Simple Summary
Natural pearls of Diplodon chilensis, a freshwater clam native to southern South America, are reported and characterized for the first time. The finding also constitutes the first record of pearls in a species of the genus Diplodon. The pearls have different shapes and sizes, and were found in both, male and female specimens. The microstructure and chemical composition of pearls is consistent with those reported in other bivalve species.
Abstract
The capability to produce pearls is widespread in the phylum Mollusca, including bivalves of the superfamily Unionoidea. Here, we identified and characterized natural pearls formed by Diplodon chilensis, a freshwater clam native to southern South America, using samples obtained from two lakes located in the Chilean Patagonia. Pearls were studied using light and scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy. Naturally formed pearls were found in both male and female D. chilensis specimens. Pearls are produced in different shapes, including spherical, ellipsoidal, buttoned, and bumpy, ranging in size from 200 µm to 1.9 mm. The internal microstructure is composed of irregular polygonal tablets, about 0.40 to 0.55 μm in thickness. EDX analysis showed that pearls are composed of calcium carbonate. FTIR and Raman spectra recorded several peaks attributable to the aragonite in pearls of this species, as has been shown in other mollusks. In addition to these results, pearls of different colors are illustrated.
1. Introduction
Mollusks represent the second group with the largest number of species after the arthropods, reaching a diversity of between 85,000 and 120,000 species [1,2]. In addition to this high diversity, mollusks have a great variety of body plans [3], which has allowed them to colonize different environments across all climatic zones. Since ancient times, these animals have been used as food sources by humans [4,5], as demonstrated by the large number of archaeological records around the world [6,7].
Traditionally, many species of mollusks have been useful for human societies in religious ceremonies, folk medicine, building tools, the production of buttons, fertilizers, cattle feed, decoration, and ornamental purposes [8,9,10,11,12,13]. Moreover, shellfishing and cultured pearl production have been and continue to be important sources of income for the inhabitants of different countries, and in many parts of the world, some species have been included in commercial aquaculture [14,15].
Several species within the classes Bivalvia, Gastropoda, and Cephalopoda can produce pearls, mainly bivalves. The soft bodies of oysters, clams, mussels, and snails are covered by the mantle, a thin epithelial tissue that covers the body organs and secretes the molluscan shell, which is comprised of calcium carbonate and made up of two to five different layers of calcite and/or aragonite [3,16]. In a similar way, molluscan pearls are produced by the mantle when a foreign body is introduced, either accidentally or deliberately, between this tissue and the shell. When this occurs, the outer mantle epithelium envelops the invading object and secretes calcium carbonate to cover it, forming the pearl [3]. In bivalves, pearl microstructure may consist of calcite, aragonite, or vaterite, three polymorphs of calcium carbonate, or a combination of these phases [17,18,19,20,21,22].
Two types of pearls have been described in mollusks: ampullae, also called “half-pearl”, “blister”, or “mabe”, a protuberance of the internal shell surface; and encysted, formed around a foreign object inside the body of the mollusk [23,24,25]. In the latter case, a donor specimen and another recipient are required to produce a pearl. The object to be implanted can be a piece of mantle tissue alone or a small bead made of different solid materials such as shells, corals, and fish scales, among others, together with a piece of mantle tissue. If the implant is successful, a non-nucleated pearl will be produced in the first case and a nucleated pearl in the second [25].
The genus Diplodon Spix, 1827, a representative of the family Hyriidae Swainson, 1840, comprises conspicuous freshwater clams that inhabit lakes, rivers, and streams in South America [26]. Although the number of species in the genus is not known with certainty, it contains more than 50 valid species [27,28,29]. To our knowledge, no pearls have been reported in any Diplodon species so far. In the study of the shells and pearls carried out in several species of unionids [30], no pearls were found in the subspecies Diplodon chilensis (Gray, 1828). In the present investigation, we apply different techniques for the characterization of pearls discovered in populations of this species sampled in Chile.
A considerable number of studies have been carried out regarding the morphology, taxonomy, ontogeny, karyology, reproduction, life cycle, toxicology, and ecology of D. chilensis, which have led to the species being considered the “best known species of Hyriidae in the continent” [31]. The objective of this study is to report the finding of pearls in populations of D. chilensis from two Patagonian lakes in southern Chile. The pearls were characterized using a light microscope, a scanning electron microscope, and FTIR and Raman spectroscopy.
2. Materials and Methods
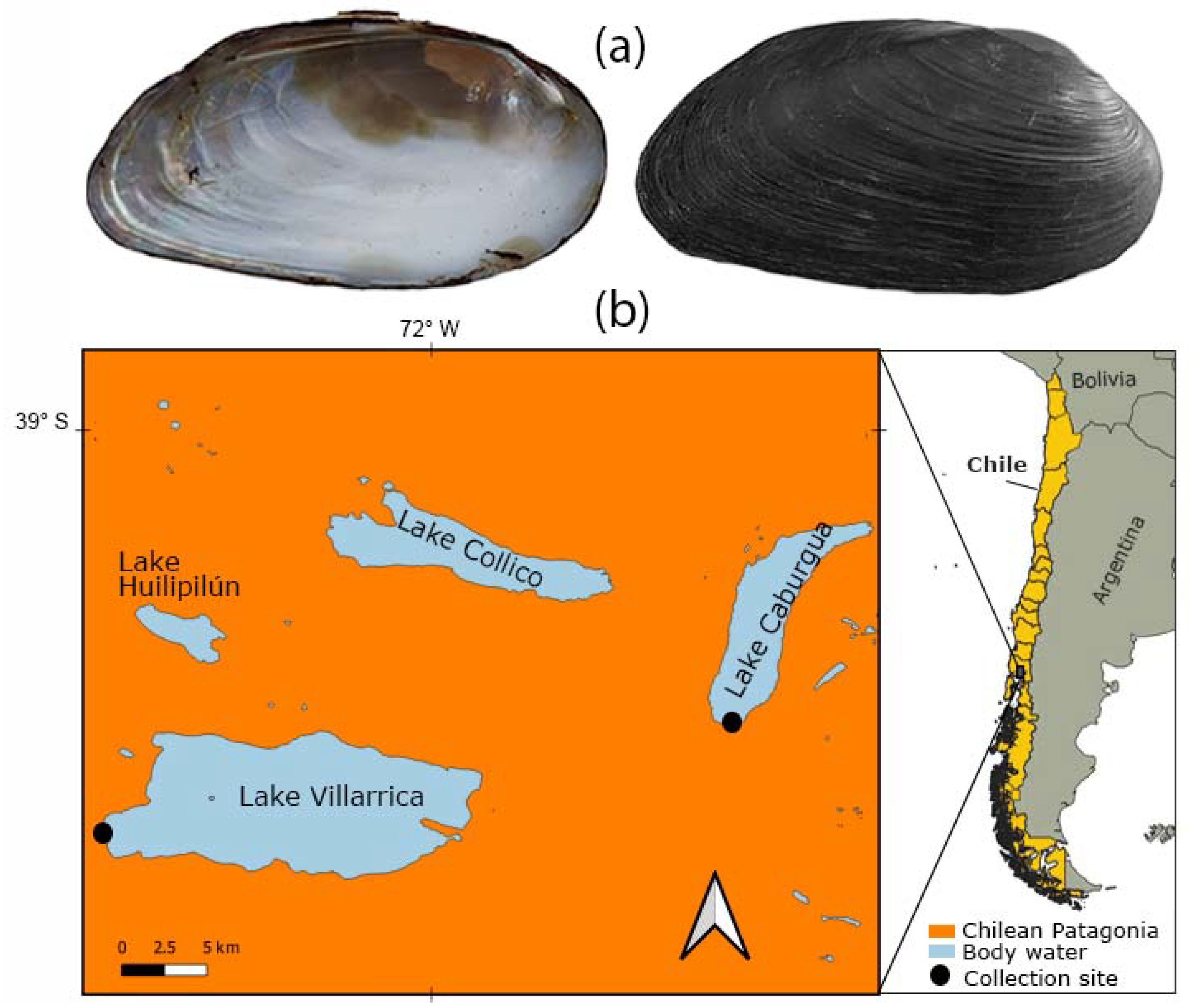
Adult D. chilensis individuals were obtained in January 2022 from a shellfisher in Playa Negra, Lake Caburgua (n = 100) and by a fisherman at the source of the Toltén River, Lake Villarrica, Southern Chile (n = 49) (Figure 1). The clams from both lakes were assigned to D. chilensis since (i) our samples were obtained within the species range, (ii) it inhabits Lake Villarrica [32,33], (iii) the current nonexistence of Diplodon chilensis patagonicus (d’Orbigny, 1846) populations in Chile [34,35,36,37], and (iv) the absence of characters that differentiate the populations of both lakes. Shell length was measured (in mm) using a vernier caliper (precision 0.01 mm). The clams were dissected using a Motic SMZ–168 stereoscopic microscope and sexed by microscopic examination of gonad smears using a Leica light microscope. The pearls were isolated from the mantle tissue using surgical material, washed with distilled water, and observed using a Hitachi 3500 scanning electron microscope (SEM). SEM was coupled with Bruker model Quantax 100 energy dispersive X-ray spectroscopy (EDX) for the chemical determination of the samples through elemental mapping. Pearls from different individuals were wrapped in paper and then broken with the blunt part of a dissecting needle to observe the internal microstructure using SEM.
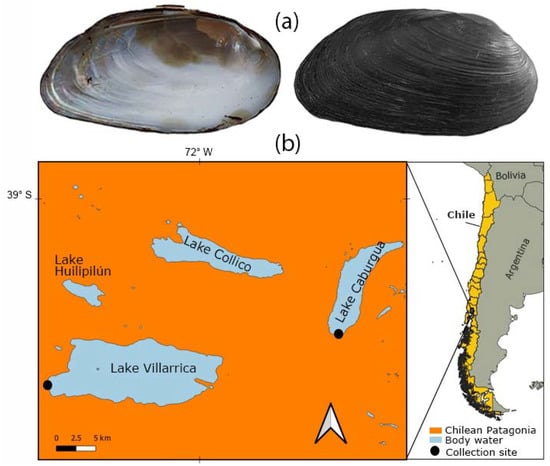
Figure 1.
The freshwater clam Diplodon chilensis in southern Chile: (a) shells (6.7 cm) in internal (left) and external (right) view; (b) collection sites. The maps were created using QGIS Geographic Information System v3.22 (http://www.qgis.org, accessed on 13 September 2022). (Maps: G.A. Collado).
Representative pearls were analyzed with a 4 cm−1 resolution using a PerkinElmer Spectrum Two FTIR spectrophotometer. Pearls were also analyzed using the Ocean Onsight Raman spectroscopy model QEPRO-RAMAN-785-PLUS to detect the type of CaCO3 polymorph present in the sample. The measurements were acquired with an excitation wavelength of 785 nm at 0.890 nW. The spectra were obtained with an 11 cm−1 optical resolution in a spectral range of 0–2000 cm−1.
3. Results
Pearls of D. chilensis were found covered by the animal’s mantle tissue (Figure 2) and were isolated by making a fine cut on it. Pearls were found in both male and female clams and were of different shapes, including spherical, elliptical, buttoned, or with bumps (“baroque”). With the electron microscope, we observed the external appearance of the pearls, indicating that most of them were smooth (Figure 3). However, some of them had small slits or holes as well as an irregular surface. Most pearls were white or grayish white in color, although a few silvers, light blue, pinkish, or brown were also found (Figure 4). In some clams, there were small pearls in the adductor muscles. The size of the pearls varied between 200 μm and 1.9 mm (Table S1). In Lake Caburgua, we recorded 1% of clams with pearls, while in Lake Villarrica, we recorded 18.4%. In this lake, the smallest individual with a pearl had a length of 41.9 mm. The largest pearl (1.9 mm) was found in a 47.2 mm individual. Only one pearl (924.0 μm) was found in Lake Caburgua in a 65.6 mm individual.
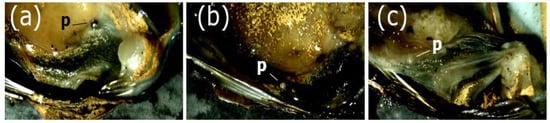
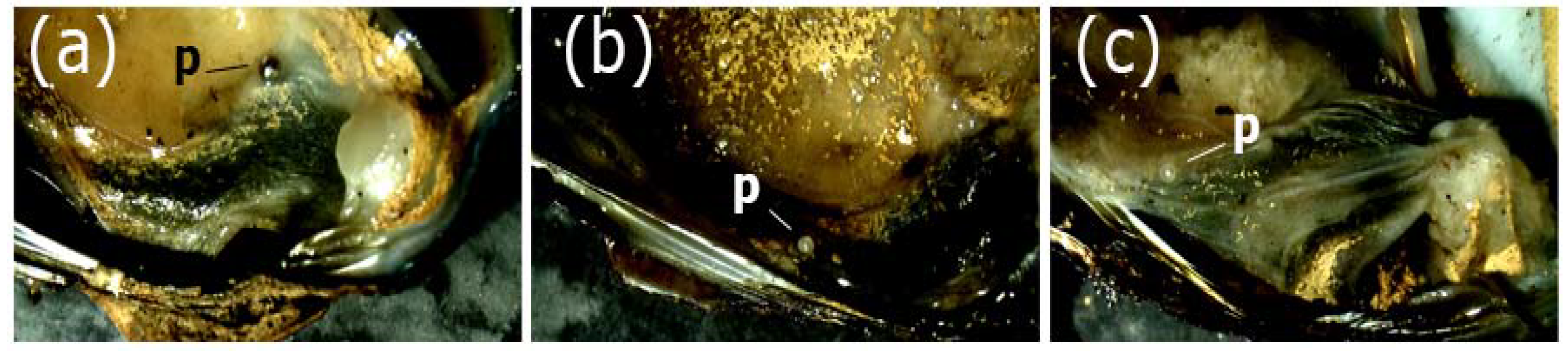
Figure 2.
Pearls (p) from the freshwater clam Diplodon chilensis from Lake Villarica: (a–c) pearls of three individuals covered by the mantle. Note a silver pearl in (a) and grayish white in (b) and (c).
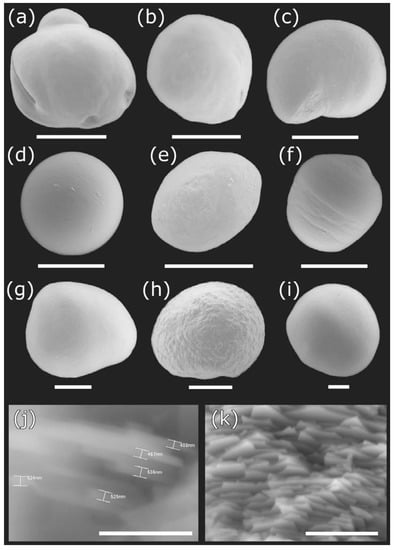
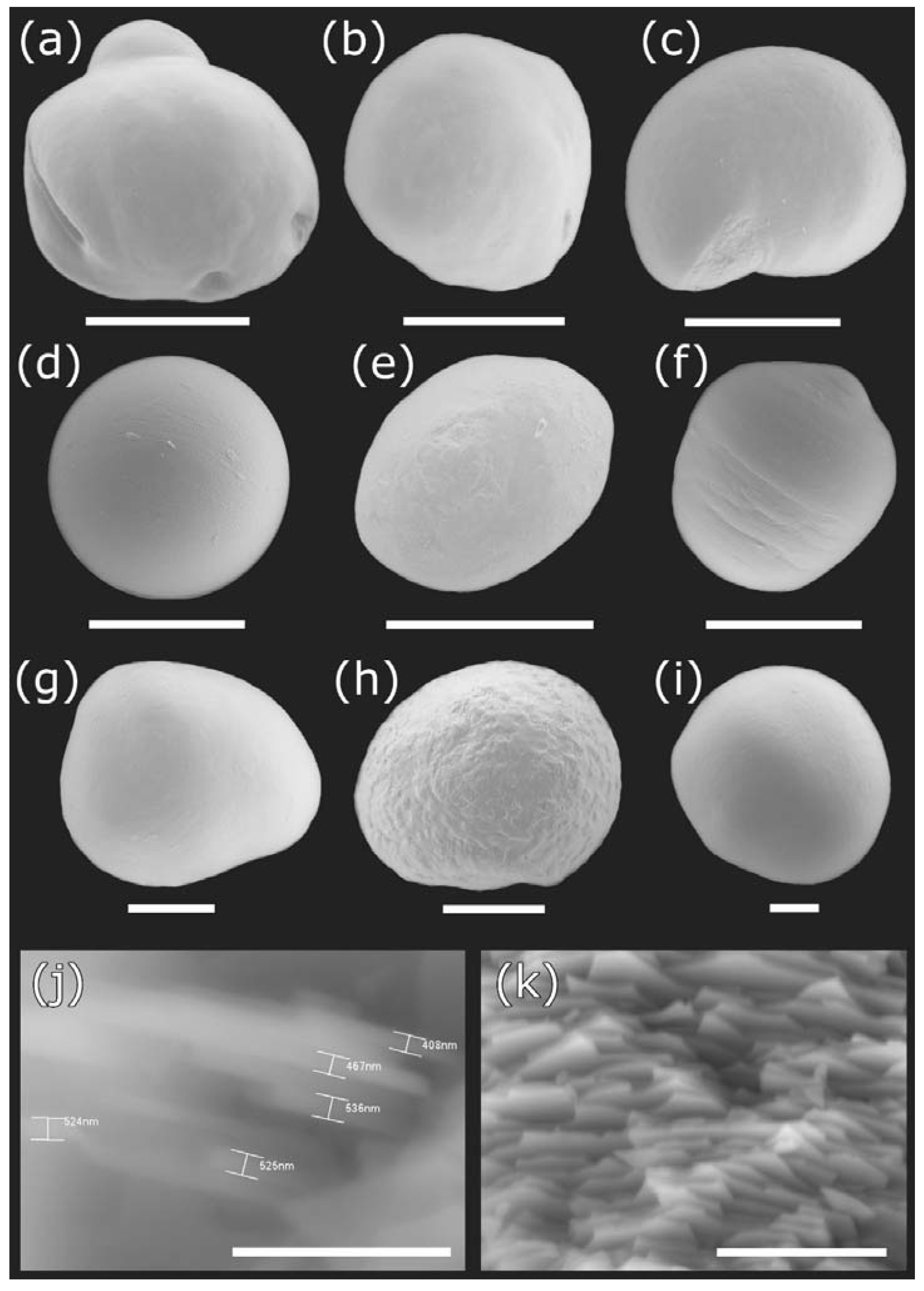
Figure 3.
Pearls produced by the freshwater clam Diplodon chilensis from southern Chile: (a–h) pearls obtained from Lake Villarrica; (i) pearls obtained from Lake Caburgua; (j,k) microstructure of pearl tablets seen at different angles and magnifications. Scale Bar: a = 1 mm; b, c = 500 µm; d–f = 300 µm; g–I = 200 µm; j = 5 µm; k = 10 µm.
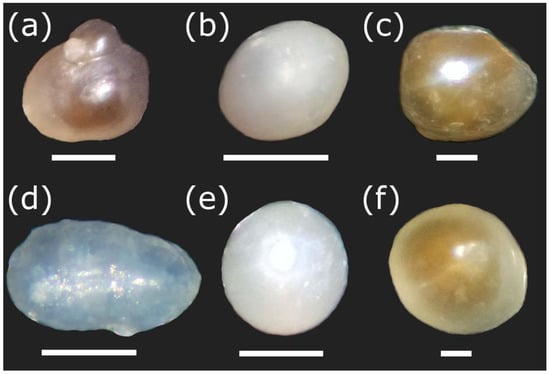
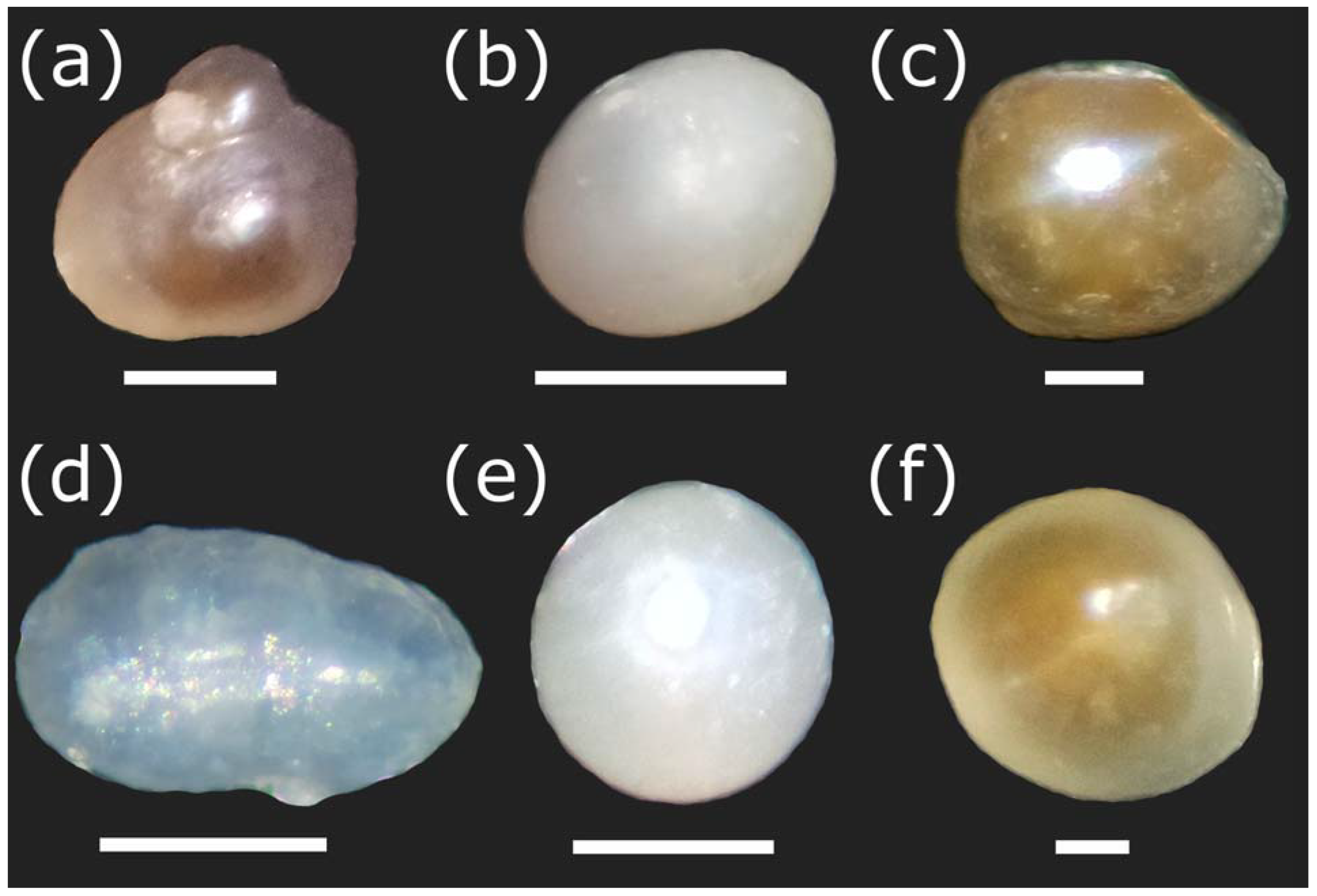
Figure 4.
Pearls of Diplodon chilensis observed with a stereoscopic microscope showing natural colors. (a–e) Pearls obtained from Lake Villarrica; (f) pearls obtained from Lake Caburgua. Note that the pearls (a–c,e,f) were imaged with SEM in Figure 3. In this case, they may have slightly varied in position because different slides were used in both sample viewing techniques. Scale Bar: a = 1 mm; b, e = 300 µm; c, f = 200 µm; d = 500 µm.
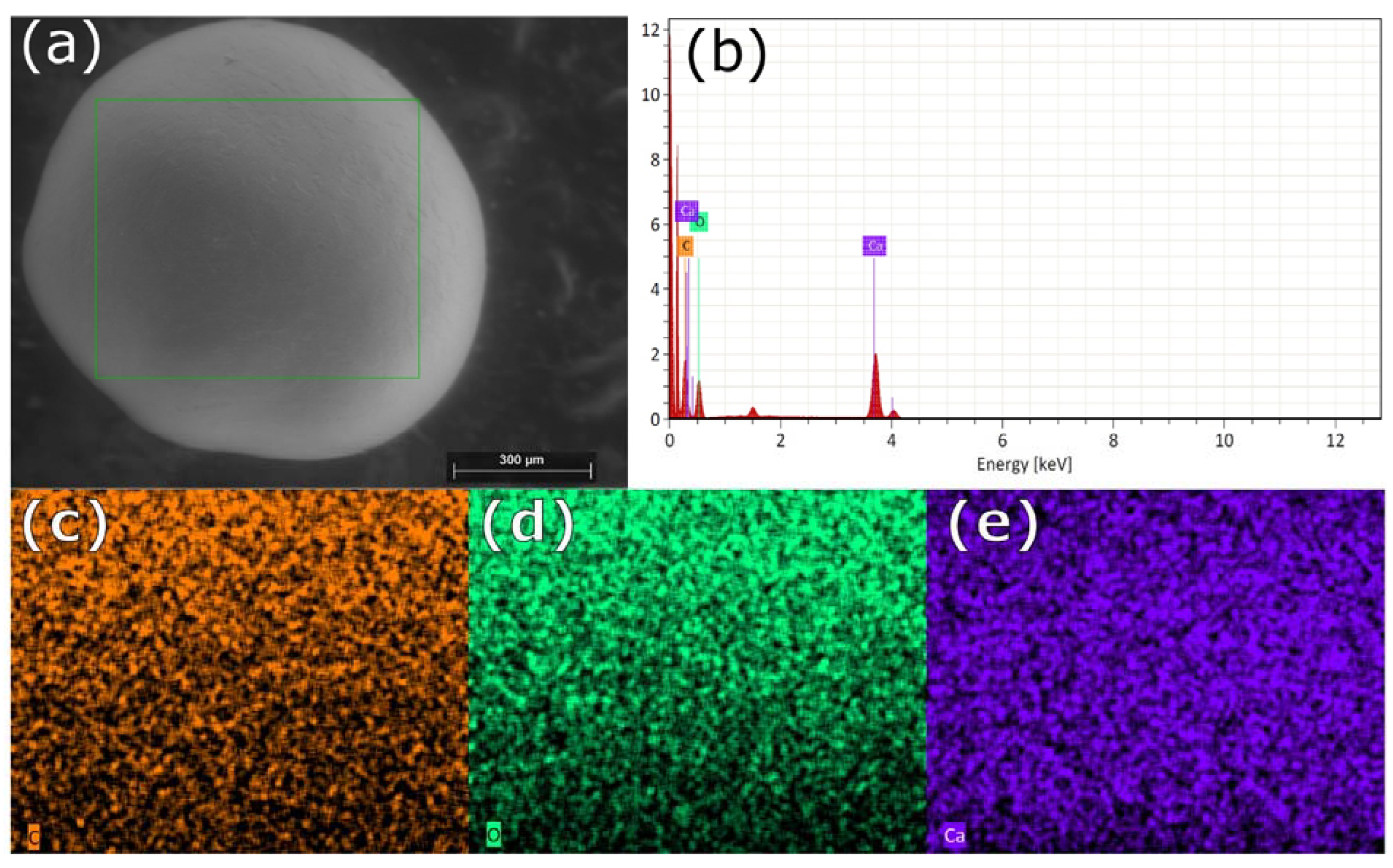
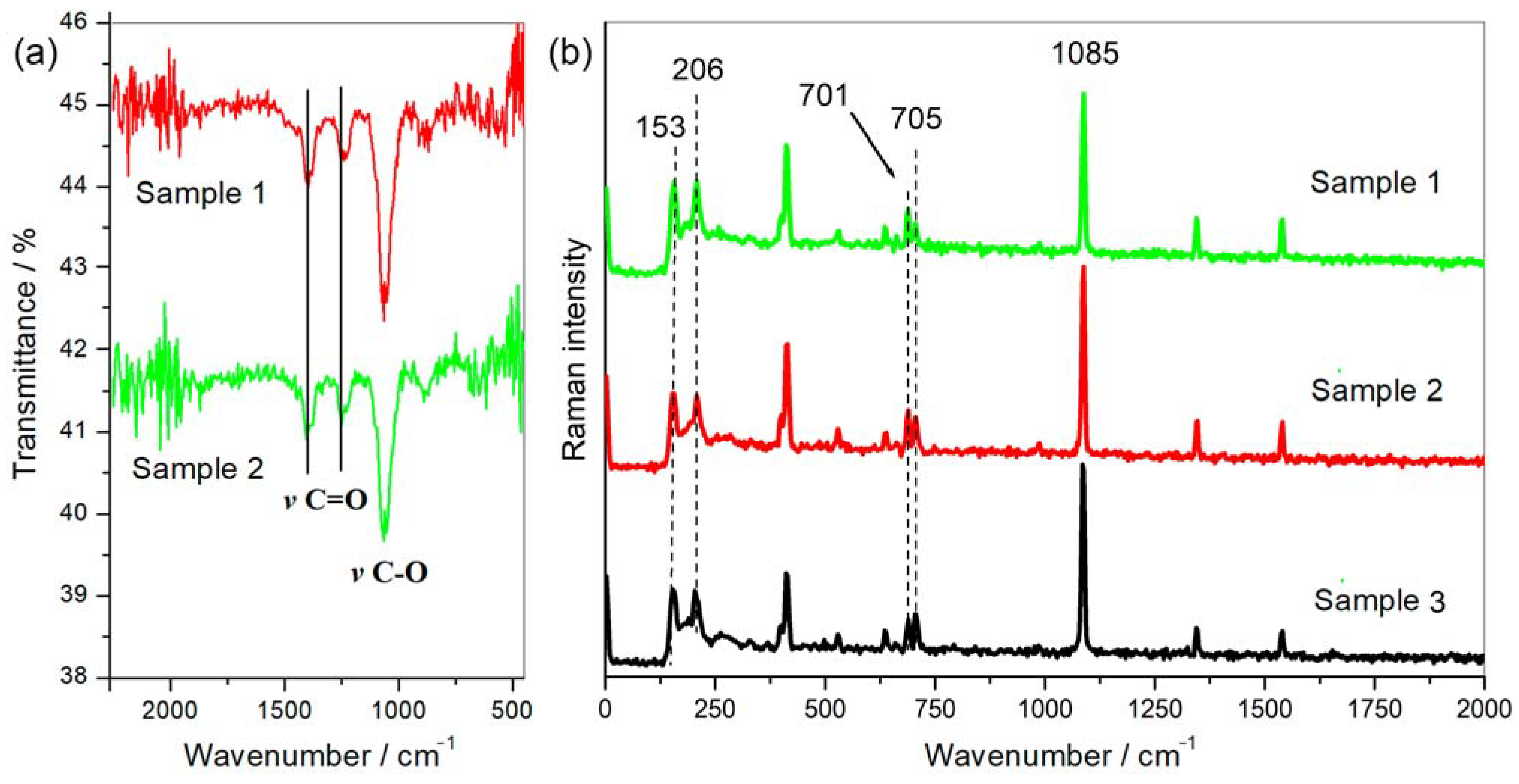
Scanning electron microscopy images indicate that the internal microstructure of D. chilensis pearls is composed of irregular polygonal tablets about 0.4 to 0.55 μm thick. EDX analysis revealed that they are composed principally of Ca, C, and O in stoichiometric amounts indicative of CaCO3 (Figure 5, Table 1 and Tables S2–S4). The FTIR spectrum showed signals located between 1410 and 1255 cm−1, at 1065 cm–1 and between 2800 and 3000 cm−1 (Figure 6). Raman spectra of pearls from different individuals present the main bands at 153, 206, 411, and 1085 cm−1 (Figure 6). Other bands of lesser intensity occurred at 701, 705, and 1340–1540 cm−1.
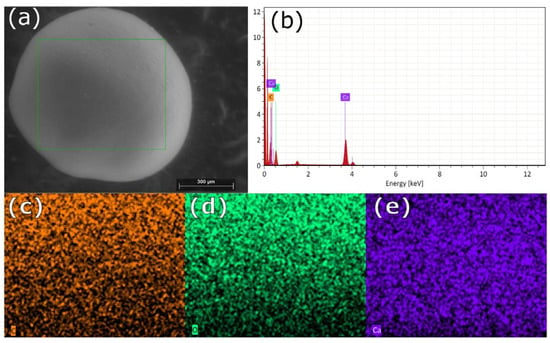
Figure 5.
SEM-EDX surface analysis of pearls from Diplodon chilensis: (a) pearl scanning using SEM; (b) EDX spectrum; (c) C distribution (orange); (d) O distribution (green); (e) Ca distribution (violet). In (a) the green line frames the analyzed area. The red color in (b) shows the energy peaks.

Table 1.
Elemental composition of the pearl surface of nine Diplodon chilensis individuals obtained by SEM-EDX analysis.
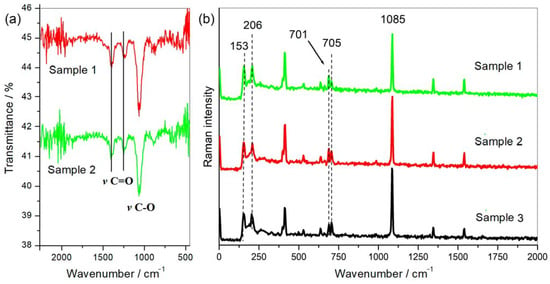
Figure 6.
Pearls spectroscopic analysis. (a) FTIR and (b) Raman spectra obtained from different pearls of Diplodon chilensis show the same qualitative and quantitative profile with the characteristic peaks attributed to aragonite [38,39].
4. Discussion
In this study, the finding of natural pearls in two lacustric populations of the freshwater clam D. chilensis from southern Chile is reported for the first time, also constituting the first report made in the genus Diplodon. Diplodon chilensis can produce pearls in a variety of shapes, sizes, and colors. Pearl production in this species is not a rare phenomenon since many species of the superfamily Unionoidea Rafinesque, 1820, are potential producers of pearls [40]. However, in South America, only a few species have been described with this capability [23,41,42].
In a way, the finding of pearls in D. chilensis is quite surprising since this species has been relatively well studied in Chile and Argentina regarding its soft body [43,44,45,46,47,48,49], but until now the finding of gems had not been reported. In our lab, dissections of clams quickly revealed the presence of conspicuous pearls in the mantle tissue of adult individuals of this species. This could be indicative of differences between D. chilensis populations in pearl production, and, in fact, our results show that natural pearl formation is more common in Lake Villarrica than Lake Caburgua. It is unknown if these differences between populations in both water bodies have a genetic basis. It has been postulated that variations in certain life history traits between D. chilensis populations from lotic and lentic environments could be due to genetic differences [49], although a karyological study contradicted this hypothesis [50]. Consistent with the latter, it has been reported that the populations of this clam from different basins near the Pacific coast in southern Chile are not genetically structured [51], and that differences found in the growth rates can be associated with geographic and limnological parameters [32].
Our results suggest that the shell size does not seem to influence the ability to produce pearls because several individuals from Lake Villarrica in the 4.0 to 5.0 cm size class produced pearls, while twice or three times as many individuals from Lake Caburgua between 5.0 and 7.0 cm did not, except for the 65.6 mm specimen. According to Hohn and Costa [42], acidic waters prevent the proper formation of pearls in bivalves, but this would not be the case since the waters of lakes Caburgua and Villarrica have very similar pH [52], although some variation has been reported in this water body [53]. Rahman et al. [54] detected seven species of pearl-producing bivalves in waters off the coast of Bangladesh with a pH of 8.1 to 8.3.
The size of the pearls varies between mollusk species. The largest natural pearl found in the present study measured 1.9 mm. Pearls with similar sizes have been found in other bivalve species from South America with relatively similar shell lengths. Triplodon corrugatus (Lamarck, 1819) produces pearls from 2 to 3 mm, Castalia ambigua Lamarck, 1819, around 2 mm, and Prisodon obliquus Schumager, 1871, from 2.5 to 4 mm [23]. In Placuna placenta Linnaeus, 1758, from India, the diameter of pearls varies from 1.5 to 4 mm [54]. The cultured pearls in Mercenaria mercenaria (Linnaeus, 1758) and Pinctada margaritifera (Linnaeus, 1758) range from 9 to 13 mm, while in Venerupis aff. Decussata (Linnaeus, 1758), they generally do not exceed 6 mm [55,56]. In the production of cultured pearls, a typical 9-mm round non-beaded pearl takes about 4 years to form [42].
Peaks of the FTIR spectrum located between 1410 and 1255 cm−1 have been associated with the vibrations of the carbonyl group (C=O) while that at 1065 cm−1 with carbon-oxygen (C-O) confirming the presence of carbonated compounds (CO3=) in the samples [57,58]. Signals located between 2800 and 3000 cm−1 correspond to carbon–hydrogen (C-H) vibrations that can be attributed to the organic moiety present in the samples [59]. The main peak of pearls in D. chilensis obtained by Raman at 1085 cm–1 is the main vibration of the CO3= molecule in carbonates [60], which is also present in pearls of other mollusk species [22]. Bands at 1084–1087 cm−1 have been attributed to aragonite or calcite in different bivalve species [61,62,63,64,65,66]. However, distinguishing between the three carbonate polymorphs (aragonite, vaterite, and calcite) using only the most intense band v1 (symmetric stretching) located approximately at 1085 cm−1 is not enough to identify a particular phase [39]. This led some authors to focus on the v2, v3, and v4 vibrational modes of CO3= depicting peaks around 850–900, 1430–1600, and 680–750 cm−1, respectively, to characterize some of the three phases [39,67]. Thus, the v4 band (a doublet) assigned to the out-of-plane vibrational modes of CO3= located at 701 and 705 cm−1 and peaks identified in bivalves at 153 and 206 cm−1 have been attributed to aragonite [22,39,63,67,68,69,70,71], all of them detected and quantified in D. chilensis pearls (Figure 5). No vaterite or calcite could be identified in this clam.
The causes of the variation in the color of pearls are still a subject of investigation [56,71,72]. Bands obtained near or between 1080, 1135, and 1530 cm−1 using Raman analysis, among other bands, suggest that the color of the pearls in several species of bivalves might be due to different chemical compounds, pigments, or optical effects of the sample [56,61,71,72,73,74,75]. However, not all species that have pearls of different colors exhibit these bands [72,76]. On the other hand, there are also other factors involved in the color of pearls, including geographics, genetics, type of mollusk (species), harvest season, water characteristics, depth, quality, and quantity of food (plankton), and the thickness of the pearl [24,42,75,77,78,79,80,81].
To improve the quality of cultured pearls, current trends show that the production of pearls has turned from the frequent improvement of traditional cultivation techniques to producing pearls through selective mollusk breeding [82]. In addition, several studies have detected genes related to the production of pearls in different species of the group, so this economic activity seems to have a very encouraging future [82,83,84]. In this context, the sequencing of the genomes and transcriptomes of some mollusk species [85,86,87,88,89,90] will be important for the identification of genes involved in shell and pearl biomineralization. To date, in D. chilensis, no progress has been made in this aspect of its biology.
Diplodon chilensis, an efficient filter feeder capable of depleting phytoplankton and bacteria from the water column [91,92,93], is one of the most abundant bivalves distributed in Patagonia [94,95,96]. However, this clam has been considered a threatened species in many Chilean water bodies since various localities in the central-southern parts of the country have experienced a decline in densities and even the disappearance of banks due to anthropic activities [32,50,96,97]. A drastic reduction in population density has also been reported in some lacustric towns in the Argentine Patagonia due to water pollution [98]. Despite these situations, the species has been classified as Least Concern (LC) by the IUNC Red List of Threatened Species as it has a widespread distribution throughout Chile and Argentina [99].
The culture of D. chilensis has not been implemented in Chile or Argentina. Although the species is not traded for food or any other purpose in the formal markets of Chile, it is edible and sold in informal urban and rural markets in the south of the country, considering the personal observation of the first author and reports by locals [100]. The use of the species as food is also evidenced by the presence of adult shells deposited in pre-Hispanic shell mounds in Patagonia [100,101,102]. The results reported in the present study may be the first step in boosting pearl production on culture farms in the country.
5. Conclusions
In this article, we provide a description of the main characteristics of the natural pearls produced by the freshwater clam Diplodon chilensis, considering shape, microstructure, and chemical composition. This is the first record of pearls produced in the genus Diplodon. Further studies are needed to investigate whether there are population differences in pearl production and culture feasibility for the species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13132231/s1. Table S1: Pearls found in Diplodon chilensis from southern Chile; Table S2: EDX analysis in a pearl of Diplodon chilensis from Lake Villarrica (individual 8, 1 in Table S1); Table S3: EDX analysis in a pearl of Diplodon chilensis from Lake Villarrica (individual 12, 2 in Table S1); Table S4: EDX analysis in a pearl of Diplodon chilensis from Lake Caburgua (individual 21, 9 in Table S1); Figure S1: SEM–EDX of the pearl’s surface (a) of Diplodon chilensis from Lake Villarrica (individual 4); Figure S2: SEM–EDX of the pearl’s surface (a) of Diplodon chilensis from Lake Villarrica (individual 12); Figure S3: SEM–EDX of the pearl’s surface (a) of Diplodon chilensis from Lake Caburgua (individual 21).
Author Contributions
Conceptualization, G.A.C. and G.C-G.; methodology, G.A.C., C.S., M.S. and G.C.-G.; writing—original draft preparation, G.A.C.; writing—review and editing, G.A.C., M.A.V. and G.C.-G.; visualization, G.A.C., M.A.V., C.S., M.S. and G.C.-G.; supervision, G.A.C., M.A.V. and G.C.-G.; project administration, G.A.C., M.A.V. and G.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was performed in agreement with the ARRIVE guidelines (https://arriveguidelines.org/, accessed on 3 February 2022). The animal study protocol was approved by the Institutional Review Board of the Universidad del Bío–Bío (21 September 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
See Figures S1–S3 and Tables S1–S4. Voucher specimens are housed at the Laboratorio de Malacología y Sistemática Molecular, Universidad del Bío–Bío, Chillán, Chile.
Acknowledgments
We acknowledge anonymous reviewers of the manuscript. We also thank CONICYT-FONDEQUIP Program (No EQM-140088) for the acquisition of Hitachi Scanning Electron Microscope (SEM). M.A.V. acknowledges a Postdoctoral Fellowship VRIP UBB No. 121859/2266/2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ponder, W.; Hutchings, P.; Chapman, R. Overview of the conservation of Australian marine invertebrates. In Report for Environment Australia; Australian Museum: Sydney, Australia, 2002; pp. 1–588. [Google Scholar]
- Rosenberg, G. A new critical estimate of named species-level diversity of the recent Mollusca. Amer. Malac. Bull. 2014, 32, 308–322. [Google Scholar] [CrossRef]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Shell, Body, and Muscles. In Biology and Evolution of the Mollusca; Ponder, W.F., Lindberg, D.R., Ponder, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–890. [Google Scholar]
- Erlandson, J.M.; Rick, T.C.; Braje, T.J.; Steinberg, A.; Vellanoweth, R.L. Human impacts on ancient shellfish: A 10,000 year record from San Miguel Island, California. J. Archaeol. Sci. 2008, 35, 2144–2152. [Google Scholar] [CrossRef]
- Klein, R.G.; Steele, T.E. Archaeological shellfish size and later human evolution in Africa. Proc. Natl. Acad. Sci. USA 2013, 110, 10910–10915. [Google Scholar] [CrossRef] [PubMed]
- Meehan, B. Shell Bed to Shell Midden; Australian Institute of Aboriginal Studies: Canberra, Australia, 1982; pp. 1–189. [Google Scholar]
- Burgos, A.; Younger, A.C.; Wolverton, S. Human mollusk interactions in a changing world. J. Ethnobiol. 2019, 39, 175–181. [Google Scholar] [CrossRef]
- Wells, S.M. International trade in ornamental corals and shells. In Proceedings of the Fourth International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981; Volume 1, pp. 323–330. [Google Scholar]
- Léo Neto, N.A.; Voeks, R.A.; Días, T.L.P.; Alves, R.R.N. Mollusks of Candomblé: Symbolic and ritualistic importance. J. Ethnobiol. Ethnomed. 2012, 8, 10. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Iol, T.L.P. Usos de invertebrados na medicina popular no Brasil e suas implicações para conservação. Trop. Conserv. Sci. 2010, 3, 159–174. [Google Scholar] [CrossRef]
- Codding, B.F.; Whitaker, A.R.; Bird, D.W. Global patterns in the exploitation of shellfish. J. Isl. Coast. Archaeol. 2014, 9, 145–149. [Google Scholar] [CrossRef]
- Clavijo, C. The pearl industry and pioneering research in biology and conservation of pearl mussels (Unionoida) in the río de La Plata Basin. Tentacle 2017, 25, 14–15. [Google Scholar]
- Alves, R.R.N.; Mota, E.L.S.; Dias, T.L.P. Use and commercialization of animals as decoration. In Ethnozoology: Animals in our Lives; Alves, R.R.N., Albuquerque, U.P., Eds.; Elsevier: London, UK, 2018; pp. 261–275. [Google Scholar]
- Viana, M.T. Abalone aquaculture, an overview. World Aquac. 2002, 33, 34–39. [Google Scholar]
- Ahmed, O.O.; Solomon, O.O. Ecological consequences of oysters culture. J. Fish. Livest. Prod. 2016, 4, 4. [Google Scholar] [CrossRef]
- Marin, F.; Le Roy, N.; Marie, B. The formation and mineralization of mollusk shell. Front. Biosci. 2012, 4, 1099–1125. [Google Scholar] [CrossRef] [PubMed]
- Satitkune, S.; Monarumit, N.; Boonmee, C.; Phlayrahan, A.; Promdee, K.; Won-in, K. Combination of FTIR and SEM for identifying freshwater-cultured pearls from different quality. Opt. Spectrosc. 2016, 120, 500–504. [Google Scholar] [CrossRef]
- Ma, H.Y.; Lee, I.-S. Characterization of vaterite in low quality freshwater-cultured pearls. Mater. Sci. Eng. C 2006, 26, 721–723. [Google Scholar] [CrossRef]
- Ma, H.; Su, A.; Zhan, B.; Li, R.-K.; Zhou, L.; Wang, B. Vaterite or aragonite observed in the prismatic layer of freshwater-cultured pearls from South China. Progr. Nat. Sci. 2009, 19, 817–820. [Google Scholar] [CrossRef]
- Ma, Y.; Berland, S.; Andrieu, J.P.; Feng, Q.; Bédouet, L. What is the difference in organic matrix of aragonite vs. vaterite polymorph in natural shell and pearl? Study of the pearl-forming freshwater bivalve mollusc Hyriopsis cumingii. Mater. Sci. Eng. C 2013, 33, 1521–1529. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Cuif, J.-P.; Dauphin, Y.; Cusack, M. Crystallography of calcite in pearls. Eur. J. Mineral. 2014, 26, 507–516. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Makhlooq, F.; Mohamed, F.; Al-Alawi, A. Raman spectroscopy of natural and cultured pearls and pearl producing mollusc shells. J. Raman Spectrosc. 2020, 51, 1813–1821. [Google Scholar] [CrossRef]
- Barros, M.R.F.; Chagas, R.A.; Santos, W.C.R.; Abreu, V.S.; Silva, R.E.O.; Herrmann, M. Capítulo 4. Bivalves límnicos da Hyriidae que um potencial para um cultivo de pérolas na região tropical do Brasil. In Aquicultura e pesca: Adversidades e resultados; Zuffo, A.M., Ed.; Ponta Grossa: Atena, Brazil, 2019; pp. 23–27. [Google Scholar] [CrossRef]
- Kanjanachatree, K.; Limsathapornkul, N.; Inthonjaroen, A.; Ritchie, R.J. Effects of mollusk size on growth and color of cultured half-pearls from Phuket, Thailand. Gems Gemol. 2019, 35, 167–175. [Google Scholar] [CrossRef]
- Taylor, J.J.; Strack, E. Pearl production. In The Pearl Oyster; Southgate, P.C., Lucas, J.S., Eds.; Elsevier: Oxford, UK, 2008; pp. 273–302. [Google Scholar]
- Walker, K.F.; Byrne, M.; Hickey, C.W.; Roper, D.S. Freshwater mussels (Hyriidae) of Australasia. In Ecology and Evolutionary Biology of the Freshwater Mussels (Unionoida); Bauer, G., Wächtler, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–31. [Google Scholar]
- Parodiz, J.J. Annotated catalogue of the genus Diplodon (Unionacea-Hyriidae). Sterkiana 1968, 30, 1–22. [Google Scholar]
- Graf, D.L.; Cummings, K.S. The Freshwater Mussels (Unionoida) of the World (and Other Less Consequential Bivalves), MUSSEL Project Web Site. 2022. Available online: http://www.mussel-project.net/ (accessed on 22 September 2022).
- MolluscaBase. Diplodon Spix. 1827. Available online: http://www.molluscabase.org/aphia.php?p=taxdetails&id=850991 (accessed on 22 September 2022).
- Jacob, D.E.; Soldati, A.L.; Wirth, R.; Huth, J.M.; Wehrmeister, U.; Hofmeister, W. Nanostructure and composition of bivalve shells. Geophys. Res. Abstr. 2009, 11, 7721. [Google Scholar]
- Pereira, D.; Mansur, M.C.D.; Duarte, L.D.S.; de Oliveira, A.S.; Mansur Pimpão, D.; Callil, C.T.; Ituarte, C.; Parada, E.; Peredo, S.; Darrigran, G.; et al. Bivalve distribution in hydrographic regions in South America: Historical overview and conservation. Hydrobiologia 2014, 735, 15–44. [Google Scholar] [CrossRef]
- Valdovinos, C.; Pedreros, P. Geographic variations in shell growth rates of the mussel Diplodon chilensis from temperate lakes of Chile: Implications for biodiversity conservation. Limnologica 2007, 37, 63–75. [Google Scholar] [CrossRef][Green Version]
- Bonetto, A.; Tassara, M.P.; Rummi, A. Australis n. subgen. of Diplodon Spix (Bivalvia, Unionacea) and its possible relationships with Australian Hyriidae. Bol. Soc. Biol. Concepc. 1986, 57, 55–61. [Google Scholar]
- Parada, E.; Peredo, S. Estado de conocimiento de los bivalvos dulceacuícolas de Chile. Gayana 2006, 70, 82–87. [Google Scholar] [CrossRef][Green Version]
- Parada, E.; Peredo, S. Diplodon patagonicus (Bivalvia: Hyriidae): To be or not to be. Gayana 2008, 72, 266–267. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Santos, S.B.; Mansur, M.C.D. Freshwater mussels from South America: State of the art of Unionida, especially Rhipidodontini. Biota Neotrop. 2017, 17, e20170341. [Google Scholar] [CrossRef]
- Dreher Mansur, M.C.; Miyahira, I.C.; Arruda, J.O.; Antoniazzi, T.N.; Pimpão, D.M. Key to Unionida. In Thorp and Covich’s Freshwater Invertebrates: Volume 5: Keys to Neotropical and Antarctic Fauna, 4th ed.; Damborenea, C., Rogers, D.C., Thorp, J.H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 409–430. [Google Scholar]
- Soldati, A.L.; Jacob, D.E.; Bianchi, M.M.; Hajduk, A. Microstructure and polymorphism of Diplodon chilensis patagonicus (d’Orbigny 1835) recent shells. Gayana 2010, 74, 61–69. [Google Scholar] [CrossRef]
- Wehrmeister, U.; Jacob, D.E.; Soldati, A.L.; Häger, T.; Hofmeister, W. Vaterite in freshwater cultured pearls from China and Japan. J. Gemm. 2007, 31, 269–276. [Google Scholar] [CrossRef]
- Alves, T.; Lima, P.; Lima, G.M.; Cunha, M.C.; Ferreira, S.; Domingues, B.; Machado, J. Phytoplankton composition of the water and gastrointestinal tract of the mussel Diplodon enno (Ortmann, 1921) from São Francisco river (Bahia, Brazil). Braz. J. Biol. 2016, 76, 352–359. [Google Scholar] [CrossRef][Green Version]
- Alves, R. Biologia de Pteria hirundo, ostra perlífera nativa do Brasil. Ph.D. Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2010. [Google Scholar]
- Hohn, H.; Costa, M.L. Ocorrência de ostras perlíferas no Marajó, rio Pará. Rev. Esc. Minas. 2002, 55, 61–64. [Google Scholar] [CrossRef]
- Peredo, S.; Parada, E. Gonadal organization and gametogenesis in the fresh-water mussel Diplodon chilensis chilensis. Veliger 1984, 27, 126–133. [Google Scholar]
- Peredo, S.; Parada, E. Reproductive cycle in the freshwater mussel Diplodon chilensis chilensis (Mollusca: Bivalvia). Veliger 1986, 28, 418–425. [Google Scholar]
- Parada, E.; Peredo, S.; Lara, G.; Valdebenito, I. Growth, age and life span of the freshwater mussel Diplodon chilensis (Gray 1828). Arch. Hydrobiol. 1989, 115, 563–573. [Google Scholar] [CrossRef]
- Parada, E.; Peredo, S.; Gallardo, C. Tácticas reproductivas y dinámica poblacional de Diplodon chilensis (Gray, 1828) (Bivalvia: Hyriidae). Rev. Chil. Hist. Nat. 1990, 63, 23–35. [Google Scholar]
- Lara, G.; Parada, E. Seasonal changes in the condition index of Diplodon chilensis chilensis (Gray, 1828) in Sandy and muddy substrata, Villarrica Lake, Chile (39°18′ S; 72°05′ W). Bol. Soc. Biol. Concepc. 1991, 62, 99–106. [Google Scholar]
- Semenas, L.; Brugni, N.; Negro, R. Características poblacionales y ciclo de vida de Diplodon chilensis (d’Orbigny, 1835) (Hyriidae, Bivalvia) en el lago Gutiérrez (Patagonia, Argentina). Ecol. Austral. 2002, 12, 29–40. [Google Scholar]
- Parada, E.; Peredo, S. Un enfoque ecológico evolutivo de las estrategias de historia de vida de los Hyriidos chilenos (Mollusca, Bivalvia). Bol. Soc. Biol. Concepc. 1994, 65, 71–80. [Google Scholar]
- Peredo, S.; Jara-Seguel, P.; Parada, E.; Palma-Rojas, C. Comparative karyology of lentic and lotic populations of Diplodon chilensis chilensis (Bivalvia: Hyriidae). Veliger 2003, 46, 314–319. [Google Scholar]
- Fuentealba, C.; Figueroa, R.; González, F.; Palma, M. Variabilidad genética local del bivalvo dulceacuícola Diplodon chilensis (Gray 1828) proveniente de tres lagos Nahuelbutanos. Gayana 2010, 74, 113–124. [Google Scholar] [CrossRef]
- Campos, H. Limnological study of Araucanian lakes (Chile). Verh. lnternat. Verein. Limnol. 1984, 22, 1319–1327. [Google Scholar] [CrossRef]
- Valenzuela Moure, A. Las aguas del lago Villarrica: Calidad y procesos fisicoquímicos de los recursos hídricos que lo alimentan. Bachelor’s thesis of Geologist, Universidad de Chile, Santiago, Chile, 2019. [Google Scholar]
- Rahman, M.A.; Parvej, M.R.; Rashid, M.H.; Hoq, E. Availability of pearl producing marine bivalves in south-eastern coast of Bangladesh and culture potentialities. J. Fish. 2015, 3, 293–296. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Notari, F. Natural pearls of the Veneridae family. Gems Gemol. 2008, 44, 374–375. [Google Scholar]
- Karampelas, S.; Fritsch, E.; Gauthier, J.P.; Hainschwang, T. UV-Vis-NIR reflectance spectroscopy of natural-color saltwater cultured pearls from Pinctada margaritifera. Gems Gemol. 2011, 47, 31–35. [Google Scholar] [CrossRef]
- Al-Hosney, H.A.; Grassian, V.H. Water, sulfur dioxide and nitric acid adsorption on calcium carbonate: A transmission and ATR-FTIR study. Phys. Chem. Chem. Phys. 2005, 7, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Williams, C.T.; Ebner, A.D.; Ritter, J.A. In situ FTIR spectroscopic analysis of carbonate transformations during adsorption and desorption of CO2 in K-promoted HTlc. Chem. Mater. 2010, 22, 3519–3526. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Thomas, D.E.; Mclnerney, B.V.; Molloy, M.P.; Jacob, D.E. Organic macromolecules in shells of Arctica islandica: Comparison with nacro-prismatic bivalve shells. Mar. Biol. 2017, 164, 208–220. [Google Scholar] [CrossRef]
- Wehrmeister, U.; Jacob, D.E.; Soldati, A.L.; Loges, N.; Häger, T.; Hofmeister, W. Amorphous, nanocrystalline and crystalline calcium carbonates in biological materials. J. Raman Spectrosc. 2011, 42, 926–935. [Google Scholar] [CrossRef]
- Gutmannsbauer, W.; Hänni, H.A. Structural and chemical investigations on shells and pearls of nacre forming salt- and fresh-water bivalve molluscs. J. Gemmol. 1994, 24, 241–252. [Google Scholar] [CrossRef]
- Liping, L.; Zhonghui, C. Cultured Pearls and colour-changed cultured pearls: Raman spectra. J. Gemmol. 2001, 27, 449–455. [Google Scholar] [CrossRef]
- Fengming, H.; Xinqiang, Y.; Mingxing, Y.; Zhonghui, C. Pearl cultivation in Donggou, Ezhou, Hubai, and cathodoluminescence of cultured pearls. J. Gemmol. 2003, 28, 449–462. [Google Scholar] [CrossRef]
- Sturman, N. Observations on pearls reportedly from the Pinnidae family (Pen Pearls). Gems Gemol. 2014, 50, 202–215. [Google Scholar] [CrossRef]
- Scarratt, K.; Hänni, H. Pearls from the lion’s paw scallop. J. Gemmol. 2004, 29, 193–203. [Google Scholar] [CrossRef]
- Kiefert, L.; McLaurin, D.; Arizmendi, E.; Hänni, H.A.; Elen, S. Cultured pearls from the Gulf of California, Mexico. Gems Gemol. 2004, 40, 26–38. [Google Scholar] [CrossRef]
- Carteret, C.; Dandeu, A.; Moussaoui, S.; Muhr, H.; Humbert, B.; Plasa, E. Polymorphism studied by lattice phonon Raman spectroscopy and statistical mixture analysis method. Application to calcium carbonate polymorphs during batch crystallization. Cryst. Growth Des. 2009, 9, 807–812. [Google Scholar] [CrossRef]
- Ma, Y.F.; Gao, Y.H.; Feng, Q.L. Effects of pH and temperature on CaCO3 crystallization in aqueous solution with water soluble matrix of pearls. J. Cryst. Growth 2010, 312, 3165–3170. [Google Scholar] [CrossRef]
- Otter, L.M.; Agbaje, O.B.A.; Huong, L.T.T.; Hanger, T.; Jacob, D.E. Akoya cultured pearl farming in eastern Australia. Gems Gemol. 2017, 53, 423–437. [Google Scholar] [CrossRef]
- Gabrielli, C.; Jaouhari, R.; Joiret, S.; Maurin, G. In situ Raman spectroscopy applied to electrochemical scaling. Determination of the structure of vaterite. J. Raman Spectrosc. 2000, 31, 497–501. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Mevellec, J.-Y.; Gauthier, J.-P.; Sklavounos, S.; Soldatos, T. Determination by Raman scattering of the nature of pigments in cultured freshwater pearls from the mollusk Hyriopsis cumingi. J. Raman Spectrosc. 2007, 38, 217–230. [Google Scholar] [CrossRef]
- Cartier, L.; Krzemnicki, M. Golden South Sea cultured pearls: Cultivation steps & gemmological investigations. J. Gemmol. Assoc. Hong Kong 2016, 37, 16–21. [Google Scholar]
- Gauthier, J.-P.; Caseiro, J.; Lasnier, B. The red pearls of Pinna nobilis. Aust. Gemmol. 1997, 19, 422–426. [Google Scholar]
- Elen, S. Spectral reflectance and fluorescence characteristics of natural-color and heat-treated “golden” South Seas cultured pearls. Gems Gemol. 2001, 37, 114–123. [Google Scholar] [CrossRef]
- Snow, M.R.; Pring, A.; Self, P.; Losic, D. The origin of the color of pearls in iridescence from nano-composite structures of the nacre. Am. Mineral. 2004, 89, 1353–1358. [Google Scholar] [CrossRef]
- Elen, S. Identification of yellow cultured pearls from the black-lipped oyster Pinctada margaritifera. Gems Gemol. 2002, 38, 66–72. [Google Scholar] [CrossRef]
- Shor, R. From single source to global free market: The transformation of the cultured pearl industry. Gems Gemol. 2007, 43, 200–226. [Google Scholar] [CrossRef]
- Ky, C.L.; Blay, C.; Sham-Koua, M.; Vanaa, V.; Lo, C.; Cabral, P. Family effect on cultured pearl quality in black-lipped pearl oyster Pinctada margaritifera and insights for genetic improvement. Aquat. Living Resour. 2013, 26, 133–145. [Google Scholar] [CrossRef]
- Ky, C.L.; Molinari, N.; Moe, E.; Pommier, S. Impact of season and grafter skill on nucleus retention and pearl oyster mortality rate in Pinctada margaritifera aquaculture. Aquac. Int. 2014, 22, 1689–1701. [Google Scholar] [CrossRef]
- Ky, C.L.; Blay, C.; Aiho, V.; Cabral, P.; Le Moullac, G.; Lo, C. Macro-geographical differences influenced by family-based expression on cultured pearl grade, shape and colour in the black-lip pearl oyster Pinctada margaritifera: A preliminary case study in French Polynesia. Aquac. Res. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Kanjanachatree, K.; Piyathamrongrut, K.; Inthonjaroen, N. Effects of sea depths and sizes of winged pearl oysters (Pteria penguin) on pearl culture. Warasan Songkhla Nakharin Sakha Witthayasat Lae Technol. 2003, 25, 659–671. [Google Scholar]
- McDougall, C.; Aguilera, F.; Moase, P.; Lucas, J.S.; Degnan, B.M. Pearls. Curr. Biol. 2013, 23, R671–R673. [Google Scholar] [CrossRef][Green Version]
- McDougall, C.; Aguilera, F.; Shokoohmand, A.; Moase, P.; Degnan, B.M. Pearl sac gene expression profiles associated with pearl attributes in the silver-lip pearl oyster, Pinctada maxima. Front. Genet. 2021, 11, 597459. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wu, W. Transcriptome analysis of the freshwater pearl mussel (Cristaria plicata) mantle unravels genes involved in the formation of shell and pearl. Mol. Genet. Genom. 2017, 292, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Blay, C.; Planes, S.; Ky, C.-L. Cultured pearl surface quality profiling by the shell matrix protein gene expression in the biomineralised pearl sac tissue of Pinctada margaritifera. Mar. Biotechnol. 2018, 20, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Mariom; Take, S.; Igarashi, Y.; Yoshitake, K.; Asakawa, S.; Maeyama, K.; Nagai, K.; Watabe, S.; Kinoshita, S. Gene expression profiles at different stages for formation of pearl sac and pearl in the pearl oyster Pinctada fucata. BMC Genom. 2019, 20, 240. [Google Scholar] [CrossRef]
- Jiao, Y.; Gu, Z.; Luo, S.; Deng, Y. Evolutionary and functional analysis of MyD88 genes in pearl oyster Pinctada fucata martensii. Fish Shellfish Immunol. 2020, 99, 322–330. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kawashima, T.; Koyanagi, R.; Gyoja, F.; Tanaka, M.; Ikuta, T.; Shoguchi, E.; Fujiwara, M.; Shinzato, C.; Hisata, K.; et al. Draft genome of the pearl oyster Pinctada fucata: A platform for understanding bivalve biology. DNA Res. 2012, 19, 117–130. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, C.; Gu, Z.; Zhan, X.; Wang, Y.; Wang, A. Characterization of the pearl oyster (Pinctada martensii) mantle transcriptome unravels biomineralization genes. Mar. Biotechnol. 2013, 15, 175–187. [Google Scholar] [CrossRef]
- Song, H.; Guo, X.; Sun, L.; Wang, Q.; Han, F.; Wang, H.; Wray, G.A.; Davidson, P.; Wang, Q.; Hu, Z.; et al. The hard clam genome reveals massive expansion and diversification of inhibitors of apoptosis in Bivalvia. BMC Biol. 2021, 19, 15. [Google Scholar] [CrossRef]
- Valdovinos, C.; Cuevas, R. Tasas de aclarancia de Diplodon chilensis (Bivalvia, Hyriidae): Un suspensívoro bentónico dulceacuícola de Chile Central. Medio Ambiente 1996, 13, 114–118. [Google Scholar]
- Soto, D.; Mena, G. Filter feeding by the freshwater mussel, Diplodon chilensis, as a biocontrol of salmon farming eutrophication. Aquaculture 1999, 171, 65–81. [Google Scholar] [CrossRef]
- Lara, G.; Contreras, A.; Encina, F. La almeja de agua dulce Diplodon chilensis (Bivalvia, Hyriidae) potencial biofiltro para disminuir los niveles de coliformes en pozos. Experimentos de laboratorio. Gayana 2002, 66, 113–118. [Google Scholar] [CrossRef]
- Torres, S.; Cao, L.; Gutiérrez Gregoric, D.E.; de Lucía, M.; Brea, F.; Darrigran, G. Distribution of the Unionida (Bivalvia, Paleoheterodonta) from Argentina and its conservation in the Southern Neotropical Region. PLoS ONE 2018, 13, e0203616. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Darrigran, G.; Damborenea, C. Distribución del género Diplodon (Mollusca: Bivalvia: Hyriidae) en la cuenca del Plata (Argentina) mediante el uso de colecciones biológicas. Número Especial I: Aguas. AUGMDOMUS 2013, 5, 90–99. [Google Scholar]
- Bonetto, A.A. Náyades de la Patagonia. Rev. Asoc. Cienc. Nat. Litoral 1973, 4, 177–185. [Google Scholar] [CrossRef]
- Peredo, S.; Parada, E.; Valdebenito, I.; Peredo, M. Relocation of the freshwater mussel Diplodon chilensis (Hyriidae) as a strategy for its conservation and management. J. Molluscan Stud. 2005, 71, 195–198. [Google Scholar] [CrossRef][Green Version]
- Rocchetta, I.; Lomovasky, B.J.; Yusseppone, M.S.; Sabatini, S.E.; Bieczynski, F.; Molina, M.D.; Luquet, C.M. Growth, abundance, morphometric and metabolic parameters of three populations of Diplodon chilensis subject to different levels of natural and anthropogenic organic matter input in a glaciar lake of North Patagonia. Limnologica 2014, 44, 72–80. [Google Scholar] [CrossRef]
- Bogan, A.E.; Cummings, K. Diplodon chilensis. The IUCN Red List of Threatened Species. 2011. Available online: https://www.iucnredlist.org/species/189076/8675023 (accessed on 22 September 2022).
- Lara, G.; Parada, E.; Peredo, S.; Inostroza, J.; Mora, H. La almeja de agua dulce Diplodon chilensis (Gray, 1828), un recurso potencial. Bol. Mus. Reg. Araucanía 1988, 3, 33–40. [Google Scholar]
- Prates, L.; Marsans, N. The use of freshwater mollusks (Diplodon chilensis patagonicus) at Angostura 1 site (General Conesa District, Río Negro province, Argentina). Intersecc. Antropol. 2007, 8, 355–359. [Google Scholar]
- Jackson, D.; Jackson, D. Antecedentes arqueológicos del género Diplodon (Spix, 1827) (Bivalvia, Hyriidae) en Chile. Gayana 2008, 72, 188–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).