Simple Summary
The harmful effects of microplastic (MP) exposure on aquatic animals have been extensively studied; however, there is a lack of research on its impact on poultry. To address this gap, the present study aimed to evaluate the effects of MP exposure on the growth performance and gut microbiota of chickens. The findings of the study revealed that MPs had a significant negative impact on the growth performance of chickens and can cause an imbalance in gut microbiota.
Abstract
As novel environmental contaminants, MPs exist widely in the environment and accumulate in organisms, which has become a global ecological problem. MP perturbations of organismal physiology and behavior have been extensively recorded in aquatic animals, but the potential effects of MPs on poultry are not well characterized. Here, we explored the adverse effects of MP exposure on the growth performance and gut microbiota of chickens. Results showed that the growth performance of chickens decreased significantly during MP exposure. Additionally, Firmicutes, Bacteroidota, and Proteobacteria were found to be dominant in the gut microbiota of MP-exposed chickens, regardless of health status. Although the types of dominant bacteria did not change, the abundances of some bacteria and the structure of the gut microbiota changed significantly. Compared with the controls, the alpha diversity of gut microbiota in chickens exposed to MPs showed a significant decrease. The results of comparative analyses of bacteria between groups showed that the levels of 1 phyla (Proteobacteria) and 18 genera dramatically decreased, whereas the levels of 1 phyla (Cyanobacteria) and 12 genera dramatically increased, during MP exposure. In summary, this study provides evidence that exposure to MPs has a significant impact on the growth performance and gut microbial composition and structure of chickens, leading to a gut microbial imbalance. This may raise widespread public concern about the health threat caused by MP contamination, which is relevant to the maintenance of environmental quality and protection of poultry health.
1. Introduction
The production of plastics has increased faster than any other material over the past few decades, and most plastics are eventually released into the environment [1,2]. Statistically, more than half of the plastics produced globally are used in non-recyclable containers, which inevitably cause serious plastic pollution [3]. These plastic products are broken down into MPs through various methods, such as UV-radiation, photodegradation, biodegradation, and mechanical abrasion [4]. MPs are listed as one of the four primary global environmental threats, in parallel with ocean acidification, climate change, and ozone depletion [5]. Land and oceans are the primary habitats of terrestrial and aquatic animals, respectively, and are the ultimate destination for plastics [5,6,7]. Notably, MPs are not only found in natural environments, such as soil, seawater, and freshwater, but are also detected in seafood and beverages, indicating the potential for MPs to be consumed by animals and humans via the food chain [8]. Previous investigations on the hazards of MPs have involved many species and revealed their negative impact on the health of host organisms. For instance, Jin et al. revealed that environmental MP exposure caused gut microbial dysbiosis in mice, accompanied by intestinal barrier dysfunction and disorders of amino acid and bile acid metabolism [9]. Moreover, recent studies on MPs demonstrated that they can lead to hepatic lipid metabolism disorder, kidney damage, and impaired quality of sperm and oocytes [10,11,12]. Although the harm of MP exposure to the environment and organisms has attracted considerable attention, the majority of current research is limited to model and aquatic animals [13]. However, research regarding the effects of MPs on poultry health remains limited [5,8].
As the main ingestion channel for MPs, the gut microbial community will inevitably be affected [14,15]. These microorganisms inhabit the intestines and play a crucial role in host growth and health, because the intestines are the major organs responsible for digestion and absorption [16,17]. Gut microbiota, which are microorganisms residing in the gut including bacteria, viruses, fungi, and protozoa, have been found to play a role in immunity, metabolism, and disease prevention [18,19]. However, gut microbiota is inevitably affected by both host- and environmental-related factors, including smoking, drinking, antibiotics, and host species [20,21,22,23]. In addition to the above-mentioned factors, environmental pollutants are an important factor that perturbs gut microbial homeostasis. Human activities, such as industrial and agricultural production, create a large amount of heavy metals, pesticides, and plastic products every year, which inevitably pollute the environment and threaten animal health [24,25,26,27,28,29]. Previous studies indicated that contaminants such as MPs found in the environment can accumulate in water and plants and can then be transferred to humans and animals by the food chain.
Broiler chickens are a vital source of global meat production [30,31]. Considering the importance of the broiler industry in the global diet, any factors that endanger the health of broilers should be given sufficient attention. However, the health of poultry may be affected by environmental MPs. Previous research reported the presence of MPs in broiler feces, which provided evidence for MP ingestion by poultry [32]. However, studies regarding the influence of environmental MP exposure on growth performance and gut microbial homeostasis in chickens remain scarce. Consequently, we hypothesized that MP exposure may affect the gut microbiota and growth performance of chickens.
2. Materials and Methods
2.1. Experimental Design
For the animal experiments, a cohort of 60 one-day-old chickens were obtained from a commercial feedlot (Jingzhou, China); these chickens were of similar weight and health status. Standard housing conditions and sufficient diet and water were provided to the chicks to ensure their growth. After three days of acclimatization, the chickens were evenly divided into control (CC) and MP-exposure (MC) groups. The chickens were raised in two cages, with 30 chickens per cage. The control chickens received a normal diet, while the treatment chickens were offered MPs (200 mg/kg) in addition to their normal diet. The MPs provided to chickens were acquired from the Duke Scientific Corporation (product ID CPMS-0.96; Palo Alto, CA, USA); their properties were reported in a previous study [1]. The whole experiment lasted for 28 days, and the dosage of MPs used was based on previous research [33,34]. After the experiment, the chickens were humanely euthanized, and the acquired cecal contents were promptly snap-frozen in liquid nitrogen to preserve their integrity for further analysis.
2.2. DNA Extraction and Illumine MiSeq Sequencing
The bacterial DNA was extracted from cecal contents of MC and CC groups using a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) based on the manufacturer’s recommendations. Afterward, 0.8% agarose gel electrophoresis and a UV–Vis spectrophotometer (NanoDrop 2000, Waltham, MA, USA) were used to evaluate the integrity and concentration of the extract, respectively. PCR amplification was performed using universal primers (338F: ACTCCTACGGGAGGCAGCA and 806R: GGACTACHVGGGTWTCTAAT) [18,21]. Following the manufacturer’s protocol, the purified products were used to construct sequencing libraries using Illumina TruSeq (Illumina, San Diego, CA, USA). The prepared libraries underwent further processing such as purification, quality control, and fluorescence quantification. The libraries that passed the quality inspection and displayed a single peak were considered qualified. Finally, the qualified libraries were diluted, denatured to single-stranded, and then subjected to 2 × 300 bp paired-end sequencing. To acquire the accurate data in subsequent bioinformatics analysis, the original sequences were preprocessed using QIIME software (Qiime1.9.1, Flagstaff, AZ, USA). Short sequences (<200 bp), mismatched primers, and chimera were removed. The effective reads were then clustered, and operational taxonomic units (OTUs) were partitioned with a 97% similarity threshold. We generated Venn diagrams to distinguish the number and distribution of OTUs in each group. Prior to performing the bacterial diversity analysis, rank abundance and rarefaction curves were constructed to investigate the sequencing depth. We calculated the microbial diversity of chicken gut microbiota by calculating Chao1, ACE, Shannon, and Simpson indices. To investigate the impact of MP on the gut microbiota of chickens, we generated PCoA plots to assess the gut microbial beta diversity. Differential taxa at different levels related to MP exposure were identified using Metastats analysis and LEfSe. The data are presented as mean ± standard error. Statistical significance was determined as a p value < 0.05.
3. Results
3.1. Growth Performance Analysis
The body weight and average daily weight gain of chickens in the MC group were significantly lower than those in the CC group (Figure 1), whereas there was no significant difference between the MC and CC groups in average daily feed intake.
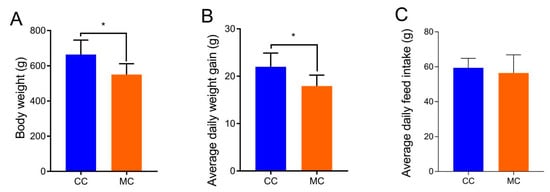
Figure 1.
Effects of MPs on growth performance (A), average daily weight gain (B), and average daily feed intake (C) of chickens. All data were represented as means ± SD. * p < 0.05.
3.2. Data Analysis
In this study, we analyzed 16 cecum samples to compare and investigate changes in the gut microbiota of chickens during MP exposure. We obtained a total of 1,279,763 (CC = 640,238, MC = 639,525) raw sequences, with each sample containing varying raw reads ranging from 79,557 to 80,523 (Table 1). There were 927,938 (CC = 473,198, MC = 454,740) valid sequences in the CC and MC groups after quality evaluation. The rarefaction and rank abundance curves demonstrated a saturation trend, suggesting that further increasing the sequencing depth is unnecessary as almost all bacterial species have already been detected (Figure 2A–C). Following taxonomic assignment, these valid sequences were recognized as 627 (CC = 547, MC = 558) OTUs, with the common OTUs in both the CC and MC groups being 100 (Figure 2D). Furthermore, the numbers of unique OTUs in the CC and MC groups were 69 and 80, respectively. Moreover, the number of OTUs in each sample ranged from 189 to 313 (Figure 2E). Among the samples, CC1 had the highest quantity of OTUs, while MC8 had the lowest.

Table 1.
Analysis of gut microbial sequence of chickens exposed to MP.
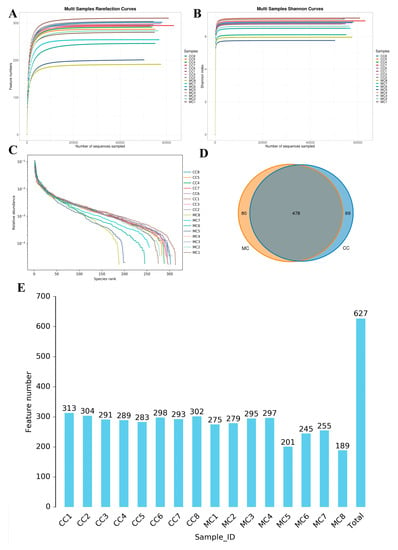
Figure 2.
Sequencing depth assessment and OTUs statistics. Sequencing depth and uniformity are assessed by rarefaction (A,B) and rank (C) abundance curves. (D): Venn diagram displays the number of shared and individual OTUs in the control and MP-exposed groups. (E): Histogram showing the number of OTUs per sample in the control and MP-exposed groups.
3.3. Significant Changes in the Gut Microbial Diversity Related to MP Exposure
Good’s coverage estimate in each sample was more than 99%, indicating that almost all bacteria could be covered. In addition, the Chao1 (297.06 ± 9.63 versus 255.06 ± 40.38, p = 0.013) and ACE (296.82 ± 9.61 versus 254.89 ± 40.66, p = 0.013) indices were significantly different between the CC and MC groups, while the Simpson (0.98 ± 0.0057 versus 0.97 ± 0.010, p = 0.20) and Shannon (6.86 ± 0.19 versus 6.48 ± 0.50, p = 0.072) indices were not statistically different (Figure 3A–D). The results of alpha diversity analysis showed that the abundance of gut microbiota in chickens decreased significantly during MP exposure, while the diversity of gut microbiota did not show a significant change. Additionally, the samples from both groups were clearly separated, suggesting significant differences in the major components of the gut microbiota (Figure 3E,F). These results demonstrate that MP exposure strongly affects the gut microbial alpha and beta diversities in chickens.
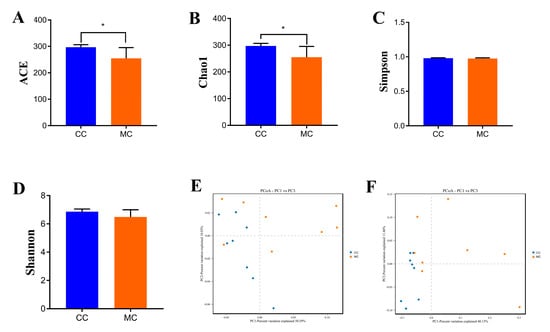
Figure 3.
MP exposure altered the alpha and beta diversities of gut microbiota in chickens. Alpha diversity could be represented by the ACE (A), Chao1 (B), Simpson (C), and Shannon (D) indices. Beta diversity could be represented by the PCoA scatterplots (E,F). CC: control group. MC: MP-exposed groups. All data were represented as mean ± SD. * p < 0.05.
3.4. Analysis of Gut Microbiota Composition Associated with MP Exposure
To investigate the impact of MP exposure on the gut microbiota, we characterized the compositions and changes of dominant bacterial phyla and genera. Results indicated that a total of 8 phyla and 124 genera were identified, varying from 5 to 8 phyla and from 70 to 99 genera per sample, respectively (Table 2). Specifically, the gut microbiota in CC and MC groups was predominated by Firmicutes (71.74% and 66.89%), Bacteroidota (23.94% and 26.08%), and Proteobacteria (3.17% and 5.90%) in descending order (Figure 4A). These three dominant phyla accounted for approximately 98% of the total bacterial composition. Other phyla such as Actinobacteriota (0.47% and 0.77%), Desulfobacterota (0.36% and 0.24%), Cyanobacteria (0.19% and 0.06%), unclassified_Bacteria (0.10% and 0.024%), and Patescibacteria (0.0011% and 0.00%) were represented with a lower abundance. Moreover, the dominant genera observed in gut microbiota in the CC group were Bacteroides (23.83%), unclassified_Lachnospiraceae (8.34%), unclassified_Oscillospiraceae (8.03%), and unclassified_Clostridia_UCG_014 (5.43%), whereas Bacteroides (25.46%), unclassified_Oscillospiraceae (7.24%), unclassified_Lachnospiraceae (6.97%), and Fournierella (6.80%) were abundantly present in the MC group (Figure 3B). Additionally, we visualized the clustering heat map to observe the differences in bacterial distribution and variation between the two groups (Figure 4C).

Table 2.
Species statistics at different taxonomic levels of samples.
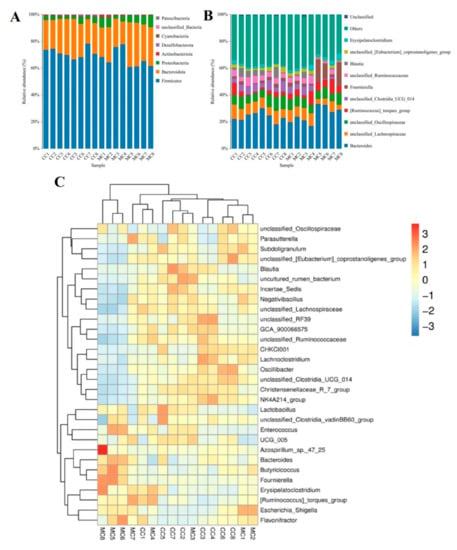
Figure 4.
The relative proportions of dominant bacteria in different taxonomical levels. (A): Dominant bacterial phyla. (B): Dominant bacterial genera. (C): The clustering heatmap was used to visualize the distribution and variability of gut microbiota.
Metastats analysis was used to identify the differential bacteria at different taxonomic levels between CC and MC groups (Table 3). Compared to the controls, the chickens exposed to MPs showed a significant increase in the abundance of Proteobacteria and a decrease in Cyanobacteria. Moreover, we also found significant changes in 30 bacterial genera with MP exposure. Among them, the relative abundances of 12 genera (Aerosphaera, Facklamia, Vagococcus, unclassified_Comamonadaceae, Bifidobacterium, Escherichia_Shigella, unclassified_Butyricicoccaceae, Sellimonas, Tyzzerella, Fournierella, Butyricicoccus, and Ruminococcus_torques_group) significantly increased. In contrast, the levels of 18 genera (unclassified_Mitochondria, Christensenellaceae_R_7_group, unclassified_UCG_010, unclassified_Anaerovoracaceae, NK4A214_group, Jeotgalibaca, Novosphingobium, Oscillibacter, unclassified_Desulfovibrionaceae, Blautia, Family_XIII_AD3011_group, Rikenella, unclassified_Oscillospirales, UCG_009, Brevibacterium, unclassified_Clostridia_UCG_014, CHKCI001, and unclassified_Hydrogenoanaerobacterium) significantly decreased with exposure to MPs. Notably, MP exposure can lead to the disappearance of some bacterial genera such as unclassified_Mitochondria, Jeotgalibaca, and Novosphingobium in the gut microbiota. Meanwhile, we also used LEfSe analysis to comprehensively identify differential taxa associated with MP exposure (Figure 5A,B). In addition to the differential taxa mentioned above, we also found that Candidatus_Soleaferrea and uncultured_rumen_bacterium was significantly overrepresented in the CC group, while Ruminococcus_torques_group and Erysipelatoclostridium were the most preponderant in the MC group.

Table 3.
The bacterial taxa with statistical differences were identified through the Metastats analysis. All the data were represented as mean ± SD.
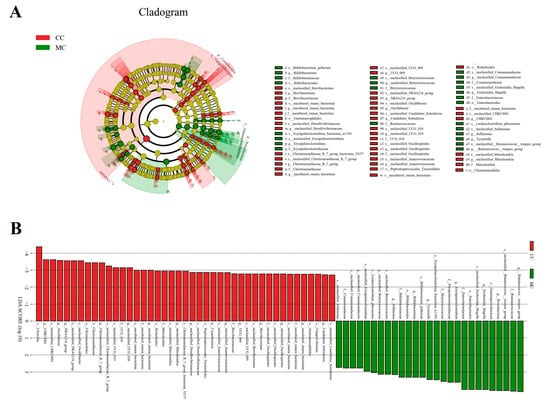
Figure 5.
The taxa with significant differences in CC and MC were identified using LEfSe. (A): Cladogram showing phylogenetic distribution of differential taxa. (B): The criterion of differential taxa was LDA scores > 2.
3.5. Correlation Network Analysis
Blautia was negatively related to Butyricicoccus (0.80) but positively associated with Christensenellaceae_R_7_group (0.82) and Angelakisella (0.7918) (Figure 6). Christensenellaceae_R_7_group was negatively related to Sellimonas (0.88) and Bifidobacterium (0.78).
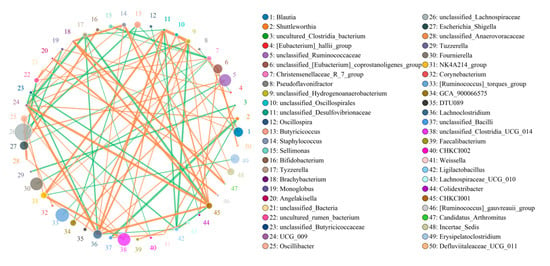
Figure 6.
Correlation analysis among bacterial genera. The correlation between bacteria is visualized by the network diagram and the strength of correlation is determined based on the color and thickness of the line. The green line represents a positive correlation, whereas the orange line indicates a negative correlation.
4. Discussion
The plastic product industry has experienced explosive growth over the past few decades owing to rapid economic development and urban expansion involving many fields of industrial and agricultural production and human life [35]. However, the environmental pollution problems and increased cost of environmental governance caused by the excessive use of plastic products have attracted mounting attention [36,37]. It should be noted that a considerable part of plastic products cannot be recycled but are processed through incineration, deep burial, and discarding which eventually enter the environment and degrade into MPs. The threat of MPs to public health and the health of the animals in husbandry industry has become a prominent issue of concern to many countries and governments. There have been reports on aquatic animals, seabirds, and waterfowl containing MPs, revealing their negative impact on host health [38,39]. The gut microbiota, as the monitor and executor of intestinal function, is inevitably affected by external factors, but information regarding the impacts of MP exposure on gut microbiota in chickens has been scarce. Therefore, we investigated the effects of MP exposure on growth performance and gut microbiota in chickens.
The gut microbiota is naturally stable because of the interaction and plasticity of the microbial community [40]. However, some factors, especially MPs, can disturb the intestinal environment and affect the survival of the microbiota [41]. Under such circumstances, the abundance or type of microorganisms may change to adapt to new intestinal environment, which may lead to the disruption of gut microbial homeostasis. Deng et al. indicated that MP exposure can cause gut microbiota dysbiosis in mice accompanied by metabolic disturbances, increased intestinal permeability, and increased inflammation [42]. Similarly, Sun et al. showed that MP exposure resulted in decreased colonic mucin production, inflammatory responses, and gut microbiota dysbiosis [1]. The indices representing the diversity and abundance include Chao1, ACE, Shannon, and Simpson, which can be used to assess gut microbial homeostasis [43]. Consistent with previous studies, we observed that MP exposure could decrease the Chao1 and ACE indices of gut microbiota in chickens, indicating that MP exposure can decrease gut microbial abundance and induce gut microbial dysbiosis [44]. Maintaining gut microbial homeostasis is crucial for the proper functioning of the intestine, including tasks such as food digestion, nutrient absorption, immune function, and barrier function [45]. However, the perturbation of gut microbial homeostasis may cause various pathological consequences such as intestinal diarrhea, increased intestinal permeability, and metabolic disorders [46,47]. Recent research on gut microbial homeostasis has also revealed its role in the development of diabetes, hypertension, and fatty liver [48]. Therefore, MPs may further cause potential harm to host metabolism, immunity, intestinal function, and health by affecting the homeostasis of gut microbiota. Meanwhile, this may also be one of the reasons for the decreasing growth performance of chickens during exposure to MPs. In addition, we observed significant changes in the major components of the gut microbiota between both the groups. These results demonstrate that gut microbial homeostasis is strongly influenced by MPs.
This study indicated that Firmicutes, Bacteroides, and Proteobacteria were abundant in the gut microbiota of chickens regardless of treatment. These bacteria were demonstrated to be the core components of gut microbiota, which are also abundantly present in ducks, geese, cattle, and pigs [49]. Although the types of dominant phyla did not change, the abundance of some dominant phyla changed dramatically during MP exposure. Proteobacteria, composed of a great deal of Gram-negative bacteria, is the largest phylum in the gut microbiota. Remarkably, some members of Proteobacteria were considered as pathogenic bacteria and opportunistic pathogens, which may seriously threaten host health [50]. In this study, the abundance of Proteobacteria was significantly increased during MP exposure. Thus, MP exposure may result in an increased risk of intestinal disease and other complications in chickens. Previous investigations indicated that environmental MP exposure could significantly change microbial composition and structure [51]. Similarly, the present research also observed significant shifts in gut microbiota of chickens exposed to MPs. Moreover, some significantly changed taxa were regarded as intestinal functional bacteria, which may play crucial roles in intestinal health and homeostasis. Christensenellaceae was considered a potentially beneficial bacterium because of the positive regulation of the hydrolytic enzyme production and intestinal environment [52]. Moreover, Christensenellaceae has been demonstrated to be negatively related to metabolic syndrome, inflammatory bowel disease, and fatty deposits [53]. Notably, some quantitatively decreased bacteria such as Oscillibacter and Blautia were potential producers of short-chain fatty acids (SCFAs). SCFAs have long been deemed as beneficial metabolites due to their vital role in preventing the colonization of pathogens and reducing oxidative stress [54]. Moreover, SCFAs have been shown to possess multiple important biological characteristics such as lowering cholesterol, regulating energy intake, and alleviating inflammation [55,56,57]. Recent investigations on SCFAs also demonstrated their positive impacts in cell proliferation, gut microbial homeostasis, and intestinal barrier function [58,59,60]. Consistent with the current study, MP exposure has also been previously reported to result in a decrease in SCFA-producing bacteria [43]. Importantly, we also found that MP exposure could increase the levels of some pathogenic bacteria, such as Facklamia and Escherichia_Shigella. Facklamia was previously demonstrated to participate in the development of invasive disease such as septicemia and meningitis [61]. Escherichia_Shigella is a potentially pathogenic bacterium associated with increased risk of intestinal infections [62]. Moreover, recently published research about Tyzzerella also indicated that it could drive the development of cardiovascular disease [63]. These bacteria have been demonstrated to play vital role in the balance of gut microbiota. Thus, we speculated that MPs may further affect gut microbial homeostasis by changing these bacteria.
It is well-established that the gut microbiota is a complex micro-ecosystem involving 1014 micro-organisms, approximately ten times the total quantity of body cells [18]. These microorganisms could interact synergistically or antagonistically to maintain gut microbial homeostasis [64]. Consequently, some changed bacteria may directly or indirectly affect the other bacterial functions, thereby further accelerating gut microbial dysbiosis. In this study, we found significant correlations between some bacteria which may be critical for gut homeostasis. This suggests that MP exposure not only directly affects the microbial composition and structure but also indirectly changes the gut microbiota through the microbial interactions, which may further affect gut microbial homeostasis and amplify the toxic effects of MPs.
5. Conclusions
In summary, the results of this research support our hypothesis that MP exposure can reduce the growth performance of chickens. Moreover, it also resulted in distinct shifts in gut microbial composition and diversity of chickens. This research is an important exploration of MP exposure on the gut health of farmed animals, suggesting that the imbalance of gut microbiota may be one of the important ways in which MPs lead to ill health.
Author Contributions
Conceptualization: W.Z. and K.L.; resources: K.L.; original draft: W.Z.; writing the manuscript: W.Z.; investigation and formal analysis: S.L., J.W., Y.X., M.A.S., M.U.S. and K.M. project administration, review and editing: S.L., J.W., Y.X., M.A.S., M.U.S. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Start-up fund of Nanjing Agricultural University (804131).
Institutional Review Board Statement
The animal study was reviewed and approved by the Ethics Committee of the Nanjing Agricultural University (NJAU. No20230413054).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original sequence data were submitted to the Sequence Read Archive (SRA) (NCBI, USA) with the accession no. PRJNA954763.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotox. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Harris, P.T.; Westerveld, L.; Nyberg, B.; Maes, T.; Macmillan-Lawler, M.; Appelquist, L.R. Exposure of coastal environments to river-sourced plastic pollution. Sci. Total Environ. 2021, 769, 145222. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Zhang, Q.; Li, Y.; Tan, S.; Li, D.; Yang, Z.; Wang, J. Cetaceans and microplastics: First report of microplastic ingestion by a coastal delphinid, Sousa chinensis. Sci. Total Environ. 2019, 659, 649–654. [Google Scholar] [CrossRef]
- Geng, X.; Wang, J.; Zhang, Y.; Jiang, Y. How do microplastics affect the marine microbial loop? Predation of microplastics by microzooplankton. Sci. Total Environ. 2021, 758, 144030. [Google Scholar] [CrossRef]
- Woodall, L.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.; Narayanaswamy, B.; Thompson, R. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef]
- Rehman, T.; Naz, S.; Hussain, R.; Chatha, A.M.M.; Ahmad, F.; Yamin, A.; Akram, R.; Naz, H.; Shaheen, A. Exposure to heavy metals causes histopathological changes and alters antioxidant enzymes in fresh water fish (Oreochromis niloticus). Asian J. Agric. Biol. 2021, 2021. [Google Scholar] [CrossRef]
- Sucharitakul, P.; Pitt, K.A.; Welsh, D.T. Assessment of microplastics in discharged treated wastewater and the utility of Chrysaora pentastoma medusae as bioindicators of microplastics. Sci. Total Environ. 2021, 790, 148076. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Yuan, Y.; Qin, Y.; Wang, M.; Xu, W.; Chen, Y.; Zheng, L.; Chen, W.; Luo, T. Microplastics from agricultural plastic mulch films: A mini-review of their impacts on the animal reproductive system. Ecotox. Environ. Saf. 2022, 244, 114030. [Google Scholar] [CrossRef]
- Xiong, X.; Gao, L.; Chen, C.; Zhu, K.; Luo, P.; Li, L. The microplastics exposure induce the kidney injury in mice revealed by RNA-seq. Ecotoxicol. Environ. Saf. 2023, 256, 114821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Y.; Zhang, W.; Shen, T.; Li, H.; Wu, J.; Zhang, L.; Qin, L.; Chen, R.; Gu, W.; et al. Lipidomics and transcriptomics insight into impacts of microplastics exposure on hepatic lipid metabolism in mice. Chemosphere 2022, 308, 136591. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jin, Y.; Luo, P.; Shi, X. Polystyrene microplastics induced male reproductive toxicity and transgenerational effects in freshwater prawn. Sci. Total Environ. 2022, 842, 156820. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, W.; Tian, H.; Zhang, K.; Li, B.; Du, Z.; Zhang, W.; Wang, J.; Wang, J.; Zhu, L. The effects of high-density polyethylene and polypropylene microplastics on the soil and earthworm Metaphire guillelmi gut microbiota. Chemosphere 2021, 267, 129219. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Y.; Yi, J.; Li, Y.; Li, Q.; Li, Y.; Shang, P.; Guo, J.; Hu, L.; Pan, J.; et al. Gut microbiota disturbance exaggerates battery waste water-induced hepatotoxicity through a gut-liver axis. Sci. Total Environ. 2022, 809, 152188. [Google Scholar] [CrossRef]
- Morris, A. Gut microbiota: Fibre restores healthy gut microbiota. Nat. Rev. Endocrinol. 2018, 14, 63. [Google Scholar] [CrossRef]
- Li, A.; Liu, B.; Li, F.; He, Y.; Wang, L.; Fakhar-E-Alam, K.M.; Li, H.; Fu, Y.; Zhu, H.; Wang, Y.; et al. Integrated Bacterial and Fungal Diversity Analysis Reveals the Gut Microbial Alterations in Diarrheic Giraffes. Front. Microbiol. 2021, 12, 712092. [Google Scholar] [CrossRef]
- Shi, X.; Xu, W.; Che, X.; Cui, J.; Shang, X.; Teng, X.; Jia, Z. Effect of arsenic stress on the intestinal structural integrity and intestinal flora abundance of Cyprinus carpio. Front. Microbiol. 2023, 14, 1179397. [Google Scholar] [CrossRef]
- Shang, X.; Xu, W.; Zhang, Y.; Sun, Q.; Li, Z.; Geng, L.; Teng, X. Transcriptome analysis revealed the mechanism of Luciobarbus capito (L. capito) adapting high salinity: Antioxidant capacity, heat shock proteins, immunity. Mar. Pollut. Bull. 2023, 192, 115017. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; He, Y.; Liu, B.; Iqbal, M.; Mehmood, K.; Jamil, T.; Chang, Y.F.; Hu, L.; Li, Y.; et al. Environmental fluoride exposure disrupts the intestinal structure and gut microbial composition in ducks. Chemosphere 2021, 277, 130222. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Ainonen, S.; Tejesvi, M.V.; Mahmud, M.R.; Paalanne, N.; Pokka, T.; Li, W.; Nelson, K.E.; Salo, J.; Renko, M.; Vanni, P.; et al. Antibiotics at birth and later antibiotic courses: Effects on gut microbiota. Pediatr. Res. 2022, 91, 154–162. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Iqbal, M.; Tang, Z.; Zhang, H. Thiram exposure in environment: A critical review on cytotoxicity. Chemosphere 2022, 295, 133928. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, X.; Hao, Z.; Yu, M.; Tang, Y.; Teng, X.; Sun, W.; Kang, L. Cadmium exposure caused cardiotoxicity in common carps (Cyprinus carpio L.): miR-9-5p, oxidative stress, energetic impairment, mitochondrial division/fusion imbalance, inflammation, and autophagy. Fish Shellfish Immun. 2023, 138, 108853. [Google Scholar] [CrossRef]
- Zhao, C.; Teng, X.; Yue, W.; Suo, A.; Zhou, W.; Ding, D. The effect of acute toxicity from tributyltin on Liza haematocheila liver: Energy metabolic disturbance, oxidative stress, and apoptosis. Aquat. Toxicol. 2023, 258, 106506. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish. Immun. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Zhong, G.L.; Wan, F.; Lan, J.; Jiang, X.X.; Wu, S.F.; Pan, J.Q.; Tang, Z.X.; Hu, L.M. Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci. Total Environ. 2021, 788, 147780. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Liu, J.; Deng, Y. Dietary High Fluorine Alters Intestinal Microbiota in Broiler Chickens. Biol. Trace Elem. Res. 2016, 173, 483–491. [Google Scholar] [CrossRef]
- Mohamed, H.; Atta, A.; Darwish, A.; Atef, M. Effect of Probiotics on the Pharmacokinetic Aspects and Tissue Residues of Difloxacin in Broiler Chickens. Pak. Vet. J. 2021, 41, 269–273. [Google Scholar] [CrossRef]
- Zain, U.; Muhammad, T.; Farzana, R.; Sajjad, U. Salutary Effects of anti-Clostridium perfringens Type A Egg Yolk Antibodies (IgY) on Growth Performance and Hemato-Biochemical Parameters in Experimentally Infected Broiler Chicken. Pak. Vet. J. 2021, 41, 562–566. [Google Scholar] [CrossRef]
- Wu, R.T.; Cai, Y.F.; Chen, Y.X.; Yang, Y.W.; Xing, S.C.; Liao, X.D. Occurrence of microplastic in livestock and poultry manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Bonyadinejad, G.; Salehi, M.; Herath, A. Investigating the sustainability of agricultural plastic products, combined influence of polymer characteristics and environmental conditions on microplastics aging. Sci. Total Environ. 2022, 839, 156385. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Behera, S.; Das, S. Environmental impacts of microplastic and role of plastisphere microbes in the biodegradation and upcycling of microplastic. Chemosphere 2023, 334, 138928. [Google Scholar] [CrossRef]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yang, C.; Zhang, T.; Tang, C.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef]
- Li, A.Y.; Wang, Y.L.; Hao, J.Y.; Wang, L.; Quan, L.T.; Duan, K.; Kulyar, M.; Ullah, K.; Zhang, J.B.; Wu, Y.; et al. Long-term hexavalent chromium exposure disturbs the gut microbial homeostasis of chickens. Ecotox. Environ. Saf. 2022, 237, 113532. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Li, A.; An, Z.; Li, C.; Cui, X.; Li, K.; Zhou, H.; Zhou, B.; Hao, P.; Kulyar, M.; Yin, W.; et al. Salt-contaminated water exposure induces gut microbial dysbiosis in chickens. Ecotox. Environ. Saf. 2023, 254, 114731. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.L.; Tian, F.W.; Zhai, Q.X.; Fan, L.P.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef]
- Liu, C.S.; Liang, X.; Wei, X.H.; Jin, Z.; Chen, F.L.; Tang, Q.F.; Tan, X.M. Gegen Qinlian Decoction Treats Diarrhea in Piglets by Modulating Gut Microbiota and Short-Chain Fatty Acids. Front. Microbiol. 2019, 10, 825. [Google Scholar] [CrossRef]
- Shen, L.; Shen, Y.; You, L.; Zhang, Y.; Su, Z.; Peng, G.; Deng, J.; Zuo, Z.; Zhong, Z.; Ren, Z.; et al. Pueraria lobata polysaccharides alleviate neonatal calf diarrhea by modulating gut microbiota and metabolites. Front. Vet. Sci. 2022, 9, 1024392. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, J.; Zhu, H.; Chen, Z.Y. Metabolites of Gut Microbiota and Possible Implication in Development of Diabetes Mellitus. J. Agr. Food Chem. 2022, 70, 5945–5960. [Google Scholar] [CrossRef]
- Li, A.; Ding, J.; Shen, T.; Han, Z.; Zhang, J.; Abadeen, Z.U.; Kulyar, M.F.; Wang, X.; Li, K. Environmental hexavalent chromium exposure induces gut microbial dysbiosis in chickens. Ecotox. Environ. Saf. 2021, 227, 112871. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Baumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Jiang, P.; Yuan, G.H.; Jiang, B.R.; Zhang, J.Y.; Wang, Y.Q.; Lv, H.J.; Zhang, Z.; Wu, J.L.; Wu, Q.; Li, L. Effects of microplastics (MPs) and tributyltin (TBT) alone and in combination on bile acids and gut microbiota crosstalk in mice. Ecotox. Environ. Saf. 2021, 220, 112345. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Chai, Z.; Zhang, C.; Zhang, Q.; Zhu, Y.; Cao, H.; Zhong, J.; Ji, Q. Comparing the Microbial Community in Four Stomach of Dairy Cattle, Yellow Cattle and Three Yak Herds in Qinghai-Tibetan Plateau. Front. Microbiol. 2019, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; He, Y.; Li, P.; Zhou, H.; Zeng, N. Regional distribution of Christensenellaceae and its associations with metabolic syndrome based on a population-level analysis. Peerj 2020, 8, e9591. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agr. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef]
- Carretta, M.D.; Quiroga, J.; Lopez, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef]
- Wu, D.; Ding, L.; Tang, X.; Wang, W.; Chen, Y.; Zhang, T. Baicalin Protects Against Hypertension-Associated Intestinal Barrier Impairment in Part Through Enhanced Microbial Production of Short-Chain Fatty Acids. Front. Pharmacol. 2019, 10, 1271. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Gahl, M.; Stockli, T.; Fahrner, R. Facklamia hominis bacteremia after transurethral resection of the prostate: A case report. BMC Urol. 2020, 20, 192. [Google Scholar] [CrossRef]
- Pang, R.; Wang, J.; Xiong, Y.; Liu, J.; Ma, X.; Gou, X.; He, X.; Cheng, C.; Wang, W.; Zheng, J.; et al. Relationship between gut microbiota and lymphocyte subsets in Chinese Han patients with spinal cord injury. Front. Microbiol. 2022, 13, 986480. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates with Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhang, W.; Kulyar, M.F.; Ullah, K.; Han, Z.; Qin, J.; Bi, C.; Wang, Y.; Li, K. Comparative analysis of gut microbiota in healthy and diarrheic yaks. Microb. Cell. Fact. 2022, 21, 111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).