Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread
Abstract
Simple Summary
Abstract
1. Introduction
2. Search Methodology
3. The Immune System as the Main Player in the Tumour’s Defence
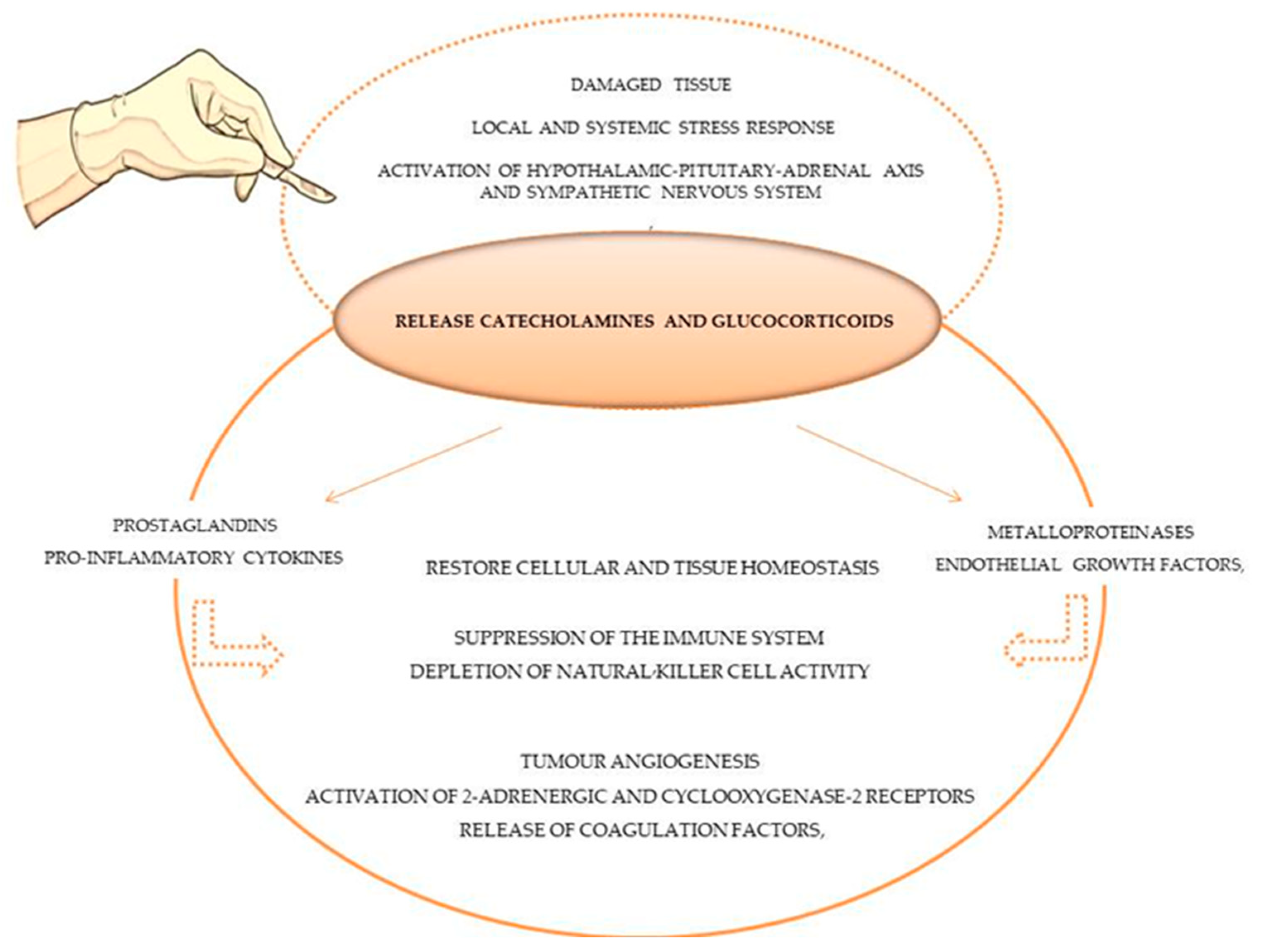
4. Tumour Surgery as a “Starting Point” for Tumour Progression
5. The Impact of the General Anaesthetics on the Immune System and Their “Anti-” and “Pro-Tumoral” Effects
5.1. Volatile Anaesthetic Agents
5.2. Intravenous Anaesthetic Agents
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wemelsfelder, F.; Mullan, S. Applying Ethological and Health Indicators to Practical Animal Welfare Assessment. Rev. Sci. Tech. OIE 2014, 33, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.E.; Colyer, A.; Haydock, R.M.; Hayek, M.G.; Park, J. Understanding How Dogs Age: Longitudinal Analysis of Markers of Inflammation, Immune Function, and Oxidative Stress. J. Gerontol. Ser. A 2018, 73, 720–728. [Google Scholar] [CrossRef]
- Pinello, K.; Amorim, I.; Pires, I.; Canadas-Sousa, A.; Catarino, J.; Faísca, P.; Branco, S.; Peleteiro, M.C.; Silva, D.; Severo, M.; et al. Vet-OncoNet: Malignancy Analysis of Neoplasms in Dogs and Cats. Vet. Sci. 2022, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Rafalko, J.M.; Kruglyak, K.M.; McCleary-Wheeler, A.L.; Goyal, V.; Phelps-Dunn, A.; Wong, L.K.; Warren, C.D.; Brandstetter, G.; Rosentel, M.C.; DiMarzio, L.; et al. Age at Cancer Diagnosis by Breed, Weight, Sex, and Cancer Type in a Cohort of More than 3,000 Dogs: Determining the Optimal Age to Initiate Cancer Screening in Canine Patients. PLoS ONE 2023, 18, e0280795. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Wang, Q.; Li, J.; Liu, H.; Meng, X.; Zhang, H. Aging, Cancer and Immunity. J. Cancer 2019, 10, 3021–3027. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Urfer, S.R.; White, M.; Megquier, K.; Shrager, S.; The Dog Aging Project Consortium; Ruple, A. Lifetime Prevalence of Malignant and Benign Tumours in Companion Dogs: Cross-sectional Analysis of Dog Aging Project Baseline Survey. Vet. Comp. Oncol. 2022, 20, 797–804. [Google Scholar] [CrossRef]
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E.L. Mortality in North American Dogs from 1984 to 2004: An Investigation into Age-, Size-, and Breed-Related Causes of Death: Mortality of Dogs in North America. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- Smith, A.N. Advances in Veterinary Oncology. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, xi–xii. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Shibata, J.; Ishihara, S.; Tada, N.; Kawai, K.; Tsuno, N.H.; Yamaguchi, H.; Sunami, E.; Kitayama, J.; Watanabe, T. Surgical Stress Response after Colorectal Resection: A Comparison of Robotic, Laparoscopic, and Open Surgery. Tech. Coloproctol. 2015, 19, 275–280. [Google Scholar] [CrossRef]
- Hume, K.R.; Johnson, J.L.; Williams, L.E. Adverse Effects of Concurrent Carboplatin Chemotherapy and Radiation Therapy in Dogs. J. Vet. Intern. Med. 2009, 23, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wendelburg, K.M.; Price, L.L.; Burgess, K.E.; Lyons, J.A.; Lew, F.H.; Berg, J. Survival Time of Dogs with Splenic Hemangiosarcoma Treated by Splenectomy with or without Adjuvant Chemotherapy: 208 Cases (2001–2012). J. Am. Vet. Med. Assoc. 2015, 247, 393–403. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Rossanese, M.; Suárez-Bonnet, A.; Hardas, A.; Yale, A.D. Urinary Bladder Hemangiosarcoma in a Cat Treated with Partial Cystectomy and Adjuvant Metronomic Cyclophosphamide and Thalidomide. Vet. Intern. Med. 2023, 37, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Riggs, J.; Adams, V.J.; Hermer, J.V.; Dobson, J.M.; Murphy, S.; Ladlow, J.F. Outcomes Following Surgical Excision or Surgical Excision Combined with Adjunctive, Hypofractionated Radiotherapy in Dogs with Oral Squamous Cell Carcinoma or Fibrosarcoma. J. Am. Vet. Med. Assoc. 2018, 253, 73–83. [Google Scholar] [CrossRef]
- Inbar, S.; Neeman, E.; Avraham, R.; Benish, M.; Rosenne, E.; Ben-Eliyahu, S. Do Stress Responses Promote Leukemia Progression? An Animal Study Suggesting a Role for Epinephrine and Prostaglandin-E2 through Reduced NK Activity. PLoS ONE 2011, 6, e19246. [Google Scholar] [CrossRef]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving Postoperative Immune Status and Resistance to Cancer Metastasis: A Combined Perioperative Approach of Immunostimulation and Prevention of Excessive Surgical Stress Responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Lee, Y.N. Effect of Anesthesia and Surgery on Immunity. J. Surg. Oncol. 1977, 9, 425–430. [Google Scholar] [CrossRef]
- Ogawa, K.; Hirai, M.; Katsube, T.; Murayama, M.; Hamaguchi, K.; Shimakawa, T.; Naritake, Y.; Hosokawa, T.; Kajiwara, T. Suppression of Cellular Immunity by Surgical Stress. Surgery 2000, 127, 329–336. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Tan, H.; Ouyang, H.; Zhang, Y.; Zeng, W. Anaesthetic Technique May Affect Prognosis for Ovarian Serous Adenocarcinoma: A Retrospective Analysis. Br. J. Anaesth. 2011, 106, 814–822. [Google Scholar] [CrossRef]
- Desborough, J.P. The Stress Response to Trauma and Surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef]
- Novitsky, Y.W.; Litwin, D.E.M.; Callery, M.P. The Net Immunologic Advantage of Laparoscopic Surgery. Surg. Endosc. 2004, 18, 1411–1419. [Google Scholar] [CrossRef]
- Dourado, A.; Gomes, A.; Teixeira, P.; Lobo, L.; Azevedo, J.T.; Dias, I.R.; Pinelas, R. Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy. Vet. Sci. 2022, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Mair, A.R.; Martinez-Taboada, F. Opioid-Free Anaesthesia in Three Dogs. Open Vet. J. 2017, 7, 104. [Google Scholar] [CrossRef]
- Tomihari, M.; Nishihara, A.; Shimada, T.; Yanagawa, M.; Miyoshi, M.; Miyahara, K.; Oishi, A. A Comparison of the Immunological Effects of Propofol and Isoflurane for Maintenance of Anesthesia in Healthy Dogs. J. Vet. Med. Sci. 2015, 77, 1227–1233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeo, J.; Park, J.S.; Choi, G.-S.; Kim, H.J.; Kim, J.K.; Oh, J.; Park, S.Y. Comparison of the Analgesic Efficacy of Opioid-Sparing Multimodal Analgesia and Morphine-Based Patient-Controlled Analgesia in Minimally Invasive Surgery for Colorectal Cancer. World J. Surg. 2022, 46, 1788–1795. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Ferreira, A.F.; Queiroga, F.L. Positive Interplay Between CD3+ T-Lymphocytes and Concurrent COX-2/EGFR Expression in Canine Malignant Mammary Tumors. Anticancer Res. 2015, 35, 2915–2920. [Google Scholar]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Lee, Y.S.; Radford, K.J. The Role of Dendritic Cells in Cancer. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 348, pp. 123–178. ISBN 978-0-12-818351-9. [Google Scholar]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New Insights into Cancer Immunoediting and Its Three Component Phases—Elimination, Equilibrium and Escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef]
- Petrucci, G.N.; Lobo, L.; Queiroga, F.; Martins, J.; Prada, J.; Pires, I.; Henriques, J. Neutrophil-to-lymphocyte Ratio Is an Independent Prognostic Marker for Feline Mammary Carcinomas. Vet. Comp. Oncol. 2021, 19, 482–491. [Google Scholar] [CrossRef]
- Marconato, L.; Martini, V.; Stefanello, D.; Moretti, P.; Ferrari, R.; Comazzi, S.; Laganga, P.; Riondato, F.; Aresu, L. Peripheral Blood Lymphocyte/Monocyte Ratio as a Useful Prognostic Factor in Dogs with Diffuse Large B-Cell Lymphoma Receiving Chemoimmunotherapy. Vet. J. 2015, 206, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8 + Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Macfarlane, M.J.; Macfarlane, L.L.; Scase, T.; Parkin, T.; Morris, J.S. Use of Neutrophil to Lymphocyte Ratio for Predicting Histopathological Grade of Canine Mast Cell Tumours. Vet. Rec. 2016, 179, 491. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Rao, S.; Lafferty, M.H.; Thamm, D.H.; Morley, P.S.; Withrow, S.J.; Dow, S.W. Association of Blood Monocyte and Lymphocyte Count and Disease-Free Interval in Dogs with Osteosarcoma: CBC Is Prognostic in Osteosarcoma. J. Vet. Intern. Med. 2010, 24, 1439–1444. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Hanazawa, A.; Nakajima, Y.; Nariai, Y.; Asanuma, H.; Kuwabara, M.; Yukawa, M.; Ito, H. T-Helper (Th) 1/Th2 Imbalance in the Peripheral Blood of Dogs with Malignant Tumor. Microbiol. Immunol. 2007, 51, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Chon, S.-K.; Im, K.-S.; Kim, N.-H.; Sur, J.-H. Correlation of Tumor-Infiltrating Lymphocytes to Histopathological Features and Molecular Phenotypes in Canine Mammary Carcinoma: A Morphologic and Immunohistochemical Morphometric Study. Can. J. Vet. Res. 2013, 77, 142–149. [Google Scholar]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A Role for T-Lymphocytes in Human Breast Cancer and in Canine Mammary Tumors. BioMed Res. Int. 2014, 2014, 130894. [Google Scholar] [CrossRef]
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages Promote the Invasion of Breast Carcinoma Cells via a Colony-Stimulating Factor-1/Epidermal Growth Factor Paracrine Loop. Cancer Res. 2005, 65, 5278–5283. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T Helper Type 2 Cell Infiltrate Correlates with Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.-H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, Ç. Comparison of the Analgesic Effects of Morphine and Tramadol after Tumor Surgery in Dogs. Open Vet. J. 2021, 11, 613. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Honma, R.; Sato, A.; Matsumoto, H.; Koyama, H.; Tagawa, M. Effect of rCaIFN-γ Pretreatment on Propofol–Isoflurane Suppression of NK Cytotoxic Activity in the Peripheral Blood of Dogs. Res. Vet. Sci. 2015, 98, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Miyata, T.; Ohkusa, T.; Teshima, T.; Koyama, H. Effects of Recombinant Canine Interferon-γ Injected before General Anesthesia with Propofol and Isoflurane on Natural Killer Cytotoxic Activity during Anesthesia in Dogs. Res. Vet. Sci. 2019, 125, 416–420. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Hashemi, V.; Maleki, L.A.; Esmaily, M.; Masjedi, A.; Ghalamfarsa, G.; Namdar, A.; Yousefi, M.; Yousefi, B.; Jadidi-Niaragh, F. Regulatory T Cells in Breast Cancer as a Potent Anti-Cancer Therapeutic Target. Int. Immunopharmacol. 2020, 78, 106087. [Google Scholar] [CrossRef]
- Terhune, J.; Berk, E.; Czerniecki, B. Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy. Vaccines 2013, 1, 527–549. [Google Scholar] [CrossRef]
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic Cells in the Cancer Microenvironment. J. Cancer 2013, 4, 36–44. [Google Scholar] [CrossRef]
- Tvedskov, T.F.; Jensen, M.-B.; Kroman, N.; Balslev, E. Iatrogenic Displacement of Tumor Cells to the Sentinel Node after Surgical Excision in Primary Breast Cancer. Breast Cancer Res. Treat. 2012, 131, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Raven, R.W. Surgical Oncology-Theory and Practice. J. Surg. Oncol. 2006, 30, 145–148. [Google Scholar] [CrossRef]
- Curtin, J.; Thomson, P.; Wong, G.; Lam, A.; Choi, S.-W. The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma. Cancers 2023, 15, 584. [Google Scholar] [CrossRef] [PubMed]
- Bogden, A.; Moreau, J.-P.; Eden, P. Proliferative Response of Human and Animal Tumours to Surgical Wounding of Normal Tissues: Onset, Duration and Inhibition. Br. J. Cancer 1997, 75, 1021–1027. [Google Scholar] [CrossRef]
- Demicheli, R.; Miceli, R.; Moliterni, A.; Zambetti, M.; Hrushesky, W.J.M.; Retsky, M.W.; Valagussa, P.; Bonadonna, G. Breast Cancer Recurrence Dynamics Following Adjuvant CMF Is Consistent with Tumor Dormancy and Mastectomy-Driven Acceleration of the Metastatic Process. Ann. Oncol. 2005, 16, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-S.; Kaneda, Y.; Ueda, K.; Hamano, K.; Zempo, N.; Esato, K. The Influence of Tumour Resection on Angiostatin Levels and Tumour Growth—An Experimental Study in Tumour-Bearing Mice. Eur. J. Cancer 2001, 37, 2283–2288. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human Prostate Cancer Metastases Target the Hematopoietic Stem Cell Niche to Establish Footholds in Mouse Bone Marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef]
- Patel, H.; Le Marer, N.; Wharton, R.Q.; Khan, Z.A.J.; Araia, R.; Glover, C.; Henry, M.M.; Allen-Mersh, T.G. Clearance of Circulating Tumor Cells after Excision of Primary Colorectal Cancer. Ann. Surg. 2002, 235, 226–231. [Google Scholar] [CrossRef]
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmüller, G.; et al. Systemic Spread Is an Early Step in Breast Cancer. Cancer Cell 2008, 13, 58–68. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Huot, J.; Auger, F.A. Mechanisms by Which E-Selectin Regulates Diapedesis of Colon Cancer Cells under Flow Conditions. Cancer Res. 2008, 68, 5167–5176. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Aigner, M.; Thorlund, K.; Mollberg, N.; Motschall, E.; Jensen, K.; Diener, M.K.; Büchler, M.W.; Koch, M.; Weitz, J. Meta-Analysis Shows That Detection of Circulating Tumor Cells Indicates Poor Prognosis in Patients with Colorectal Cancer. Gastroenterology 2010, 138, 1714–1726.e13. [Google Scholar] [CrossRef]
- Seth, R.; Tai, L.H.; Falls, T.; De Souza, C.T.; Bell, J.C.; Carrier, M.; Atkins, H.; Boushey, R.; Auer, R.A. Surgical Stress Promotes the Development of Cancer Metastases by a Coagulation-Dependent Mechanism Involving Natural Killer Cells in a Murine Model. Ann. Surg. 2013, 258, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.L.; Hanly, E.J.; Nwanko, J.I.; Lamb, J.; Herring, A.E.; Marohn, M.R.; De Maio, A.; Talamini, M.A. The Effect of Timing of Pneumoperitoneum on the Inflammatory Response. Surg. Endosc. Other Interv. Tech. 2004, 18, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Cummings III, K.C.; Zimmerman, N.M.; Maheshwari, K.; Cooper, G.S.; Cummings, L.C. Epidural Compared with Non-Epidural Analgesia and Cardiopulmonary Complications after Colectomy: A Retrospective Cohort Study of 20,880 Patients Using a National Quality Database. J. Clin. Anesth. 2018, 47, 12–18. [Google Scholar] [CrossRef]
- Sakai, E.M.; Connolly, L.A.; Klauck, J.A. Inhalation Anesthesiology and Volatile Liquid Anesthetics: Focus on Isoflurane, Desflurane, and Sevoflurane. Pharmacotherapy 2005, 25, 1773–1788. [Google Scholar] [CrossRef]
- Park, Y.; Ha, J.W. Comparison of One-Level Posterior Lumbar Interbody Fusion Performed With a Minimally Invasive Approach or a Traditional Open Approach. Spine 2007, 32, 537–543. [Google Scholar] [CrossRef]
- Zappalà, G.; McDonald, P.G.; Cole, S.W. Tumor Dormancy and the Neuroendocrine System: An Undisclosed Connection? Cancer Metastasis Rev. 2013, 32, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ghiso, J.A. Models, Mechanisms and Clinical Evidence for Cancer Dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef]
- Schmidt-Kittler, O.; Ragg, T.; Daskalakis, A.; Granzow, M.; Ahr, A.; Blankenstein, T.J.F.; Kaufmann, M.; Diebold, J.; Arnholdt, H.; Müller, P.; et al. From Latent Disseminated Cells to Overt Metastasis: Genetic Analysis of Systemic Breast Cancer Progression. Proc. Natl. Acad. Sci. USA 2003, 100, 7737–7742. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.H.; Folkman, J. Angiostatin: A Novel Angiogenesis Inhibitor That Mediates the Suppression of Metastases by a Lewis Lung Carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef]
- Varani, J.; Lovett, E.J.; Lundy, J. A Model of Tumor Cell Dormancy: Effects of Anesthesia and Surgery. J. Surg. Oncol. 1981, 17, 9–14. [Google Scholar] [CrossRef]
- Yang, H.; Lee, S.; Lee, S.; Kim, K.; Yang, Y.; Kim, J.H.; Adams, R.H.; Wells, J.M.; Morrison, S.J.; Koh, G.Y.; et al. Sox17 Promotes Tumor Angiogenesis and Destabilizes Tumor Vessels in Mice. J. Clin. Investig. 2013, 123, 418–431. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Pires, I.; Parente, M.; Gregório, H.; Lopes, C.S. COX-2 over-Expression Correlates with VEGF and Tumour Angiogenesis in Canine Mammary Cancer. Vet. J. 2011, 189, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sui, W.; Zhang, Y.; Wang, Z.; Wang, Z.; Jia, Q.; Wu, L.; Zhang, W. Antitumor Effect of a Selective COX-2 Inhibitor, Celecoxib, May Be Attributed to Angiogenesis Inhibition through Modulating the PTEN/PI3K/Akt/HIF-1 Pathway in an H22 Murine Hepatocarcinoma Model. Oncol. Rep. 2014, 31, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.Y.S.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular Normalizing Doses of Antiangiogenic Treatment Reprogram the Immunosuppressive Tumor Microenvironment and Enhance Immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, A.; Collivignarelli, F.; Paolini, A.; Falerno, I.; Rinaldi, V.; Tamburro, R.; Bianchi, A.; Terragni, R.; Gianfelici, J.; Frescura, P.; et al. Sentinel Lymph Node Mapping with Indirect Lymphangiography for Canine Mast Cell Tumour. Vet. Sci. 2022, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Karayannopoulou, M.; Anagnostou, T.; Margariti, A.; Kritsepi-Konstantinou, M.; Psalla, D.; Savvas, I.; Kazakos, G. Effect of Anaesthesia on Cell-Mediated Immunity in Dogs Undergoing Mastectomy for Mammary Cancer. Vet. Anaesth. Analg. 2022, 49, 265–274. [Google Scholar] [CrossRef]
- Gaynor, J.S. Control of Cancer Pain in Veterinary Patients. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1429–1448. [Google Scholar] [CrossRef]
- Te Boveldt, N.; Vernooij-Dassen, M.; Burger, N.; Vissers, K.; Engels, Y. Pain and Its Interference with Daily Activitiesin Medical Oncology Outpatients. Pain Phys. 2013, 16, 379–389. [Google Scholar] [CrossRef]
- Miyata, T.; Kodama, T.; Honma, R.; Nezu, Y.; Harada, Y.; Yogo, T.; Hara, Y.; Tagawa, M. Influence of General Anesthesia with Isoflurane Following Propofol-Induction on Natural Killer Cell Cytotoxic Activities of Peripheral Blood Lymphocytes in Dogs. J. Vet. Med. Sci. 2013, 75, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.-J.; Jo, J.-Y.; Kim, J.-I.; Chin, J.-H.; Kim, W.-J.; Kim, H.R.; Lee, E.-H.; Choi, I.-C. Impact of Anesthetic Agents on Overall and Recurrence-Free Survival in Patients Undergoing Esophageal Cancer Surgery: A Retrospective Observational Study. Sci. Rep. 2017, 7, 14020. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, T.J.; Jhanji, S. Long-Term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery. Retrospective Analysis. Anesthesiology 2016, 124, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Kirkby Shaw, K. An Extended Release Local Anaesthetic: Potential for Future Use in Veterinary Surgical Patients? Vet. Med. Sci. 2016, 2, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Portela, D.A.; Breghi, G.; Otero, P.E. Stress-Related Biomarkers in Dogs Administered Regional Anaesthesia or Fentanyl for Analgesia during Stifle Surgery. Vet. Anaesth. Analg. 2016, 43, 44–54. [Google Scholar] [CrossRef]
- Grubb, T.; Lobprise, H. Local and Regional Anaesthesia in Dogs and Cats: Overview of Concepts and Drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef]
- Gargiulo, S.; Greco, A.; Gramanzini, M.; Esposito, S.; Affuso, A.; Brunetti, A.; Vesce, G. Mice Anesthesia, Analgesia, and Care, Part I: Anesthetic Considerations in Preclinical Research. ILAR J. 2012, 53, E55–E69. [Google Scholar] [CrossRef]
- Mahmoud, K.; Ammar, A. Immunomodulatory Effects of Anesthetics during Thoracic Surgery. Anesthesiol. Res. Pract. 2011, 2011, 317410. [Google Scholar] [CrossRef][Green Version]
- Mitsuhata, H.; Shimizu, R.; Yokoyama, M.M. Suppressive Effects of Volatile Anesthetics on Cytokine Release in Human Peripheral Blood Mononuclear Cells. Int. J. Immunopharmacol. 1995, 17, 529–534. [Google Scholar] [CrossRef]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of Natural Killer Cell Activity and Promotion of Tumor Metastasis by Ketamine, Thiopental, and Halothane, but Not by Propofol: Mediating Mechanisms and Prophylactic Measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef]
- Huang, H.; Benzonana, L.L.; Zhao, H.; Watts, H.R.; Perry, N.J.S.; Bevan, C.; Brown, R.; Ma, D. Prostate Cancer Cell Malignancy via Modulation of HIF-1α Pathway with Isoflurane and Propofol Alone and in Combination. Br. J. Cancer 2014, 111, 1338–1349. [Google Scholar] [CrossRef]
- Deng, X.; Vipani, M.; Liang, G.; Gouda, D.; Wang, B.; Wei, H. Sevoflurane Modulates Breast Cancer Cell Survival via Modulation of Intracellular Calcium Homeostasis. BMC Anesth. 2020, 20, 253. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, X. Isoflurane Promotes Non-Small Cell Lung Cancer Malignancy by Activating the Akt-Mammalian Target of Rapamycin (mTOR) Signaling Pathway. Med. Sci. Monit. 2016, 22, 4644–4650. [Google Scholar] [CrossRef]
- Boost, K.A.; Flondor, M.; Hofstetter, C.; Platacis, I.; Stegewerth, K.; Hoegl, S.; Nguyen, T.; Muhl, H.; Zwissler, B. The Beta-Adrenoceptor Antagonist Propranolol Counteracts Anti-Inflammatory Effects of Isoflurane in Rat Endotoxemia. Acta Anaesthesiol. Scand. 2007, 51, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.; Gleed, R.; British Small Animal Veterinary Association. BSAVA Manual of Small Animal Anaesthesia and Analgesia, 2nd ed.; BSAVA: Gloucester, UK, 1999. [Google Scholar]
- Brand, J.-M.; Kirchner, H.; Poppe, C.; Schmucker, P. The Effects of General Anesthesia on Human Peripheral Immune Cell Distribution and Cytokine Production. Clin. Immunol. Immunopathol. 1997, 83, 190–194. [Google Scholar] [CrossRef]
- Wei, H.; Sun, T.; Liu, J.; Wang, X.; Zhao, G.; Shi, J.; Chen, Y. Isoflurane Activates AMP-Activated Protein Kinase to Inhibit Proliferation, and Promote Apoptosis and Autophagy in Cervical Carcinoma Both in vitro and in vivo. J. Recept. Signal Transduct. 2021, 41, 538–545. [Google Scholar] [CrossRef]
- Markovic, S.N.; Murasko, D.M. Inhibition of Induction of Natural Killer Activity in Mice by General Anesthesia (Avertin): Role of Interferon. Clin. Immunol. Immunopathol. 1991, 60, 181–189. [Google Scholar] [CrossRef]
- Inada, T.; Yamanouchi, Y.; Jomura, S.; Sakamoto, S.; Takahashi, M.; Kambara, T.; Shingu, K. Effect of Propofol and Isoflurane Anaesthesia on the Immune Response to Surgery. Anaesthesia 2004, 59, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Faller, S.; Strosing, K.M.; Ryter, S.W.; Buerkle, H.; Loop, T.; Schmidt, R.; Hoetzel, A. The Volatile Anesthetic Isoflurane Prevents Ventilator-Induced Lung Injury via Phosphoinositide 3-Kinase/Akt Signaling in Mice. Anesth. Analg. 2012, 114, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Kawaraguchi, Y.; Horikawa, Y.T.; Murphy, A.N.; Murray, F.; Miyanohara, A.; Ali, S.S.; Head, B.P.; Patel, P.M.; Roth, D.M.; Patel, H.H. Volatile Anesthetics Protect Cancer Cells against Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis via Caveolins. Anesthesiology 2011, 115, 499–508. [Google Scholar] [CrossRef]
- Benzonana, L.L.; Perry, N.J.S.; Watts, H.R.; Yang, B.; Perry, I.A.; Coombes, C.; Takata, M.; Ma, D. Isoflurane, a Commonly Used Volatile Anesthetic, Enhances Renal Cancer Growth and Malignant Potential via the Hypoxia-Inducible Factor Cellular Signaling Pathway in vitro. Anesthesiology 2013, 119, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, M.; Zhou, Y.; Dangelmajer, S.; Kahlert, U.D.; Xie, R.; Xi, Q.; Shahveranov, A.; Ye, D.; Lei, T. Isoflurane Enhances the Malignant Potential of Glioblastoma Stem Cells by Promoting Their Viability, Mobility in Vitro and Migratory Capacity in Vivo. Br. J. Anaesth. 2016, 116, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhao, H.; Hennah, L.; Ning, J.; Liu, J.; Tu, H.; Ma, D. Impact of Isoflurane on Malignant Capability of Ovarian Cancer in vitro. Br. J. Anaesth. 2015, 114, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Minguet, G.; Franck, T.; Joris, J.; Ceusters, J.; Mouithys-Mickalad, A.; Serteyn, D.; Sandersen, C. Effects of Isoflurane and Sevoflurane on the Neutrophil Myeloperoxidase System of Horses. Vet. Immunol. Immunopathol. 2015, 165, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, Z.; Marcinkiewicz, J.; Katz, D.R.; Chain, B.M. Neutrophil Myeloperoxidase: Soldier and Statesman. Arch. Immunol. Ther. Exp. 2012, 60, 43–54. [Google Scholar] [CrossRef]
- Meier, A.; Gross, E.T.E.; Schilling, J.M.; Seelige, R.; Jung, Y.; Santosa, E.; Searles, S.; Lin, T.; Tu, X.M.; Patel, H.H.; et al. Isoflurane Impacts Murine Melanoma Growth in a Sex-Specific, Immune-Dependent Manner: A Brief Report. Anesth. Analg. 2018, 126, 1910–1913. [Google Scholar] [CrossRef]
- Tyther, R.; O’Brien, J.; Wang, J.; Redmond, H.P.; Shorten, G. Effect of Sevoflurane on Human Neutrophil Apoptosis. Eur. J. Anaesthesiol. 2005, 20, 111–115. [Google Scholar] [CrossRef]
- Tyther, R.; Fanning, N.; Halligan, M.; Wang, J.; Redmond, H.P.; Shorten, G. The Effect of the Anaesthetic Agent Isoflurane on the Rate of Neutrophil Apoptosis in vitro. Ir. J. Med. Sci. 2001, 170, 41. [Google Scholar] [CrossRef]
- Morisaki, H.; Aoyama, Y.; Shimada, M.; Ochiai, R.; Takeda, J. Leucocyte Distribution during Sevoflurane Anaesthesia. Br. J. Anaesth. 1998, 80, 502–503. [Google Scholar] [CrossRef][Green Version]
- Loop, T.; Dovi-Akue, D.; Frick, M.; Roesslein, M.; Egger, L.; Humar, M.; Hoetzel, A.; Schmidt, R.; Borner, C.; Pahl, H.L.; et al. Volatile Anesthetics Induce Caspase-Dependent, Mitochondria-Mediated Apoptosis in Human T Lymphocytes in vitro. Anesthesiology 2005, 102, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Levins, K.J.; Prendeville, S.; Conlon, S.; Buggy, D.J. The Effect of Anesthetic Technique on Μ-Opioid Receptor Expression and Immune Cell Infiltration in Breast Cancer. J. Anesth. 2018, 32, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Elena, G.; Amerio, N.; Ferrero, P.; Bay, M.L.; Valenti, J.; Colucci, D.; Puig, N.R. Effects of Repetitive Sevoflurane Anaesthesia on Immune Response, Select Biochemical Parameters and Organ Histology in Mice. Lab. Anim. 2003, 37, 193–203. [Google Scholar] [CrossRef]
- Wei, H.; Liang, G.; Yang, H.; Wang, Q.; Hawkins, B.; Madesh, M.; Wang, S.; Eckenhoff, R.G. The Common Inhalational Anesthetic Isoflurane Induces Apoptosis via Activation of Inositol 1,4,5-Trisphosphate Receptors. Anesthesiology 2008, 108, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Beck-Schimmer, B.; Baumann, L.; Restin, T.; Eugster, P.; Hasler, M.; Booy, C.; Schläpfer, M. Sevoflurane Attenuates Systemic Inflammation Compared with Propofol, but Does Not Modulate Neuro-Inflammation: A Laboratory Rat Study. Eur. J. Anaesthesiol. 2017, 34, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Tavare, A.N.; Perry, N.J.S.; Benzonana, L.L.; Takata, M.; Ma, D. Cancer Recurrence after Surgery: Direct and Indirect Effects of Anesthetic Agents*. Int. J. Cancer 2012, 130, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Su, Z.; Liu, H.; Liu, Y. Retraction Notice to “Anti-Proliferation and Anti-Metastatic Effects of Sevoflurane on Human Osteosarcoma U2OS and Saos-2 Cells” [Experimental and Molecular Pathology 108 (2019) 121–130]. Exp. Mol. Pathol. 2022, 127, 104784. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S. Anesthesia in Patients with Cancer Disorders. Curr. Opin. Anaesthesiol. 2012, 25, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Seki, S.; Takahashi, T.; Kawarabayashi, N.; Higuchi, H.; Habu, Y.; Sugahara, S.; Kazama, T. Combined Spinal and General Anesthesia Attenuates Liver Metastasis by Preserving Th1/Th2 Cytokine Balance. Anesthesiology 2007, 106, 499–506. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tanaka, S.; Arai, M.; Genda, Y.; Sakamoto, A. Differences in microRNA Changes of Healthy Rat Liver between Sevoflurane and Propofol Anesthesia. Anesthesiology 2012, 117, 1245–1252. [Google Scholar] [CrossRef]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef] [PubMed]
- Nesek Adam, V.; Marin, D.; Popović, M.; Berić Lerotić, S.; Gudan Kurilj, A.; Matičić, D.; Vnuk, D. The Effect of Repeated Sevoflurane and Nitrous Oxide Exposure on Immunity in Rabbits. Vet. Arh. 2018, 88, 37–48. [Google Scholar] [CrossRef]
- Argano, M.; De Maria, R.; Vogl, C.; Rodlsberger, K.; Buracco, P.; Larenza Menzies, M.P. Canine Mammary Tumour Cells Exposure to Sevoflurane: Effects on Cell Proliferation and Neuroepithelial Transforming Gene 1 Expression. Vet. Anaesth. Analg. 2019, 46, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Cattai, A.; Rabozzi, R.; Ferasin, H.; Isola, M.; Franci, P. Haemodynamic Changes during Propofol Induction in Dogs: New Findings and Approach of Monitoring. BMC Vet. Res. 2018, 14, 282. [Google Scholar] [CrossRef]
- Kato, K.; Itami, T.; Nomoto, K.; Endo, Y.; Tamura, J.; Oyama, N.; Sano, T.; Yamashita, K. The Anesthetic Effects of Intramuscular Alfaxalone in Dogs Premedicated with Low-Dose Medetomidine and/or Butorphanol. J. Vet. Med. Sci. 2021, 83, 53–61. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Moon, C.; Park, J.; Lee, H.; Jeong, S.M. Anesthetic Effect of Different Ratio of Ketamine and Propofol in Dogs. J. Vet. Clin. 2017, 34, 234–240. [Google Scholar] [CrossRef]
- Ilkiw, J.E. Balanced Anesthetic Techniques in Dogs and Cats. Clin. Tech. Small Anim. Pract. 1999, 14, 27–37. [Google Scholar] [CrossRef]
- Eden, C.; Esses, G.; Katz, D.; DeMaria, S. Effects of Anesthetic Interventions on Breast Cancer Behavior, Cancer-Related Patient Outcomes, and Postoperative Recovery. Surg. Oncol. 2018, 27, 266–274. [Google Scholar] [CrossRef]
- Barr, C.A.; Alvarado, F.; Chang, Y.-M.; Luo, J.; Garden, O.A. The Impact of Alfaxalone, Propofol and Ketamine on Canine Peripheral Blood Lymphocyte Cytotoxicity in vitro. Res. Vet. Sci. 2021, 136, 182–184. [Google Scholar] [CrossRef]
- Li, C.; Xia, M.; Wang, H.; Li, W.; Peng, J.; Jiang, H. Propofol Facilitates Migration and Invasion of Oral Squamous Cell Carcinoma Cells by Upregulating SNAI1 Expression. Life Sci. 2020, 241, 117143. [Google Scholar] [CrossRef]
- Meng, C.; Song, L.; Wang, J.; Li, D.; Liu, Y.; Cui, X. Propofol Induces Proliferation Partially via Downregulation of P53 Protein and Promotes Migration via Activation of the Nrf2 Pathway in Human Breast Cancer Cell Line MDA-MB-231. Oncol. Rep. 2017, 37, 841–848. [Google Scholar] [CrossRef]
- Feng, C.; Qian, D.; Chen, C. A Meta-Analysis and Systematic Review of Propofol on Liver Ischemia-Reperfusion Injury Protection during Hepatocellular Carcinoma Anesthesia Surgery. Ann. Palliat. Med. 2021, 10, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hu, Y.; Li, W.; Li, L.; Li, S.; Zhang, M.; Li, Q. The Neuroprotective Effect of Propofol against Brain Ischemia Mediated by the Glutamatergic Signaling Pathway in Rats. Neurochem. Res. 2011, 36, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G.; Yu, Y.; Wang, Y. The Role of Phosphoinositide-3-Kinase/Akt Pathway in Propofol-Induced Postconditioning against Focal Cerebral Ischemia-Reperfusion Injury in Rats. Brain Res. 2009, 1297, 177–184. [Google Scholar] [CrossRef]
- Kubo, K.; Inada, T.; Shingu, K. Possible Role of Propofol’s Cyclooxygenase-Inhibiting Property in Alleviating Dopaminergic Neuronal Loss in the Substantia Nigra in an MPTP-Induced Murine Model of Parkinson’s Disease. Brain Res. 2011, 1387, 125–133. [Google Scholar] [CrossRef]
- Nyssen, P.; Franck, T.; Serteyn, D.; Mouithys-Mickalad, A.; Hoebeke, M. Propofol Metabolites and Derivatives Inhibit the Oxidant Activities of Neutrophils and Myeloperoxidase. Free Radic. Biol. Med. 2022, 191, 164–175. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Huang, L.; Zhang, F.; Kang, R. Effects of Propofol on Cancer Development and Chemotherapy: Potential Mechanisms. Eur. J. Pharmacol. 2018, 831, 46–51. [Google Scholar] [CrossRef]
- Ma, X.; Wang, T.; Zhao, Z.-L.; Jiang, Y.; Ye, S. Propofol Suppresses Proinflammatory Cytokine Production by Increasing ABCA1 Expression via Mediation by the Long Noncoding RNA LOC286367. Mediat. Inflamm. 2018, 2018, 8907143. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Liang, F.; Liu, G.; Zhao, G. Anesthetic Drug Propofol Inhibits the Expression of Interleukin-6, Interleukin-8 and Cyclooxygenase-2, a Potential Mechanism for Propofol in Suppressing Tumor Development and Metastasis. Oncol. Lett. 2018, 15, 9523–9528. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, W.; Xie, S.; Xue, F.; Zhu, X. The Role of Inhaled Anesthetics in Tumorigenesis and Tumor Immunity. Cancer Manag. Res. 2020, 12, 1601–1609. [Google Scholar] [CrossRef]
- Sen, Y.; Xiyang, H.; Yu, H. Effect of Thoracic Paraspinal Block-Propofol Intravenous General Anesthesia on VEGF and TGF-β in Patients Receiving Radical Resection of Lung Cancer. Medicine 2019, 98, e18088. [Google Scholar] [CrossRef] [PubMed]
- Pirttikangas, C.-O.; Salo, M.; Mansikka, M.; Grönroos, J.; Pulkki, K.; Peltola, O. The Influence of Anaesthetic Technique upon the Immune Response to Hysterectomy: A Comparison of Propofol Infusion and Isoflurane. Anaesthesia 1995, 50, 1056–1061. [Google Scholar] [CrossRef]
- Yamada, R.; Tsuchida, S.; Hara, Y.; Tagawa, M.; Ogawa, R. Apoptotic Lymphocytes Induced by Surgical Trauma in Dogs. J. Anesth. 2002, 16, 131–137. [Google Scholar] [CrossRef]
- Faroni, E.; Sabattini, S.; Lenzi, J.; Guerra, D.; Comazzi, S.; Aresu, L.; Mazzanti, A.; Zanardi, S.; Cola, V.; Lotito, E.; et al. Sleeping Beauty: Anesthesia May Promote Relapse in Dogs with Diffuse Large B-Cell Lymphoma in Complete Remission After Chemo-Immunotherapy. Front. Vet. Sci. 2021, 8, 760603. [Google Scholar] [CrossRef]
- Inada, T.; Kubo, K.; Shingu, K. Promotion of Interferon-Gamma Production by Natural Killer Cells via Suppression of Murine Peritoneal Macrophage Prostaglandin E2 Production Using Intravenous Anesthetic Propofol. Int. Immunopharmacol. 2010, 10, 1200–1208. [Google Scholar] [CrossRef]
- Kambara, T.; Inada, T.; Kubo, K.; Shingu, K. Propofol Suppresses Prostaglandin E2 Production in Human Peripheral Monocytes. Immunopharmacol. Immunotoxicol. 2009, 31, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Inada, T.; Shingu, K. Enhancement of Antitumor Immunity after Propofol Treatment in Mice. Immunopharmacol. Immunotoxicol. 2007, 29, 477–486. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative Events Influence Cancer Recurrence Risk after Surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Mao, L.; Lin, S.; Lin, J. The Effects of Anesthetics on Tumor Progression. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 1–10. [Google Scholar]
- Cui, C.; Zhang, D.; Sun, K.; Zhu, Y.; Xu, J.; Kang, Y.; Zhang, G.; Cai, Y.; Mao, S.; Long, R.; et al. Propofol Maintains Th17/Treg Cell Balance in Elderly Patients Undergoing Lung Cancer Surgery through GABAA Receptor. BMC Immunol. 2022, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When Worlds Collide: Th17 and Treg Cells in Cancer and Autoimmunity. Cell Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.J.; Moreno-Bueno, G.; Sarrio, D.; Locascio, A.; Cano, A.; Palacios, J.; Nieto, M.A. Correlation of Snail Expression with Histological Grade and Lymph Node Status in Breast Carcinomas. Oncogene 2002, 21, 3241–3246. [Google Scholar] [CrossRef]
- Nimmo, A.F.; Absalom, A.R.; Bagshaw, O.; Biswas, A.; Cook, T.M.; Costello, A.; Grimes, S.; Mulvey, D.; Shinde, S.; Whitehouse, T.; et al. Guidelines for the Safe Practice of Total Intravenous Anaesthesia (TIVA): Joint Guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia. Anaesthesia 2019, 74, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, R.; Canfrán, S.; Gómez de Segura, I.A.; Aguado, D. Intraoperative Effect of Low Doses of Ketamine or Dexmedetomidine Continuous Rate Infusions in Healthy Dogs Receiving Propofol Total Intravenous Anaesthesia and Epidural Anaesthesia: A Prospective, Randomised Clinical Study. Res. Vet. Sci. 2022, 143, 4–12. [Google Scholar] [CrossRef]
- Nolan, A.; Reid, J. Pharmacokinetics of Propofol Administered by Infusion in Dogs Undergoing Surgery. Br. J. Anaesth. 1993, 70, 546–551. [Google Scholar] [CrossRef][Green Version]
- Guzel, O.; Sevim, G.; Aydin Kaya, D.; Sezer, D.; Erek, M.; Esen Gursel, F.; Atmaca, G.; Demirtas, B.; Matur, E. Ketamine or Propofol Anesthesia in Dogs: How Do They Affect Cytokines, Antioxidants and Neutrophil Functions? J. Hell. Vet. Med. Soc. 2022, 73, 3783–3792. [Google Scholar] [CrossRef]
- Schneemilch, C.E.; Ittenson, A.; Ansorge, S.; Hachenberg, T.; Bank, U. Effect of 2 Anesthetic Techniques on the Postoperative Proinflammatory and Anti-Inflammatory Cytokine Response and Cellular Immune Function to Minor Surgery. J. Clin. Anesth. 2005, 17, 517–527. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wu, M.-Y.; Chien, Y.-J.; Su, I.-M.; Wang, S.-C.; Kao, M.-C. Anesthesia and Long-Term Oncological Outcomes: A Systematic Review and Meta-Analysis. Anesth. Analg. 2021, 132, 623–634. [Google Scholar] [CrossRef]
- Ferré, P.J.; Pasloske, K.; Whittem, T.; Ranasinghe, M.G.; Li, Q.; Lefebvre, H.P. Plasma Pharmacokinetics of Alfaxalone in Dogs after an Intravenous Bolus of Alfaxan-CD RTU. Vet. Anaesth. Analg. 2006, 33, 229–236. [Google Scholar] [CrossRef]
- Murison, P.J.; Taboada, F.M. Effect of Propofol and Alfaxalone on Pain after Ovariohysterectomy in Cats. Vet. Rec. 2010, 166, 334–335. [Google Scholar] [CrossRef]
- Mathis, A.; Pinelas, R.; Brodbelt, D.C.; Alibhai, H.I. Comparison of Quality of Recovery from Anaesthesia in Cats Induced with Propofol or Alfaxalone. Vet. Anaesth. Analg. 2012, 39, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.P.; Mathis, A.; Mora, S.S.; Brodbelt, D.; Alibhai, H. Evaluation of the Quality of the Recovery after Administration of Propofol or Alfaxalone for Induction of Anaesthesia in Dogs Anaesthetized for Magnetic Resonance Imaging. Vet. Anaesth. Analg. 2012, 39, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zheng, X.; Zhou, Y.; Zhu, W.; Ou, Y.; Shu, M.; Gao, X.; Leng, T.; Qiu, P.; Yan, G. Alphaxalone Inhibits Growth, Migration and Invasion of Rat C6 Malignant Glioma Cells. Steroids 2013, 78, 1041–1045. [Google Scholar] [CrossRef]
- Suzuki, T.; Tomioka, M.; Uchida, M.K. Inhibitory Effects of Steroidal Anesthetics on Histamine Release from Rat Mast Cells Stimulated by Concanavalin A, Compound 48/80 and A23187. Gen. Pharmacol. Vasc. Syst. 1988, 19, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, K.A.; Lippold, C.; Rice, A.L.; Nobrega, R.; Finkel, J.C.; Quezado, Z.M. Subanesthetic Ketamine for Pain Management in Hospitalized Children, Adolescents, and Young Adults: A Single-Center Cohort Study. J. Pain Res. 2017, 10, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lilius, T.O.; Jokinen, V.; Neuvonen, M.S.; Niemi, M.; Kalso, E.A.; Rauhala, P.V. Ketamine Coadministration Attenuates Morphine Tolerance and Leads to Increased Brain Concentrations of Both Drugs in the Rat. Br. J. Pharmacol. 2015, 172, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Loix, S.; De Kock, M.; Henin, P. The Anti-Inflammatory Effects of Ketamine: State of the Art. Acta Anaesthesiol. Belg. 2011, 62, 47–58. [Google Scholar]
- Spencer, H.F.; Berman, R.Y.; Boese, M.; Zhang, M.; Kim, S.Y.; Radford, K.D.; Choi, K.H. Effects of an Intravenous Ketamine Infusion on Inflammatory Cytokine Levels in Male and Female Sprague–Dawley Rats. J. Neuroinflamm. 2022, 19, 75. [Google Scholar] [CrossRef]
- Lu, W.; Wang, L.; Wo, C.; Yao, J. Ketamine Attenuates Osteoarthritis of the Knee via Modulation of Inflammatory Responses in a Rabbit Model. Mol. Med. Rep. 2016, 13, 5013–5020. [Google Scholar] [CrossRef]
- Brinck, E.; Tiippana, E.; Heesen, M.; Bell, R.F.; Straube, S.; Moore, R.A.; Kontinen, V. Perioperative Intravenous Ketamine for Acute Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2018, 68, 110071. [Google Scholar] [CrossRef]
- Plein, L.M.; Rittner, H.L. Opioids and the Immune System—Friend or Foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Hardy, J.; Quinn, S.; Fazekas, B.; Plummer, J.; Eckermann, S.; Agar, M.; Spruyt, O.; Rowett, D.; Currow, D.C. Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Toxicity of Subcutaneous Ketamine in the Management of Cancer Pain. J. Clin. Oncol. 2012, 30, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Lankveld, D.P.K.; Bull, S.; Van Dijk, P.; Fink-Gremmels, J.; Hellebrekers, L.J. Ketamine Inhibits LPS-Induced Tumour Necrosis Factor-Alpha and Interleukin-6 in an Equine Macrophage Cell Line. Vet. Res. 2005, 36, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Ohashi, Y.; Fujino, Y. Ketamine Inhibits Maturation of Bone Marrow-Derived Dendritic Cells and Priming of the Th1-Type Immune Response. Anesth. Analg. 2009, 109, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Beilin, B.; Rusabrov, Y.; Shapira, Y.; Roytblat, L.; Greemberg, L.; Yardeni, I.Z.; Bessler, H. Low-Dose Ketamine Affects Immune Responses in Humans during the Early Postoperative Period. Br. J. Anaesth. 2007, 99, 522–527. [Google Scholar] [CrossRef]
- Braun, S.; Gaza, N.; Werdehausen, R.; Hermanns, H.; Bauer, I.; Durieux, M.E.; Hollmann, M.W.; Stevens, M.F. Ketamine Induces Apoptosis via the Mitochondrial Pathway in Human Lymphocytes and Neuronal Cells. Br. J. Anaesth. 2010, 105, 347–354. [Google Scholar] [CrossRef]
- Hirota, K.; Lambert, D.G. Ketamine: New Uses for an Old Drug? Br. J. Anaesth. 2011, 107, 123–126. [Google Scholar] [CrossRef]
- Laudanski, K.; Qing, M.; Oszkiel, H.; Zawadka, M.; Lapko, N.; Nowak, Z.; Worthen, G.S. Ketamine Affects In Vitro Differentiation of Monocyte into Immature Dendritic Cells. Anesthesiology 2015, 123, 628–641. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, A.V.; Petrucci, G.N.; Dourado, A.; Pires, I. Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread. Animals 2023, 13, 3392. https://doi.org/10.3390/ani13213392
Pinheiro AV, Petrucci GN, Dourado A, Pires I. Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread. Animals. 2023; 13(21):3392. https://doi.org/10.3390/ani13213392
Chicago/Turabian StylePinheiro, Ana Vidal, Gonçalo N. Petrucci, Amândio Dourado, and Isabel Pires. 2023. "Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread" Animals 13, no. 21: 3392. https://doi.org/10.3390/ani13213392
APA StylePinheiro, A. V., Petrucci, G. N., Dourado, A., & Pires, I. (2023). Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread. Animals, 13(21), 3392. https://doi.org/10.3390/ani13213392





