, 335–344. [Google Scholar] [CrossRef]
- Allais, S.; Hennequet-Antier, C.; Berri, C.; Salles, L.; Demeure, O.; Le Bihan-Duval, E. Mapping of QTL for Chicken Body Weight, Carcass Composition, and Meat Quality Traits in a Slow-Growing Line. Poult. Sci. 2019, 98, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, J.; Li, J.; Bao, H. Identification of Candidate Genes Associated with Slaughter Traits in F2 Chicken Population Using Genome-Wide Association Study. Anim. Genet. 2021, 52, 532–535. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, L.-Y.; Xu, Z.-C.; Kramer, L.M.; Yu, J.-Q.; Zhang, X.-Y.; Na, W.; Yang, L.-L.; Cao, Z.-P.; Luan, P.; et al. Haplotype-Based Genome-Wide Association Studies for Carcass and Growth Traits in Chicken. Poult. Sci. 2020, 99, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.M.; El-Gendy, E.A. Marker-Assisted Selection for Improving Body Weight in Local Chickens in Egypt. J. Agric. Sci. 2023, 161, 135–147. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yu, J.; Shao, F.; Zhang, Y.; Lu, X.; Gu, Z. MiR-122 Targets the Vanin 1 Gene to Regulate Its Expression in Chickens. Poult. Sci. 2016, 95, 1145–1150. [Google Scholar] [CrossRef]
- van Diepen, J.A.; Jansen, P.A.; Ballak, D.B.; Hijmans, A.; Hooiveld, G.J.; Rommelaere, S.; Galland, F.; Naquet, P.; Rutjes, F.P.J.T.; Mensink, R.P.; et al. PPAR-Alpha Dependent Regulation of Vanin-1 Mediates Hepatic Lipid Metabolism. J. Hepatol. 2014, 61, 366–372. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Tang, C.; Tang, X.; Liu, L.; Liu, C. Vanin-1 Is a Key Activator for Hepatic Gluconeogenesis. Diabetes 2014, 63, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Li, J.; Yu, J.; Wang, Z.; Xu, L.; Yao, W. PSIV-19 Identification of VNN1-Regulated Genes Involved in Lipid Metabolism in Chicken Hepatocytes. J. Anim. Sci. 2018, 96, 131–132. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Hua, N.; Li, J.; Xu, L.; Yao, W.; Gu, Z. Regulation of Chicken Vanin1 Gene Expression by Peroxisome Proliferators Activated Receptor α and miRNA-181a-5p. Anim. Biosci. 2021, 34, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Z.; Liu, S.; Wei, Z.; Yu, J.; Li, J.; Li, J.; Yao, W.; Gu, Z. CRISPR/Cas9-Mediated Knockout of the Vanin-1 Gene in the Leghorn Male Hepatoma Cell Line and Its Effects on Lipid Metabolism. Anim. Biosci. 2024, 37, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Wegner, R.-M. Experience with the Get-Away Cage System. World’s Poult. Sci. J. 1990, 46, 41–47. [Google Scholar] [CrossRef]
- Yang, X.; Xian, Y.; Li, Z.; Wang, Z.; Nie, Q. G0S2 Gene Polymorphism and Its Relationship with Carcass Traits in Chicken. Animals 2022, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaScience 2015, 4, s13742-015. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Perdry, H.; Dandine-Roulland, C.; Banddyopadhyay, D.; Kettner, L. Gaston: Genetic Data Handling (QC, GRM, LD, PCA) & Linear Mixed Models. R Package 2018, 83, 1–29. [Google Scholar]
- El-Attrouny, M.M.; Iraqi, M.M.; Sabike, I.I.; Abdelatty, A.M.; Moustafa, M.M.; Badr, O.A. Comparative Evaluation of Growth Performance, Carcass Characteristics and Timed Series Gene Expression Profile of GH and IGF-1 in Two Egyptian Indigenous Chicken Breeds versus Rhode Island Red. J. Anim. Breed. Genet. 2021, 138, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dong, X.; Mao, H.; Xu, N.; Yin, Z. Expression Analysis of the PITX2 Gene and Associations between Its Polymorphisms and Body Size and Carcass Traits in Chickens. Animals 2019, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.P.; Fan, Y.X.; Ma, T.W.; Wang, Z.; Tan Tai, W.J.; Nie, H.T.; Guo, Y.X.; Yu, X.Q.; Sun, L.W.; Wang, F. Carcass Traits, Meat Quality, Antioxidant Status and Antioxidant Gene Expression in Muscle and Liver of Hu Lambs Fed Perilla Seed. J. Anim. Physiol. Anim. Nutri. 2018, 102, e828–e837. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Dou, T.F.; Li, Q.H.; Rong, H.; Tong, H.Q.; Xu, Z.Q.; Huang, Y.; Gu, D.H.; Chen, X.B.; Ge, C.R.; et al. Myostatin mRNA Expression and Its Association with Body Weight and Carcass Traits in Yunnan Wuding Chicken. Genet. Mol. Res. 2016, 15, gmr15048967. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Y.; Wang, Y.; Liu, Y.-P.; Wang, J.; Zhu, Q. Polymorphisms and Expression of the Chicken POU1F1 Gene Associated with Carcass Traits. Mol. Biol. Rep. 2012, 39, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Li, N.; Leng, L.; Wang, Y. Tissue Expression and Association with Fatness Traits of Liver Fatty Acid-Binding Protein Gene in Chicken. Poult. Sci. 2006, 85, 1890–1895. [Google Scholar] [CrossRef]
- Mayr, C. Regulation by 3’-Untranslated Regions. Annu. Rev. Genet. 2017, 51, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Leppek, K.; Das, R.; Barna, M. Functional 5′ UTR mRNA Structures in Eukaryotic Translation Regulation and How to Find Them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lu, J. Function and Evolution of Upstream ORFs in Eukaryotes. Trends Biochem. Sci. 2019, 44, 782–794. [Google Scholar] [CrossRef]
- Cui, H.X.; Yang, S.Y.; Wang, H.Y.; Zhao, J.P.; Jiang, R.R.; Zhao, G.P.; Chen, J.L.; Zheng, M.Q.; Li, X.H.; Wen, J. The Effect of a Mutation in the 3-UTR Region of the HMGCR Gene on Cholesterol in Beijing-You Chickens. Anim. Biotechnol. 2010, 21, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Shen, X.; Zhang, X.; Li, F.; Amevor, F.K.; Zhu, Q.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; et al. A Functional Polymorphism of Inhibin Alpha Subunit at miR-181b-1-3p-Binding Site Regulates Proliferation and Apoptosis of Chicken Ovarian Granular Cells. Cell Tissue Res. 2021, 384, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, U.; Kerje, S.; Bed’hom, B.; Sahlqvist, A.-S.; Ekwall, O.; Tixier-Boichard, M.; Kämpe, O.; Andersson, L. The Dark Brown Plumage Color in Chickens Is Caused by an 8.3-Kb Deletion Upstream of SOX10. Pigment Cell Melanoma Res. 2011, 24, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dai, G.; Wang, J.; Wei, Y.; Ding, F.; Li, Z.; Zhao, X.; Xie, K.; Wang, W. Polymorphisms in 5’-Upstream Region of the Myostatin Gene in Four Chicken Breeds and Its Relationship with Growth Traits in the Bian Chicken. Afr. J. Biotechnol. 2012, 11, 9677–9682. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, H.; Lin, S.; Liang, L.; Ye, S.; Xu, Z.; Ji, C.; Zhang, Z.; Zhang, D.; Zhang, X. Polymorphisms of AMY1A Gene and Their Association with Growth, Carcass Traits and Feed Intake Efficiency in Chickens. Genomics 2021, 113, 583–594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).