Simple Summary
European sea bass is a species with high economic and societal value in the Mediterranean due to its intensive use in aquaculture. However, it is a species characterized by high cortisol levels that show high variation. The present systematic review and meta-analysis collected and examined all the published data on circulating cortisol in this species. The aim of the study was to analyze all published values in order to provide normal values and ranges of plasma cortisol in this species, both in basal and post-acute stress conditions. Results revealed a very high between-study heterogeneity, while it also calculated the pooled levels of cortisol and their confidence intervals for both basal and post-stress conditions. Moreover, results were analyzed based on various parameters that can potentially affect cortisol levels, including technical, such as assay type and rearing unit, as well as biological, such as body size and anesthesia, influences.
Abstract
Background: European sea bass is a species characterized by high and dispersed cortisol levels. The aim of the present study was to analyze all published data on basal and post-acute stress cortisol levels in this species. Methods: For this systematic review and meta-analysis the Web of Science and Scopus databases were searched for papers reporting plasma or serum cortisol levels in E. sea bass, without language or date restrictions. Data were extracted directly for the reported results and were analyzed separately for basal and post-acute stress levels, as well their standardized mean differences (SMD) using random-effects meta-analyses. Results: Of 407 unique records identified, 69 were eligible. Basal cortisol levels had a pooled effect of 88.7 ng mL−1 (n = 57), while post-acute stress levels were 385.9 ng mL−1 (n = 34). The average SMD between basal and post-stress was calculated to be 3.02 (n = 22). All analyses had a high between-study heterogeneity. Results for basal and post-stress levels were affected by the assay type and anesthesia prior to blood sampling. Conclusions: Cortisol levels in E. sea bass are higher than most studied fish species and display large heterogeneity. Application of stress led to elevated cortisol levels in all studies examined. In all cases, sources of between-studies heterogeneity were identified.
Keywords:
aquaculture; cortisol; European sea bass; meta-analysis; reference values; stress; welfare 1. Introduction
Cortisol is the major stress hormone in fish [1,2,3]. It is the final product of the action of the Hypothalamus–Pituitary–Interrenal tissue (HPI) axis, and it has been reported to respond with increased concentrations to various types of stress in order to regulate stress responses [1,2,3]. Apart from controlling the stress responses, cortisol is a regulatory hormone for both metabolism and osmoregulation in fish [3]. Therefore, it is a crucial hormone in the physiology and biology of fish.
European sea bass, Dicentrarchus labrax, is a fish species with high economic value due to the fact that it is one of the main marine aquaculture fish species in the Mediterranean. Although widely cultured, this species shows high cortisol responses to stress compared to other species widely cultivated in the Mediterranean, such as gilthead seabream, Sparus aurata, and meage, Argyrosomus regius [4,5], as well as high variation in cortisol concentrations in basal (pre-stress) and post-stress conditions [6]. A decade ago, Ellis et al. (2012) [6] reported that a high between-study variation in the basal and post-stress cortisol concentration can be observed in this species, proposing five possible sources of variation. Recent research has shown that most of these sources indeed can add variation, while other factors remain untested still (Table 1).

Table 1.
Suggested sources of variation in cortisol levels of E. sea bass presented by Ellis et al. (2012) [6], and the respective factors that have been studied thereinafter.
1.1. Rationale for Meta-Analysis
As discussed earlier, cortisol variation in E. sea bass is large, both in the same study (within population [8]) and between different studies. This makes the generalization of conclusions based on a single measurement impossible, since under such conditions it is hard to define accurate reference values. However, cortisol measurement is an important indicator of the physiological status of fish in terms of stress, osmoregulatory, and metabolic regulations [2]. A meta-analysis of data from different independent studies can provide a quantitative statistical way to combine their results. Especially in cases with data that show high heterogeneity, such as cortisol in E. sea bass, a meta-analysis using random effects models allows for important conclusions to be drawn. Moreover, certain meta-analysis statistical tools provide the means to incorporate other effects, such as environmental (husbandry, water quality etc.), biological (body weight), and technical (method used to quantify cortisol), in the analysis. For instance, the assay type used to measure cortisol in each study (ELISA, RIA, HPLC) can be included in a sub-group meta-analysis to provide information on whether the assay type affects the outcome of the study.
1.2. Objectives
The objective of the present study was to provide normal values and ranges of plasma cortisol in E. sea bass, both in basal (i.e., without experimental exposure to any stressors) and post-acute stress conditions, as well as quantify the standardized mean difference between basal and post- stress (both acute and chronic) cortisol levels. Moreover, this study aimed at investigating the effects of (1) cortisol measurement assay type, (2) type of rearing unit, (3) use of anesthesia during the blood sampling, (4) water parameters such as temperature, salinity, dissolved oxygen, pH, (5) fish body weight, (6) stocking density, and, in the cases of post-stress levels, (7) the time after stress that the blood sampling took place.
2. Materials and Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses Statement (PRISMA) guidelines to plan, implement, and report this systematic review and meta-analysis have been followed in this study [25]. The PRISMA checklist is available in Supplementary Table S1.
2.1. Identification of Studies
The databases Web of Science and Scopus were assessed using the search terms (“European sea bass” OR “sea bass” OR dicentrarchus OR labrax) AND (cortisol OR glucocorticoid OR corticosteroid) to find peer-reviewed articles reporting cortisol levels in E. sea bass, until the date of search (7 March 2022). Figure 1 shows a flow diagram that summarizes all stages of the systematic review process, including the numbers of studies identified at each stage and any reasons for exclusion. This workflow has not been published and no protocol was prepared.
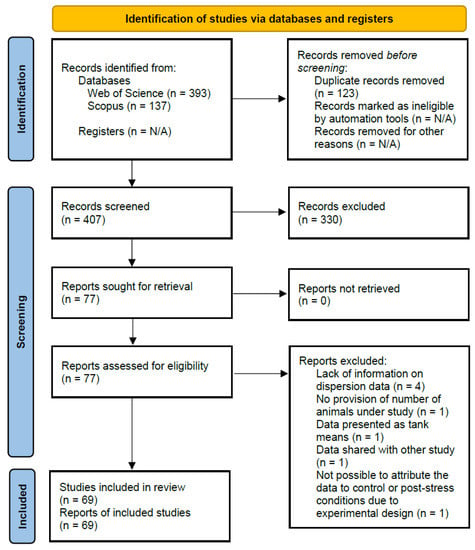
Figure 1.
Flow diagram showing phases of systematic review and study selection for meta-analysis.
2.2. Eligibility Criteria
Out of the 407 research items retrieved from the database search after duplicate removal, the criteria used for screening the articles were that: (Scr1) the study concerned E. sea bass, (Scr2) examined fully developed fish, therefore excluding larvae, and (Scr3) reported plasma, or serum cortisol levels with their concentration. At this stage, 330 research items were excluded, and the remaining 77 were subjected to more detailed inspection for eligibility, including only studies that: (Eli1) provided information on the number of animals used in each experimental group, (Eli2) provided information regarding the dispersion of the data, either as Standard Deviation (S.D.) or as Standard Error of the Mean (S.E.M.), (Eli3) used the individuals and not the tanks as experimental units and, therefore, presented the results as an average of individuals and not tanks, and (Eli4) it was possible to attribute the data to control or post-stress conditions. After this stage, 8 research items were excluded ([16,26,27,28,29,30,31,32]; Table S2), and the remaining 69 items were used in the meta-analysis.
2.3. Data Extraction
All qualitative information of the studies, such as assay type, rearing system, fish size, and water parameters, were retrieved from the text and tables of the research items. Data from the research items were extracted either from tables or graphs reporting the mean value ± S.D. or S.E.M. In the latter case, the software ImageJ was used for image analysis based on measuring the length of the y-axis and the length of the projections of the cortisol mean ± S.D. or S.E.M. In cases where various post-stress time points were presented, the time of the peak response was used. Moreover, when more than one control group was presented and in order not to violate the assumption of independency of data for the meta-analysis by analyzing data from the same research item more than once, the control groups were pooled. All cortisol data were analyzed as ng mL−1. The vast majority of the studies reported this measuring unit (62 out of 69 studies) or its derivatives ug/dL, ug/mL and ng/dL (4 out of 69 studies). There were 3 studies that reported the results as nmol L−1 (nM), and their data were converted to ng mL−1 by multiplying with the conversion factor 0.36245.
2.4. Coding of Data
Each study was coded for quantitative and qualitative data. Quantitative data included (1) fish body size, (2) water temperature, (3) dissolved oxygen, (4) water salinity, (5) water pH, and (6) stocking density. The qualitative data included (7) assay type used to measure cortisol, (8) rearing system, (9) anesthesia type, and, (10) in cases of post-stress samplings, total time between the application of stress and sampling, defined as classes (e.g., 0–30 min, 30–60 min etc.).
In cases where the quantitative data were reported as a range, the mean value was calculated. For instance, in the study by Tintos et al. 2006 [33] where the body weight was reported to range between 15–20 g, the weight was recorded as the mean between the two values, i.e., 17.5 g.
2.5. Statistical Analysis
All statistical analysis was performed in RStudio [34], using the packages “meta” [35], “dmetar” [36], and “tidyverse” [37]. Since considerable between-study heterogeneity was expected, a random-effects model was used to pool effect sizes. The heterogeneity variance τ2 was calculated using the restricted maximum likelihood estimator, while the confidence interval around the pooled effect was calculated using the Knapp–Hartung adjustments [38].
Specifically, for the basal and post-stress analysis, the pre-calculated effect sizes were analyzed under the “metagen” function, while analysis of the standardized mean differences (SMD) and their 95% confidence intervals (CIs) between basal and post-stress levels were analyzed under the “metacont” function, using Hedges method to calculate the SMD due to the small number of subjects in most studies [35,39]. Subgroup analysis for the qualitative treatment data was performed using the “byvar” argument and was based on calculating different τ2 for each subgroup, and subgroups were tested for significant differences using the Q test. Meta-regression on the quantitative influence data was performed using the “metareg” function and risk of bias was assessed by Egger’s test using the “metabias” function. The forest plot was created using the “forest” function.
3. Results
3.1. Study Characteristics
The final outcome of the literature search was 69 peer-reviewed studies that concerned circulating cortisol levels in E. sea bass and provided sufficient information on the number of animals used and the population mean values and dispersion (Figure 1). Out of these, 35 studies reported only basal, 12 only post-stress, and 22 both basal and post-stress cortisol concentrations, thus making a total of 57 studies reporting basal levels and 34 studies reporting post-stress levels. Summary characteristics for the research items included in the meta-analysis are presented (Table 2). All 69 studies had reported the weight of the fish.

Table 2.
Studies included in the meta-analysis (n = 69). In Dissolved oxygen, Salinity, pH, and Stocking density a “+” symbol signifies the presence, while a “−“ symbol the absence of information in the respective study.
3.2. Basal Levels
Frequency distribution analysis on the basal cortisol levels resulted in a positively skewed distribution (Figure 2a). The pooled effect size was calculated at 88.7 ng mL−1 [95%-CI: 65.5–109.8], with a high between-study heterogeneity (Table 3). Egger’s test showed that no significant publication bias was present in the dataset (intercept = 13.5, df = 55; p = 0.525).
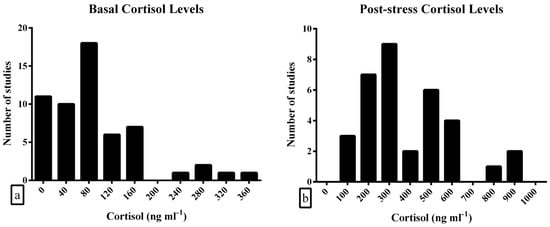
Figure 2.
Frequency distribution of meta-analysis data for (a) basal and (b) post-stress cortisol levels.

Table 3.
Results of the meta-analysis regarding basal cortisol levels, including subgroup analysis. ES: effect size; 95%-CI: 95% confidence intervals; I2: Higgin’s and Thompson’s between-study heterogeneity statistic; τ2: heterogeneity variance.
Subgroup analysis based on the assay type revealed differences between groups. Due to the low number of studies using HPLC and chemiluminescence/electrochemiluminescence assays, an analysis including only the studies using ELISA and RIA assays was performed and showed that the difference between them was significant (Q1 = 5.36; p = 0.021), being higher in ELISA than RIA. The use of anesthetic also had a significant effect on cortisol (Q5 = 12.69; p = 0.026), while no differences were observed between the rearing unit systems (Q3 = 1.41; p = 0.703).
Meta-regression analysis between effect sizes and quantitative characteristics showed that none of the examined parameters, i.e., fish body weight, water temperature, dissolved oxygen concentration, salinity, pH, and stocking density, affected the results.
3.3. Post-Stress Levels
Frequency distribution analysis on the post-stress cortisol levels resulted in a positively skewed distribution (Figure 2b). The pooled effect size was calculated at 385.9 ng mL−1 [95%-CI: 310.8–460.9], with a high between-study heterogeneity (Table 4). Egger’s test resulted in a marginally significant publication bias in the dataset (intercept = 6.4, df = 32; p = 0.047).

Table 4.
Results of the meta-analysis regarding post-stress cortisol levels, including subgroup analysis. ES: effect size; 95%-CI: 95% confidence intervals; I2: Higgin’s and Thompson’s between-study heterogeneity statistic; τ2: heterogeneity variance.
Subgroup analysis based on the assay type revealed differences between groups (Q1 = 7.76; p = 0.005), being higher in ELISA than RIA. The use of anesthetic also had a significant effect on cortisol (Q3 = 17.13; p < 0.001), excluding the “none”, “ice/cold water”, and “decap/blow to head” groups from the analysis due to the small number of studies in each group. On the other hand, no differences were observed between rearing unit systems (Q2 = 5.36; p = 0.069), excluding the ponds due to their small number. Time after stress, excluding the “>240” group due to the low number of studies, showed a significant effect (Q4 = 9.99; p = 0.041). Finally, none of the quantitative parameters was related to the effect sizes when the respective meta-regression analysis was performed.
3.4. Standardized Mean Difference between Basal and Post-Stress Cortisol
To assess the difference between basal and post-stress cortisol, 22 studies that included both pre- and post- exposure to acute stress data were used. The pooled SMD was calculated to be 3.02 [95%-CI: 2.46–3.58] (Table 5). The between-study heterogeneity was lower than the ones in the previous analysis, but still significantly large (I2 = 81.7%, τ2 = 1.12). Egger’s test using the Pustejovsky and Rodgers modification to avoid false positive results that arise with the classical Egger’s test on SMDs [94], resulted in a significant publication bias in the dataset (intercept = 4.7, df = 20; p = 0.010).

Table 5.
Results of the meta-analysis regarding the standardized mean difference (SMD) between basal and post-stress cortisol levels, including subgroup analysis. ES: effect size; 95%-CI: 95% confidence intervals; I2: Higgin’s and Thompson’s between-study heterogeneity statistic; τ2: heterogeneity variance.
It is obvious that stress had an overall effect on cortisol, a result that was observed in every study (Figure 3). The high heterogeneity between the studies can also be observed (Figure 3).
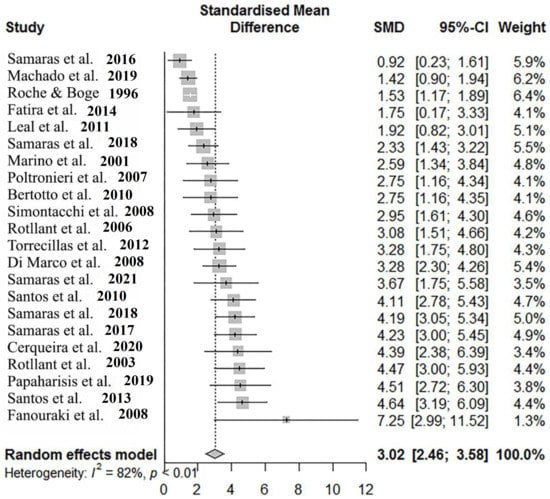
Figure 3.
Forest plot depicting the SMD, 95%-CI, and weight of each study included in the analysis. Numbers in the brackets indicate the citation number of each study in the references section. Studies used in this meta-analysis are cited in the references [5,7,10,14,44,54,56,58,61,62,65,68,71,72,74,75,76,77,79,80,82,86].
Subgroup analysis based on the assay type revealed no significant differences between groups (Q1 = 0.03; p = 0.870), being similar between studies using ELISA rather than RIA assays. No differences were observed between rearing unit systems, excluding ponds due to the low number of studies in this group (Q2 = 0.11; p = 0.945). The same was true for the use of anesthetics, analyzing only phenoxyethanol and MS222 (Q1 = 0.65; p = 0.421). Finally, time after stress had no significant effect on cortisol response (Q2 = 1.56; p = 0.458) excluding the “0–30”, “120–240”, and “<240” due to low number of studies. Finally, meta-regression analysis between effect sizes and quantitative characteristics showed that none of the examined parameters, i.e., fish body weight, water temperature, dissolved oxygen concentration, salinity, pH, and stocking density, affected the results.
4. Discussion
The study of circulating cortisol concentration in E. sea bass is intriguing due to the fact that this species shows high basal and post-stress levels of cortisol, as well as high variation both within the same population and between different studies [4,6,8]. In fact, out of the studied teleost species, E. sea bass is among the ones with the highest reported cortisol levels, together with the chub, Leuciscus cephalus, the latter having been characterized as a cortisol resistant species [95]. This high stress susceptibility has been suggested to be co-responsible for disease outbreaks in this species [96].
In this context, a systemic review of the published cortisol levels of E. sea bass could assist in better understanding whether cortisol levels in this species are indeed high as well as to define possible sources of variation between studies. This meta-analysis led to the conclusion that a high between-studies heterogeneity exists in both basal and post-stress concentrations. The reported basal concentrations were calculated to have a pooled effect size of 88.7 ng mL−1 with an 95% confidence interval between 65.5–109.8 ng mL−1, while post-stress concentration had a pooled effect size of 385.9 ng mL−1 [95%-CI: 310.8–460.9].
Subgroup meta-analysis revealed some interesting findings. However, these findings should be interpreted with care since there are constraints in the use of subgroups meta-analyses. The most important ones are the small number of studies in a subgroup and the high between-study heterogeneity, since both reduce the statistical power of the analysis. In order to reduce the “small number of studies” effect in the current analyses, subgroups with lower than three studies were excluded from subgroup meta-analysis. On the other hand, although high heterogeneity was observed in the current study, there are no available tools to mitigate its effect on the subgroups analysis.
Having the above constrains in mind, one of the major factors that seemed to affect the heterogeneity was the assay type. In both basal and post-stress levels, the pooled effect size of studies using ELISA assays was significantly higher than studies using RIA assays. Ιt is generally accepted that RIA has a higher efficiency in measuring cortisol compared to ELISA. In many studies, RIA assays are considered as more accurate when it comes to the analysis of fish cortisol [97], human salivary cortisol [98], as well as mice [99] and bird [100] corticosterone. However, there are also studies in mammals that show equal results between ELISA and RIA [101], or even better performance in the ELISA assays [102]. Therefore, it is difficult to definitely conclude which assay type is more accurate in reporting cortisol levels, but the current study supports the notion that the cortisol assay type should be taken into careful consideration when designing a study and when interpreting the results. On the other hand, when the standardized mean differences between basal and post-stress cortisol levels were analyzed, no difference between assay types was observed. This result indicates that although ELISA assays tend to over-estimate cortisol levels, they do so in a similar manner in basal and post-stress concentrations. In other words, both assay types record the magnitude of the response in the same way although ELISA overestimates the absolute values.
The rearing system, on the other hand, seemed not to affect cortisol levels. The most commonly used systems were the open-flow and the RAS, consisting of approximately 3/4 of the total number of studies. To the best of our knowledge, there are no published studies in E. sea bass to directly compare fish welfare between these rearing systems, though the effects of increased stocking density seem to be the same in fish reared in RAS and open flow systems [55,78]. What has been shown to affect welfare in this species is the size of the rearing unit, in either open flow tanks in larval stages [103] or sea cages during on-growing [76].
Regarding means of anesthesia, most studies used chemical anesthetics, mainly phenoxyethanol, followed by MS222. In both basal and post-stress conditions anesthesia treatment significantly affected the results, showing lower values in decapitated or percussively blown-in-the-head fish. It is well known that E. sea bass is a species with a rapid cortisol response [75], and, therefore, immediate killing does not allow cortisol to rise. In that way, minimum cortisol levels are reported when using this method. In basal conditions, the highest levels were reported in fish that were not under anesthesia, i.e., they were conscious, during blood sampling. When anesthetics were used, phenoxyethanol and MS222 resulted in lower levels than the other anesthetics indicating that, when E. sea bass is sampled for cortisol levels under anesthesia, it is preferable to use one of the aforementioned anesthetics. However, it should be noted that studies assessing direct comparisons between conscious and chemically anesthetized fish, using either phenoxyethanol or clove-oil [59] and MS222 [65], have not reported such differences.
In terms of magnitude of the stress response in relation to the time after stress, it is known that, in E. sea bass, cortisol starts to rise at least 6 min after the application of stress [75], reaching maximum levels at 60 to 120 min post-stress when recovery starts to take place [4,7,14]. Grouping of studies based on the post-stress time at which fish were sampled revealed a similar outcome although it should be noted that the differences were not significant. However, this synthesis of results reflected the typical, more-or less, time-course cortisol response of this species [4,5,14,75], even though different stressors, in terms of nature, intensity, and duration were used.
It is acknowledged that there are some limitations in the conclusions of the current meta-analysis. As mentioned before, the first is due to the high between-studies heterogeneity. This is a result of various reasons, including the different aims of the studies, the different assays used, the rearing methods, temperature, and so on. The second lies to the fact that E. sea bass responds very fast to handling [75], and it is, therefore, difficult to ascertain that the reported basal levels have been obtained under similar sampling stress between studies. This is similar to the source of variation #4 proposed by Ellis et al. [6] (Table 1). Third, there was a scarcity of information in environmental data that could affect the cortisol response, such as water temperature, salinity, pH, and dissolved oxygen concentration. Finally, the circadian rhythm [7,19] the seasonality [5,18], and the sex of the fish [104] are additional potential sources of variation in cortisol levels which are hardly taken into consideration—and subsequently not reported—in most published studies, and, therefore, the current study could not include them in the analysis.
5. Conclusions
In conclusion, taking into consideration the limitations discussed above, the current meta-analysis examined 69 studies and calculated a pooled effect for basal and post-stress cortisol levels for E. sea bass. A high between-studies heterogeneity was recorded, with the factors assay type and anesthesia affecting cortisol levels and adding variance to the results. Moreover, a significant effect of acute stress on cortisol levels was observed in all studies examined. On the contrary, no association between cortisol and fish body weight or environmental conditions such as water temperature, dissolved oxygen, salinity pH, stocking density, or the rearing unit was observed. Finally, although it was not possible to directly test for genetic differences between fish, seasonality, circadian rhythms, and sex due to the lack of data in the published studies, the high between-study heterogeneity indicates that these factors may be additional factors causing variation between studies in the examined species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13081340/s1, Table S1: PRISMA 202 Checklist; Table S2: Excluded studies due to violation of exclusion criteria.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon reasonable request.
Acknowledgments
The author would like to thank Irini Tsikopoulou for her advice in software use.
Conflicts of Interest
The author declares no conflict of interest.
References
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.; Flik, G. The endocrinology of the stress response in fish—An adaptation-physiological view. In Fish Physiology Biology of Stress in Fish; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 75–111. [Google Scholar]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Fanouraki, E.; Mylonas, C.C.; Papandroulakis, N.; Pavlidis, M. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen. Comp. Endocrinol. 2011, 173, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Papandroulakis, N.; Costari, M.; Pavlidis, M. Stress and metabolic indicators in a relatively high (European sea bass, Dicentrarchus labrax) and a low (meagre, Argyrosomus regius) cortisol responsive species, in different water temperatures. Aquac. Res. 2016, 47, 3501–3515. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I.M. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Fatira, E.; Papandroulakis, N.; Pavlidis, M. Diel changes in plasma cortisol and effects of size and stress duration on the cortisol response in European sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 911–919. [Google Scholar] [CrossRef]
- Samaras, A.; Dimitroglou, A.; Sarropoulou, E.; Papaharisis, L.; Kottaras, L.; Pavlidis, M. Repeatability of cortisol stress response in the European sea bass (Dicentrarchus labrax) and transcription differences between individuals with divergent responses. Sci. Rep. 2016, 6, 34858. [Google Scholar] [CrossRef]
- Samaras, A.; Pavlidis, M. Regulation of divergent cortisol responsiveness in European sea bass, Dicentrarchus labrax L. PLoS ONE 2018, 13, e0202195. [Google Scholar] [CrossRef]
- Samaras, A.; Dimitroglou, A.; Kollias, S.; Skouradakis, G.; Papadakis, I.E.; Pavlidis, M. Cortisol concentration in scales is a valid indicator for the assessment of chronic stress in European sea bass, Dicentrarchus labrax L. Aquaculture 2021, 545, 737257. [Google Scholar] [CrossRef]
- Chatziplis, D.; Oikonomou, S.; Loukovitis, D.; Tsiokos, D.; Samaras, A.; Dimitroglou, A.; Kottaras, L.; Papanna, K.; Papaharisis, L.; Tsigenopoulos, C.; et al. QTL for stress and disease resistance in European sea bass, Dicentrarchus labrax L. Animals 2020, 10, 1668. [Google Scholar] [CrossRef]
- Ferrari, S.; Millot, S.; Leguay, D.; Chatain, B.; Bégout, M.L. Consistency in European seabass coping styles: A life-history approach. Appl. Anim. Behav. Sci. 2015, 167, 74–88. [Google Scholar] [CrossRef]
- Ferrari, S.; Rey, S.; Høglund, E.; Øverli, Ø.; Chatain, B.; MacKenzie, S.; Bégout, M.L. Physiological responses during acute stress recovery depend on stress coping style in European sea bass, Dicentrarchus labrax. Physiol. Behav. 2020, 216, 112801. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Papandroulakis, N.; Lika, K.; Pavlidis, M. Water temperature modifies the acute stress response of European sea bass, Dicentrarchus labrax L. (1758). J. Therm. Biol. 2018, 78, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Kunzmann, A.; Bögner, M.; Meyer, A.; Thiele, R.; James Slater, M. Metabolic and molecular stress responses of European seabass, Dicentrarchus labrax at low and high temperature extremes. Ecol. Indic. 2020, 112, 106118. [Google Scholar] [CrossRef]
- Islam, M.J.; Slater, M.J.; Kunzmann, A. What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: The case of European seabass, Dicentrarchus labrax. Sci. Total Environ. 2020, 749, 141458. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Extreme winter cold-induced osmoregulatory, metabolic, and physiological responses in European seabass (Dicentrarchus labrax) acclimatized at different salinities. Sci. Total Environ. 2021, 771, 145202. [Google Scholar] [CrossRef]
- Pascoli, F.; Lanzano, G.S.; Negrato, E.; Poltronieri, C.; Trocino, A.; Radaelli, G.; Bertotto, D. Seasonal effects on hematological and innate immune parameters in sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 2011, 31, 1081–1087. [Google Scholar] [CrossRef]
- Planas, J.; Gutierrez, J.; Fernandez, J.; Carrillo, M.; Canals, P. Annual and daily variations of plasma cortisol in sea bass, Dicentrarchus labrax L. Aquaculture 1990, 91, 171–178. [Google Scholar] [CrossRef]
- Benhaïm, D.; Bégout, M.L.; Péan, S.; Manca, M.; Prunet, P.; Chatain, B. Impact of a plant-based diet on behavioural and physiological traits in sea bass (Dicentrarchus labrax). Aquat. Living Resour. 2013, 26, 121–131. [Google Scholar] [CrossRef]
- Carbonara, P.; Zupa, W.; Bitetto, I.; Alfonso, S.; Dara, M.; Cammarata, M. Evaluation of the effects of the enriched-organic diets composition on european sea bass welfare through a multi-parametric approach. J. Mar. Sci. Eng. 2020, 8, 934. [Google Scholar] [CrossRef]
- Serradell, A.; Torrecillas, S.; Makol, A.; Valdenegro, V.; Fernández-Montero, A.; Acosta, F.; Izquierdo, M.S.; Montero, D. Prebiotics and phytogenics functional additives in low fish meal and fish oil based diets for European sea bass (Dicentrarchus labrax): Effects on stress and immune responses. Fish Shellfish Immunol. 2020, 100, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, R.; Machado, M.; Afonso, A.; Fierro-Castro, C.; Reyes-López, F.E.; Tort, L.; Gesto, M.; Conde-Sieira, M.; Míguez, J.M.; Soengas, J.L.; et al. Neuroendocrine and immune responses undertake different fates following tryptophan or methionine dietary treatment: Tales from a teleost model. Front. Immunol. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Alfonso, S.; Cousin, X.; Prunet, P.; Bégout, M.L.; Leguen, I. Global assessment of the response to chronic stress in European sea bass. Aquaculture 2021, 544, 737072. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Karakatsouli, N.; Katsakoulis, P.; Leondaritis, G.; Kalogiannis, D.; Papoutsoglou, S.E.; Chadio, S.; Sakellaridis, N. Acute stress response of European sea bass Dicentrarchus labrax under blue and white light. Aquaculture 2012, 364–365, 48–52. [Google Scholar] [CrossRef]
- Lupatsch, I.; Santos, G.A.; Schrama, J.W.; Verreth, J.A.J. Effect of stocking density and feeding level on energy expenditure and stress responsiveness in European sea bass Dicentrarchus labrax. Aquaculture 2010, 298, 245–250. [Google Scholar] [CrossRef]
- Santulli, A.; Modica, A.; Messina, C.; Ceffa, L.; Curatolo, A.; Rivas, G.; Fabi, G.; D’Amelio, V. Biochemical responses of European sea bass (Dicentrarchus labrax L.) to the stress induced by off shore experimental seismic prospecting. Mar. Pollut. Bull. 1999, 38, 1105–1114. [Google Scholar] [CrossRef]
- Caruso, G.; Genovese, L.; Maricchiolo, G.; Modica, A. Haematological, biochemical and immunological parameters as stress indicators in Dicentrarchus labrax and Sparus aurata farmed in off-shore cages. Aquac. Int. 2005, 13, 67–73. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Altunay, S. Physiological stress and innate immune response in gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) exposed to combination of trimethoprim and sulfamethoxazole (TMP-SMX). Fish Physiol. Biochem. 2011, 37, 401–409. [Google Scholar] [CrossRef]
- Goda, A.M.A.S.; Srour, T.M.; Mansour, A.T.; Baromh, M.Z.; Sallam, G.R.; Baromh, A.Z. Assessment of stresful ambient water salinity on growth, feed utilization and hematological indices of european sea bass, Dicentrarchus labrax, juveniles. AACL Bioflux 2019, 12, 553–563. [Google Scholar]
- Gornati, R.; Papis, E.; Rimoldi, S.; Terova, G.; Saroglia, M.; Bernardini, G. Rearing density influences the expression of stress-related genes in sea bass (Dicentrarchus labrax, L.). Gene 2004, 341, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tintos, A.; Míguez, J.M.; Mancera, J.M.; Soengas, J.L. Development of a microtitre plate indirect ELISA for measuring cortisol in teleosts, and evaluation of stress responses in rainbow trout and gilthead sea bream. J. Fish Biol. 2006, 68, 251–263. [Google Scholar] [CrossRef]
- RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, USA. Available online: http://www.rstudio.com/ (accessed on 2 February 2023).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Heal. 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpes, P.; Furukawa, T.; Ebert, D.D. dmetar: Companion R Package for the Guide “Doing Meta-Analysis in R” 2019. Available online: https://dmetar.protectlab.org/ (accessed on 2 February 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpes, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-on Guide; Chapmann & Hall: London, UK; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-61007-4. [Google Scholar]
- Acerete, L.; Reig, L.; Alvarez, D.; Flos, R.; Tort, L. Comparison of two stunning/slaughtering methods on stress response and quality indicators of European sea bass (Dicentrarchus labrax). Aquaculture 2009, 287, 139–144. [Google Scholar] [CrossRef]
- Alfonso, S.; Sadoul, B.; Gesto, M.; Joassard, L.; Chatain, B.; Geffroy, B.; Bégout, M.L. Coping styles in European sea bass: The link between boldness, stress response and neurogenesis. Physiol. Behav. 2019, 207, 76–85. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2020, 98, 1496–1508. [Google Scholar] [CrossRef]
- Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Dioguardi, M.; Dara, M.; Lembo, G.; Carbonara, P.; Cammarata, M.; Cammarata, M. Surgical implantation of electronic tags does not induce medium-term effect: Insights from growth and stress physiological profile in two marine fish species. Anim. Biotelemetry 2020, 8, 4–9. [Google Scholar] [CrossRef]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Majolini, D.; Radaelli, G.; Simontacchi, C. Alternative matrices for cortisol measurement in fish. Aquac. Res. 2010, 41, 1261–1267. [Google Scholar] [CrossRef]
- Bodur, T.; León-Bernabeu, S.; Navarro, A.; Tort, L.; Afonso, J.M.; Montero, D. Effects of new plant based anesthetics Origanum sp. and Eucalyptus sp. oils on stress and welfare parameters in Dicentrarchus labrax and their comparison with clove oil. Aquaculture 2018, 495, 402–408. [Google Scholar] [CrossRef]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen peroxide oxygenation and disinfection capacity in recirculating aquaculture systems. Aquac. Eng. 2021, 92, 102140. [Google Scholar] [CrossRef]
- Brandts, I.; Balasch, J.C.; Gonçalves, A.P.; Martins, M.A.; Pereira, M.L.; Tvarijonaviciute, A.; Teles, M.; Oliveira, M. Immuno-modulatory effects of nanoplastics and humic acids in the European seabass (Dicentrarchus labrax). J. Hazard. Mater. 2021, 414, 125562. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, P.; Corsi, I.; Focardi, S.; Lembo, G.; Rochira, S.; Scolamacchia, M.; Spedicato, M.T.; Mckinley, R.S. The effects of stress induced by cortisol administration on the repeatability of swimming performance tests in the European sea bass (Dicentrarchus labrax L.). Mar. Freshw. Behav. Physiol. 2010, 43, 283–296. [Google Scholar] [CrossRef]
- Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; Zupa, W.; Mckinley, R.S.; Lembo, G. Muscle activity as a key indicator of welfare in farmed European sea bass (Dicentrarchus labrax L. 1758). Aquac. Res. 2015, 46, 2133–2146. [Google Scholar] [CrossRef]
- Carbonara, P.; Dioguardi, M.; Cammarata, M.; Zupa, W.; Vazzana, M.; Spedicato, M.T.; Lembo, G. Basic knowledge of social hierarchies and physiological profile of reared sea bass Dicentrarchus labrax (L.). PLoS ONE 2019, 14, e0208688. [Google Scholar] [CrossRef]
- Caruso, G.; Denaro, M.G.; Caruso, R.; Mancari, F.; Genovese, L.; Maricchiolo, G. Response to short term starvation of growth, haematological, biochemical and non-specific immune parameters in European sea bass (Dicentrarchus labrax) and blackspot sea bream (Pagellus bogaraveo). Mar. Environ. Res. 2011, 72, 46–52. [Google Scholar] [CrossRef]
- Celi, M.; Vazzana, M.; Sanfratello, M.A.; Parrinello, N. Elevated cortisol modulates Hsp70 and Hsp90 gene expression and protein in sea bass head kidney and isolated leukocytes. Gen. Comp. Endocrinol. 2012, 175, 424–431. [Google Scholar] [CrossRef]
- Rubio, V.C.; Sánchez, E.; Cerdá-Reverter, J.M. Compensatory feeding in the sea bass after fasting and physical stress. Aquaculture 2010, 298, 332–337. [Google Scholar] [CrossRef]
- Cerqueira, M.; Millot, S.; Felix, A.; Silva, T.; Oliveira, G.A.; Oliveira, C.C.V.; Rey, S.; MacKenzie, S.; Oliveira, R. Cognitive appraisal in fish: Stressor predictability modulates the physiological and neurobehavioural stress response in sea bass. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192922. [Google Scholar] [CrossRef]
- D’Orbcastel, E.R.; Lemarié, G.; Breuil, G.; Petochi, T.; Marino, G.; Triplet, S.; Dutto, G.; Fivelstad, S.; Coeurdacier, J.L.; Blancheton, J.P. Effects of rearing density on sea bass (Dicentrarchus labrax) biological performance, blood parameters and disease resistance in a flow through system. Aquat. Living Resour. 2010, 23, 109–117. [Google Scholar] [CrossRef]
- Di Marco, P.; Priori, A.; Finoia, M.G.; Massari, A.; Mandich, A.; Marino, G. Physiological responses of European sea bass Dicentrarchus labrax to different stocking densities and acute stress challenge. Aquaculture 2008, 275, 319–328. [Google Scholar] [CrossRef]
- Faggion, S.; Bertotto, D.; Babbucci, M.; Dalla Rovere, G.; Franch, R.; Bovolenta, M.; Laureau, S.; Pascoli, F.; Toffan, A.; Bargelloni, L.; et al. Resistance to viral nervous necrosis in European sea bass (Dicentrarchus labrax L.): Heritability and relationships with body weight, cortisol concentration, and antibody titer. Genet. Sel. Evol. 2021, 53, 32. [Google Scholar] [CrossRef] [PubMed]
- Fanouraki, E.; Papandroulakis, N.; Ellis, T.; Mylonas, C.C.; Scott, A.P.; Pavlidis, M. Water cortisol is a reliable indicator of stress in European sea bass, Dicentrarchus labrax. Behaviour 2008, 145, 1267–1281. [Google Scholar]
- Filiciotto, F.; Buscaino, G.; Buffa, G.; Bellante, A.; Maccarrone, V.; Mazzola, S. Anaesthetic qualities of eugenol and 2-phenoxyethanol and their effect on same haematological parameters in farmed European sea bass (Dicentrarchus labrax L.). J. Anim. Vet. Adv. 2012, 11, 494–502. [Google Scholar] [CrossRef]
- Herrero, M.J.; Martínez, F.J.; Míguez, J.M.; Madrid, J.A. Response of plasma and gastrointestinal melatonin, plasma cortisol and activity rhythms of European sea bass (Dicentrarchus labrax) to dietary supplementation with tryptophan and melatonin. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007, 177, 319–326. [Google Scholar] [CrossRef]
- Leal, E.; Fernández-Durán, B.; Guillot, R.; Ríos, D.; Cerdá-Reverter, J.M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2011, 181, 1035–1044. [Google Scholar] [CrossRef]
- Machado, M.; Castro, C.; Oliva-Teles, A.; Costas, B. Interactive effects of dietary vegetable oil and carbohydrate incorporation on the innate immune response of European seabass (Dicentrarchus labrax) juveniles subjected to acute stress. Aquaculture 2019, 498, 171–180. [Google Scholar] [CrossRef]
- Machado, M.; Azeredo, R.; Domingues, A.; Fernandez-Boo, S.; Dias, J.; Conceição, L.E.C.; Costas, B. Dietary tryptophan deficiency and its supplementation compromises inflammatory mechanisms and disease resistance in a teleost fish. Sci. Rep. 2019, 9, 7689. [Google Scholar] [CrossRef]
- Maricchiolo, G.; Mirto, S.; Caruso, G.; Caruso, T.; Bonaventura, R.; Celi, M.; Matranga, V.; Genovese, L. Welfare status of cage farmed European sea bass (Dicentrarchus labrax): A comparison between submerged and surface cages. Aquaculture 2011, 314, 173–181. [Google Scholar] [CrossRef]
- Marino, G.; Di Marco, P.; Mandich, A.; Finoia, M.G.; Cataudella, S. Changes in serum cortisol, metabolites, osmotic pressure and electrolytes in response to different blood sampling procedures in cultured sea bass (Dicentrarchus labrax L.). J. Appl. Ichthyol. 2001, 17, 115–120. [Google Scholar] [CrossRef]
- Millot, S.; Cerqueira, M.; Castanheira, M.-F.; Øverli, Ø.; Oliveira, R.F.; Martins, C.I.M. Behavioural Stress Responses Predict Environmental Perception in European Sea Bass (Dicentrarchus labrax). PLoS ONE 2014, 9, e108800. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Grande, B.; Guerreiro, P.M.; Sanahuja, I.; Fernández-Alacid, L.; Ibarz, A. Evaluation of an acute osmotic stress in european sea bass via skin mucus biomarkers. Animals 2020, 10, 1546. [Google Scholar] [CrossRef]
- Papaharisis, L.; Tsironi, T.; Dimitroglou, A.; Taoukis, P.; Pavlidis, M. Stress assessment, quality indicators and shelf life of three aquaculture important marine fish, in relation to harvest practices, water temperature and slaughter method. Aquac. Res. 2019, 50, 2608–2620. [Google Scholar] [CrossRef]
- Peruzzi, S.; Varsamos, S.; Chatain, B.; Fauvel, C.; Menu, B.; Falguière, J.C.; Sévère, A.; Flik, G. Haematological and physiological characteristics of diploid and triploid sea bass, Dicentrarchus labrax L. Aquaculture 2005, 244, 359–367. [Google Scholar] [CrossRef]
- Petochi, T.; Di Marco, P.; Priori, A.; Finoia, M.G.; Mercatali, I.; Marino, G. Coping strategy and stress response of European sea bass Dicentrarchus labrax to acute and chronic environmental hypercapnia under hyperoxic conditions. Aquaculture 2011, 315, 312–320. [Google Scholar] [CrossRef]
- Poltronieri, C.; Maccatrozzo, L.; Simontacchi, C.; Bertotto, D.; Funkenstein, B.; Patruno, M.; Radaelli, G. Quantitative RT-PCR analysis and immunohistochemical localization of HSP70 in sea bass Dicentrarchus labrax exposed to transport stress. Eur. J. Histochem. 2007, 51, 125–136. [Google Scholar] [CrossRef]
- Roche, H.; Boge, G. Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar. Environ. Res. 1996, 41, 27–43. [Google Scholar] [CrossRef]
- Roque, A.; Yildiz, H.Y.; Carazo, I.; Duncan, N. Physiological stress responses of sea bass (Dicentrarchus labrax) to hydrogen peroxide (H2O2) exposure. Aquaculture 2010, 304, 104–107. [Google Scholar] [CrossRef]
- Rotllant, J.; Ruane, N.M.; Dinis, M.T.; Canario, A.V.M.; Power, D.M. Intra-adrenal interactions in fish: Catecholamine stimulated cortisol release in sea bass (Dicentrarchus labrax L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 375–381. [Google Scholar] [CrossRef]
- Rotllant, J.; Ruane, N.M.; Caballero, M.J.; Montero, D.; Tort, L. Response to confinement in sea bass (Dicentrarchus labrax) is characterised by an increased biosynthetic capacity of interrenal tissue with no effect on ACTH sensitivity. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Pavlidis, M.; Lika, K.; Theodoridi, A.; Papandroulakis, N. Scale matters: Performance of European sea bass, Dicentrarchus labrax, L. (1758), reared in cages of different volumes. Aquac. Res. 2017, 48, 990–1005. [Google Scholar] [CrossRef]
- Samaras, A.; Santo, C.E.; Papandroulakis, N.; Mitrizakis, N.; Pavlidis, M.; Höglund, E.; Pelgrim, T.N.M.; Zethof, J.; Spanings, F.A.T.; Vindas, M.A.; et al. Allostatic load and stress physiology in European seabass (Dicentrarchus labrax L.) and gilthead seabream (Sparus aurata L.). Front. Endocrinol. 2018, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Sammouth, S.; d’Orbcastel, E.R.; Gasset, E.; Lemarié, G.; Breuil, G.; Marino, G.; Coeurdacier, J.L.; Fivelstad, S.; Blancheton, J.P. The effect of density on sea bass (Dicentrarchus labrax) performance in a tank-based recirculating system. Aquac. Eng. 2009, 40, 72–78. [Google Scholar] [CrossRef]
- Santos, G.A.; Schrama, J.W.; Capelle, J.; Rombout, J.H.W.M.; Verreth, J.A.J. Effects of dissolved carbon dioxide on energy metabolism and stress responses in European seabass (Dicentrarchus labrax). Aquac. Res. 2013, 44, 1370–1382. [Google Scholar] [CrossRef]
- Santos, G.A.; Schrama, J.W.; Mamauag, R.E.P.; Rombout, J.H.W.M.; Verreth, J.A.J. Chronic stress impairs performance, energy metabolism and welfare indicators in European seabass (Dicentrarchus labrax): The combined effects of fish crowding and water quality deterioration. Aquaculture 2010, 299, 73–80. [Google Scholar] [CrossRef]
- Silva-Brito, F.; Timóteo, F.; Esteves, Â.; Peixoto, M.J.; Ozorio, R.; Magnoni, L. Impact of the replacement of dietary fish oil by animal fats and environmental salinity on the metabolic response of European Seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part-B Biochem. Mol. Biol. 2019, 233, 46–59. [Google Scholar] [CrossRef]
- Simontacchi, C.; Poltronieri, C.; Carraro, C.; Bertotto, D.; Xiccato, G.; Trocino, A.; Radaelli, G. Alternative stress indicators in sea bass Dicentrarchus labrax, L.J. Fish Biol. 2008, 72, 747–752. [Google Scholar] [CrossRef]
- Teles, M.; Pacheco, M.; Santos, M.A. Biotransformation, stress and genotoxic effects of 17β-estradiol in juvenile sea bass (Dicentrarchus labrax L.). Environ. Int. 2006, 32, 470–477. [Google Scholar] [CrossRef]
- Teles, M.; Gravato, C.; Pacheco, M.; Santos, M.A. Juvenile sea bass biotransformation, genotoxic and endocrine responses to β-naphthoflavone, 4-nonylphenol and 17β-estradiol individual and combined exposures. Chemosphere 2004, 57, 147–158. [Google Scholar] [CrossRef]
- Terova, G.; Gornati, R.; Rimoldi, S.; Bernardini, G.; Saroglia, M. Quantification of a glucocorticoid receptor in sea bass (Dicentrarchus labrax, L.) reared at high stocking density. Gene 2005, 357, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Dhanasiri, A.K.S.; Sweetman, J.; Izquierdo, M. Effects on mortality and stress response in European sea bass, Dicentrarchus labrax (L.), fed mannan oligosaccharides (MOS) after Vibrio anguillarum exposure. J. Fish Dis. 2012, 35, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Tsalafouta, A.; Papandroulakis, N.; Pavlidis, M. Early life stress and effects at subsequent stages of development in European sea bass (D. labrax). Aquaculture 2015, 436, 27–33. [Google Scholar] [CrossRef]
- Varsamos, S.; Flik, G.; Pepin, J.F.; Wendelaar Bonga, S.E.; Breuil, G. Husbandry stress during early life stages affects the stress response and health status of juvenile sea bass, Dicentrarchus labrax. Fish Shellfish Immunol. 2006, 20, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Varsamos, S.; Xuereb, B.; Commes, T.; Flik, G.; Spanings-Pierrot, C. Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J. Endocrinol. 2006, 191, 473–480. [Google Scholar] [CrossRef]
- Vazzana, M.; Vizzini, A.; Sanfratello, M.A.; Celi, M.; Salerno, G.; Parrinello, N. Differential expression of two glucocorticoid receptors in seabass (teleost fish) head kidney after exogeneous cortisol inoculation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 49–54. [Google Scholar] [CrossRef]
- Vera, L.M.; Migaud, H. Continuous high light intensity can induce retinal degeneration in Atlantic salmon, Atlantic cod and European sea bass. Aquaculture 2009, 296, 150–158. [Google Scholar] [CrossRef]
- Volckaert, F.a.M.; Hellemans, B.; Batargias, C.; Louro, B.; Massault, C.; Van Houdt, J.K.J.; Haley, C.; de Koning, D.-J.; Canario, A.V.M. Heritability of cortisol response to confinement stress in European sea bass Dicentrarchus labrax. Genet. Sel. Evol. 2012, 44, 15. [Google Scholar] [CrossRef]
- Yildiz, H.; Ergonul, M.B. Is prophylactic formalin exposure a stress source for gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax)? Ankara Univ. Vet. Fak. Derg. 2010, 57, 113–118. [Google Scholar] [CrossRef]
- Pustejovsky, J.E.; Rodgers, M.A. Testing for funnel plot asymmetry of standardized mean differences. Res. Synth. Methods 2019, 10, 57–71. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Carrick, T.R.; Appleby, A.; Yeomans, W.E. High blood cortisol levels and low cortisol receptor affinity: Is the chub, Leuciscus cephalus, a cortisol-resistant teleost? Gen. Comp. Endocrinol. 2000, 120, 108–117. [Google Scholar] [CrossRef] [PubMed]
- FAO Cultured Aquatic Species Information Programme: Dicentrarchus labrax (Linnaeus, 1758). Available online: http://www.fao.org/fishery/culturedspecies/Dicentrarchus_labrax/en#tcNA0112 (accessed on 24 September 2021).
- Sink, T.D.; Lochmann, R.T.; Fecteau, K.A. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol. Biochem. 2008, 34, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Raff, H.; Homar, P.J.; Burns, E.A. Comparison of two methods for measuring salivary cortisol. Clin. Chem. 2002, 48, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Rod, A.M.K.; Harkestad, N.; Jellestad, F.K.; Murison, R. Comparison of commercial ELISA assays for quantification of corticosterone in serum. Sci. Rep. 2017, 7, 6748. [Google Scholar] [CrossRef]
- Glucs, Z.E.; Smith, D.R.; Tubbs, C.W.; Scherbisnki, J.J.; Welch, A.; Burnett, J.; Clark, M.; Eng, C.; Finkelstein, M.E. Glucocorticoid measurement in plasma, urates and feathers from California condors (Gymnogyps califorianus) in response to a human-induced stressor. PLoS ONE 2018, 13, e0205565. [Google Scholar] [CrossRef]
- Bekhbat, M.; Glasper, E.R.; Rowson, S.A.; Kelly, S.D.; Gretchen, N.N. Measuring corticosterone concentrations over a physiological dynamic range in female rats. Physiol. Behav. 2018, 194, 73–76. [Google Scholar] [CrossRef]
- Nejad, J.G.; Park, K.H.; Forghani, F.; Lee, H.G.; Lee, J.S.; Sung, K.I. Measuring hair and blood cortisol in sheep and dairy cattle using RIA and ELISA assay: A comparison. Biol. Rhythm Res. 2020, 51, 887–897. [Google Scholar] [CrossRef]
- Lika, K.; Pavlidis, M.; Mitrizakis, N.; Samaras, A.; Papandroulakis, N. Do experimental units of different scale affect the biological performance of European sea bass Dicentrarchus labrax larvae? J. Fish Biol. 2015, 86, 1271–1285. [Google Scholar] [CrossRef]
- Haddy, J.A.; Pankhurst, N.W. Stress-induced changes in concentrations of plasma sex steroids in black bream. J. Fish Biol. 1999, 55, 1304–1316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).