Simple Summary
In the post-antibiotic era, the attainment of higher feed efficiency has become the primary goal of the poultry farming. Bacillus subtilis is commonly used as a substitute for antibiotics in animal and poultry feed. The results demonstrated that dietary 5 × 108 cfu/kg Bacillus subtilis HC6 increased the feed efficiency, antioxidant capacity, and mRNA expression of pro-inflammatory cytokines in the jejunal mucosa, and decreased the activity of diamine oxidase in serum, which might be attributed to the modulation of community composition and functions of cecal microbiota in white-feathered broilers. Our results provide new insights and evidence for the application of probiotics in broiler breeding.
Abstract
This study aimed to investigate the impact of Bacillus subtilis HC6 on the growth performance, immunity, antioxidant capacity, and intestinal health of broilers. A total of 180 one-day-old white feather broilers were randomly divided into two experimental groups, each comprising six replicates of fifteen chicks from 1 to 50 d of age. The groups were either fed a basal diet (CON) or the same diet supplemented with 5 × 108 cfu/kg of Bacillus subtilis HC6 (BS). Our results indicated that compared with the CON, dietary supplementation with BS increased feed efficiency during d 21–50 and d 1–50 (p < 0.05). Moreover, BS supplementation enhanced antioxidant capacity in the serum and liver, and also decreased the activity of diamine oxidase and the level of endotoxins (p < 0.05). Additionally, BS treatment increased the villi height in the jejunum and ileum, increased the ratio of villus height/crypt depth in the ileum, upregulated the expression of tight junction proteins in the jejunal mucosa, and downregulated the levels of IL-22 and IFN-γ on day 50 (p < 0.05). Principal coordinates analysis yielded clear clustering of two groups; dietary BS increased the relative abundance of Bacteroidales_unclassified (genus) and Olsenella (genus), and decreased the abundance of genera Alistipes on day 50, which identified a strong correlation with FCR, serum differential metabolites, or differential gene expression in the jejunal mucosa by spearman correlation analysis. The PICRUSt2 analysis revealed that supplementation with BS enriched the pathways related to xenobiotics biodegradation and metabolism, carbohydrate metabolism, energy metabolism, signaling molecules and interaction, the digestive system, and transport and catabolism. These results demonstrated that dietary BS increased feed efficiency, antioxidant capacity, and the mRNA expression of pro-inflammatory cytokines in the jejunal mucosa; and decreased the activity of diamine oxidase in serum, which might be attributed to the modulation of community composition and the functions of cecal microbiota in white-feathered broilers.
1. Introduction
In recent years, chicken has emerged as the predominant protein source worldwide due to its increasing demand in the poultry industry, surpassing beef and pork. However, both the intensification of chicken production practices and the ban on antibiotic usage have presented challenges and vulnerabilities for broiler breeders and enterprises [1,2]. Consequently, scholars and experts in livestock production and poultry science have been actively exploring alternative strategies to effectively address these issues, given the escalating demand for antibiotic-free meat products. Feed additives such as antibacterial agents, probiotics, and prebiotics have been extensively administered to improve the growth performance of birds. Among these products, there is a growing interest in characterizing probiotics, which have been shown to improve immunity, antioxidant status, gut function, health and growth parameters in broiler chickens [3,4].
Bacillus-based probiotics are widely abundant in nature and are commonly employed as a substitute for antibiotics in animal and poultry feed. They have the ability to form spores, which confer resistance against high temperatures, pH, bile, and enzymes in the gastrointestinal tract [5,6]. There is mounting evidence suggesting that probiotic supplementation can sustain intestinal health by modifying the gut microbiota; boosting immune regulation, barrier function, and nutrient digestibility; and ultimately enhancing growth performance [7]. Previous studies have demonstrated that the use of Bacillus-based probiotics as a supplement improved growth performance, immunity, gut homeostasis, and microbiota in various animals such as fish, piglets, and chickens [8,9,10]. Meanwhile, other studies evaluated the effects of B. amyloliquefaciens LFB112 and B. subtilis on the performance of broilers, and observed that feeding broilers with B. amyloliquefaciens at a concentration of 5 × 105 cfu/g resulted in improved body weight gain and feed conversion ratio [11].
In this study, 817 white feather broilers were used as a model to investigate the potential positive impact of B. subtilis HC6 supplementation on growth performance, immune function, antioxidant capacity, and intestinal health in broilers, which might provide more potential product and evidence for the application of probiotics in broiler breeding.
2. Materials and Methods
2.1. Bacterial Preparation
Bacillus subtilis HC6 (BS) was isolated from our laboratory, and the bacteria were cultured overnight in Lu-ria-Bertani broth at 37 °C, followed by centrifugation at 5000 rpm for 15 min. The resulting pellet was washed twice with sterile phosphate-buffered saline (PBS; pH = 7.4), and then BS powder was prepared at a concentration of 2.0 × 108 cfu/g.
2.2. Birds, Experimental Design, and Diets
A total of 180 healthy one-day-old male white feather broilers chicks with an average initial body weight of 36.20 ± 0.01 g were purchased from Muyuan Animal Husbandry Co., Ltd. (Guangzhou, China), and randomly allocated to two groups, with six replicates of 15 birds per replicate. Birds were fed a basal diet, or fed a basal diet supplemented with BS (5.0 × 108 cfu/kg). Pre-experiments before the formal test and previous research results of the members of the research group determined the dosage of probiotics [12]. The diet was formulated according to the nutrient requirements of white-feathered broiler chickens (Table 1), and nutrients met or exceeded nutrient requirements for chickens (NRC, 1994). This study involved housing chicks in a controlled environment with free access to water and feed. The chicks were subjected to twelve-hour light-dark cycles and maintained at different room temperatures throughout the study period. Specifically, the temperature was maintained at 34 °C for the first week, 32 °C for the second week, and 24 °C for the rest of the study period. The humidity was kept at around 62%.

Table 1.
Composition and nutrient content of experimental diets.
2.3. Sample Collection
On day 21 and 50, after 8 h of feeding deprivation, the body weight and the feed residues within each replicate was recorded, then the growth performance parameters, such as the average daily feed intake (ADFI), average daily gain (ADG), and the ratio of feed to gain (FCR), were calculated on a replicate basis and adjusted for mortality during both different phases (1~21 d and 22~50 d) and the whole experiment (1~50 d).
After weighting at d 21 and 50, one broiler chicken that was closest to the average weight of each replicate was selected for blood and tissue sample collection. Briefly, about 3–5 mL of blood was drawn from the brachial vein and allowed to rest before being centrifuged at 3000× g for 15 min at 4 °C, and the extracted serum was then preserved at −80 °C to facilitate subsequent determinations. After blood collection, the selected chickens were sacrificed by exsanguination and tissue samples were collected, including the liver, jejunum, ileum, jejunal mucosa, and cecal contents. Liver tissue samples were uniformly collected from the left-side of each chicken, placed in liquid nitrogen for a period, and eventually stored at −80 °C to aid in determining antioxidant capacity. Finally, the jejunum, jejunal mucosa, and the contents of cecum were methodically gathered and preserved using different techniques. Both jejunal mucosa and jejunal tissue samples were obtained from the middle section of the intestinal segment. The jejunal mucosa was collected by scraping the jejunum using a scalpel. Additionally, the scraped jejunal tissue, measuring approximately 3–4 cm in length, was preserved for further analysis. Specifically, the jejunal mucosa and cecal contents were rapidly cryopreserved in liquid nitrogen before being finally stored at −80 °C. Overall, the rigorous sampling and preservation procedures employed in this study laid a solid foundation for reliable and accurate analyses.
2.4. Measured Serum Biomarkers
In this study, we utilized assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) to detect various serum biomarkers. These included triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, albumin, uric acid, aspartate transaminase, alanine transaminase, alkaline phosphatase, and lactate dehydrogenase. Additionally, the corresponding diamine oxidase (DAO) assay kit and endotoxin kit were used to measure DAO activity and endotoxin level, respectively. The secretory immunoglobulin A (sIgA) and inflammatory factors, including interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) concentrations were determined using a chicken-specific ELISA kit (Nanjing Jiancheng, Bioengineering Institute, Nanjing, China). The assay was performed according to the manufacturer’s instructions (Jiancheng Biomedicine, Shanghai, China).
2.5. Serum and Liver Antioxidant Markers
The serum antioxidant index was evaluated using a kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China), which was comprised of biomarkers such as total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), malondialdehyde (MDA), catalase (CAT), and glutathione peroxidase (GSH-Px). After being thawed on ice, liver samples were weighed and homogenized with nine times normal saline at a weight (g):volume (mL) ratio of 1:9. The preparation of homogenate samples from liver tissue follows the experimental methodology provided in the technical support section of the Nanjing Jiancheng Kit website (http://www.njjcbio.com/contents.asp?cid=2&wid=2&id=507, accessed on 7 December 2022). The resulting mixture was then centrifuged, and the supernatant was collected for subsequent measurements. The serum and liver antioxidant indexes were assessed by measuring T-SOD, T-AOC, MDA, CAT, and GSH-Px biomarkers according to the instructions provided by the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Intestinal Tissue Morphology
Approximately 1 cm of jejunum and ileum tissue specimens were preserved in a 4% formaldehyde solution for fixation, and they underwent paraffin embedding. Five serial sections were obtained after tissue dehydration and clearing, with the first four sections rehydrated and stained with hematoxylin and eosin. Tissue sections were observed using an optical microscope with a 10× objective lens connected to a LEICA (DFC290 HD system digital camera, Heilbrugger, Switzerland). Villus height and corresponding crypt depth measurements were taken on straight and relatively intact villi, villus height was measured from the crypt-villus junction to the tip of the villus, and crypt depth was measured from the base of the crypt to the crypt-villus junction. The villus/crypt ratio was calculated by dividing the villus height by the crypt depth.
2.7. RNA Extraction, cDNA Synthesis and Quantitative PCR
The jejunal mucosa total RNA was extracted using TransZol reagent (TransGen Biotech, Beijing, China), and the concentration, OD 260/280 ratio, and OD 260/230 ratio were determined with high accuracy using a VWRI732-2534 spectrophotometer (Avantor, Radnor, PA, USA) and a mere 1 µL of extracted RNA solution. Subsequently, 1 µg of RNA underwent meticulous reverse transcription and cDNA synthesis utilizing the RT EasyTMII Kit (Foregene, Chengdu, China) to eliminate any genomic DNA contamination. These primers (the primers are presented in Table 2) were synthesized by Qingke Biological Co., Ltd. (Beijing, China), and cDNA gene expression assays were executed via real-time quantitative PCR (QuantStudio 3, Thermo Fisher Scientific, Shanghai, China) utilizing the Real-Time PCR Easy TM-SYBR Green I kit (Foregene, Chengdu, China). The total volume of PCR reaction system was 20 µL, and PCR cycling conditions were 95 °C for 30 s to denature the cDNA template, followed by 40 cycles at 95 °C for 10 s and 60 °C for 30 s. The primer pairs for specific genes, such as immune-related genes and intestinal barrier related genes, were designed using the NCBI primer tool (https://www.ncbi.nlm.nih.gov, accessed on 17 December 2022), and their specificity was verified. All primer pairs were validated, and they exhibited approximately 100% amplification efficiency. Real-time quantitative PCR using the 2−∆∆Ct method was employed to determine the relative gene expression with β-actin as an internal reference.

Table 2.
The primer sequence of the gene.
2.8. 16S Sequencing and Cecal Microbiota Analysis
Total genomic DNA from cecal contents was extracted using the Cetyltrimethylammonium Bromide method (CTAB) method (Sample mixing, thermal lysis, chloroform extraction, ethanol washing, and dissolution). Agarose gel electrophoresis was used to assess the DNA extraction quality, and the DNA was quantified using an ultraviolet spectrophotometer. Two primers (V3-forward: 5′-CCTACGGGNGGCWGCAG′ and V4-reverse: 5′-GACTACHVGGGTATCTAATCC-3′) were designed and synthesized for amplifying the V3–V4 region of the 16S rDNA gene. PCR products were purified using AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA) and quantified with Qubit (Invitrogen, Waltham, MA, USA). The purified PCR products were evaluated using the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and Illumina’s library quantification kit (KapaBiosciences, Woburn, MA, USA). The concentration of the qualified library should be above 2 nM. The NovaSeq 6000 sequencer was used for 2 × 250 bp double-ended sequencing with the NovaSeq 6000 SP Reagent Kit (500 cycles). For the double-ended data obtained by sequencing, the samples were first separated based on barcode information, and the joint and barcode sequences were removed. The following steps were then performed for data cleaning and quality control: 1. Primer sequences were removed and the base sequence of RawData was balanced using cutadapt (v 1.9) software; 2. Paired-end reads were combined into longer tags based on the overlap area using FLASH (v 1.2.8) software; 3. Sequencing reads were quality scanned using the window method, with a default scanning window size of 100 bp. If the average quality value within the window was lower than 20, the read part from the beginning of the window to the 3′ end was cut off using fqtrim software (v 0.94); 4. Sequences with a length less than 100 bp were removed; 5. Sequences with a N (uncertain fuzzy bases) content above 5% after truncation were removed; 6. Chimeric sequences were removed using Vsearch (v 2.3.4) software. The Dada 2 tool was invoked with qiime DADA 2 denoise-paired for length filtering and denoising. The ASV (feature) sequence and ASV (feature) abundance table were obtained, and singleton ASVs were removed. Alpha diversity analysis and beta diversity analysis were conducted based on the obtained ASV (feature) sequence and ASV (feature) abundance table. The alpha diversity analysis assesses domestic diversity using six indexes: observed_species, shannon, simpson, chao1, goods_coverage, and pielou_e. Additionally, four types of distances (unweighted_unifrac, weighted_unifrac, jaccard, bray_curtis) were calculated to evaluate the diversity between habitats (samples/groups). The ASV (feature) sequence file, based on the SILVA database (Release 138, available at https://www.arb-SILVA.de/documentation/release138/, accessed on 27 December 2022), was used to annotate the NT-16s database for species. The abundance of each species in each sample was then analyzed using the ASV (feature) abundance table. Spearman’s correlations were analyzed using the R packages heatmap (R 3.6.3). To determine the possible functional profile of the cecal microbiota affected by dietary treatments, we utilized PICRUSt2 (https://github.com/picrust/picrust2, accessed on 7 January 2023), an improved version of the original method. The predicted Kyoto Encyclopedia of Genes and Genomes (KEGG) orthologs were then categorized into Level-2 functional categories.
2.9. Statistical Analysis
Independent sample t-tests were used for all analyses, and the data were reported as mean ± standard deviation (SD). Statistical analysis and result presentation utilized SPSS software version 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8 (GraphPad Software, Inc., San Diego, CA, USA), respectively. Statistical significance was defined as a p value < 0.05 (p < 0.05).
3. Results
3.1. Growth Performance
The growth performance of the broilers is summarized in Table 3. Compared with the control group, the BS group decreased the feed conversion ratio of chickens during periods of 21–50 d (p = 0.015) and 1–50 d (p = 0.020). Nonetheless, body weight (BW), average daily gain (ADG), and average daily feed intake (ADFI) were comparable between the two groups across all stages (p > 0.05).

Table 3.
Effects of BS on growth performance in broilers.
3.2. Intestinal Morphology
Table 4 details a significant rise in the height of jejunal (p = 0.001) and ileal (p = 0.005) villi, along with an increased villus-to-crypt ratio (p = 0.011) in the ileum on day 50 in broilers of the BS-administered group compared to those of the basal diet group.

Table 4.
Effects of treatments on jejunal and ileal morphology.
3.3. Jejunal Mucosal Barrier Function
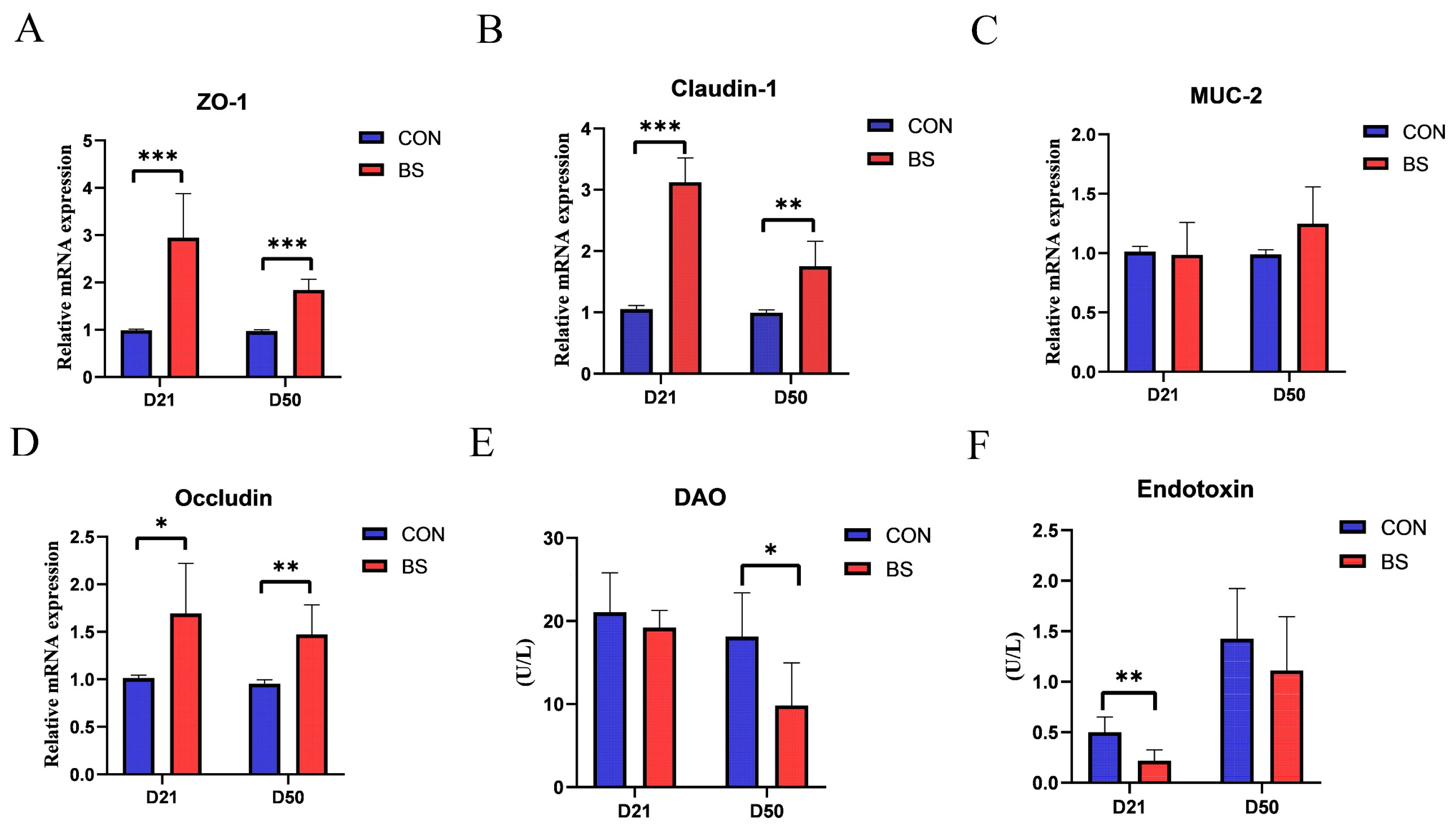
As presented in Figure 1, the effects of BS on the mRNA expression of tight junction proteins, as well as the activity of DAO and the endotoxin of broilers at 21 and 50 days of age, were investigated. The results showed that the mRNA expression levels of ZO-1, Claudin-1, and Occludin were significantly higher in the BS group than those in the CON group on both day 21 and day 50 (p < 0.05). However, there was no significant difference in MUC-2 levels between the two groups at either stage (p > 0.05). Moreover, serum endotoxin levels and DAO activity were measured to assess intestinal mucosal damage. Broilers in the BS group displayed a significant decrease in endotoxin levels on day 21 and DAO activity on day 50 compared to those in the CON group (p < 0.05).
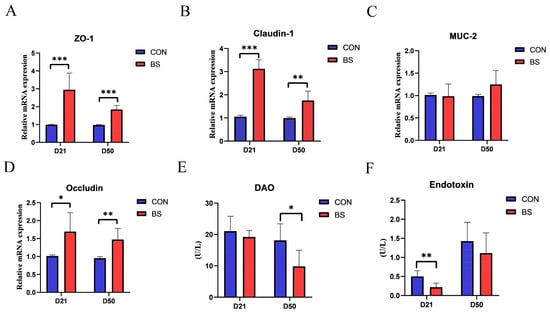
Figure 1.
Effects of BS on tight junction protein mRNA expression, serum DAO activity, and endotoxin activity of broilers on day 21 and day 50. (A) ZO-1; (B) MUC-2; (C) Claudin-1; (D) Occludin; (E) DAO; (F) Endotoxin; * p < 0.05, ** p < 0.01, and *** p < 0.001 compared with CON group. CON, control; BS, Bacillus subtilis HC6; DAO, diamine oxidase.
3.4. Immune Factor
The levels of serum immune factors for both dietary interventions at days 21 and 50 are listed in Table 5. Compared to the CON group, dietary BS supplementation decreased serum levels of IL-6 (p = 0.024) on day 21 and TNF-α (p = 0.040) on day 50. However, there was no significant difference in remaining parameters. Table 5 also portrays the impact of BS on immunomodulatory factors in the jejunal mucosa of broilers. Compared with the control group, the addition of BS resulted in an upregulation of IL-10 (p = 0.009), IL-22 (p = 0.019), and IFN-γ (p = 0.035) at the mRNA expression levels on day 21, as well as a downregulation of IL-22 (p = 0.001) and IFN-γ (p = 0.049) on day 50.

Table 5.
Effects of BS on the serum and immune jejunal mucosa factors of broilers.
3.5. Serum and Liver Antioxidant Index
As shown in Table 6, compared with the CON group, supplementation with BS resulted in significant increases in the serum T-AOC (p = 0.016) level, as well as in serum CAT (p = 0.006) and T-SOD (p = 0.030) activities after 21 days. Moreover, the level of T-AOC (p = 0.039), and activities of CAT (p = 0.031), T-SOD (p = 0.026), and GSH-Px (p = 0.003) in serum were elevated after 50 days of treatment with BS. The results also demonstrated that the hepatic activities of T-SOD (p = 0.019) and GSH-Px (p = 0.006) were significantly increased after 21 days of BS supplementation compared to the control group. Similarly, after 50 days, the level of T-AOC (p = 0.029), and hepatic activities of CAT (p = 0.034), T-SOD (p = 0.025) and GSH-Px (p = 0.047) were also significantly increased in the BS supplementation group compared to the control group. In contrast, no significant differences in MDA (p > 0.05) content were observed between the different two phases.

Table 6.
Effects of BS on serum and liver antioxidant index of broilers.
3.6. Serum Biochemical Index
Table 7 shows the effects of BS on serum biochemical index on 21st and 50th day. The results revealed that, compared to broilers in the CON group, those in the BS group manifested a significant increase in triglyceride content (p = 0.004) and aspartate aminotransferase (p = 0.043) activity, coupled with a significant decrease in albumin (p = 0.049) level on day 21 and a significant increase in HDL-C (p = 0.010) level on day 50.

Table 7.
Effects of BS on serum physiological and biochemical indexes of broilers.
3.7. Gut Microbiota
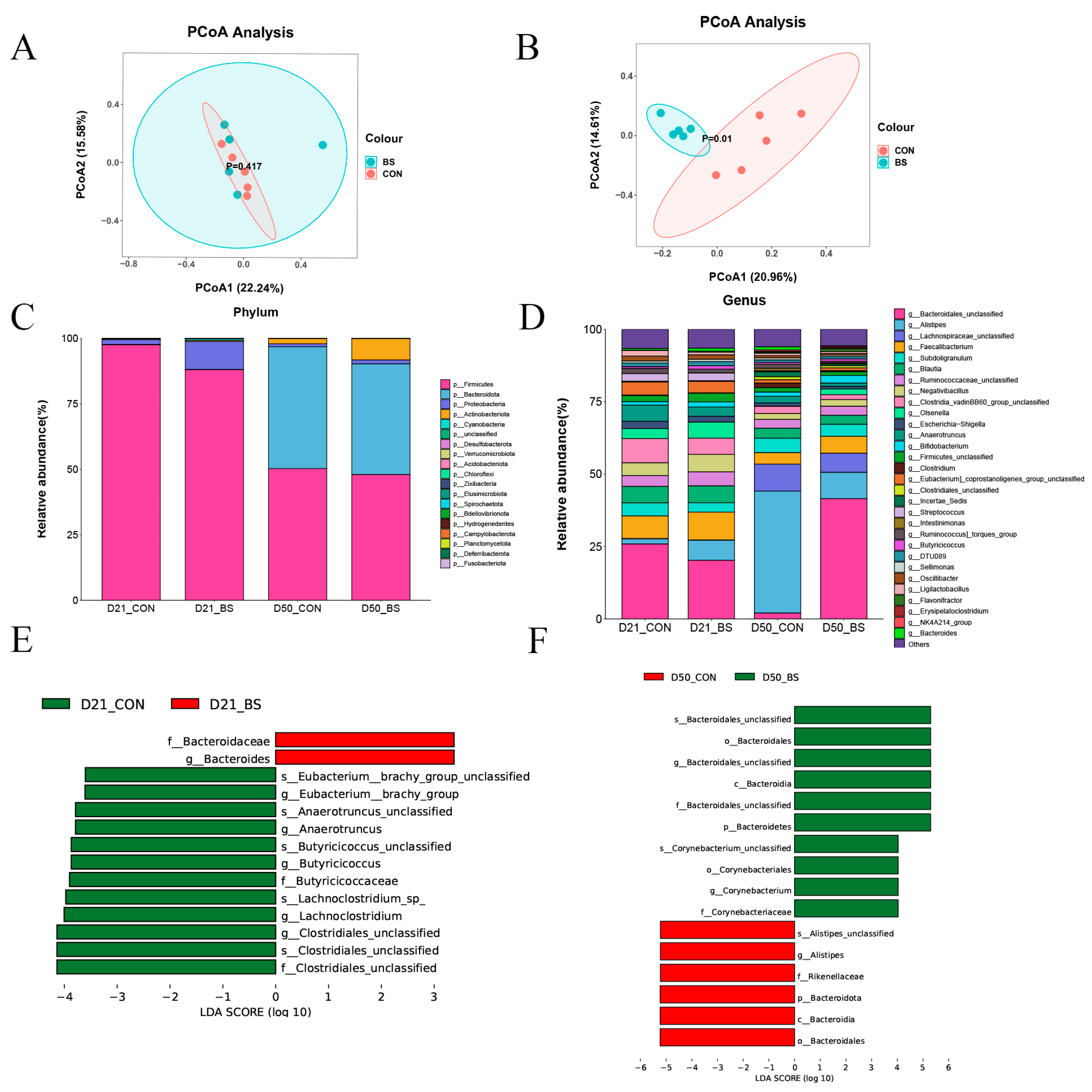
To assess the influence of BS supplementation on the cecal microbiota of broilers, bacterial 16S rDNA amplicon sequencing was performed on the gut microbiota. Table 8 shows that supplementation with BS did not significantly impact the alpha diversity of the bacterial community. As illustrated in Figure 2A,B, the principal coordinate analysis (PCoA) of the weighted UniFrac distances demonstrated distinct segregation between the two groups on day 50, with the first principal component (PCoA1) accounting for 20.96% and the second principal component (PCoA2) accounting for 14.61%, but there was no significant separation on day 21.

Table 8.
Alpha diversity indexes of cecal microbiota.
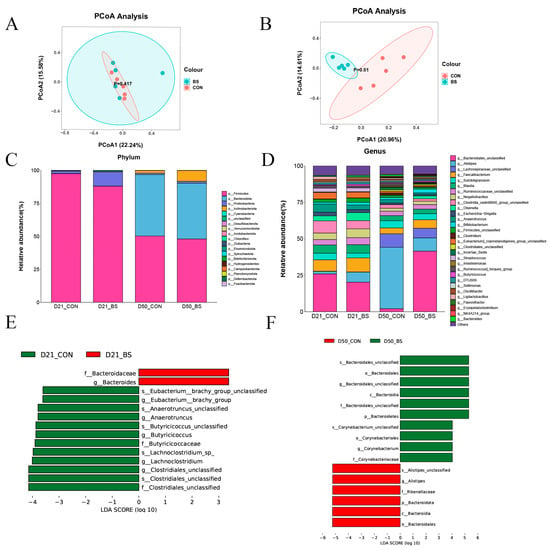
Figure 2.
Relative abundance of the chicken cecal microbiota; (A) D 21 principal coordinate analysis plot; (B) D 50 principal coordinate analysis plot; (C) Phylum-level taxonomic composition of the cecal microbiota; (D) Genus-level taxonomic composition and relative abundance of the cecal microbiota; (E) Lefse identified the most differential genera on 21 days; (F) Lefse identified the most differential genera on 50 days. CON, control; BS, Bacillus subtilis HC6.
The compositions of the top 20 phyla and top 30 genera in the cecal contents of broilers are presented in Figure 2C,D. The dominant phyla were identified as Firmicutes on day 21, whereas the dominant phyla were Firmicutes and Bacteroidota on day 50. There were no significant differences between the CON and BS groups in terms of the abundance of Firmicutes and Bacteroidetes, or the Bacteroidetes/Firmicutes ratios in the two stages (Figure 2C). At the genus level, the composition of the microbiota differed among the two groups at different phases (Figure 2D). Compared with the CON group, BS group increased the abundance of Bacteroidales_unclassified (p = 0.001) and Olsenella (p = 0.001), while decreasing the abundance of Alistipes (p = 0.035). As shown in Figure 2E,F, the effect size measurements (LEfSe) analysis identified 14 and 16 biomarkers with linear discriminant analysis (LDA) scores greater than three in 21 days and 50 days, respectively. Furthermore, two bacterial taxa, namely Bacteroidaceae (family) and Bacteroides (genus), were found to be enriched in the BS group on day 21, whereas Clostridiales_unclassified (family), Clostridiales_unclassified (species), Clostridiales_unclassified (genus), Lachnoclostridium (genus), Lachnoclostridium_sp_ (species), Butyricicoccaceae (family), Butyricicoccus (genus), Butyricicoccus_unclassified (species), Anaerotruncus (genus), Anaerotruncus_unclassified (species), Eubacterium__brachy_group (genus), and Eubacterium_brachy_group_unclassified (species) were enriched in the CON group. Furthermore, on day 50, ten bacterial taxa were found to be enriched in the BS group, including Corynebacteriaceae (family), Corynebacterium (genus), Corynebacteriales (order), Corynebacterium_unclassified (species), Bacteroidetes (phylum), Bacteroidales_unclassified (family), Bacteroidia (class), Bacteroidales_unclassified (genus), Bacteroidales (order), and Bacteroidales_unclassified (species), wheseas Bacteroidales (order), Bacteroidia (class), Bacteroidota (phylum), Rikenellaceae (family), Alistipes (genus), and Alistipes_unclassified (species) were abundant in the CON groups.
3.8. Spearman Correlation Analysis of Cecal Microbiota
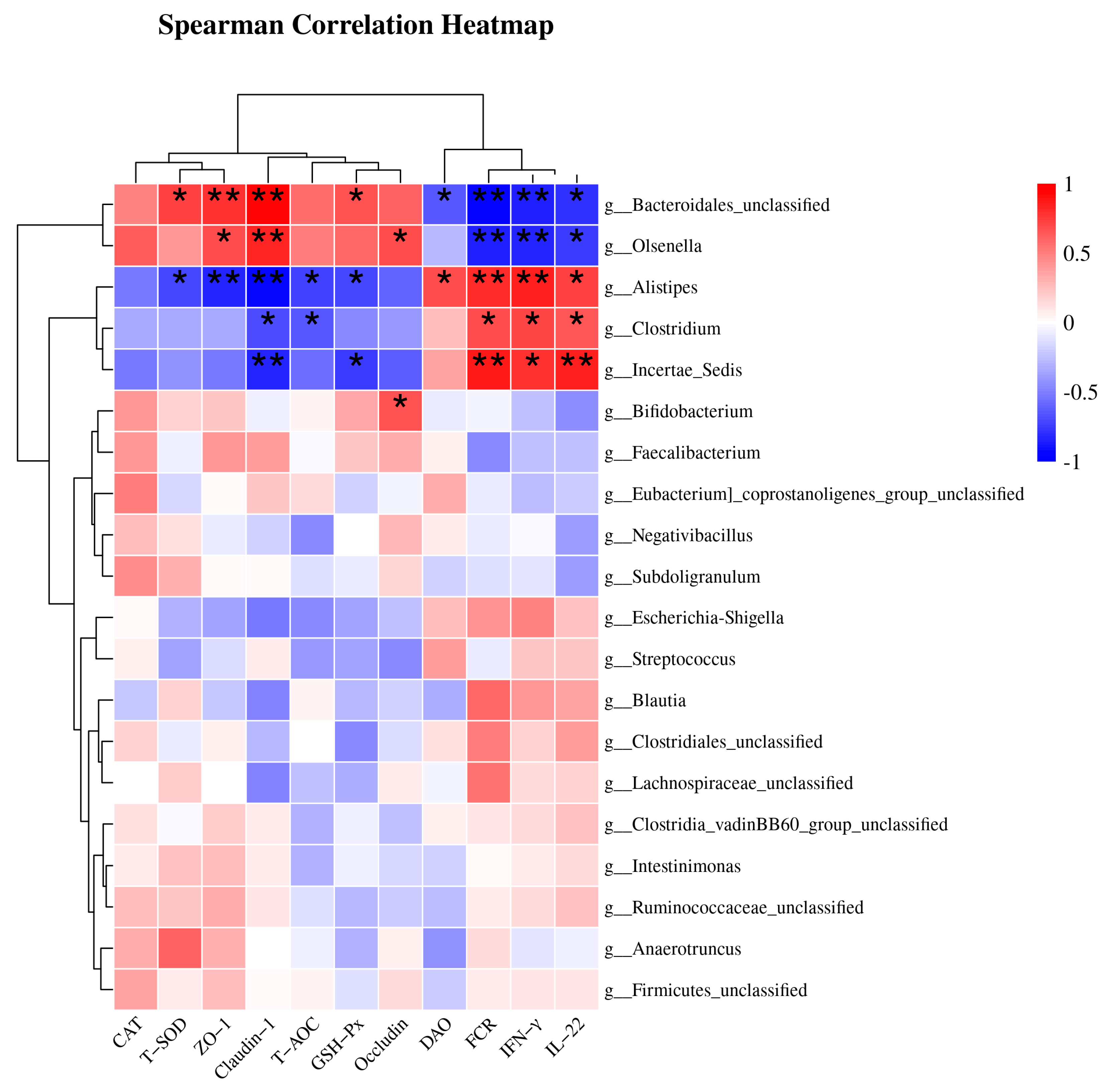
As shown in Figure 3, the top 20 most abundant species at the genus level were selected, and Spearman correlation analysis was performed between bacterial taxa and differential indicators to explore potential links, such as FCR, antioxidant parameters (CAT, T-SOD, T-AOC and GSH-Px), inflammation biomarkers (IFN-γ and IL-22), and intestinal mucosal barrier function (endotoxin, DAO, ZO-1, Occludin and Claudin-1). There were two distinct groups based on the strong correlation between the genera and the parameters. Group 1, including Bacteroidales_unclassified and Olsenella, was positively correlated with T-SOD, T-AOC, GSH-Px, ZO-1, Occludin, and Claudin-1, and was negatively correlated with FCR, DAO, IFN-γ, and IL-22. Group 2, including Clostridium, Alistipes, and Incertae_Sedis, was positively correlated with FCR, DAO, IFN-γ, and IL-22, and negatively correlated with T-SOD, T-AOC, GSH-Px, ZO-1, Occludin, and Claudin-1.
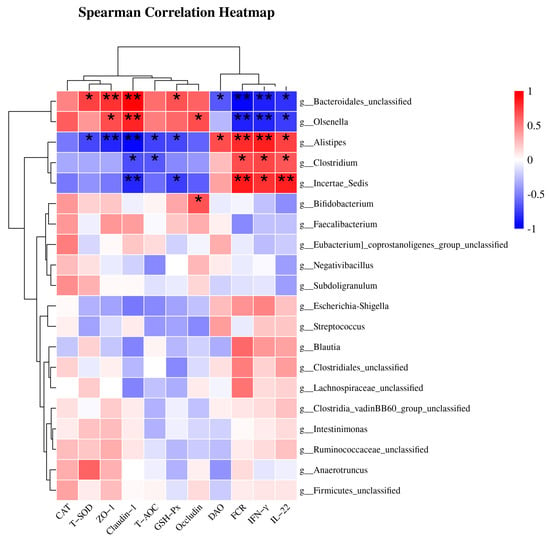
Figure 3.
This heatmap displays the results of Spearman correlation analysis between specific bacterial taxa, FCR, and biochemical parameters. Statistically significant associations (p < 0.05) were observed between all indicators and bacterial genera. The red blocks indicate positive correlations, while the blue blocks indicate negative correlations. The intensity of the colors reflects the strength of the correlations. * p < 0.05 and ** p < 0.01 indicate significant differences compared to the CON group; DAO, diamine oxidase; CON, control; BS, Bacillus subtilis HC6.
3.9. Predicted Microbial Functional Analysis by PICRUSt2
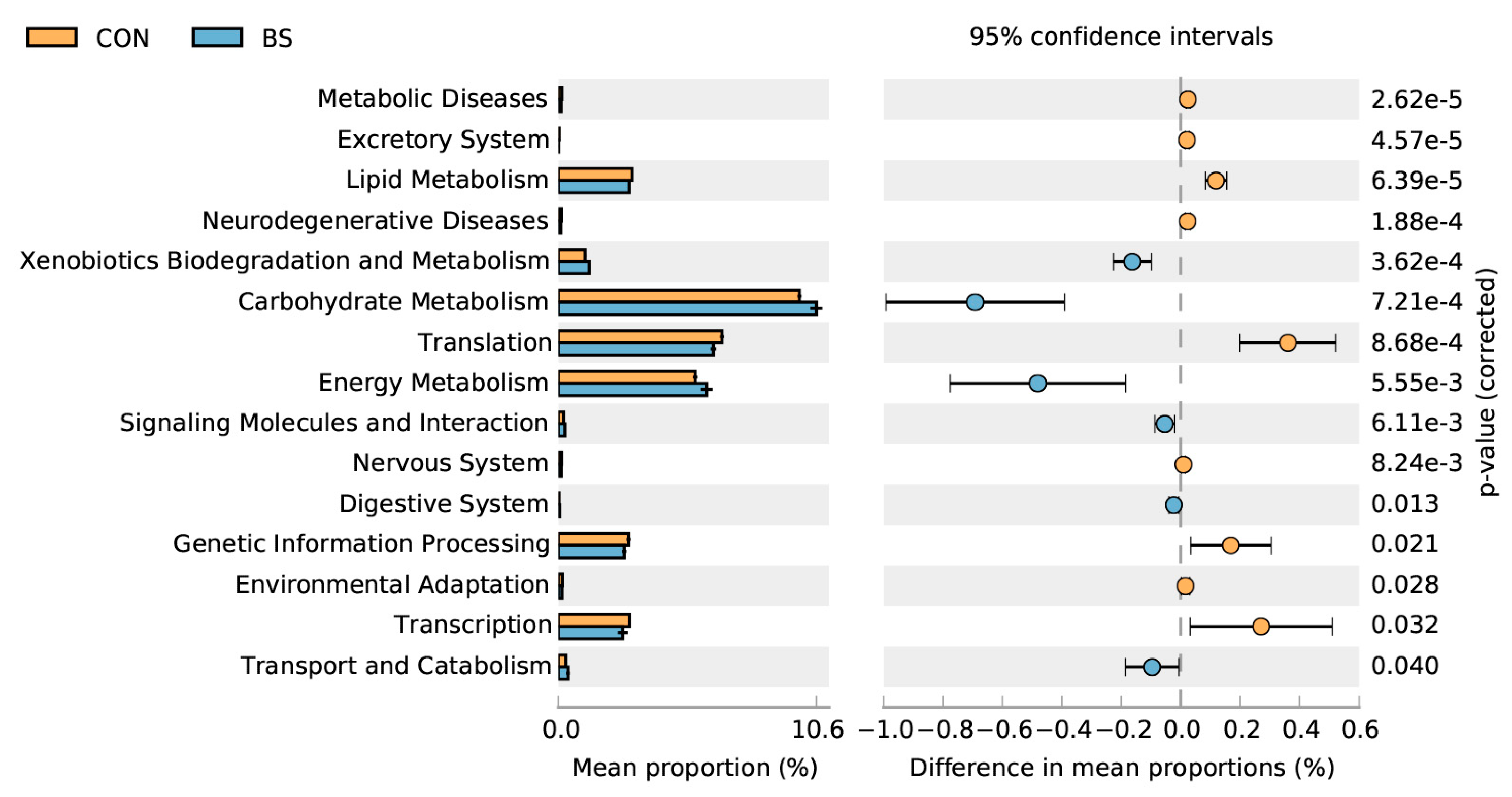
PICRUSt 2 microbial functional analysis results determined that BS treatment significantly (p < 0.05) enriched six metabolic pathways, including xenobiotics biodegradation and metabolism, carbohydrate metabolism, energy metabolism, signaling molecules and interaction, the digestive system, transport, and catabolism, and downregulated nine metabolic pathways, namely metabolic diseases, the excretory system, lipid metabolism, neurodegenerative diseases, translation, the nervous system, genetic information processing, environmental adaptation, and transcription.
4. Discussion
The feed conversion ratio (FCR), an extensively employed metric, reflects the efficiency of feed utilization and production benefits in animal husbandry. The results of this study showed that supplementation with BS increased the feed conversion rate from 21 to 50 days, and throughout the trial period. In line with our results, a previous study pointed out that the introduction of Bacillus TL to poultry feed resulted in a significant increase in broiler weight at 21 days and a decrease in feed conversion ratio [13]. Furthermore, Spearman correlation analysis also indicated that the genus Bacteroidales_unclassified was negatively correlated with FCR, indicating that Bacteroidales_unclassified could improve the efficiency of feed utilization. This may be ascribed to Bacteroides being frequently involved in the decomposition of polysaccharides, especially starch and glucan [14]. Taken together, these findings indicate that the administration of BS supplements can decrease FCR, potentially attributable to the influence on cecal microorganisms. Prior research has documented that dietary supplementation with B. subtilis probiotics did not affect broiler growth performance during the d 1 to 21 period; instead, it increased average daily gain (ADG) and feed intake, as well as decreased FCR from day 22 to day 42 compared to the control group [15]. In contrast, other studies reported that Bacillus amyloliquefaciens and Saccharomyces cerevisiae feed supplements did not significantly enhance growth performance at the age of nine weeks, but increased growth performance at the age of 11 weeks in red-feathered native chickens [16]. However, no significant changes in body weight or ADG were observed herein. This discrepancy could be ascribed to multiple factors, including differences in strains, age, breed, testing period, and experimental conditions.
Maintaining a proper balance of intestinal microorganisms is essential for ensuring the health and performance of animals at every stage of their growth [17]. While various segments of the gastrointestinal tract serve distinct functions in host organisms, the cecum houses the most heterogeneous microbial populations, which play a pivotal role in energy extraction and nutrient breakdown, ultimately influencing the intestinal well-being and growth efficiency of chickens [18,19]. Consistent with the findings of previous research, our results demonstrated that Firmicutes and Bacteroidota were the predominant bacterial phyla [20,21]. Compared with the CON group, the BS group increased the abundance of Bacteroidales_unclassified and Olsenell, while decreasing the abundance of Alistipes. Moreover, Lefse results showed that Bacteroides was the biomarker after BS addition at 21 d, and Bacteroidales_unclassified at 50 d (Figure 2D–F). Bacteroides undergo anaerobic respiration to generate acetic acid, isovaleric acid, and succinic acid, which are released into the colon to exert various metabolic effects [22]. Bacteroidaceae have the ability to stimulate cytokine production, and an abundance of Bacteroidaceae operational taxonomic units (OTUs) may be associated with elevated levels of peripheral cytokines [23]. Bacteroidales_unclassified is associated with multiple health-promoting mechanisms, including stimulating the production of SCFAs, regulating the immune system, and enhancing the growth of probiotic microorganisms [24]. These effects might be linked to the expression of ZO-1 and Claudin-1, which contribute to the integrity and stability of the intestinal mucosal barrier. In addition, Bacteroidale may stimulate the intestinal immune system and various important metabolic pathways by activating signaling pathways in intestinal epithelial cells [25], including mechanisms such as T-SOD and GSH-Px-related pathways. Bacteroidales can also reduce the number and activity of inflammatory cells in the intestinal tract, such as TH1 cells and macrophages, in order to minimize the synthesis of inflammatory mediators [26]. Furthermore, Alistipes infections are closely associated with intestinal barrier function and systemic inflammation [27]. This may account for the lower DAO activity in the BS group compared to that in the CON group. In summary, BS increased the abundance of Bacteroides and Olsenell, which possess the ability to synthesize metabolites such as short-chain fatty acids that can exert antioxidative effects and activate anti-inflammatory signaling pathways.
Moreover, the results showed that BS had no effect on the α-diversity index, but significantly improved the β-diversity index of cecal microbiota. The predictive microbial functional analysis of microbiota using PICRUSt2 indicated that there was an increase in xenobiotics biodegradation and metabolism, carbohydrate metabolism, energy metabolism, signaling molecules and interaction, the digestive system, transport, and catabolism in the BS group, implying improvements in the digestibility of carbohydrates and proteins, as well as a potential amelioration in the homeostatic mechanism (Figure 4). Of note, carbohydrate-metabolism-related pathways encompass a range of metabolic processes related to energy metabolism, such as those involving glycogen, glucose, disaccharides, pentose phosphate, and glycosaminoglycan [28]. The addition of live BS to the diet resulted in changes to the composition and diversity of the intestinal microbiota. Specifically, there was an increase in pathways related to carbohydrate metabolism and energy metabolism [29]. Altogether, these findings are consistent with the results of the current study.
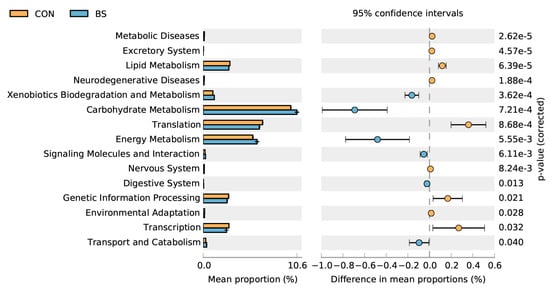
Figure 4.
Comparison of predicted pathway abundances on day 50 between two groups by statistical analysis of taxonomic and functional profiles (STAMP). CON, control; BS, Bacillus subtilis HC6.
In the small intestine, over 90% of nutrient digestion and absorption occur, underscoring the paramount importance of its epithelial integrity in sustaining gut health [30]. Villus height and crypt depth are known to play a vital role in nutrient absorption and digestion. Better feed digestion and nutrient absorption capability may also be indirectly related to the immunostimulatory and antioxidative effects of Bacillus strains, which can significantly improve the intestinal morphology of the intestinal tract. Moreover, the immune-modulatory action enhances the mucosal barrier integrity, and the intestinal mucosal structure increases the villus height and the villus height to crypt depth ratio [31,32]. Prior research has demonstrated that probiotics led to increases in villus height and the villus height to crypt depth ratio, and decreases in the crypt depth of broiler chicken [33,34]. Similarly, BS increased the villus height of the jejunum and ileum, as well as the ileal villus height to crypt depth ratio on day 50, but had no significant effect on intestinal morphology on day 21 (Table 4). We speculate that BS supplementation exerts a positive impact on nutrient absorption and digestion by increasing intestinal morphology, which is partly reflected by the lower FCR during periods of 21–50 d and 1–50 d.
The intestinal epithelial barrier plays a regulatory role in mucosal homeostasis and defense against foreign invasion [35]. Intestinal epithelial cells rely on tight junctions (TJs) and adhesion junctions (AJs) to execute their barrier function [36]. Impaired intestinal physical barrier function is characterized by increased intestinal permeability and the downregulated expression of AJs and TJs. Furthermore, the endotoxin level and DAO activity are positively correlated with intestinal permeability [37]. Noteworthily, elevated intestinal permeability can compromise the integrity of the intestinal barrier, facilitating the translocation of bacteria and other detrimental agents into the bloodstream, thereby inciting inflammation and other health complications. Our results showed that BS significantly reduced the endotoxin level on day 21 and decreased DAO activity on day 50, accompanied by upregulated mRNA expression levels of ZO-1, Occludin, and Claudin-1 in the jejunal mucosa (Figure 1). In agreement with our findings, some studies have found that supplementation with probiotics reduced the serum endotoxin level and DAO activity in mice and E. coli-infected birds [38,39]. Dietary supplementation with BL-S6 significantly elevated both the mRNA and protein levels of tight junction proteins (ZO-1 and Occludin) in the intestinal epithelial cells of weaned piglets [20]. Additionally, B. licheniformis was found to upregulate intestinal MUC-2 expression in chickens and pigs, emphasizing its role in fortifying the mucus layer as a chemical barrier against bacterial infections [40,41,42]. However, our results indicate that Bacillus subtilis does not affect MUC-2 expression, which is consistent with previous findings [43]. Moreover, Spearman correlation analysis also indicated that the genus Bacteroidales_unclassified was positively correlated with the mRNA expression levels of tight junction proteins ZO-1, Occludin, and Claudin-1, which are essential for preserving intestinal integrity and barrier function. Based on the results of this study, we can gain a better understanding of the beneficial effects of BS on the intestinal epithelial barrier function of chickens.
Immunosuppression in the poultry industry, attributed to factors such as over-crowding, stress, and pathogenic infections, can significantly compromise growth performance, decrease the quality of poultry products, and result in diminished economic returns [44,45,46]. Pro-inflammatory cytokines (such as IFN-γ and TNF-α) and anti-inflammatory cytokines (such as IL-10) assume central roles in bolstering cellular immunity and preserving immune homeostasis during the process of inflammation [47]. Hence, it is crucial to establish and sustain adequate immunological function to promote healthy growth in broilers [48]. Numerous studies have reported the ability of probiotics to modulate host immune function, and they have thus been extensively utilized in both animal and human populations to boost disease resistance capacity [49,50,51]. Our results revealed that BS supplementation increased IL-6 levels at 21 d, and concurrently decreased TNF-α at 50 d in serum, as well as upregulated the mRNA expression of IL-10, IL-22, and IFN-γ in the jejunal mucosa, collectively signifying that probiotic B. subtilis might serve as a promising immune modulator for mediating the intestinal immune function of broilers. Studies conducted on mice have shown that certain Bacillus strains possess immunomodulatory properties. In particular, B. subtilis strains (B. subtilis B10, B. subtilis BS02, and B. subtilis (natto) B4 spores) have been found to activate macrophages and induce the production of pro-inflammatory cytokines [52]. However, its efficacy may vary owing to the dynamic equilibrium of the immune system and other factors such as environment and nutrition. Furthermore, Spearman correlation analysis also indicated that the levels of IL-22 and IFN-γ were negatively correlated with the Bacteroidales_unclassified and Olsenella genera, but positively correlated with the Alistipes genus. This observation offers insights into the underlying rationale for the decrease in the proportion of immune-regulatory signaling molecules in the jejunal mucosa of 50-day-old broilers. Nevertheless, further research is warranted to comprehensively elucidate this phenomenon. Hence, these results suggest that BS supplementation modulates immune function in broilers by improving gut microbiota composition.
Apart from their immunomodulatory effect, certain Bacillus strains have been found to exhibit antioxidant activities, which are hypothesized to be facilitated through the synthesis of antioxidant enzymes that confer protection to the host against oxidative stress [53]. Among the antioxidant enzymes, Bacillus spp. are known to synthesize superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and glutathione reductase (GR). As is well documented, free radicals can damage the lipids in cell membranes and generate MDA, which affects polyunsaturated fatty acids found in biological cell membranes, further boosting lipid peroxidation, which in turn exacerbates oxidative stress [54]. Antioxidant levels reflect the body’s ability to scavenge oxygen-free radicals and maintain homeostasis. T-AOC is typically to estimate antioxidative potential, while SOD and GSH-Px are regarded as crucial enzymatic antioxidants. In addition, GSH acts as an essential nonenzymatic antioxidant that eliminates free radicals [55]. The present study revealed that the inclusion of Bacillus subtilis resulted in elevated levels of antioxidant biomarkers, namely T-AOC, T-SOD, CAT, and GSH-Px, in both serum and hepatic samples at two distinct stages. Earlier research outcomes parallel our results in that B. subtilis fmbJ can increase the levels of glutathione (GSH), GR, glutathione peroxidase (GSH-Px), and SOD activity in broiler serum and liver, leading to decreased mitochondrial ROS content in the liver [15]. Likewise, the findings of multiple studies are consistent with those of our trial [56,57]. Furthermore, Spearman correlation analysis also indicated that the abundance of the Bacteroidales_unclassified genus was positively correlated with T-SOD and GSH-Px activities, potentially accounting for why the higher antioxidant activity in the BS group was higher than that in the CON group. In summary, these outcomes signify that Bacillus may play a beneficial role in oxidative defense, owing to its inherent antioxidant activity.
5. Conclusions
The results of the present study suggested that dietary supplementation with 5 × 108 cfu/kg Bacillus subtilis HC6 improves the feed conversion ratio and antioxidant capacity in serum and liver, increases the levels of high-density lipoprotein and aspartate aminotransferase, and decreases the activities of diamine oxidase and endotoxin. Likewise, dietary Bacillus subtilis upregulated the mRNA expression of tight junction proteins in the jejunal mucosa, and changed the cecal microflora composition of broilers. Meanwhile, Spearman correlation analysis identified a correlation between cecal microbiota composition and FCR, serum differential metabolites or differential gene expression in the jejunal mucosa, which might be attributed to the increased Bacteroidales_unclassified and Olsenella abundance, and decreased Alistipes abundance in broilers. The aforementioned results offer novel insights into the utilization of Bacillus subtilis, specifically by placing emphasis on its influence on the composition, functional capacity, and diversity of gut microbiota. Further research endeavors are necessary to clarify the potential mechanisms underlying host-microbe interactions in metabolism and physiology, ultimately holding the potential to improve the overall performance and well-being of broilers.
Author Contributions
Conceptualization, L.G.; methodology, S.L. and G.X.; software, S.L., Q.W. and Q.Z.; investigation, Q.W., Q.Z. and J.T.; resources, W.L.; data curation, S.L., Q.W. and G.X.; writing—original draft preparation, S.L., L.G. and G.X.; writing—review and editing, S.L., L.G. and W.L.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515110819) and the National Natural Science Foundation of China (No. 32202718).
Institutional Review Board Statement
The experimental procedures were approved by the Animal Care and Use Committee of Foshan University (approval ID: FOSU#19-025).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, Y.; Li, Q.; Zeng, X.; Xu, Y.; Jin, K.; Liu, J.; Cao, G. Effects of Probiotics on the Growth Performance, Antioxidant Functions, Immune Responses, and Caecal Microbiota of Broilers Challenged by Lipopolysaccharide. Front. Vet. Sci. 2022, 9, 846649. [Google Scholar] [CrossRef]
- Tong, Z.; Lei, F.; Liu, L.; Wang, F.; Guo, A. Effects of Plotytarya strohilacea Sieb. et Zuce Tannin on the Growth Performance, Oxidation Resistance, Intestinal Morphology and Cecal Microbial Composition of Broilers. Front. Vet. Sci. 2021, 8, 806105. [Google Scholar] [CrossRef]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef]
- Park, I.; Lee, Y.; Goo, D.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.; Lillehoj, H.S. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020, 99, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.I.; Ahmad, N.; Miah, M.A. Comparative analysis of body weight and serum biochemistry in broilers supplemented with some selected probiotics and antibiotic growth promoters. J. Adv. Vet. Anim. Res. 2017, 4, 288–294. [Google Scholar] [CrossRef]
- Elleithy, E.M.M.; Bawish, B.M.; Kamel, S.; Ismael, E.; Bashir, D.W.; Hamza, D.; Fahmy, K.N.E. Influence of dietary Bacillus coagulans and/or Bacillus licheniformis-based probiotics on performance, gut health, gene expression, and litter quality of broiler chickens. Trop. Anim. Health Prod. 2023, 55, 38. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Wang, Y.; Li, W. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish. Physiol. Biochem. 2010, 36, 501–509. [Google Scholar] [CrossRef]
- Lee, S.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lokhande, A.; Kim, E.K.; Kwon, I.K.; Kim, Y.H.; Chae, B.J. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim. Feed. Sci. Technol. 2014, 188, 102–110. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Sun, W.; Bumbie, G.Z.; Elokil, A.A.; Mohammed, K.A.F.; Zebin, R.; Hu, P.; Wu, L.; Tang, Z. Feeding Bacillus subtilis ATCC19659 to Broiler Chickens Enhances Growth Performance and Immune Function by Modulating Intestinal Morphology and Cecum Microbiota. Front. Microbiol. 2021, 12, 798350. [Google Scholar] [CrossRef]
- Ahmat, M.; Cheng, J.; Abbas, Z.; Cheng, Q.; Fan, Z.; Ahmad, B.; Hou, M.; Osman, G.; Guo, H.; Wang, J. Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics 2021, 10, 1427. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Tang, L.; Zeng, Z.; Gong, L.; Wu, Y.; Li, W.F. Effects of Bacillus amyloliquefaciens Instead of Antibiotics on Growth Performance, Intestinal Health, and Intestinal Microbiota of Broilers. Front. Vet. Sci. 2021, 8, 679368. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Cheng, Y.; Guan, L.; Zhou, Z.; Li, X.; Shi, D.; Xiao, Y. Bacillus amyloliquefaciens TL Downregulates the Ileal Expression of Genes Involved in Immune Responses in Broiler Chickens to Improve Growth Performance. Microorganisms 2021, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, L.; Simon, O.; Vahjen, W. Isolation and identification of mixed linked β-glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-β-glucanase activities. J. Basic Microbiol. 2006, 46, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Qiang, H.; Zhang, J.; He, J.; Tian, W. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef]
- Lee, T.T.; Chou, C.H.; Wang, C.; Lu, H.Y.; Yang, W.Y. Bacillus amyloliquefaciens and Saccharomyces cerevisiae feed supplements improve growth performance and gut mucosal architecture with modulations on cecal microbiota in red-feathered native chickens. Anim. Biosci. 2022, 35, 869–883. [Google Scholar] [CrossRef]
- Trevisi, P.; Luise, D.; Correa, F.; Bosi, P. Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms 2021, 9, 313. [Google Scholar] [CrossRef]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef]
- Porter, N.T.; Luis, A.S.; Martens, E.C. Bacteroides thetaiotaomicron. Trends Microbiol. 2018, 26, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1902862. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shen, B.; Huang, R.; Liu, H.; Zhang, W.; Song, M.; Liu, K.; Lin, X.; Chen, S.; Liu, Y.; et al. Bacteroides fragilis strain ZY-312 promotes intestinal barrier integrity via upregulating the STAT3 pathway in a radiation-induced intestinal injury mouse model. Front. Nutr. 2022, 9, 1063699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xia, X.; Wang, P.; Chen, S.; Yu, C.; Huang, R.; Yang, L. Induction and Amelioration of Methotrexate-Induced Gastrointestinal Toxicity are Related to Immune Response and Gut Microbiota. EBioMedicine 2018, 33, 122–133. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e1416. [Google Scholar] [CrossRef]
- Choi, H.; Na, K.J. Pan-cancer analysis of tumor metabolic landscape associated with genomic alterations. Mol. Cancer 2018, 17, 150. [Google Scholar] [CrossRef]
- Zhu, C.; Gong, L.; Huang, K.; Li, F.; Tong, D.; Zhang, H. Effect of Heat-Inactivated Compound Probiotics on Growth Performance, Plasma Biochemical Indices, and Cecal Microbiome in Yellow-Feathered Broilers. Front. Microbiol. 2020, 11, 585623. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, J.; Xiao, X.; Wang, F.; Jin, M.; Fang, W.; Wang, Y.; Zong, X. Faecal microbiota transplantation-mediated jejunal microbiota changes halt high-fat diet-induced obesity in mice via retarding intestinal fat absorption. Microb. Biotechnol. 2022, 15, 337–352. [Google Scholar] [CrossRef]
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 52. [Google Scholar] [CrossRef]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets1. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef] [PubMed]
- Gunal, M.; Yayli, G.; Kaya, O.; Karahan, N.; Sulak, O. The Effects of Antibiotic Growth Promoter, Probiotic or Organic Acid Supplementation on Performance, Intestinal Microflora and Tissue of Broilers. Int. J. Poult. Sci. 2006, 5, 605–609. [Google Scholar] [CrossRef]
- Samli, H.E.; Senkoylu, N.; Koc, F.; Kanter, M.; Agma, A. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 2007, 61, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, I.; Abdulllah, N.; Azrin, N.M.; Ho, Y.W. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br. Poult. Sci. 2000, 41, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Xu, C.; Xie, X.; Jiang, S.; Peng, J. Maternal Soluble Fiber Diet during Pregnancy Changes the Intestinal Microbiota, Improves Growth Performance, and Reduces Intestinal Permeability in Piglets. Appl. Environ. Microbiol. 2018, 84, e01047-18. [Google Scholar] [CrossRef]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Zhang, M.; Wang, J.; Wang, S.; Ran, Y.; Ren, Z.; et al. Bacillus toyonensis SAU-19 and SAU-20 Isolated from Ageratina adenophora Alleviates the Intestinal Structure and Integrity Damage Associated with Gut Dysbiosis in Mice Fed High Fat Diet. Front. Microbiol. 2022, 13, 820236. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.F. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function in newly weaned MUC4 resistant pigs following Escherichia coli challenge. Appl. Environ. Microbiol. 2016, 83, AEM.02747-02716. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Lei, K.; Wang, B.; Li, W. Effects of Dietary Bacillus licheniformis on Gut Physical Barrier, Immunity, and Reproductive Hormones of Laying Hens. Probiotics Antimicrob. Proteins 2017, 9, 292–299. [Google Scholar] [CrossRef]
- Kan, L.; Guo, F.; Liu, Y.; Pham, V.H.; Guo, Y.; Wang, Z. Probiotics Bacillus licheniformis Improves Intestinal Health of Subclinical Necrotic Enteritis-Challenged Broilers. Front. Microbiol. 2021, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.M.; Podolsky, D.K. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G613. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M.; Karaca, K.; Pertile, T. Virus-induced immunosuppression in chickens. Poult. Sci. 1994, 73, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Huff, G.R.; Shini, A.; Kaiser, P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010, 115, 117–123. [Google Scholar] [CrossRef]
- Gomes, A.V.S.; Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Baskeville, E.; Akamine, A.T.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Brain Behav. Immun. 2014, 40, e30–e31. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Hsu, T.S.; Chang, Y.J.; Lai, M.Z. IL-6 receptor blockade corrects defects of XIAP-deficient regulatory T cells. Nat. Commun. 2018, 9, 463. [Google Scholar] [CrossRef]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef]
- Isolauri, E.; Sütas, Y.; Kankaanp??, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444S. [Google Scholar] [CrossRef]
- Borchers, A.T.; Selmi, C.; Meyers, F.J.; Keen, C.L.; Gershwin, M.E. Probiotics and immunity. J. Gastroenterol. 2009, 44, 26–46. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Q.; Mao, Y.; Cui, Z.; Li, W. Immunomodulatory effects of Bacillus subtilis (natto) B4 spores on murine macrophages. Microbiol. Immunol. 2012, 56, 817–824. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.F.; An, L.J.; Jiang, B.; Guan, S.; Bao, Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zeng, X.; Tan, F.; Li, W.; Li, C.; Song, Y.; Zhao, X. Effect of insect tea on D-galactose-induced oxidation in mice and its mechanisms. Food Sci. Nutr. 2019, 7, 4105–4115. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, D.; Wang, H.; Qing, X.; Sun, N.; Xin, J.; Luo, M.; Khalique, A.; Pan, K.; Shu, G.; et al. Dietary Probiotic Bacillus licheniformis H2 Enhanced Growth Performance, Morphology of Small Intestine and Liver, and Antioxidant Capacity of Broiler Chickens against Clostridium perfringens-Induced Subclinical Necrotic Enteritis. Probiotics Antimicrob. Proteins 2020, 12, 883–895. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).