Simple Summary
Sarcophaga crassipalpis Macquart, 1839 (Diptera: Sarcophagidae) is a flesh fly species of medical, veterinary, and forensic importance. It is often used in the laboratory for several biological studies. In the current research, we investigate its life history under changing temperatures ranging from 15.7 to 31.1 °C, with an average of 24.55 °C, and the relative humidity ranges from 31.4 to 82.8% and at six fixed temperatures of 15, 20, 25, 30, 32, and then 35 °C. Also, pteridine from the head was used to assess adult age grading. Our results revealed that the life history and development rate of S. crassipalpis under changing temperatures were very close to those observed at a fixed temperature of 25 °C. The longest and shortest growing times were found at a low rearing temperature of 15 °C and higher temperature of 32 °C, respectively. The pattern of pteridine increase differed depending on the temperature. By employing a changing temperature model in the current study, we aim to provide information on the life history of S. crassipalpis that will be useful in future research.
Abstract
Flesh flies (Diptera: Sarcophagidae) are regarded as significant in medical and veterinary entomology, and their development models can be utilized as considerable markers to ascertain the minimum postmortem interval (PMImin). In this research, we explored the growth cycle and larval body length of Sarcophaga crassipalpis Macquart 1839 (Diptera: Sarcophagidae) reared under variable temperatures ranging from 15.7 to 31.1 °C, with an average of 24.55 °C and relative humidity ranges from 31.4 to 82.8% and at six fixed temperatures of 15, 20, 25, 30, 32, and then 35 °C. Moreover, pteridine from the head was used to assess adult age grading. Our results allowed us to provide three development models: the isomorphen chart, the isomegalen chart, and the thermal summation models. The time taken for S. crassipalpis to complete its development from larviposition to adult emergence at constant temperatures of 15, 20, 25, 30, 32, and 35 °C was 1256.3 ± 124.2, 698.6 ± 15.1, 481.8 ± 35.7, 366.0 ± 13.5, and 295.8 ± 20.5 h, respectively, except 35 °C, where all pupae were unable to attain adulthood. They lasted 485.8 ± 5.4 h under variable temperatures. The minimum developmental limit (D0) temperature and the thermal summation constant (K) of S. crassipalpis were 9.31 ± 0.55 °C and 7290.0 ± 388.4 degree hours, respectively. The increase in pteridine content exhibited variations across different temperatures. There was quite a considerable distinction in the pteridine contents of male and female S. crassipalpis at 15 °C (p = 0.0075) and 25 °C (p = 0.0213). At 32 °C and variable temperatures, the pteridine content between female and male S. crassipalpis was not statistically divergent. However, temperature and gender remain the main factors influencing the pteridine content in the head of S. crassipalpis. We aim to provide detailed developmental data on S. crassipalpis that can be used as a valuable resource for future research and PMI estimation.
1. Introduction
Sarcophaga crassipalpis Macquart, 1839 (Diptera: Sarcophagidae) is a hemisynanthropic Sarcophagidae species of medical and veterinary significance. Since 2003, its life cycle has been studied [1]. This species has been known to cause myiasis [2] and has been used in medicolegal entomology in estimating the minimum postmortem interval [3]. It is widely distributed, especially in temperate and tropical regions [4,5]. It is frequently used in the laboratory for a lot of physiological research, including life history, diapause, genetic expression [6,7,8,9,10], and behavior patterns [11], as well as circadian rhythms [12,13,14]. Temperature governs entire aspects of ectothermic organisms, especially their growth and development [15]. Since insects live in thermally variable environments, they are subjected to various thermal regimes, including extremely high and low temperatures, which can impact their physiology and life story [15,16,17,18]. Nonetheless, the mechanisms regulating the development of S. crassipalpis under varying temperature conditions remain poorly understood. Little research has been performed on the life history of this species, including its development under non-diapause conditions [19,20], constant temperatures [21], reproductive time [22,23] and fecundity [24], eclosion rhythm [8], and pupal diapause [8,25,26], as well as the effects of food and weather conditions on its growth processes [10]. The findings show developmental plasticity in S. crassipalpis with different development patterns in populations from different geographical locations in the USA, China, and Turkey.
Most of the species belonging to the Sarcophagidae family exhibit a slow growth rate at low temperatures and a fast growth rate at high temperatures, as evidenced by the development patterns exhibited by Sarcophaga dux Thomson, 1869 (Diptera: Sarcophagidae) [27], Sarcophaga peregrina (Robineau-Desvoidy, 1830) (Diptera: Sarcophagidae) [28], Sarcophaga (Liopygia) argyrostoma Robineau-Desvoidy, 1830 [29], Sarcophaga ruficornis (Fabricius, 1794) [30,31], and Wohlfahrtia nubia Wiedemann, 1830 (Diptera: Sarcophagidae) [32]. Sarcophaga africa had a slow life cycle, with a 1.66-day difference between the rainy and winter seasons and a rapid one during the summer [33]. Shang et al. found that, under fluctuating temperatures (18–36 °C and 22–30 °C), S. peregrina had a longer development and a lower pupariation rate and emergence rate than those observed at constant temperatures [34].
Some discrepancy was observed in the growth rates of insects maintained under controlled laboratory conditions with a constant temperature and those subjected to variable temperatures in their natural habitat. For instance, it has been reported that Protophormia terraenovae, Robineau-Desvoidy, 1830 (Diptera: Calliphoridae) developed quicker at a higher changing temperature and slower at the lowest temperature range [35]. Niederegger et al. recorded, under daily low and optimum fluctuating temperatures (5–29 °C), the faster development of S. argyrostoma (Diptera: Sarcophagidae) and Lucilia illustris (Meigen, 1926) (Diptera: Calliphoridae) and lower development of Calliphora vicina (Robineau-Desvoidy) and Calliphora vomitoria (Linnaeus, 1758) (Diptera: Calliphoridae) [36], whereas Chen and collaborators reported that Aldrichina grahami (Aldrich, 1930) (Diptera: Calliphoridae) had a cold thermal preference with a low thermal threshold of 3.41 ± 0.48 °C [37]. Byrd and Butler reported that, under fluctuating temperatures, Sarcophaga haemorrhoidalis (Fallen) (Diptera: Sarcophagidae) had a maximal thermal preference of 30 °C [38]. Brent and Spurgeon [39] found non-significant developmental durations of Lygus Hesperus (Hemiptera: Miridae) observed at moderate and high fluctuating temperatures of 22 °C and 29 °C, respectively. Mironidis [40] observed that the longevity and fecundity of adult Helicoverpa armigera (Lepidoptera: Noctuidae) were decreased at variable temperatures ranging from 17.5 to 32.5 °C. One of the reasons for these various effects is that fluctuating temperatures may delay the development process at all stages, with survival and longevity gradually decreasing as temperatures decrease and increase [41]. Moreover, significant deviations from the standard temperature range may adversely impact the thermophysiological capabilities of insects [41,42]. It is vital to recognize that the frequency of extreme weather events is projected to rise in the future, with potentially significant consequences for insect populations [43]. Consequently, more research is required to elucidate the developmental processes of insects under conditions of temperature variability.
Pteridines are heterocyclic substances derived from the pyrimidine-pyrazine and are 2-amino-4-hydroxy derivatives that accumulated in the eyes of adult flies over time [44]. They are commonly known as ocular pigments and serve as a filter for UV protection [44,45] and as a pathway of nitrite elimination [44,46]. In the other words, these are biochemical metabolics that can be quantified and utilized for calculating the ages of insects [47,48]. Temperature is a factor that affects pteridine levels. It promotes an increase in the amount of pteridine as adult flies age [44,49]. To date, the quantitative pteridine levels of forensically important dipteran species, including C. vicina (Robineau-Desvoidy) (Diptera: Calliphoridae) [45], Musca domestica Linnaeus, (Diptera: Muscidae) [50], Boettcherisca peregrina (Robineau-Desvoidy) [51], Lucilia sericata (Meigen) (Diptera: Calliphoridae) [44], and Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) [52], have been investigated.
In this research, the baseline developmental data for S. crassipalpis were obtained at six fixed and variable temperatures. In addition, the pteridine content in the head of S. crassipalpis was quantified and analyzed at the same variable temperature conditions and three constant temperatures (15, 25, and 32 °C) to assess adult age grading. The findings have significant implications for utilizing this species in estimating the minimum postmortem intervals during forensic examinations.
2. Materials and Methods
2.1. Settlement of Laboratory Specimen and SAMPLING
Wild adults of S. crassipalpis were captured on pork bait using Nylon nets for trapping flies in Xi Hu Park in Changsha City (28°12′ N; 112°58′ E), Hunan Province, China, in September and October 2021. Before raising, they were anesthetized at −20 °C for 1–2 min and identified under Zeiss AxioCam 208 color microscopy using morphological keys by a medicolegal entomologist expert [53] and confirmed using molecular techniques by performing a polymerase chain reaction (PCR) of the long cytochrome oxidase subunit I (COI) gene [54]. The species were reared according to the fly culture methodology previously described by Zhang et al. [55]. Adult S. crassipalpis were cultivated in a rearing nylon box and placed in an artificial climate cage (250A GPL, Shen Zhen Ren Gong. Ltd., Tianjin, China). The weather conditions were 25.0 °C temperature, 70% relative humidity, and 12:12 h of light/dark photoperiod cycles. The flies were fed a diet consisting of water and milk powder and were reared to the fifth generation before starting the experiments. Before the commencement of the study, the total number of adult S. crassipalpis was maintained at 1000–2000 specimens.
Six artificial climate incubators (LRH-250-GSI, Taihong Co., Ltd., Shaoguan, China) were set at fixed temperatures of 15 °C, 20 °C 25 °C, 30 °C, 32 °C, and then 35 °C, with 70% RH and a photoperiod 12:12 h L/D cycle, while the flies did not undergo eclosion at a temperature of 35 °C. As in the variable temperature conditions the rearing occurs outside, the variable temperatures were determined based on meteorological conditions in Hunan Province, Southeast China. A GPS temperature and humidity data logger (GPS-6, Elitech Co., Ltd., Jiangsu, China) was used to record the minimum, average, and maximum ambient temperatures of 15.7 °C, 24.55 °C, and 31.1 °C, respectively, during September and October 2022. The relative minimum and maximum humidity ranged from 31.4% to 82.8% (Figure 1).
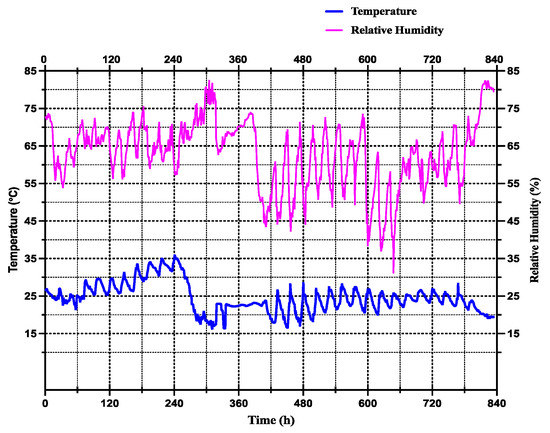
Figure 1.
Variable temperatures and relative humidity were recorded based on the meteorologic conditions occurring in Hunan Province, China, using a GPS temperature and moisture data logger (GPS-6, Elitech Co., Ltd., Jiangsu, China) in the months of September and October 2022. The blue line represented the average daily fluctuating temperatures, while the purple one symbolized the average daily fluctuating relative humidity.
2.2. Evaluation of Lifespan and Measurement of Larval Body Length
Approximately 50 g of pig lungs were put into Petri dishes and introduced into a fly-rearing box to stimulate larviposition. After 2 h, approximately 2000–3000 larvae deposited by gravid female S. crassipalpis were collected. Each batch containing 400–500 larvae was reared in a plastic bowl with a relative quantity of pig lungs [56]. The bowls were then introduced into fly-breeding cages covered with 2 cm of wet sand until pupation [56]. Larvae rearing and sampling were conducted under both variable (outside) and constant temperatures, as previously described.
Larvae were monitored every 8 h from the first instar to wandering and every 12 h from pupation to adult eclosion. Fresh pieces of pork lungs were provided 1 to 3 times per day based on consumption [56]. Eight or ten larvae were collected every 8 h until wandering and then treated in hot water at 90 °C for thirty seconds, as previously described [57]. The larvae were conserved in a centrifuge tube containing 75% ethanol and stored at −20 °C [58]. The larval instar was established by examining the number of splits in the posterior spiracle using Zeiss AxioCam 208 color microscopy [59]. The body lengths were measured using an electronic Vernier caliper (Meinaite, Shanghai, China). The different stages of development, including wandering, pupation, and eclosion, were noted during the experiment [56]. Each experiment was repeated with five biological replicates. A total of 4090 larvae were sampled: 1250 in 15 °C, 680 in 20 °C, 600 in 25 °C, 450 in 30 °C, 320 in 32 °C, 350 in 35°C, and 440 in the variable temperature group.
2.3. Pteridine Extraction
Adult S. crassipalpis were collected at three constant (15, 25, and 32 °C) and variable (15.7 °C to 31.1 °C) temperatures when approximately 50% of the pupae emerged into adults and were designated as zero days. Eight adults (four males and four females) were then sampled every 48 h for fourteen days. The samples were preserved in 2 mL centrifuge tubes at −80 °C for the subsequent analysis. A global number of 32 adult males and 32 adult females were collected per group. The experiment was repeated with three biological replicates.
Pteridine extraction and fluorescence analysis were performed using the modified methods previously described [44,49,51,60,61,62]. The flies were taken out from the −80 °C refrigerator and unfrozen, and the sex of each individual was noted. Their heads were decapitated, detached from their bodies, and transferred into 2 mL centrifuge tubes. The mouthpieces were carefully removed from each head using dissecting tweezers (Code No. TST-11; R’DEER tools, Hong Kong Robust Deer Tools Co. Limited, Hong Kong) under an AxioCam 208 color microscope (Carl Zeiss Microscopy GmbH, Jena, Germany). Each head capsule was weighed utilizing precision scales (Code No. TP-100D; Xiangyi Balance Equipment, Changsha, China) and returned to the 2 mL centrifuge tubes. The head capsules were mixed in 600 μL of 1 M Tris-HCl buffer at pH 8.0 [45]. A maceration bead was added to each 2 mL microcentrifuge tube containing a head capsule and macerated using a Tissuelyser-24 (Ling Xin Industrial Development Co. Ltd., Shanghai, China) at 50.00 hertz for 35 s. The tubes were then centrifuged in a Microfuge® 20R Centrifuge (Ref. B31614; Beckman Coulter Euro Center SA, Nylon 1, Nyon, Switzerland) and centrifugated at 6000× g for 5 min in 4 °C; then, 150 μL of the supernatant was loaded into a 96-well cell culture plate (Code No. 11510; LABSELECT®, Beijing Labgic Technology Co., Ltd., Beijing, China) and covered with aluminium foil. The fluorescence intensity was gauged instantly after stimulation. The pteridine fluorescence was gauged in an EnSpire® Multimode Plate Reader (PerkinElmer, Waltham, MA, USA) at a transmitter frequency of 482 nanometers with the agitation set at 360–450 nanometers. The capacity of the released light was expressed in the relative fluorescence units (RFU) [45,52].
2.4. Statistical Analysis
GraphPad Prism 9.4.1 and IBM SPSS Statistics 26 were used to perform the data analysis; One-way ANOVA examined the consequence of the temperature on the total duration of the life story [37]. Nonlinear regression was utilized for analyzing the correlation between the larval body length and feeding period [63,64]. To define the equation for the PMImin estimation, the larval body length was used as the independent variable while the time after larviposition was the dependent variable and inversely [56]. The reviewed regression pattern outlined by Ikemoto and Takai [65], as reported by Mukesh et al. [66]: D = K + Tm, where D represents development time, K the thermal constant, and Tm the low thermal threshold, was utilized for the analysis of the correlation between the developmental rate and accumulation degree hours (ADH) at each developmental stage and the total developmental times [27]. The mathematical equations were obtained using GraphPad Prism version 9.4.1, Boston, MA, USA. The slope and y-intercept of the linear regression equation for each stage were both utilized to define the developmental threshold temperature D0 and thermal accumulated constant K, respectively [67].
The age of the adults of S. crassipalpis was defined based on the methods prior described [45,49]. The adult age was expressed as the time after eclosion in accumulated degree days (ADD) (ADD = temperature in Celsius degrees × age in days) [68] without subtraction of the minimum temperature threshold [45]. Using IBM SPSS Statistics version 26, a log10 transformation of pteridine fluorescence and ADD was utilized to perform a linear regression and write the regression equations for females and males in each temperature group [45]. Paired t-tests and two-way ANOVA were utilized to analyze differences in the pteridine levels between different temperatures and sexes.
3. Results
3.1. Development Time at Fixed and Changing Temperatures
The developmental cycle of S. crassipalpis was observed under variable temperatures ranging from 15.7 to 31.1 °C (mean: 24.55 °C) (Figure 1) and at 6 fixed temperatures (15, 20, 25, 30, 32, and 35 °C). The results indicate that the length of the developmental cycle reduced with the rising temperature from the early immature stage to pupae (Table 1).

Table 1.
Mean (SD ±) development time (h) of S. crassipalpis at six fixed and variable temperatures (VT).
A one-way ANOVA analysis at a p-value inferior to 0.05 demonstrated a considerable distinction between the means of total developmental durations for constant vs. variable temperatures (F = 200.2, df = 28, p < 0.0001, R2 = 0.9775). However, comparisons between each group of constants vs. variable temperatures (VT) showed varying results. According to Tukey’s multiple comparison test and one-way ANOVA at alpha = 0.05, there was no statistical significance between the total developmental duration at 25 °C vs. VT (df = 23, p = 0.9999). In contrast, significant differences were found between total developmental durations at 15 °C and VT (df = 23, p < 0.0001), 20 °C and VT (df = 23, p <0.0001), 30 °C and VT (df = 23, p = 0.0377), and 32 °C vs. variable temperature (df = 23, p = 0.0005) (Supplementary Figure S1).
3.2. Lifespan and Isomorphen Diagrams
However, at 35 °C, while larvae completed the active feeding, wandering, and pupariation stages, they did not emerge as adult flies. This indicates that 35 °C is a higher lethal thermal for S. crassipalpis (Table 1). Additionally, the life cycle duration was reduced from 1256.3 h at 15 °C to 295.8 h at 32 °C and was recorded as 485.8 h at VT (Table 1). According to Tukey’s multiple comparison test and one-way ANOVA at alpha = 0.05, there is a considerable variation between the development times at 25 °C and 15 °C (df = 23, p < 0.0001), at 25 °C and 20 °C (df = 23, p < 0.0001), at 25 °C and 30 °C (df = 23, p = 0.0314), and at 25 °C and 32 °C (df = 23, p = 0.0003) (Supplementary Figure S2).
The isomorphen diagram was designed based on the time of all developmental phases from larviposition to adult emergence (x-axis) vs. different fixed temperatures (y-axis), where each line characterizes larval body changes, and the distances between lines describe the developmental stages [69]. An increase in temperature from 15 to 32 °C caused the time between each growth stage (first ecdysis, second ecdysis, wandering, pupation, and eclosion) to shrink and the distances between lines to decrease (Figure 2).
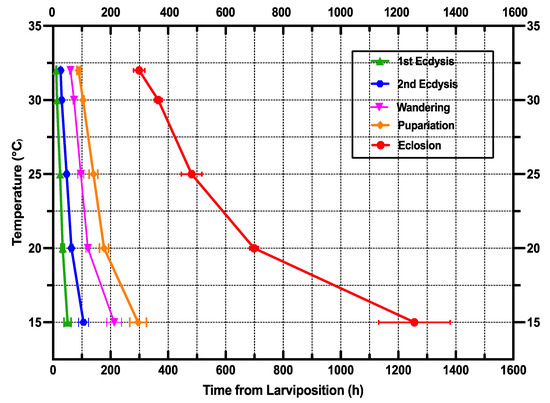
Figure 2.
Isomorphen diagram of S. crassipalpis. The development time of each stage (first ecdysis, second ecdysis, wandering, pupation, and eclosion) was charted with the time from larviposition to the onset of each development time. Each curve represents a developmental event, and the error bar corresponds to the standard deviation of each event.
3.3. Thermal Accumulated Models
According to the correlation between the developmental times of S. crassipalpis from larviposition to adult emergence (x-axis) and accumulated degree hours (y-axis), six thermal summation patterns were plotted using linear regression examination (Figure 3). The coefficient of determination (R2) of all thermal accumulated models was ≥0.90, demonstrating that all data matched relatively similarly to the linear models (Table 2). Utilizing the revised regression model suggested by Ikemoto and Takai [65], the developmental threshold temperature (D0) and thermal summation constants (K) were estimated to be 9.31 ± 0.55 °C and 7290.0 ± 388.4 degree hours, respectively. In addition, the thermal requirements (K) were 293.0 ± 58.1, 355.5 ± 13.9, 904.2 ± 100.7, 774.4 ± 56.8, and 5066.0 ± 272.4 degree hours at the first, second, and third immature stages, as well as wandering and pupal stages, respectively (Table 2). The development of larvae to adult emergence was slowest at 15 °C and quickest at 32 °C, as is usual in other sarcophagids.
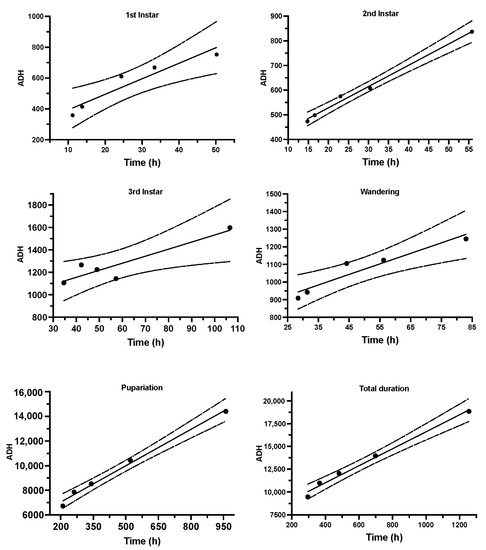
Figure 3.
Thermal accumulated models for five developmental phases and total developmental duration of S. crassipalpis. The strong line corresponds to the regression line, and the dashed line corresponds to a 95% confidence interval.

Table 2.
Mean (±SE) of the developmental threshold temperature (D0) and thermal summation constants (K) for five developmental stages and the total developmental period of S. crassipalpis and the coefficient of determination (R2) of the thermal summation models.
3.4. Variations in Larval Body Length Measurement and Isomegalen Graphs
Figure 4 displays the data of larval body length changes over time after larviposition in different constant and variable temperatures. Larvae grew faster at increasing temperatures (15–32 °C). The disparity in growth rates between 15 °C and 25 °C was significant, but it reduced as the temperatures reached 30 °C to 32 °C. The fourth-order polynomials models at each temperature group were used to define the equations characterizing the larval body length changes over time after larviposition. The larval body length was used as the independent variable, while the time after larviposition was the dependent variable. At 15 °C, 20 °C, 25 °C, 30 °C, 32 °C, and then VT, the average optimum larval body length was 20.8, 20.1, 20.2, 20.1, 20.2, and 20.7 mm, respectively.
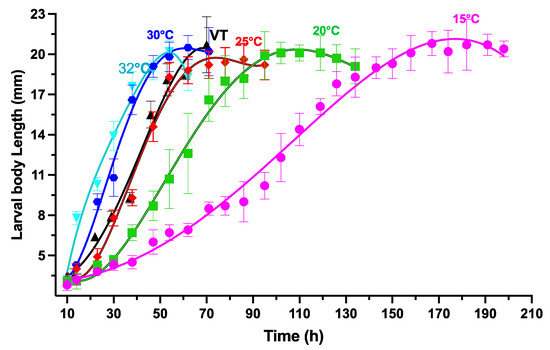
Figure 4.
Variations in larval body length of S. crassipalpis over time (h) after larviposition at diverse fixed temperatures. The vertical bars symbolize the standard deviation.
The equations in Table 3 exhibit the fluctuations in larval body length (L) with the elapse of time (T), with larval body length as the dependent variable and time after larviposition as the independent variable. The coefficient of determination (R2), F value, and p-value suggest that the models have a good correlation with the data. The equations in Table 4 display the connection between time (T) and larval body length (L) by using time after larviposition as the dependent factor and the larval body length as the independent factor.

Table 3.
Equations, F values, p-values, and coefficient of determination (R2) of the relationship between the body length (L, mm) of S. crassipalpis larvae and the time after larviposition (T, h) at constant and variable temperatures.

Table 4.
Equations, F values, p-values, and coefficient of determination (R2) of the relationship between the time after larviposition (T, h) and the body length (L, mm) of S. crassipalpis larvae at constant and variable temperatures.
The Isomegalen diagram (Figure 5) was constructed by plotting the time elapsed from larviposition to the peak feeding stage on the x-axis and z-axis, while the constant temperatures were on the y-axis. The lower boundary of each contour displays a range of larval body lengths from 3 mm to 20 mm.
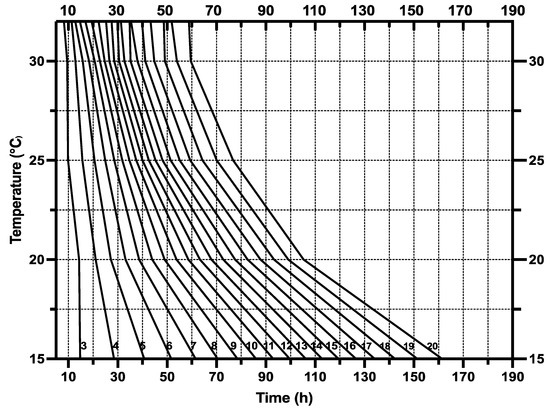
Figure 5.
Isomegalen graph of S. crassipalpis larvae from larviposition to peak feeding phase. Time (h) was charted against temperature, and each line corresponds to the developmental larval length of 3–20 mm, with size indicated by the number at the lower left of each contour.
3.5. Effect of Temperature Variation on the Content of Pteridine
The pteridine concentrations exhibited statistically significant differences in 15 °C against 25 °C (p = 0.0019, t = 4.847, df = 7), 15 °C against 32 °C (t = 6.375, df = 7, p = 0.0004), 15 °C against VT (t = 3.419, df = 7, p = 0.0112), 25 °C against 32 °C (t = 3.412, df = 7, p = 0.0113), and 32 °C against VT (t = 2.667, df = 7, p = 0.0321). However, there was no considerable distinction between 25 °C and VT (t = 2.065, df = 7, p = 0.0778) (Figure 6).
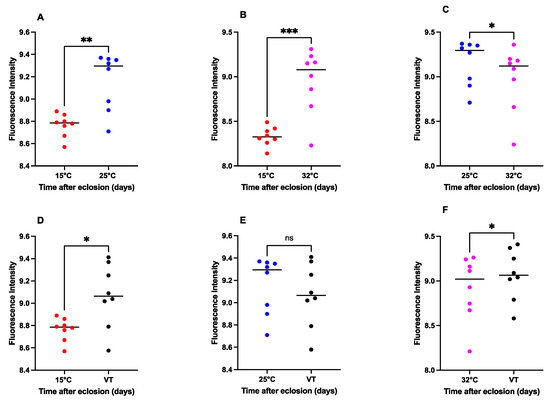
Figure 6.
Comparison of the pteridine content of S. crassipalpis with different temperatures ((A): 15 °C vs. 25 °C; (B): 15 °C vs. 32 °C; (C): 25 °C vs. 32 °C; (D): 15 °C vs. VT; (E): 25 °C vs. VT, and (F): 32 °C vs. VT). The number of asterisks indicates the significant difference between different temperature groups: ***: p < 0.001; **: p < 0.01; *: p < 0.05; ns: not significant.
An increase in the pteridine content was observed over time following eclosion, as illustrated in Figure 7. Nevertheless, the pattern of this increase varied across different temperatures.
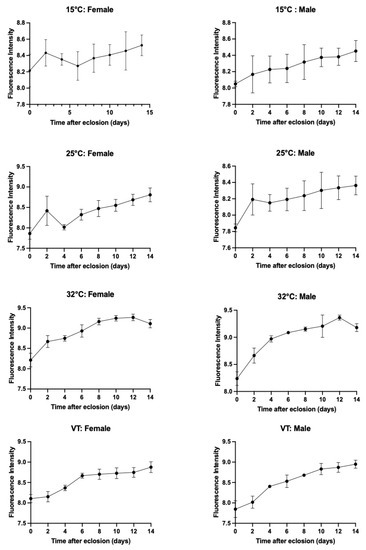
Figure 7.
Accumulated pteridines in the heads of females and males of S. crassipalpis when raised to three different constant and variable temperatures. The pteridine fluorescence intensity (RFU) for each sex (y-axis) was plotted against time after eclosion (x-axis) in days.
There was a substantial difference in the pteridine concentrations between females and males of S. crassipaplis at 15 °C (t = 3.719, df = 7, p = 0.0075) and 25 °C (t = 2.952, df = 7, p = 0.0213). Nevertheless, there was no statistical variation in the pteridine level between males and females at 32 °C (p = 0.0827, t = 2.023, df = 7) and under variable temperatures (p = 0.619, t = 0.5205, df = 7) (Figure 8).
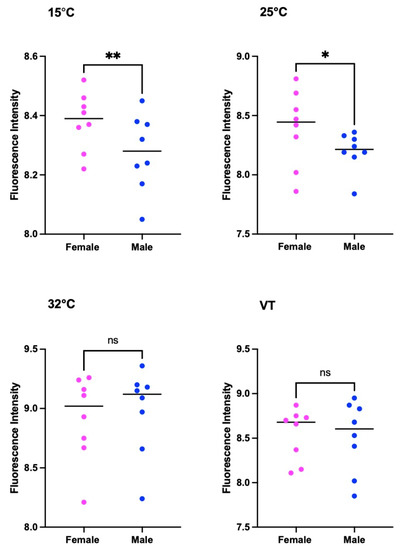
Figure 8.
Comparison of the pteridine content of S. crassipalpis between females and males under different temperatures. The number of asterisks indicates the significant difference between different temperature groups. **: p < 0.01; *: p < 0.05; ns: not significant.
In addition, the scrutiny of the pteridine content in the heads of adult male and female S crassipalpis exhibited a linear regression that correlated with the rising log10 of ADD and, therefore, with time after eclosion (Figure S4). The accumulated pteridine concentration could be used to predict the age of adult S. crassipalpis. Across all experiment conditions, the head capsule weight did not have a statistically reliable effect; the p-value was over 0.05. Except for females collected at 15 °C (R2 = 0.503), the index of determination of all regression patterns was R2 ≥ 0.700, indicating that all data fit relatively well to the linear models (Table 5). Thus, the age of male and female adults of S. crassipalpis can be determined by applying the equations from Table 5.

Table 5.
Regression analysis of the pteridine fluorescence intensity (RFU) against times (ADD) for female and male S. crassipalpis at three constant (15 °C, 25 °C, and 32 °C) and variable (mean = 24.55 °C) temperatures. Times in accumulated degree per day (ADD) were used as the dependent variable and pteridines fluorescence intensity as the independent variable. F = female, M = male, y = predicted age hours, and x = pteridines fluorescence intensity.
4. Discussion
This study provides the examination of developmental times for the flesh fly species S. crassipalpis, which holds medical and veterinary significance, particularly in forensic medicine. The investigation was conducted under variable and constant laboratory temperatures in Changsha City, Hunan Province, China. We provided developmental data, including isomegalen and isomorphen diagrams, thermal summation models, and a pteridine content analysis of adult heads, to facilitate the use of this species in estimating PMImin.
The investigational results showed that the variable temperature group generated a moderated life cycle duration and a moderated rate in comparison with the control group of a fixed temperature. Under variable temperatures, the total developmental duration of S. crassipalpis was 485.8 ± 5.4 h, relatively similar to the developmental duration observed under a constant temperature of 25 °C (469.8 ± 27.1 h). However, the development was slower under constant temperatures of 15 °C (1256.3 ± 124.2 h) and 20 °C (698.6 ± 15.1) than under variable temperature conditions (485.8 ± 5.4 h), while development was relatively long under variable temperatures compared with the developmental duration under 30 °C (366.0 ± 13.5 h) and 32 °C (295.8 ± 20.5), with significant differences. These data indicated that the mean temperature of variable temperatures was nearer to the optimal constant temperature. In a comparison to the effect of variable temperatures vs. constant temperatures on S. crassipalpis, it appeared that, under the low constant temperature groups, S. crassipalpis grew more slowly, moderately under the optimum temperature and greater under high temperatures.
Since there is not yet a published study on the consequence of variable temperatures on the lifespan of S. crassipalpis, the present research findings were discussed with the available literature on other taxa. In the current study, we found that variable temperatures can influence the development rates and durations of S. crassipalpis. Analogously, Dadour et al. [70] found that Hydrotaea rostrata (Diptera: Muscidae) had slower developmental rates under winter conditions than under a summer regime. Clarkson et al. [71] reported that P. terraenovae (Diptera: Calliphoridae) developed more quickly in the early immature stages under fixed temperatures than under fluctuating temperatures.
Similarly, an investigation on the development of larvae of different forensic flies was conducted by Niederegger et al. [36] under naturally changing (5–29 °C) and invariable (13 °C) temperatures, with invariable temperatures designed as the average of changing temperatures. They discovered that, at variable temperatures, S. argyrostoma and L. illustris developed more rapidly than C. vicina and C. vomitoria. In 2013, Warren and Anderson [35] conducted other research on the influence of unstable temperatures on the life history of P. terraenovae and found that its growth was accelerating at 4–28 °C higher fluctuating temperatures, reasonable at 9–23 °C changing temperatures, and slowest at 16 °C stable temperatures. Interestingly, this discrepancy in development rate is thought to have been caused by the rate summation effect, as temperature variations above the mean tend to raise the rate relatively more than they can lower it. Recently, Chen et al. [37] investigated the life history parameters of A. grahami (Diptera: Calliphoridae) under stable and changing temperatures. They found that A. grahami developed slowly under changing temperatures ranging from 6 to 20 °C, according to the natural meteorological conditions, than under stable temperatures ranging from 8 to 36 °C. These correlated with our findings in the present study. Sert et al. [72] analyzed the impact of fixed and changing temperatures on the post-feeding time of S. argyrostoma (Diptera: Sarcophagidae) and found a similar intrapuparial development with a non-significant difference between adult emergence times at fixed temperatures (25 °C) and variable temperature conditions. Although this result concerned exclusively the intrapuparial stage, it was similar to the observation made on the growth time of S. crassipalpis under variable temperatures vs. 25 °C in the current research.
A thorough understanding of insect development parameters under fluctuating temperatures can aid in addressing various challenges, including accelerated, delayed, or unchanged developmental rates [37]. The results of experiments on the impact of variable temperatures on insect development can be related to the climatic conditions under which the models were chosen and the thermophysiological capacity of each species to adapt to fluctuating temperatures. Even though data from studies on the development of insects under different constant temperatures have been successfully used to estimate PMI, several studies found it inconceivable to use this model to investigate the developmental parameters of insects that naturally live under different variable temperature conditions [42,73,74]. Thus, ignoring variable temperatures in real investigations can result in significantly incorrect estimates of the PMI [36]. Furthermore, variable temperatures should be factored into growth prediction models [37].
Several studies on various diapause parameters in insects have been conducted on S. crassipalpis [6,26,75,76,77]. However, very few studies have examined its development time [10,19]. In our study, S. crassipalpis developed successfully at 15 to 32 °C and ceased to accomplish its growth at 35 °C, similar to the thermal conditions chosen by Bulut et al. [10] to analyze the impacts of tissue type and invariable temperatures on the life story of S. crassipalpis in Turkey. In contrast, the developmental rate of Turkey’s colony was lower at 32 °C for specimens fed bovine minced meat, bovine tongue, and chicken heart, and not all pupae reached adult emergence when fed minced meat [10]. This is different from the current study colony, which developed successfully at 32 °C while being reared on pig lung tissue. This disparity could be attributed to the rearing tissues [10].
The growth time of the immature stages of S. crassipalpis in the present study at 15 °C is shorter than those fed with bovine tongues and bovine minced meat in Turkey [10]. However, those reared on chicken heart tissue at 15 °C developed 1.81 days faster than the Chinese colony (our study). At 20 °C, the larvae of our colony developed faster than those of the Turkish colony [10]. Interestingly, when comparing the duration larval stage at 25 °C and 30 °C, the results from the present study are very similar to the Turkish study [10], except when the larvae were reared on chicken heart tissue. In contrast, at 32 °C, the growth period of the maggots in China, the present study was significantly shorter than in Turkey [10]. The successful development of S. crassipalpis in low temperature conditions can be attributed to its cold tolerance capacity. Chen et al. [78] found that a short acclimation at 0 °C allowed its larvae, pupae, and adults to subsist at −10 °C by increasing their hemolymph osmolality and glycerol levels, as well as the membrane fatty acids, necessary for low temperature tolerance [79].
In the current investigation, the developmental period of the pupal phase decreased with the growing temperatures, similar to the study carried out by Bulut et al. [10]. However, the pupa’s development times were slower than in Turkey, except for 30 °C, which was similar to the Turkish specimen fed with chicken heart tissue [10]. At 32 °C, the present investigation found that the period of the pupal phase was shorter than in Turkey. The developmental events (from larvae to pupae) for S. crassipalpis varied among the temperatures [19]. These disparities reflect the plasticity of developmental rates across populations, as well as the different food and feeding techniques, which may influence the development time of insects [56].
In our study, the higher constant temperatures we exposed S. crassipalpis to were 30, 32, and 35 °C. Although the larvae accomplished their development at 30 °C and 32 °C with total growing durations of 366 h and 295.8 h, respectively, unfortunately, all pupae ceased to reach the adult phase at 35 °C. This suggests that the longer exposure time at this temperature resulted in insufficient development of muscle contraction motor patterns for successful adult emergence [80]. Similarly, Bulut et al. [10] found that S. crassipalpis can complete their developmental time at 30 °C, with lower average developmental times to achieve the developmental cycle than at 32 °C and at 35 °C, where all larvae failed to reach the pupal phase. These findings suggested that 32 °C could be close to the upper growing threshold temperature for S. crassipalpis. These observations supported those made by Nassu et al. [31] on the development time of Microcerella halli (Engel 1913) (Diptera: Sarcophagidae) raised under diverse constant temperatures. They found that larvae of M. halli raised at 35 °C attained the pupal phase much later than other groups but never reached the adult stage. Contrary, Joseph et al. [24] discovered that an exposition for one or two hours at the suboptimal (−10 °C) and supraoptimal (40 °C and 45 °C) temperatures decreased the fecundity in both male and female S. crassipalpis but did not affect the larvae and pupae.
Our study found that temperature significantly impacted the development period of S. crassipalpis from the immature phases to adult emergence. These findings supported those of Bulut et al., who discovered that temperature and tissue type had a significant impact on the immature stage and pupal survival, as well as the adult weight of S. crassipalpis [10]. Also, Chen et al. [19] noted that the span from leaving the larval nutritional source to the beginning of pharate adult development showed the highest response to temperature variations, showing that physiological processes occurring at this time are particularly susceptible to temperature control [81]. Research has indicated that laboratory equipment and conditions, moisture, and photoperiods may have an impact on the development process, but the temperature is the predominant factor that determines the growth and development of insects [27]. Temperature plays a significant role in phenotypical variations, which may lead to geographical variations. Furthermore, the temperature favors adaptation to the geographical area. Therefore, it is important to prioritize assessing the aforementioned factors, recording data precisely, and designing the experiment cautiously to form a reliable benchmark for the period of fly development [82].
The pteridine content in the heads of S. crassipalpis is a reliable technique for determining the age of adult necrophagous species for the PMI estimation. In the current study, there is a logical correlation between the time after eclosion, thus the age of the adult S. crassipalpis, and the pteridine intensity, as well as between an increased pteridine concentration and different temperatures. Previous studies have proven a positive interaction between the pteridine content and the age of necrophagous flies, including Stomoxy calcitrant [83,84], Glossina morsitrans [61], Cochliomyia hominivorax [60], Chrysomya bezziana [85], Lucilia sericata [44,86], Aldrichina grahami [87], Musca domestica [50], Chrysomya megacephala [52], Cochliomyia macellaria, Phormia regina [49], Boettcherisca peregrina [51], and Calliphora vicina [45].
Our findings indicate that temperature is a crucial factor in the increase of pteridine. The pattern of this increase varied across different temperatures, suggesting that the metabolic activity of pteridine increased at higher temperatures. This is coherent with the results of Zhu et al. [52], who found that the content of pteridine was twice as high in the high-temperature group as opposed to the low-temperature group. This affirmative thermal impact on the pteridine concentration is consistent in B. peregrina, Stomoxys calcitrans, and M. domestica as well [50,51,84]. Contrary to our results, Bernhardt et al. [45] found a non-significant relationship between temperature and the rise of the pteridine levels in the head of C. vicina. We additionally observed that the head capsule had no impact on the pteridine levels, which is in agreement with the findings of Bernhardt et al. [45], who found that the head weight did not influence the pteridine fluorescence.
Our research found a substantial linear correlation between the pteridine content and age of male and female adults of S. crassipalpis at different constant and variable temperatures. This corresponds to the conclusion of Zhu et al. [51], who observed a significant linear interaction between pteridine fluorescence and age in male and female adults of B. peregrina (Diptera: Sarcophagidae) raised in different constant temperatures. In our study, there was a considerable variation in the content of pteridine between female and male S. crassipalpis at 15 °C and 25 °C. Nevertheless, we found no statistical distinction in the pteridine content between female and male S. crassipalpis at 32 °C and variable temperatures. This contrasts with previous research, which showed that male flies exhibited higher pteridine levels than female flies (L. sericata, M. domestica, and Calliphora erythrocephala) [50,88]. We suspect that the lack of gender difference in the morphology of S. crassipalpis eyes and increasing temperature may be crucial factors in our findings (Figure S3 and Figure 8). The low R2 values observed in females sampled at 15 °C may be related to the decreased metabolic activity of the specimens raised at this temperature. Similarly, Cammack et al. [49] also discovered low indexes of determination in both male and female Phormia regina reared at 5.40 °C and suggested that this finding can be linked to reduced metabolic activity, which is the result of poor physical activity, and the reduced consumption of food and water of this species. Because pteridines are a byproduct of purine metabolism, a decrease in metabolic activity would culminate in a decrease in the pteridine concentration levels [49]. For a more precise estimation of the PMI, it is suggested that the pteridine content should be used in combination with other aging methods [45].
Our results allowed us to generate the following development patterns, including isomorphen diagram (Figure 2), isomegalen diagram (Figure 5), and the thermal accumulated model (Figure 3), commonly used by forensic entomologists to determine the minimum period after death. An isomorphen graph provides data on age ranges based on the times of the developmental stages (x-axis) against the temperatures (y-axis) to estimate the PMImin [37]. Hence, the oldest individual’s age found on the corpse and the average ambient temperature close to the experimental temperatures are essential for using this method to estimate the PMImin [89,90]. Nevertheless, the simplicity of this method may compromise the precision of the results [37,91]. Some researchers discovered that an isomegalen diagram is more accurate to estimate the PMImin than an isomorphen diagram [91,92], because it is the only method that sets out changes in the larval body length between the larviposition and peak feeding [64]; unfortunately, it does not apply when the larvae shrink naturally [37,91]. The method to kill and preserve larvae collected should be taken into account when using the larval body length. In this study, larvae were killed in boiled water at 90° for 30 s to cause the larvae to expand to their full extent and preserved in 75% ethanol, as recommended by Adams and Martin [57]. However, the larva should be measured immediately after killing in boiling water before preservation [57,93]. Therefore, some authors have suggested that the accumulated degree hours (ADH) technique is preferable to the two earlier methods, given its applicability in natural settings with varying temperatures [89,94]. Nonetheless, the weakness of this method is related to the large variations between the higher and lower developmental temperature limits [37,91]. In addition, Wu et al. [73] found that, when making quantitative predictions, it is not possible to use ADH models, since the connection between temperature and the rate of development is not totally correlative.
All of these models could be used to estimate the PMImin; however, the biology of the insects and the climatic parameters under which they are dynamic should be considered [37]. In addition, to provide an accurate PMI, Shang et al. [28] suggested the use of a multimethod combination, as well as a combination of multidisciplinary specialists, to analyze and interpret data from forensic investigations [95]. Therefore, further research is still required to test other methods using this species.
5. Conclusions
This research offers the initial experimental laboratory information on the lifespan of S. crassipalpis at variable and constant temperatures in China for the minimum postmortem interval estimation (PMImin). Our findings showed that the life history and development rate of S. crassipalpis under variable temperatures were very close to those observed at a fixed temperature of 25 °C. The longest and shortest growing times were found at a low rearing temperature of 15 °C and higher temperature of 32 °C, respectively. However, all pupae failed to emerge at 35 °C, suggesting that the upper developmental threshold temperature for S. crassipalpis may be close to this temperature. The pattern of pteridine increase varied across different temperatures. In addition, most of the total developmental durations exhibited significant differences between variable and constant temperatures. By utilizing a fluctuating temperature model in our study, we aimed to provide detailed developmental data on S. crassipalpis that can serve as a valuable resource for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13152402/s1: Figure S1. Tukey’s multiple comparison test, one-way ANOVA at alpha = 0.05 of the total developmental durations between constant vs. variable temperatures. The significant differences among developmental durations are indicated with asterisks. Figure S2. Tukey’s multiple comparison test, one-way ANOVA at alpha = 0.05 of the total developmental durations between the constant temperature control group (25 °C) vs. other temperature groups. The significant differences among the developmental durations are indicated with asterisks. Figure S3. Sex differences in the morphology of S. crassipalpis eyes: (A) female and (B) male. Figure S4. Log10 of time (ADD) after eclosion plotted against log10 of the mean pteridine fluorescence (RFU) for males and females at different temperatures ((A) 15 °C, (B) 25 °C, (C) 32 °C, and (D) mean variable temperatures (24.55 °C).
Author Contributions
Conceptualization, F.J.N., X.Z., C.Z., L.R. and Y. G.; Methodology, F.J.N., X.Z. and C.Z.; Software, F.J.N., X.Z., H.Q. and F.Y.; Validation, C.Z., L.R. and Y.G.; Formal Analysis, F.J.N., X.Z., Y.F. and Y.S.; Investigation F.J.N., H.Q., S.C., and X.Z.; Resource, F.J.N. and X.Z.; Data Curation, F.J.N., X.Z., F.Y. and C.Z.; Writing—Original Draft Preparation, F.J.N.; Writing—Review and Editing, F.J.N. and X.Z.; Visualization, F.J.N. and X.Z.; Supervision, L.R.; Project Administration, Y.G.; and Funding Acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (grant number 82072114) and the Fundamental Research Funds for the Central Universities of Central South University (2023ZZTS0546).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and code presented in this study are contained in the manuscript and available on request from the corresponding author; therefore, they are not filed in a public repository.
Acknowledgments
We thank Lushi Chen (Guizhou Police Officer Vocational College) for his expertise in species identification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kruger, R.; Ribeiro, P.; Costa, P. Ciclo de vida de Sarcophaga (Liopygia) crassipalpis (Macquart) (Diptera, Sarcophagidae). Entomol. Vectores 2003, 10, 85–98. [Google Scholar]
- Giangaspero, A.; Marangi, M.; Balotta, A.; Venturelli, C.; Szpila, K.; Di Palma, A. Wound Myiasis Caused by Sarcophaga (Liopygia) Argyrostoma (Robineau-Desvoidy) (Diptera: Sarcophagidae): Additional Evidences of the Morphological Identification Dilemma and Molecular Investigation. Sci. World J. 2017, 2017, 9064531. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, T.; Greco, S.; Berardo, C.; Brandmayr, P.; Vercillo, V. The Flesh Fly Sarcophaga (Liopygia) crassipalpis Macquart 1839 as an Invader of a Corpse in Calabria (Southern Italy). J. Forensic Sci. Criminol. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Pape, T. Catalogue of the Sarcophagidae of the World (Insecta: Diptera); Memoirs of Entomology International: Gainesville, FL, USA, 1996; Volume 8, pp. 1–558. [Google Scholar]
- Aslan, A.; Çalışkan, H. Fauna of Eskişehir Sarcophagidae (Insecta, Diptera), and new records for Turkey. Sak. Üniversitesi Fen. Edeb. Derg. 2009, 11, 15–27. [Google Scholar]
- Flannagan, R.D.; Tammariello, S.P.; Joplin, K.H.; Cikra-Ireland, R.A.; Yocum, G.D.; Denlinger, D.L. Diapause-specific gene expression in pupae of the flesh fly Sarcophaga crassipalpis. Proc. Natl. Acad. Sci. USA 1998, 95, 5616–5620. [Google Scholar] [CrossRef]
- Tammariello, S.P.; Denlinger, D.L. G0/G1 cell cycle arrest in the brain of Sarcophaga crassipalpis during pupal diapause and the expression pattern of the cell cycle regulator, proliferating cell nuclear antigen. Insect Biochem. Mol. Biol. 1998, 28, 83–89. [Google Scholar] [CrossRef]
- Yocum, G.D.; Joplin, K.H.; Denlinger, D.L. Upregulation of a 23 kDa small heat shock protein transcript during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem. Mol. Biol. 1998, 28, 677–682. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Denlinger, D.L. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol. Biol. 2000, 9, 641–645. [Google Scholar] [CrossRef]
- Bulut, M.; Zeybekoğlu, Ü.; Kökdener, M. Effects of Tissue Type and Temperature on Selected Life-History Traits of the Flesh Fly, Sarcophaga crassipalpis (Macquart, 1839) (Diptera: Sarcophagidae). J. Med. Entomol. 2022, 59, 1921–1927. [Google Scholar] [CrossRef]
- Dylan Shropshire, J.; Moore, D.; Seier, E.; Joplin, K.H. Male aggression, limited female choice and the ontogeny of mating behaviour in the flesh fly Sarcophaga crassipalpis. Physiol. Entomol. 2015, 40, 325–335. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Goto, S.G.; Tanaka, K.; Saito, O.; Watari, Y. Thermoperiodic regulation of the circadian eclosion rhythm in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2011, 57, 1249–1258. [Google Scholar] [CrossRef]
- Kostál, V.; Závodská, R.; Denlinger, D. Clock genes period and timeless are rhythmically expressed in brains of newly hatched, photosensitive larvae of the fly, Sarcophaga crassipalpis. J. Insect Physiol. 2009, 55, 408–414. [Google Scholar] [CrossRef]
- Prohaska, F.; Joplin, K.H.; Moore, D. Effects of gender, age, and nutrition on circadian locomotor activity rhythms in the flesh fly Sarcophaga crassipalpis. J. Insect Physiol. 2018, 107, 265–275. [Google Scholar] [CrossRef]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a Warming Climate: Insect Responses to Extreme High Temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.H.; Gil, H.; Para, A.; Gallio, M. A thermometer circuit for hot temperature adjusts Drosophila behavior to persistent heat. Curr. Biol. 2022, 32, 4079–4087.e4. [Google Scholar] [CrossRef]
- Stejskal, V.; Vendl, T.; Li, Z.; Aulicky, R. Minimal Thermal Requirements for Development and Activity of Stored Product and Food Industry Pests (Acari, Coleoptera, Lepidoptera, Psocoptera, Diptera and Blattodea): A Review. Insects 2019, 10, 149. [Google Scholar] [CrossRef]
- Chen, C.-P.; Denlinger, D.; Lee, R. Responses of Nondiapausing Flesh Flies (Diptera: Sarcophagidae) to Low Rearing Temperatures: Developmental Rate, Cold Tolerance, and Glycerol Concentrations. Ann. Entomol. Soc. Am. 1987, 80, 790–796. [Google Scholar] [CrossRef]
- Goto, S.G.; Denlinger, D.L. Short-day and long-day expression patterns of genes involved in the flesh fly clock mechanism: Period, timeless, cycle and cryptochrome. J. Insect Physiol. 2002, 48, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, C.; Min, J. Effects of temperature on the growth and development of four common necrophagous flies and their significance in forensic medicine. Chin. J. Forensic Med. 1998, 13, 81–84. [Google Scholar]
- Hahn, D.; James, L.; Milne, K.; Hatle, J. Life-history plasticity after attaining a dietary threshold for reproduction is associated with protein storage in flesh flies. Funct. Ecol. 2008, 22, 1081–1090. [Google Scholar] [CrossRef]
- Hahn, D.A.; Rourke, M.N.; Milne, K.R. Mating affects reproductive investment into eggs, but not the timing of oogenesis in the flesh fly Sarcophaga crassipalpis. J. Comp. Physiol. B 2008, 178, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.; Yocum, G.; Denlinger, D. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 2000, 25, 330–336. [Google Scholar] [CrossRef]
- Denlinger, D. Diapause among the flesh flies (Diptera: Sarcophagidae). Eur. J. Entomol. 2022, 119, 170–182. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Yocum, G.D.; Denlinger, D.L. Developmental upregulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Ssarcophaga crassipalpis. Insect Biochem. Mol. Biol. 2000, 30, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Shang, Y.; Ren, L.; Chen, W.; Wang, S.; Guo, Y. Development of Sarcophaga dux (diptera: Sarcophagidae) at constant temperatures and differential gene expression for age estimation of the pupae. J. Therm. Biol. 2020, 93, 102735. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Amendt, J.; Wang, Y.; Ren, L.; Yang, F.; Zhang, X.; Zhang, C.; Guo, Y. Multimethod combination for age estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) with implications for estimation of the postmortem interval. Int. J. Leg. Med. 2022, 137, 329–344. [Google Scholar] [CrossRef]
- Sert, O.; Örsel, G.M.; Şabanoğlu, B.; Özdemir, S. A Study of the pupal developments of Sarcophaga argyrostoma (Robineau-Desvoidy, 1830). Forensic Sci. Med. Pathol. 2020, 16, 12–19. [Google Scholar] [CrossRef]
- Amoudi, M.A.; Diab, F.M.; Abou-Fannah, S.S. Development rate and mortality of immature Parasarcophaga (Liopygia) ruficornis (Diptera: Sarcophagidae) at constant laboratory temperatures. J. Med. Entomol. 1994, 31, 168–170. [Google Scholar] [CrossRef]
- Nassu, M.P.; Thyssen, P.J.; Linhares, A.X. Developmental rate of immatures of two fly species of forensic importance: Sarcophaga (Liopygia) ruficornis and Microcerella halli (Diptera: Sarcophagidae). Parasitol. Res. 2014, 113, 217–222. [Google Scholar] [CrossRef]
- Amoudi, M.A. Effect of temperature on the developmental stages of Wohlfahrtia nuba (Diptera: Sarcophagidae). J. Egypt. Soc. Parasitol. 1993, 23, 697–705. [Google Scholar]
- Bhosale, P.A. Significance of Forensic Fly and Seasonal Variation in the Temperature of Developmental Stages in Lifecycle for Family Sarcophagidae, Sarcophaga africa. Int. J. Adv. Res. Sci. Commun. Technol. 2023, 3, 153–156. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, F.; Ngando, F.J.; Zhang, X.; Feng, Y.; Ren, L.; Guo, Y. Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures. Animals 2023, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.A.; Anderson, G.S. Effect of fluctuating temperatures on the development of a forensically important blow fly, Protophormia terraenovae (Diptera: Calliphoridae). Env. Entomol. 2013, 42, 167–172. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef]
- Chen, W.; Yang, L.; Ren, L.; Shang, Y.; Wang, S.; Guo, Y. Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of Aldrichina grahami (Diptera: Calliphoridae). Insects 2019, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.; Butler, J.F. Effects of temperature on Sarcophaga haemorrhoidalis (Diptera: Sarcophagidae) development. J. Med. Entomol. 1998, 35, 694–698. [Google Scholar] [CrossRef]
- Brent, C.S.; Spurgeon, D.W. Reproductive Development of Lygus hesperus (Hemiptera: Miridae) Adults Under Constant and Variable Temperatures. J. Insect Sci. 2019, 19, 24. [Google Scholar] [CrossRef]
- Mironidis, G.K. Development, survivorship and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under fluctuating temperatures. Bull. Entomol. Res. 2014, 104, 751–764. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, R.; Zhou, W.; Pan, Y.; Tian, H.; Yin, Z.; Chen, W. Fruit Fly in a Challenging Environment: Impact of Short-Term Temperature Stress on the Survival, Development, Reproduction, and Trehalose Metabolism of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2022, 13, 753. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Filazzola, A.; Matter, S.F.; MacIvor, J.S. The direct and indirect effects of extreme climate events on insects. Sci. Total Environ. 2021, 769, 145161. [Google Scholar] [CrossRef] [PubMed]
- Estévez Dimitrov, R.; Amendt, J.; Rothweiler, F.; Zehner, R. Age determination of the adult blow fly Lucilia sericata (Diptera: Calliphoridae) through quantitative pteridine fluorescence analysis. Forensic Sci. Med. Pathol. 2020, 16, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, V.; Hannig, L.; Kinast, R.; Verhoff, M.A.; Rothweiler, F.; Zehner, R.; Amendt, J. Quantitative pteridine fluorescence analysis: A possible age-grading technique for the adult stages of the blow fly Calliphora vicina (Diptera: Calliphoridae). J. Insect Physiol. 2017, 98, 356–359. [Google Scholar] [CrossRef]
- Harmsen, R. The excretory role of pteridines in insects. J. Exp. Biol. 1966, 45, 1–13. [Google Scholar] [CrossRef]
- Amendt, J.; Bugelli, V.; Bernhardt, V. Time Flies-Age Grading of Adult Flies for the Estimation of the Post-Mortem Interval. Diagnostics 2021, 11, 152. [Google Scholar] [CrossRef]
- Croce, A.C.; Scolari, F. Autofluorescent Biomolecules in Diptera: From Structure to Metabolism and Behavior. Molecules 2022, 27, 4458. [Google Scholar] [CrossRef]
- Cammack, J.A.; Reiskind, M.H.; Guisewite, L.M.; Denning, S.S.; Watson, D.W. Quantifying pteridines in the heads of blow flies (Diptera: Calliphoridae): Application for forensic entomology. Forensic Sci. Int. 2017, 280, 44–48. [Google Scholar] [CrossRef]
- McIntyre, G.S.; Gooding, R.H. Pteridine accumulation in Musca domestica. J. Insect Physiol. 1995, 41, 357–368. [Google Scholar] [CrossRef]
- Zhu, G.H.; Ye, G.Y.; Li, K.; Hu, C.; Xu, X.H. Determining the age of adult flesh flies, Boettcherisca peregrina, using pteridine fluorescence. Med. Vet. Entomol. 2013, 27, 59–63. [Google Scholar] [CrossRef]
- Zhu, G.H.; Ye, G.Y.; Hu, C. Determining the adult age of the oriental latrine fly, Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) by pteridine fluorescence analysis. Insect Sci. 2003, 10, 245–255. [Google Scholar] [CrossRef]
- Chen, L. Necrophagous Flies in China; Guizhou Science and Technology Press: Guiyang, China, 2013. [Google Scholar]
- Guo, Y.D.; Cai, J.F.; Meng, F.M.; Chang, Y.F.; Gu, Y.; Lan, L.M.; Liang, L.; Wen, J.F. Identification of forensically important flesh flies based on a shorter fragment of the cytochrome oxidase subunit I gene in China. Med. Vet. Entomol. 2012, 26, 307–313. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, Y.; Ren, L.; Qu, H.; Zhu, G.; Guo, Y. A Study of Cuticular Hydrocarbons of All Life Stages in Sarcophaga peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 2022, 59, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Yang, L.; Tao, L.; Wang, J. Development of Chrysomya megacephala at constant temperatures within its colony range in Yangtze River Delta region of China. Forensic Sci. Res. 2018, 3, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Adams, Z.J.; Hall, M.J. Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci. Int. 2003, 138, 50–61. [Google Scholar] [CrossRef]
- Day, D.M.; Wallman, J.F. Effect of preservative solutions on preservation of Calliphora augur and Lucilia cuprina larvae (Diptera: Calliphoridae) with implications for post-mortem interval estimates. Forensic Sci. Int. 2008, 179, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, X.; Peng, X.; Tao, M.; Peng, L.; Chen, G.; Zhang, X. Effect of Short-Term Low Temperature on the Growth, Development, and Reproduction of Bactrocera tau (Diptera: Tephritidae) and Bactrocera cucurbitae. J. Econ. Entomol. 2020, 113, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.B.; Chen, A.C. Age determination in the adult screwworm (Diptera: Calliphoridae) by pteridine levels. J. Econ. Entomol. 1989, 82, 1140–1144. [Google Scholar] [CrossRef]
- Lehane, M.; Mail, T. Determining the age of adult male and female Glossina morsitans morsitans using a new technique. Ecol. Entomol. 1985, 10, 219–224. [Google Scholar] [CrossRef]
- Roziah, A.; Rostlawatt, R.; Nazni, W.; Norazizah, A.; Khairul Asuad, M.; Lee, H. Pteridine fluorescence in age-determination of immature Chrysomya megacephla (Fabricius) and Chrysomya rufifacies (Macquart) (Diptera: Calliphoridae). Trop. Biomed. 2019, 36, 488–494. [Google Scholar]
- Grzywacz, A. Thermal requirements for the development of immature stages of Fannia canicularis (Linnaeus) (Diptera: Fanniidae). Forensic Sci. Int. 2019, 297, 16–26. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.L.; Wang, J.F.; Wang, M.; Yang, L.J.; Tao, L.Y.; Zhang, Y.N.; Hou, Y.D.; Chu, J.; Hou, Z.L. Development of the green bottle fly Lucilia illustris at constant temperatures. Forensic Sci. Int. 2016, 267, 136–144. [Google Scholar] [CrossRef]
- Ikemoto, T.; Takai, K. A New Linearized Formula for the Law of Total Effective Temperature and the Evaluation of Line-Fitting Methods with Both Variables Subject to Error. Environ. Entomol. 2000, 29, 671–682. [Google Scholar] [CrossRef]
- Dhillon, M.K.; Hasan, F. Temperature-dependent development of diapausing larvae of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae). J. Therm. Biol. 2017, 69, 213–220. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.F.; Zhang, Y.N.; Tao, L.Y.; Wang, M. Forensically Important Boettcherisca peregrina (Diptera: Sarcophagidae) in China: Development Pattern and Significance for Estimating Postmortem Interval. J. Med. Entomol. 2017, 54, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Catts, E. Problems in Estimating the Postmortem Interval in Death Investigations. J. Agric. Entomol. 1992, 9, 245–255. [Google Scholar]
- Yanmanee, S.; Husemann, M.; Benbow, M.E.; Suwannapong, G. Larval development rates of Chrysomya rufifacies Macquart, 1842 (Diptera: Calliphoridae) within its native range in South-East Asia. Forensic Sci. Int. 2016, 266, 63–67. [Google Scholar] [CrossRef]
- Dadour, I.R.; Cook, D.F.; Wirth, N. Rate of development of Hydrotaea rostrata under summer and winter (cyclic and constant) temperature regimes. Med. Vet. Entomol. 2001, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.A.; Hobischak, N.R.; Anderson, G. A Comparison of the Development Rate of Protophormia Terraenovae (Robineau-Desvoidy) Raised under Constant and Fluctuating Temperature Regimes. Can. Soc. Forensic Sci. J. 2004, 37, 95–101. [Google Scholar] [CrossRef]
- Sert, O.; Özdemir, S.; Şabanoğlu, B. Effect of constant and fluctuating temperature on the intrapuparial development of Sarcophaga argyrostoma (Robineau-Desvoidy, 1830; Diptera: Sarcophagidae). J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Shiao, S.F.; Okuyama, T. Development of insects under fluctuating temperature: A review and case study. J. Appl. Entomol. 2015, 139, 592–599. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Hagenbucher, S.; Romeis, J.; Grabenweger, G.; Meissle, M. Fluctuating temperatures influence the susceptibility of pest insects to biological control agents. J. Pest. Sci. 2020, 93, 1007–1018. [Google Scholar] [CrossRef]
- Pavlides, S.C.; Pavlides, S.A.; Tammariello, S.P. Proteomic and phosphoproteomic profiling during diapause entrance in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2011, 57, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Denlinger, D.L. High temperature and hexane break pupal diapause in the flesh fly, Sarcophaga crassipalpis, by activating ERK/MAPK. J. Insect Physiol. 2007, 53, 1276–1282. [Google Scholar] [CrossRef]
- Sláma, K.; Denlinger, D.L. Transitions in the heartbeat pattern during pupal diapause and adult development in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2013, 59, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P.; Denlinger, D.; Lee, R. Cold-Shock Injury and Rapid Cold Hardening in the Flesh Fly Sarcophaga crassipalpis. Physiol. Zool. 1987, 60, 297–304. [Google Scholar] [CrossRef]
- Michaud, M.; Denlinger, D. Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2006, 52, 1073–1082. [Google Scholar] [CrossRef]
- Yocum, G.; Žďárek, J.; Joplin, K.; Lee, R.; Smith, D.C.; Manter, D.; Denlinger, D. Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J. Insect Physiol. 1994, 40, 13–21. [Google Scholar] [CrossRef]
- Jafari, S.; Fathipour, Y.; Faraji, F. Temperature-dependent development of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) at seven constant temperatures. Insect Sci. 2012, 19, 220–228. [Google Scholar] [CrossRef]
- Villet, M.; Amendt, J. Advances in Entomological Methods for Death Time Estimation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 213–237. [Google Scholar]
- Mail, T.; Chadwick, J.; Lehane, M. Determining the age of adults of Stomoxys calcitrans (L.) (Diptera: Muscidae). Bull. Entomol. Res. 1983, 73, 501–525. [Google Scholar] [CrossRef]
- Lehane, M.; Chadwick, J.; Howe, M.; Mail, T. Improvements in the Pteridine Method for Determining Age in Adult Male and Female Stomoxys calcitrans (Diptera: Muscidae). J. Econ. Entomol. 1986, 79, 1714–1719. [Google Scholar] [CrossRef]
- Wall, R.; Langley, P.A.; Stevens, J.; Clarke, G.M. Age-determination in the old-world screw-worm fly Chrysomya bezziana by pteridine fluorescence. J. Insect Physiol. 1990, 36, 213–218. [Google Scholar] [CrossRef]
- Wall, R.; Langley, P.A.; Morgan, K. Ovarian development and pteridine accumulation for age determination in the blowfly Lucilia sericata. J. Insect Physiol. 1991, 37, 863–868. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, G.; Hu, C.; Li, K. Determining the age of adult Aldrichina grahami by pteridine fluorescence. Chin. J. Appl. Environ. Biol. 2007, 13, 224–227. [Google Scholar]
- Patat, U. Über das Pterinmuster der Facettenaugen von Calliphora erythrocephala: Ein Beitrag zur Funktion und Stabilität der Pterine. Z. Vgl. Physiol. 1965, 51, 103–134. [Google Scholar] [CrossRef]
- Acosta, X.; González-Reyes, A.X.; Corronca, J.A.; Centeno, N.D. Estimation of the Postmortem Interval Through the Use of Development Time of Two South American Species of Forensic Importance of the Genus Lucilia (Diptera: Calliphoridae). J. Med. Entomol. 2021, 58, 1064–1073. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Sun, Y.; Zhang, Y.; Wang, M.; Wang, J. Development of Chrysomya rufifacies (Diptera: Calliphoridae) at Constant Temperatures Within its Colony Range in Yangtze River Delta Region of China. J. Med. Entomol. 2019, 56, 1215–1224. [Google Scholar] [CrossRef]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef]
- Reiter, C. Growth behavior of the blue blowfly Calliphora vicina maggots. Z. Rechtsmed. 1984, 91, 295–308. [Google Scholar] [CrossRef]
- Matthes, K.; Zehner, R.; Amendt, J. Influence of storage on larval length and age determination of the forensically important blow fly Lucilia sericata (Diptera: Calliphoridae). Sci. Justice 2021, 61, 579–585. [Google Scholar] [CrossRef]
- Donovan, S.E.; Hall, M.J.; Turner, B.D.; Moncrieff, C.B. Larval growth rates of the blowfly, Calliphora vicina, over a range of temperatures. Med. Vet. Entomol. 2006, 20, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, L.; Pradelli, J.; Tuccia, F.; Giordani, G.; Cattaneo, C.; Vanin, S. Comparison of Accumulated Degree-Days and Entomological Approaches in Post Mortem Interval Estimation. Insects 2021, 12, 264. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).