Simple Summary
Captive breeding is important for ex-situ conservation and future reintroduction. This research aims to conduct a population viability analysis (PVA) for a sustainable reintroduction program for bantengs. The monthly development of 23 founder individuals was assessed. The PVA showed that the time required to reach the maximum population in a captive banteng program is dependent on the carrying capacity of the habitat. The reduction of a small banteng founder group by the reintroduction of animals into the wild can negatively affect the population growth of the captive group. This information can be used to maintain the population viability of bantengs and sustain ex-situ conservation and the reintroduction program in Thailand and elsewhere.
Abstract
Captive breeding is important for ex-situ conservation and the future reintroduction of bovids that become extinct in the wild. The age structure, development, and viability of captive-bred bantengs (Bos javanicus) are important to sustain the long-term reintroduction program in Salakphra Wildlife Sanctuary (SWF) and other areas. This research conducted a long-term population viability analysis (PVA) using height, weight, body condition scores (BSC), age structure, and development in captivity for a sustainable reintroduction program of bantengs in Thailand. Monthly development photographs of 23 founder individuals (12 males and 11 females) were assessed by three banteng experts, two researchers, and three members of the general public. The assessments of weight and BCS were not significantly different among the three groups, while height was underestimated by the general public. The PVA showed that the time to reach the maximum population in a captive banteng program is dependent on the carrying capacity of the habitat. The reduction of a small banteng founder group by the reintroduction of animals into the wild can negatively affect the population growth of the captive group. This information can be used to maintain the population viability of bantengs and sustain ex-situ conservation and the reintroduction program in Thailand and elsewhere.
1. Introduction
Habitat fragmentation, poaching, illegal logging, and mining have reduced the global and national population of the banteng (Bos javanicus) [1]. The construction of roads and agricultural areas, as well as poaching, are the biggest threats to the banteng in Thailand [2]. Globally, bantengs are listed as Endangered by The International Union for Conservation of Nature (IUCN) [3] and listed as Critically Endangered in Thailand [4]. The global population is approximately 5000–8000 individuals [5]. Bantengs are found in parts of southeast Asia, such as Myanmar, Thailand, Indochina, and Java [6]. In Thailand, there were 470 individuals in 1995 [7]. Recently, the populations have been increasing in many areas, although the exact numbers are not known. Bantengs were extirpated in the Salakphra Wildlife Sanctuary when it was first established as a nature preserve. After more than 30 years of local extinction, a reintroduction program under the guideline of IUCN/SSC [8] was established between 2014 and 2022. Since then, 16 captively bred bantengs have been reintroduced into the wild [9].
Bantengs prefer open dry deciduous forests and avoid evergreen rainforests but occupy secondary forest formations that are a result of logging and fires and enter tracts of sub-humid forest on occasion in the more humid areas of Java and Borneo (Wharton, 1968). However, their predominant habitat type is the tropical lowland dipterocarp forest in Sabah, Malaysia [10], at elevations lower than 900 m [11]. In Thailand, the highest density was found in Huai Kha Kheng Wildlife Sanctuary. Sometimes they have been found feeding in agricultural areas such as cassava and coconut plantations [12].
In Thailand, bantengs feed on 150 species of food plants [13], with 23 of these species found in Khao Khiao-Khao Chomphu Wildlife Sanctuary [12] and 24 species in Salakphra Wildlife Sanctuary [14]. They are often found near saltlicks and water sources [13]. Bantengs can be found living together with other mammals, such as sambar (Rusa unicolor) and wild boar (Sus scrofa) [15]. Bantengs are highly active in the late afternoon and at dusk. They prefer resting under shrubs, and seasonal changes do not affect their activities [16]. They forage in groups containing between 2 and 30 individuals. Herds mostly comprise adult and subadult females, with adult males joining the herd during mating season [2]. Solitary animals tend to be mature bulls or sometimes old cows. The composition of small groups of cows with calves or juveniles and the solitary state of old individuals may remain the same for months or even years.
The bantengs found in this study were collected from diverse areas and kept at the Khao Nam Phu Nature and Wildlife Education Center (KNP). A female banteng calf named Pongtong was found and captured by local people at the Pongtong saltlick located in Salakphra Wildlife Sanctuary. This calf was sent to the Special Warfare Command, Lopburi Province, before being sent to KNP for breeding. One male was found in the forest area of Kamphaeng Phet Province. Two other males came from Kaeng Krachan National Park and Huai Kha Khaeng Wildlife Sanctuary (S.N.; head of KNP, personal communication) [17]. The life history of each animal at KNP, such as name, sex, date of birth, identifying characteristics, vaccination, and pedigree, were recorded along with monthly photographs. This information was used to select suitable bantengs for reintroduction to the Salakphra Wildlife Sanctuary [9,14,18].
The objective of this study was to determine the age structure, growth (weight and height), and body condition score to develop banteng body metrics and assessment. We also projected the number of captive individuals and their age structure into the future to assess the ability of the captive population to indicate the continued viability of the captive-bred banteng population and the ability of the program to yield animals for reintroduction to ex-situ populations in Thailand and elsewhere.
2. Materials and Methods
2.1. Life History of Bantengs in Khao Nam Phu Nature and Wildlife Education Center
The ages of 23 bantengs (12 males and 11 females) kept in KNP were identified from photographs and studbook data between 2007–2019 (Table 1).

Table 1.
The scoring criteria for body composition of the bantengs in captivity.
2.2. Relationship among Age, Height, Weight, and Body Condition Score of Captive Banteng
The photographs of each individual banteng were used to assess age (year), weight (kg), height (m) and BSC monthly. The height and weight were described using morphology as for gaur [19]. Only sets of photos in which all parts of the body were visible were selected for analysis. Each individual was identified by obvious morphological characteristics such as scars, the shape of horns, and collars [18]. The weights and heights of the captive bantengs were assessed by comparison with a pole of known height in the enclosure. Three groups of assessors (specialists, researchers and untrained assessors) recorded the individual heights and weights monthly. The results in height and weight estimated by the researchers were validated by comparing them with the specialists and untrained assessors. The correlation between the age, weight and height of the captive bantengs was determined using general linear models in an R package [20]. The results were validated with the Bornean banteng [5].
The condition of seven body parts was rated using a five-level scoring system [18] (Table 1). The Spearman’s rank correlation between categorical body composition and the BCS of 23 bantengs was calculated using an R package [20] to assess changes in their condition [21]. Chi-square was used to compare the BCS of the bantengs between categorical factors such as wet and dry seasons. A Mann–Whitney–Wilcoxon rank of the BCS categories of the banteng was determined using SPSS [22]. The differences between treatments were tested at a p < 0.05 significance level.
2.3. Prediction of Population Number of Captive Banteng
We used Vortex v10 [23], parameterized with a combination of expert opinion and empirical data. The PVA was performed using various pieces of information to maximize the reliability of the model based on the IUCN/SSC Asian Wild Cattle Specialist Group (AWCSG), Gardner et al. [5] and KNP studbook (Table 2). The captive population used for population viability modelling in this study originally had 23 individuals and was reduced to 16 individuals when seven bantengs were reintroduced into SWF. The population models were designed to extend for 100 years (approximately 10 generations). The correlation between reproduction and survival as a function of environmental variance was 0.2 and was expected to be relatively low due to the K-selected nature of the species (i.e., low years for reproduction are not always low years for survival). The reproductive system was categorized as short-term polygyny [5]. The ages of females and males of the first offspring were 3 and 4 years, respectively (KNP studbook). The maximum age for reproductive females and males was 15 years (KNP studbook). The maximum lifespan was 26 years (KNP studbook), and the max number of calving events per year was 1 calf per year (KNP studbook). The sex ratio at birth was 50:50 [5]. The intrinsic model of percentage breeding adult females per year (interbirth interval) was 50% (KNP studbook). The percentage mortality of females was 20 ± 10% at age 0–1 year, 2 ± 5% at 1–2 years, 4 ± 5% at 2–3 years and 11.9 ± 2% at >3 years, with a maximum lifespan of ~28 years. The percentage mortality of males was 26 ± 10% at age 0–1 year, 8 ± 5% at 1–2 years, 11.9 ± 2% at 2–3 years and 11.9 ± 2% at >3 years, with a maximum lifespan of ~28 years (AWCSG). The intrinsic model of males in the breeding pool showed the highest potential at 100% (KNP studbook). The initial population size was 23 or 16 individuals, and the initial carrying capacity was 50 individuals (KNP studbook). All population modelling was carried out using Vortex v10 [23]. A key piece of literature used to guide this process was Lacy et al. [24]. Sensitivity testing based on the percentage of females breeding per year was carried out using parameters from Table 1 and 1000 simulations.

Table 2.
Population parameters for bantengs in Khao Nam Phu Nature and Wildlife Education Center.
3. Results
3.1. Growth Rate of Captive Banteng
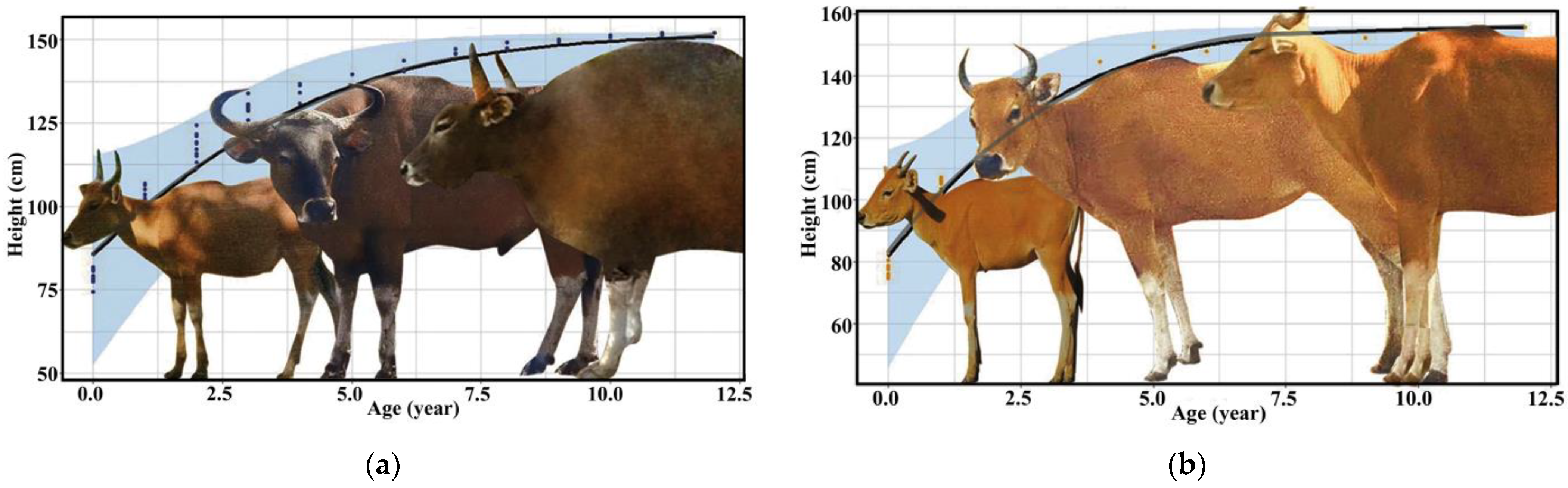
The development of bantengs was different between males and females; this is called sexual dimorphism. The morphology of male bantengs differed among age classes, especially after three years old, and especially their horn curvature, body size, skin color and dewlap. The male banteng’s color is generally dark brown, and its body is larger than the female’s. The male’s height may extend up to 160 cm, and its weight from 600–800 kg. The morphology of the female banteng only changes slightly after three years old. The females are thinner and usually pale brown or chestnut red. The female’s height may extend up to 140 cm, with a weight of 590–670 kg (Figure 1).
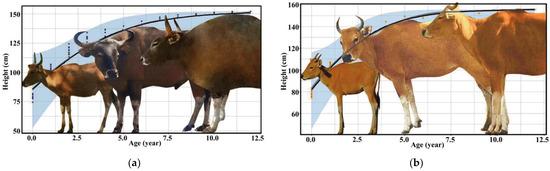
Figure 1.
Development of morphology and height of (a) male and (b) female banteng in captivity.
3.2. Relationship among Age, Height, Weight and Body Condition Score of Captive Banteng
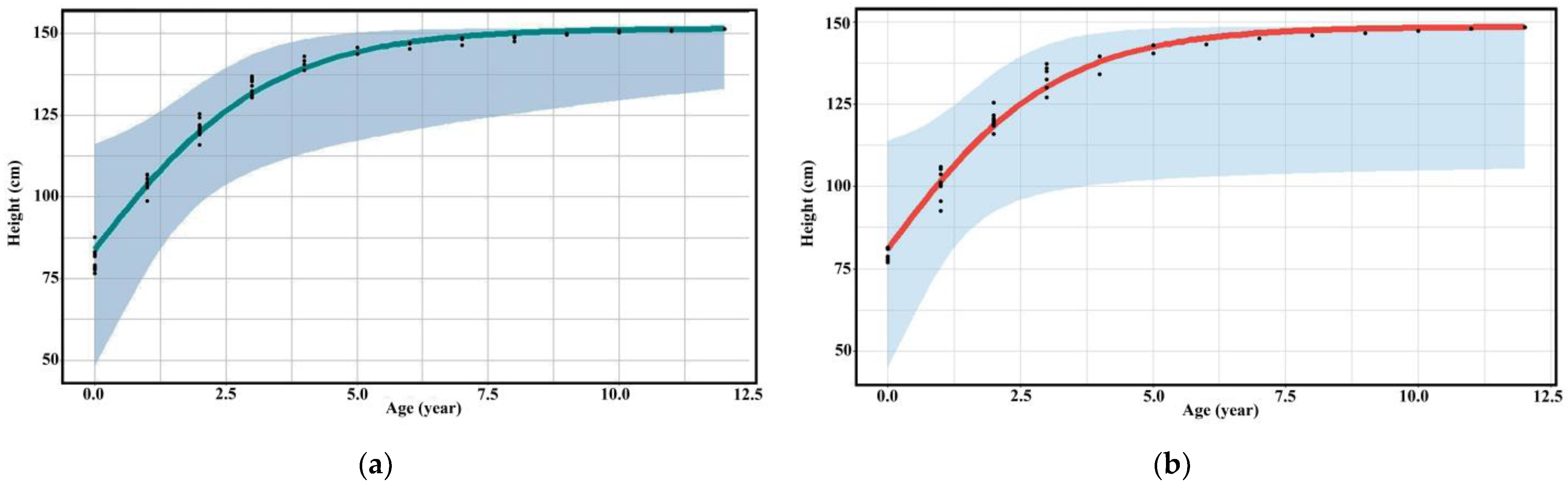
The three groups of assessors evaluated the relationship between age and height of captive bantengs with the same trend as logistic growth into three phases: (1) 1–4 years was highly increased; (2) 4–6 years was slowly increased, and (3) 6–12 years was a steady state increase. The height assessment of the specialists was higher than researchers and untrained assessors (Table 3, Figure 2, Supplementary Table S1 and Figures S1 and S2).

Table 3.
Relationship between age (y), height, weight and body condition score (BCS) of captive banteng.

Figure 2.
Scatter diagram (black dots) with regression line of the relationship between age and height of (a) male and (b) female captive banteng by researchers.
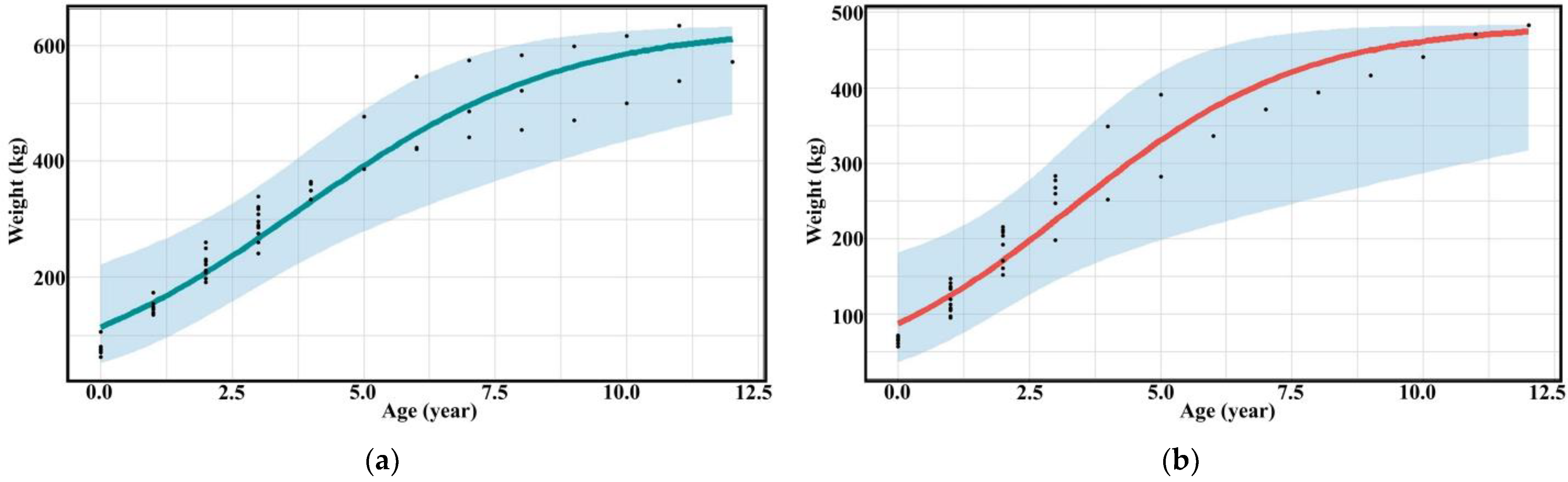
The three groups of assessors evaluated the relationship between the age and weight of captive bantengs. The assessment between professionals and researchers was not significantly different (p > 0.05), while the untrained assessors provided different results (p < 0.001) (Table 3, Figure 3, and Supplementary Figures S3 and S4).
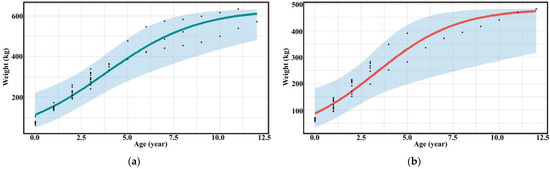
Figure 3.
Scatter diagram (black dots) with regression line of the relationship between age and weight of (a) male and (b) female captive banteng by researchers.
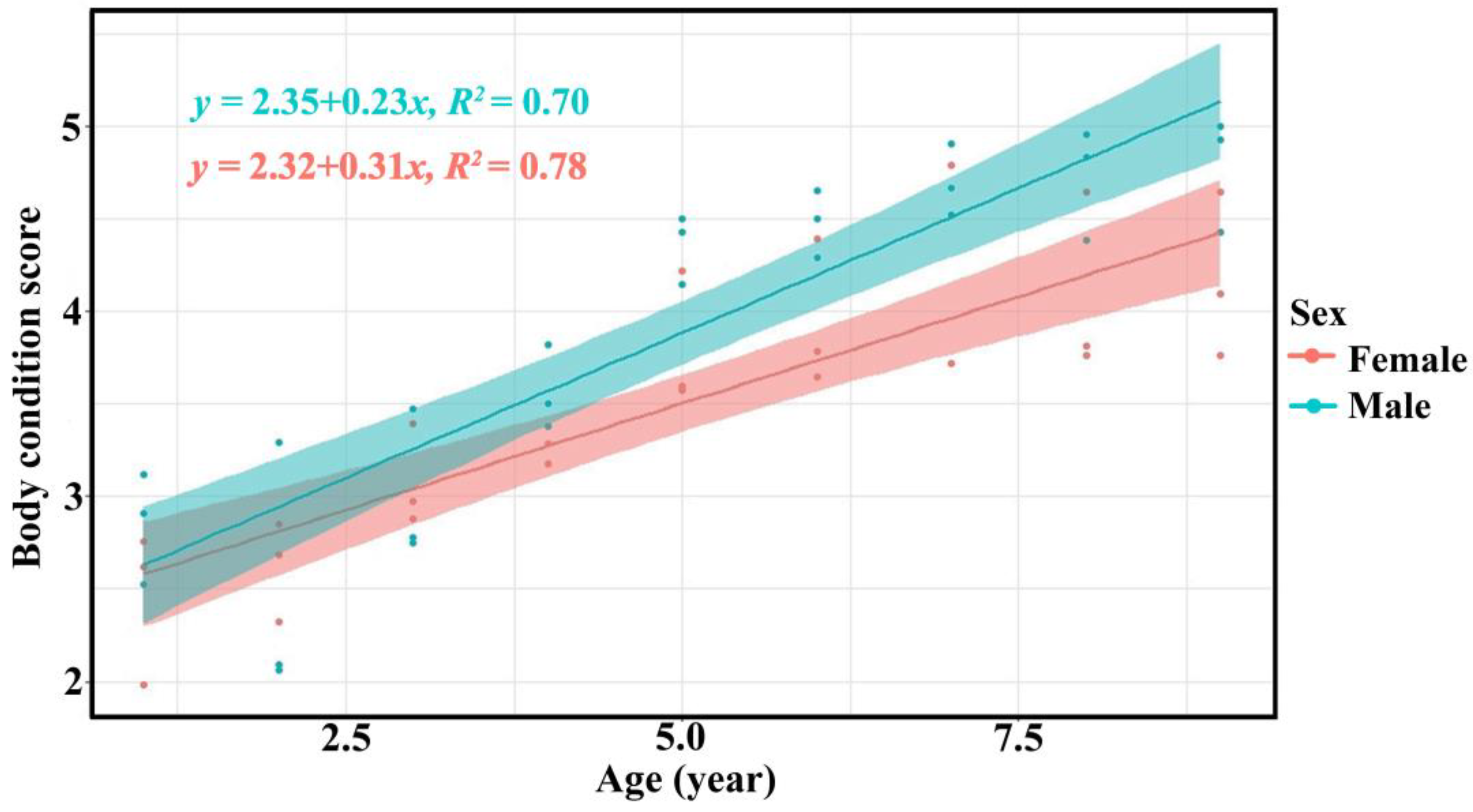
The body condition scores of bantengs increased with age, as evaluated by the three assessor groups (Table 3, Figure 4 and Supplementary Figure S5).
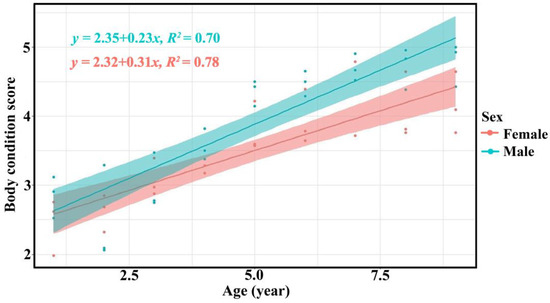
Figure 4.
Relationship between age and body condition score of male (green) and female (red) captive bantengs by researchers.
3.3. Prediction of Population Number of Captive Banteng
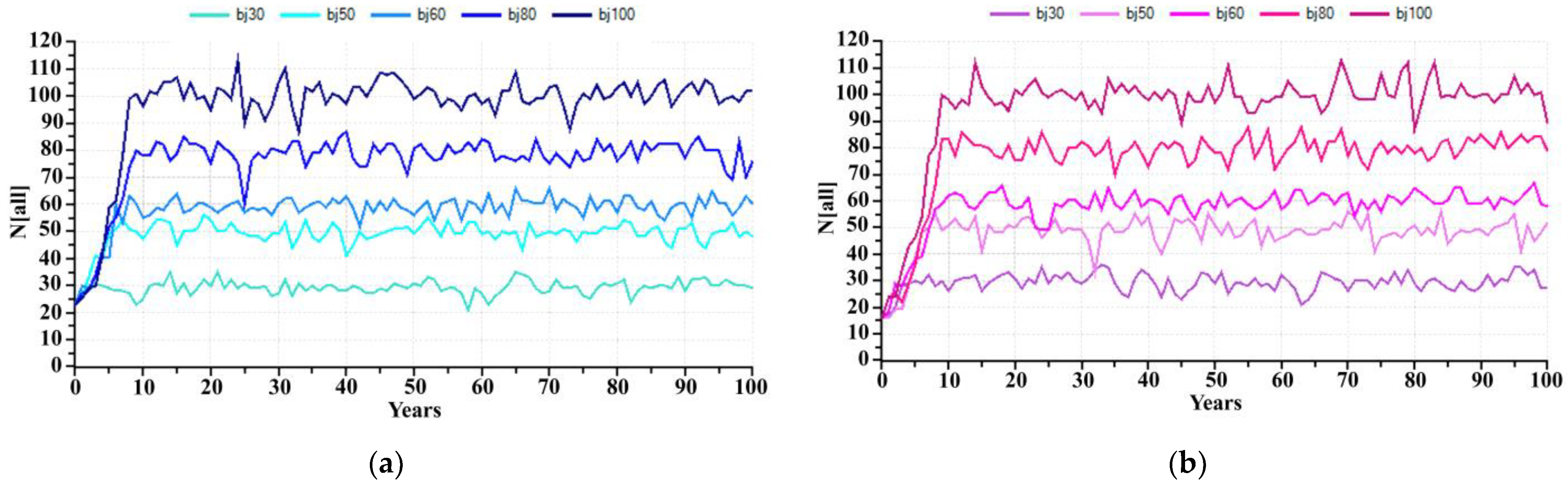
At present, the captive breeding facility of KNP has 30 individuals, but if KNP wants to increase and sustain captive breeding capacity at 50, 60, 80 and 100 individuals in the next 100 years, what is the possible hypothetical carrying capacity that it should be? The prediction for the banteng population in captivity in the next 100 years, if the carrying capacity estimates by KNP were 30, 50, 60, 80 and 100 individuals and assuming there is no removal of individuals from the captive population during this population growth, was predicted. PVA estimated a banteng founder with 23 individuals would initially reach these populations in 2, 8, 4, 13 and 6 years, respectively (Figure 5a), while a starting population of 16 bantengs will reach the carrying capacity in 5, 6, 9, 11 and 13 years, respectively (Figure 5b). These models did not include unexpected stochastic events such as disease outbreaks, natural disasters, etc.
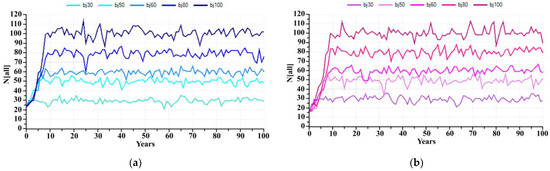
Figure 5.
The number of captive banteng in 100 years with the carrying capacity at 30, 50, 60, 80 and 100 individuals; (a) 23 founders and (b) 16 founders.
4. Discussion
4.1. Growth Rate of Captive Banteng
There were differences between the development of male and female bantengs. The morphology of male bantengs differed in horn shape, body size, skin color and dewlap at all ages, especially after three years old [2], while the morphology of female bantengs had only slight differences after three years old, as reported by Copland [25].
4.2. Relationship among Age, Height, Weight, and Body Condition Score of Captive Banteng
The height assessment of specialists was higher than researchers and untrained assessors due to the specialists being more familiar with the banteng than the researchers and the untrained group. It has been previously noted that variations in assessments may reduce the ability to assess the characteristics of the banteng [26]. However, relative changes in height assessed by all three groups in this study were similar.
The assessments of body condition by the three assessor groups were similar. The body condition score of bantengs increased with age in both males and females, as found in a previous study of reintroduced bantengs in Salakphra Wildlife Sanctuary [18]. This finding indicated that the food and nutrition supplied were suitable even though it was lower than the diet available in Salakphra Wildlife Sanctuary [14]. The males had higher BCS than females as male bantengs used a high BCS for courtship, while the BCS of females was reduced when they were lactating [18]. This study did not note any diseases or other factors affecting BCS as reported in wild bantengs in Indonesia [27,28,29].
4.3. Prediction of Population Number of Captive Banteng
Starting with the founder group of 23 individuals, if the KNP expands its carrying capacity to 30, 50, 60, 80, and 100 individuals, the banteng will reach these numbers in 2, 8, 4, 13 and 6 years, respectively. However, when the number of founders is reduced to 16 due to reintroductions, reaching carrying capacity is expected to be delayed to 5, 6, 9, 11 and 13 years, respectively. A small captive founder group is affected more by reductions in size through the reintroduction program. A small founder group may also lead to inbreeding. The severity of inbreeding depression varies widely among species [30,31]. To reach this possible hypothetical, KNP needs to prepare for captive breeding capacity, such as increasing the carrying capacity of captivity, increasing staff, increasing the quality and quantity of forages, and finding long-term funding support. The limitation of these models was to assume there is no removal of individuals from the captive population during this population growth and lack of genetic information of KNP. For future works, these models should include unexpected stochastic events, such as disease outbreaks, natural disasters, genetic considerations, etc., as the parameters.
4.4. Conservation and Management
If a captive banteng population is lower than ~20 individuals, increasing the population to an expected stable population for 100 years may take longer than a group of higher than 20 individuals. This possible hypothetical will be successful if the Department of National Parks, Wildlife and Plant Conservation (DNP) agrees, facilitate, and supports the KNP to continue the sustainable banteng captive breeding program. In general speculation, a captive breeding program with approximately 20 founder individuals can result in inbreeding depression and a decline in the captive population after 40 years if no individuals are translocated to the wild [5]. However, starting with a small group can be an optimal interim strategy if it allows time to establish recruitment mechanisms [32]. Breeding technology such as artificial insemination (AI) and embryo transfer [33,34,35] may be appropriate to increase the breeding banteng population. A translocation strategy of wild individuals to increase the gene pool of the captive program in KNP is important to increase the population genetics and recovery of the population. This activity will be a more effective use of biological and financial resources to prevent the extinction of the reintroduced banteng in Salakphra Wildlife Sanctuary and other areas.
5. Conclusions
Therefore, the correct management for breeding pairs was to monitor growth and body metrics to raise healthy captive individuals and predict the population viability of captive breeding bantengs to manage the suitable number of captive bantengs. For future studies, their genetic diversity is integral to minimizing inbreeding depression within a captive setting. Low genetic variability is currently an issue for captive-bred bantengs in Thailand [31] and other wildlife species [36,37], such as those found in the Malayan gaur (Bos gaurus hubbacki) where only a few founder individuals resulted in multiple progenies with shared parents. This affects the survival rate of newborn calves [38,39]. More than one ex-situ breeding facility supplied with multiple wild-caught individuals originating from different management units would be preferable. This methodology can not only minimize mortality arising from disease transmission but also maintain wildlife security and avoid unintentional reintroduction, such as when a captive-bred banteng escapes from its enclosure [12].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13020198/s1, Figure S1: Relationship between age and height of individual captive bantengs from three assessor groups: bjm = male Bos javanicus; bjf = female Bos javanicus; Figure S2: Relationship between age and weight of individual captive bantengs from three assessor groups: bjm = male Bos javanicus; bjf = female Bos javanicus; Figure S3: Relationship between age and weight of individual captive bantengs from three assessor groups: bjm = male Bos javanicus; bjf = female Bos javanicus; Figure S4: Relationship between age and weight of (a) male and (b) female captive bantengs by researchers; Figure S5: Relationship between age and body condition score by (a) specialists (b) researchers and (c) untrained assessors. Table S1: Relationship between age (y), height (h), weight (w) and body condition score (b) of captive bantengs.
Author Contributions
Conceptualization, R.C., N.S. and P.K.; methodology, R.C.; software, N.Y.; validation, R.C., N.S. and P.K.; formal analysis, N.S. and P.K.; investigation, S.N.; resources, R.C. and S.N.; data curation, N.S. and P.K.; writing—original draft preparation, N.S. and P.K.; writing—review and editing, R.C.; visualization, N.Y.; supervision, R.C.; project administration, R.C.; funding acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
“This research was funded by Mahidol University (Basic Research Fund: fiscal year 2022), grant number BRF1-071-2565” and “The APC was funded by Mahidol University (Basic Research Fund: fiscal year 2022), grant number BRF1-071-2565”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Mahidol University-Institute Animal Care and Use (F02-65-006, 1 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study under the permission of the Department of National Parks, Wildlife and Plant Conservation (DNP), Ministry of Natural Resources and Environment of the Kingdom of Thailand (MNRE-0907.4/13065).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Supanya Khongphetsak, Pongsak Boonthong, Sopot Mankhong, and the staff of Khao Nam Phu Nature and Wildlife Education Center for their data collection support. This research project is supported by Mahidol University (Basic Research Fund: fiscal year 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garsetiasih, R. Daya Dukung Padang Perumputan banteng (Bos javanicus d’Alton 1832): Studi kasus di Sadengan dan Sumber Gedang, Jawa Timur. J. Penelit. Hutan Dan Konserv. Alam 2013, 10, 229–240. [Google Scholar] [CrossRef][Green Version]
- Lekagul, B.; McNeely, J.A. Mammals of Thailand; Association for the Conservation of Wildlife: Bangkok, Thailand, 1977. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2018-2. 2019. Available online: http://www.iucnredlist.org (accessed on 28 December 2018).
- Office of Natural Resources and Environmental Policy and Planning (ONEP). Thailand Red Data: Vertebrates; Ministry of Natural Resource and Environment: Bangkok, Thailand, 2017.
- Gardner, P.C.; Goossens, B.; Bin Abu Bakar, S.; Bruford, M.W. Hunting pressure is a key contributor to the impending extinction of Bornean wild cattle. Endanger. Species Res. 2021, 45, 225–235. [Google Scholar] [CrossRef]
- Timmins, R.J.; Duckworth, J.W.; Hedges, S.; Steinmetz, R.; Pattanavibool, A. Bos javanicus. The IUCN Red List of Threatened Species. 2016. Available online: https://www.researchgate.net/profile/Simon-Hedges-2/publication/301746958_Bos_javanicus_The_IUCN_Red_List_of_Threatened_Species_2008/links/5724c48d08aee491cb3a9a54/Bos-javanicus-The-IUCN-Red-List-of-Threatened-Species-2008.pdf (accessed on 1 April 2020).
- Srikosamatara, S.; Suteethorn, V. Populations of gaur and banteng and their management in Thailand. Nat. His. Bull. Siam Soc. 1995, 43, 55–83. [Google Scholar]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013. [Google Scholar]
- Chaiyarat, R.; Youngpoy, N.; Kongsurakan, P.; Nakbun, S. Habitat preferences of reintroduced banteng (Bos javanicus) into the Salakphra Wildlife Sanctuary, Thailand. Wildl. Res. 2019, 46, 573. [Google Scholar] [CrossRef]
- Gardner, P.C. The Natural History, Non-Invasive Sampling, Activity Patterns and Population Genetic Structure of the Bornean Banteng Bos javanicus Lowi in Sabah, Malaysian Borneo. Ph.D. Thesis, Cardiff University, Cardiff, NY, USA, 2014. [Google Scholar]
- Corbett, G.B.; Hill, J.E. The Mammals of the Indomalay Region: A Systematic Review; Natural History Museum Publications, Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Chaiyarat, R.; Saengpong, S.; Tunwattana, W.; Dunriddach, P. Habitat and food utilization by banteng (Bos javanicus d’Alton, 1823) accidentally introduced into the Khao Khieo-Khao Chomphu Wildlife Sanctuary, Thailand. Mammalia 2017, 82, 23–34. [Google Scholar] [CrossRef]
- Melletti, M.; Burton, J. Ecology, Evolution and Behaviour of Wild Cattle: Implications for Conservation; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Chaiyarat, R.; Sakchan, P.; Panprayun, G.; Thongthip, N.; Nakbun, S. Monitoring of forage and nutrition before and after reintroduction of banteng (Bos javanicus d’ Alton, 1823) to Salakphra Wildlife Sanctuary, Thailand. Sci. Rep. 2020, 10, 11135. [Google Scholar] [CrossRef] [PubMed]
- Prakobphon, N. Behaviour of Banteng (Bos javanicus) in Chiang Mai Zoo Changwat Chiang Mai and Khao Kheow Open Zoo Changwat Chonburi. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1988. [Google Scholar]
- Rahman, D.A.; Herliansyah, R.; Rianti, P.; Rahmat, U.M.; Firdaus, A.Y.; Syamsudin, M. Ecology and Conservation of the Endangered Banteng (Bos javanicus) in Indonesia Tropical Lowland Forest. HAYATI J. Biosci. 2019, 26, 68. [Google Scholar] [CrossRef]
- Nakbun, S. (Khao Nampu Nature and Wildlife Education Center, Department of National Parks, Wildlife and Plant Conservation, Kanchanaburi, Thailand). Personal communication, 2022.
- Kongsurakan, P.; Chaiyarat, R.; Nakbun, S.; Thongthip, N.; Anuracpreeda, P. Monitoring body condition score of reintro-duced banteng (Bos javanicus D’Alton, 1923) into Salakphra Wildlife Sanctuary, Thailand. PeerJ 2020, 8, e9041. [Google Scholar] [CrossRef]
- Ahrestani, F.S.; Prins, H.H. Age and sex determination of gaur Bos gaurus (Bovidae). Mammalia 2011, 75, 151–155. [Google Scholar] [CrossRef]
- Niedballa, J.; Sollmann, R.; Bin Mohamed, A.; Bender, J.; Wilting, A. Defining habitat covariates in camera-trap based occupancy studies. Sci. Rep. 2015, 5, 17041. [Google Scholar] [CrossRef]
- Wayne, W.D. Spearman Rank Correlation Coefficient. In Applied Nonparametric Statistics, 2nd ed.; PWS-Kent: Boston, MA, USA, 1990; pp. 358–365. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Lacy, R.C.; Pollak, J.P. Vortex: A Stochastic Simulation for the Extinction Process; Version 10.2.6; Chicago Zoological Society: Brookfield, IL, USA, 2017. [Google Scholar]
- Lacy, R.C.; Miller, P.S.; Traylor-Holzer, K. An Overview of Population Viability Analysis Using VORTEX, Vortex 10 User’s Manual; 19 January 2015 Update; IUCN SSC Conservation Breeding Specialist Group, Chicago Zoological Society: Apple Valley, MN, USA, 2015. [Google Scholar]
- Copland, R.S. Observations on Banteng cattle in Sabah. Trop. Anim. Health Prod. 1974, 6, 89–94. [Google Scholar] [CrossRef]
- Hedges, S.; Meijaard, E. Reconnaissance Survey for Banteng (Bos javanicus) and Banteng Survey Methods Training Project, Kayan-Mentarang National Park, East Kalimantan, Indonesia; World Wide Fund for Nature-Indonesia (WWF) and Centre for International Forestry Research (CIFOR): East Kalimantan, Indonesia, 1999. [Google Scholar]
- Soares, F.S.; Dryden, G.M. A Body Condition Scoring System for Bali Cattle. Asian-Australas. J. Anim. Sci. 2011, 24, 1587–1594. [Google Scholar] [CrossRef]
- Prosser, N.S.; Gardner, P.C.; Smith, J.A.; Wern, J.G.E.; Ambu, L.N.; Goossens, B. Body condition scoring of Bornean banteng in logged forests. BMC Zool. 2016, 1, 8. [Google Scholar] [CrossRef]
- Jelantik, I.G.N.; Benu, I.; Malelak, G.E.M. Influence of Body Condition Score on Carcass Characteristics of Cull Bali Cows. IOP Conf. Ser. Earth Environ. Sci. 2020, 478, 012011. [Google Scholar] [CrossRef]
- Lacy, R.C. Lessons from 30 years of population viability analysis of wildlife populations. Zoo Biol. 2018, 38, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Chaichanathong, S.; Klinsawat, W.; Sukmak, M.; Sakulthai, A.; Wajjwalku, W.; Sripiboon, S.; Kaolim, N.; Nakbhun, S.; Tun-pradit, B.; Nipanunt, T.; et al. Genetic characterization of banteng (Bos javanicus) populations in Thailand for conservation. Thai. J. Vet. Med. 2021, 51, 647–654. [Google Scholar]
- McCann, N.P.; Wheeler, P.M.; Coles, T.; Bruford, M.W. Rapid ongoing decline of Baird’s tapir in Cusuco National Park, Honduras. Integr. Zool. 2012, 7, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Humblot, P.; Le Bourhis, D.; Fritz, S.; Colleau, J.J.; Gonzalez, C.; Joly, C.G.; Malafosse, A.; Heyman, Y.; Amigues, Y.; Tissier, M.; et al. Reproductive Technologies and Genomic Selection in Cattle. Veter-Med. Int. 2010, 2010, 192787. [Google Scholar] [CrossRef] [PubMed]
- Kidie, H.A. Review on growth and development of multiple ovulation and embryo transfer technology in cattle. World Sci. News Int. Sci. J. 2019, 127, 191–211. [Google Scholar]
- Mastromonaco, G.F.; Gonzalez-Grajales, A.L. Reproduction in female wild cattle: Influence of seasonality on ARTs. Theriogenology 2020, 150, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Leberg, P.L.; Firmin, B.D. Role of inbreeding depression and purging in captive breeding and restoration programmes. Mol. Ecol. 2008, 17, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, K.A.; Hogg, C.J.; Grueber, C.E. A meta-analysis of birth-origin effects on reproduction in diverse captive environments. Nat. Commun. 2018, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.; Ithnin, H.; Rovie-Ryan, J.J. Inbreeding depression of captive Malayan gaur (Bos gaurus hubbacki) at Jenderak Selatan Wildlife Conservation Centre, Pahang. J. Wildl. Parks. 2016, 31, 71–79. [Google Scholar]
- Md-Zain, B.M.; Abdul-Aziz, A.; Aifat, N.R.; Mohd-Yusof, N.S.; Zulkifli, N.A.; Japning, J.R.R.; Rosli, N.; Yaakop, S. Sequence variation data of the mitochondrial DNA D-loop region of the captive Malayan Gaur (Bos gaurus hubbacki). Data Brief. 2018, 24, 103532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).