Simple Summary
Haemosporidian parasites infect terrestrial vertebrates as well as avian species worldwide. However, for resident birds of the Rallidae family, such as White-breasted Waterhens, few data are available regarding haemosporidian infection. Therefore, this study aimed to detect haemosporidian infection in White-breasted Waterhens, microscopic and genetic examinations of blood samples were performed. Four species of haemosporidians were identified; however, the morphological features of only Haemoproteus gallinulae are presented. Phylogenetic analysis of standard DNA sequences revealed a close relationship between Haemoproteus species and their classification into the subgenus Parahaemoproteus. However, three Plasmodium species were found to have a wide range of hosts. The phylogenetic tree of this parasite clearly showed individual clusters. For one of them, the species was not identified; however, it matched a previously reported species that was isolated from mosquitoes. Two of the Plasmodium species were identified as well-known, generalist parasites that cause severe diseases in hosts: P. collidatum and P. elongatum. This study revealed the role of White-breasted Waterhens as carriers of haemosporidian parasites. Our findings provide potential information for further research.
Abstract
Haemosporidian parasites are vector-borne parasites infecting terrestrial vertebrates as well as avian species, such as the White-breasted Waterhen, a Gruiformes waterbird found in lowlands near wetlands and distributed throughout Thailand. However, information regarding haemosporidia infection in this species is lacking. To establish regional information, 17 blood samples were collected from White-breasted Waterhens. Four haemoparasite lineages were identified in six blood samples: Haemoproteus gallinulae, Plasmodium collidatum, Plasmodium elongatum, and an unidentified Plasmodium species. H. gallinulae was characterized with morphological features in White-breasted Waterhens for the first time; the morphological characteristics were consistent with previous descriptions. H. gallinulae was more closely related to Haemoproteus species of Passeriformes birds than to those of Gruiformes birds. The Plasmodium parasites infecting these White-breasted Waterhens previously caused severe avian malaria in other host species. The unidentified Plasmodium species had rarely been documented, although it was reported in the Culex vector and was possibly associated with specialist parasites either as host or habitat. Our findings reveal multiple haemosporidian species reflecting the role of this avian host as a carrier of haemosporidians. This study offers species records and molecular materials that may provide critical information for further targeted research into these haemosporidia.
1. Introduction
Haemosporidian parasites in all terrestrial vertebrate, likewise in avian species, are vector-borne blood parasites classified into four genera: Haemoproteus, Plasmodium, Leucocytozoon, and Fallisia [1]. These parasites are not a frequent cause of death, although the resulting infection can be mild to severe, dependent on the degree of immunity to the parasitic infection [2] and of the infected host species (tolerant or resistant) [3]. Moreover, avian blood parasite infections affect body conditions and growth rate of tail feather, thereby possibly reducing reproductive success in migratory birds and wild passerines [4,5]. However, haemosporidian infection was found to be occasionally highly virulent, such as infections caused by Plasmodium homocircumflexum, which further caused anemia and cerebral paralysis and led to the death of experimentally infected canaries [6]. Highly pathogenic infections caused by P. elongatum (GRW6) and P. matutinum (LINN1) in New Zealand Kiwi (Apteryx spp.) have also been reported [7]. Furthermore, infection with haemosporidian parasites contributed to population decline in avian species such as European Turtle Dove (Streptopelia turtur) [8] and Skylark (Alauda arvensis) [9]. Therefore, a survey of pathogens—expanding on the knowledge of causes of morbidity and mortality—can be implemented to manage and prevent haemosporidian infections.
The White-breasted Waterhen (Amaurornis phoenicurus) is a waterbird of the family Rallidae in the order Gruiformes that is resident in south and southeast Asia. In Thailand, these birds are generally found near freshwater in agriculture fields [10] and are considerable an indicator of the successful restoration of wetland biodiversity [11]. In residential regions, a survey of parasites in White-breasted Waterhens previously revealed helminth parasites in Japan and Thailand [12,13] and an unidentified Plasmodium parasite in Malaysia [14]. However, the White-breasted Waterhen was found to be an additional host of Haemoproteus gallinule, similar to other Rallidae such as Red-legged Crake (Rallina fasciata) and Ruddy-breasted Crake (Porzana fusca) [15]. Haemoproteus parasites have rarely been reported in wild birds of Thailand. Only eight morphospecies have been solely isolated from passerines [16]. Furthermore, in a previous study, Haemoproteus parasites of Gruiformes were found to have only four morphospecies, and singular H. antigonis was available for molecular detection and identification (barcoding) [17]. A survey of haemosporidian parasites in White-breasted Waterhens can provide potential information on haemosporidia infections in Rallidae; furthermore, it can provide insights into the regional distribution of wildlife parasites in White-breasted Waterhens. Therefore, this survey aimed to detect and describe the haemosporidian parasites found in White-breasted Waterhens based on morphological and molecular examinations.
2. Materials and Methods
2.1. Study Sites and Sample Collection
White-breasted Waterhens (Amaurornis phoenicurus) are a resident bird that frequently forages around lowland paddy fields where abundant with insects, small fish, aquatic invertebrates, and grains. This bird nestled in dry locations and colonized nearly freshwater. During the 2021 dry season, birds were decoy-trapped individually using bird sounds and mist nets in the Salaya suburb, Phutthamonthon district, Thailand (13°48′7″ N, 100°19′18″ E). This location is represented and relates to the White-breasted Waterhen habitat. Seventeen birds were trapped and transported to The Monitoring and Surveillance Center for Zoonotic Diseases in Wildlife and Exotic Animals, Faculty of Veterinary Science, Mahidol University. Approximately 0.5 mL of blood was collected from the brachial vein and a thin blood smear was immediately prepared, allowed to air-dry, and fixed with absolute methanol. Duplicate or triplicate smears were prepared for each bird. The remainder of the blood sample was transferred into a 1.5-mL EDTA tube and stored at −20 °C until DNA extraction was performed. Then, all birds were released back into their original habitat at the sampling site. All procedures involving White-breasted Waterhens were reviewed and approved by the Faculty of Veterinary Science, Animal Care and Use Committee of Mahidol University (protocol no. MUVS-2020-11-55).
2.2. Staining and Microscopic Examination
Thin blood smears were stained with Giemsa’s azure–eosin–methylene blue solution (Merk KGaA, Darmstadt, Germany) in accordance with the manufacturer’s recommended protocol with phosphate buffer (pH 7.2) at 5% concentration for 1 h. Microscopic examinations of at least 100 fields were performed under low magnification (×400) for 5–10 min and high magnification (×1000) using a Nikon/ECIPSE Ti2 Inverted Microscope (Nikon Instruments Inc., New York, NY, USA). The intensity of infection was estimated from the number of infected erythrocytes per 10,000 cells [18]. The morphological and morphometric characteristics of the uninfected and infected erythrocytes and parasite gametocytes were imaged and measured using the NIS Elements AR 5.41.00 software (Nikon Instruments Inc., New York, NY, USA). Haemoproteus species were identified using the key morphologic characteristics based on “Keys to the avian Haemoproteus parasites” [17]. To estimate the difference in percentage of white blood cells (WBC) between infected and uninfected birds, the differential WBC count percentage was measured using blood smears of better quality following a previously reported protocol [19].
2.3. DNA Extraction and Mitochondrial Cytochrome b Gene Amplification
DNA was extracted from whole blood samples using the Genomic DNA Mini Kit (blood/cultured cells) (Geneaid Biotech, New Taipei City, Taiwan) in accordance with the manufacturer’s recommended protocol. The partial cytochrome b (cytb) gene was amplified using nested polymerase chain reaction (PCR), as previously described [20], with the outer primers HaemNFI and HaemNR3 and the inner primers HaemF and HaemR2. The initial PCR conditions were as follows: initial denaturation at 94 °C for 30 s; 20 cycles of amplification at 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 45 s; and final extension at 72 °C for 10 min. The nested steps included the same PCRs with 35 cycles of amplification. The PCR reactions were performed in a 20 µL reaction volume containing 1 µL of DNA template or PCR product, 10 µL of 2 × PCR master mix solution (i-Taq™), 1 µL of each primer (10 µM), and up to 20 µL of ultrapure water. Ultrapure water and DNA samples extracted from positive-microscopy slides were used as negative and positive controls, respectively. Subsequently, 5 µL of the PCR product was used to determine the size of the amplicon by gel electrophoresis on 2% agarose gel using GelRed® (Biotium, Fremont, CA, USA) and visualized using a BioSens SC-Series 710 gel documentation system (GenXpress, Wiener Neudorf, Austria). The positive PCR products were subjected to nucleotide sequencing based on the Sanger sequencing method by a commercial company (Bionics, Seoul, Republic of Korea) using both strands of inner primers. The electropherograms of all obtained sequences were carefully checked for double bases, which indicate co-infection, using BioEdit version 7.0.5.3 (Ibis Biosciences, Carlsbad, CA, USA) [21]. Sequences with ambiguous sites for multiple infections were rechecked using DnaSP version 6.12.03 [22]. Subsequently, the sequences were subjected to BLASTn searching using the NCBI GenBank and MalAvi databases [23]. The obtained nucleotides of cytb sequences with no match found to those previously published were considered to be a new lineage of haemosporidia and named according to the MalAvi nomenclature [23]. The obtained cytb sequences of all detected haemosporidian parasites were submitted and deposited to GenBank under accession numbers OQ565584–OQ565589.
2.4. Phylogenetic Analyses of the Partial Cytochrome b Gene Sequences
A phylogenetic tree was constructed to determine the relationships between the newly obtained molecular characteristics of H. gallinulae and other Plasmodium parasites and the previously reported morphospecies of haemosporidian parasites in Asia and Oceania, which is the habitat of the avian host White-breasted Waterhen. The alignment was constructed with 27 partial cytb sequences (479 bp) of well-known morphospecies of Haemoproteus and Plasmodium species, 13 individual lineages, and Leucocytozoon majoris (as the outgroup) using MAFFT version 7.520 software [24]. A Bayesian inference phylogeny was constructed using MrBayes version 3.2 software [25]. The best-fit substitution model was a general time-reversible model with gamma-distributed substitution rates and a proportion of invariant sites (GTR + I + G) selected using the Akaike information criterion in MrModeltest 2.4 software [26]. A Bayesian inference analysis was run for three million (×106) generations, with sampling every hundredth generation. The first 25% of the tree was discarded as burn-in. The remaining 22,500 trees were calculated to determine the majority rule consensus tree, which was then visualized using FigTree.v1.4.4 [27]. The sequence divergence between sequences was calculated using MEGA X [28] with the Jukes–Cantor model [29].
2.5. Statistical Analysis
The heterophil-to-lymphocyte (H:L) ratio between uninfected and infected White-breasted Waterhens was compared using the Mann–Whitney U test. Comparisons of mean morphometric measurements between uninfected and infected erythrocytes as well as between macrogametocytes and microgametocytes were performed using Student’s independent t-test, after normal distribution and homogeneity of variance were confirmed using Shapiro–Wilk’s test and Levene’s test, respectively. A p-value of ≤0.05 was considered statistically significant.
3. Results
The overall prevalence of haemosporidian infection was 35% (6/17). No birds in this study had signs of pathogenicity. The differential WBC count of haemosporidian infection is shown in Table 1, although it was difficult to determine the stress indicator from the percentage of individual WBCs. However, the H:L ratio of differential WBCs was significantly higher (U = 27, p < 0.001) in infected White-breasted Waterhens than in uninfected ones (Table 1). Two birds were infected with a new lineage of the Haemoproteus parasite. However, the characteristic morphology of the new Haemoproteus lineage was identical to H. gallinule that was previously described in another host species, Slaty-legged Crake (Rallina eurizonoides) [15]. The intensity of parasitemia was extremely low, ranging 0.0001–0.003%. Four out of six partial cytb genes were amplified and the BLAST results were 100% identical to two known species: P. elongatum (lineage GRW06) and P. collidatum (lineage FANTAIL01) found in individual birds and an unknown Plasmodium species (GenBank no.: AB601445) found in two birds. Furthermore, the partial cytb sequences were deposited in GenBank under the accession numbers OQ565584–OQ565589 and one newly obtained sequence of H. gallinulae was assigned the lineage name AMPHO01 in the MalAvi database. Voucher specimen (accession no. G466274) of H. gallinulae from White-breasted Waterhens were deposited in the Queensland Museum, Queensland, Australia.

Table 1.
Comparison of differential white blood cell (WBC) counts and heterophil-to-lymphocyte (H:L) ratio in White-breasted Waterhens (Amaurornis phoenicurus) from representative four of eleven uninfected (negative) and six infected (positive) with haemosporidian parasites. Data are expressed as mean and range (in parentheses).
3.1. Morphological Characterization of Haemoproteus gallinulae de Mello, 1935 from Amaurornis phoenicurus in Thailand
Young gametocytes (Table 2; Figure 1A–D): These were found in mature erythrocytes. The initial stage was found anywhere in the cytoplasm of infected erythrocytes. The developmental stage was usually found in a subpolar position relative to erythrocyte nuclei, touching neither nuclei nor the erythrocyte membrane. The outline of growing gametocytes (Figure 1B–D) showed an ameboid or irregular form covering both sides of the erythrocyte nuclei. Small vacuoles possibly appeared. Small-sized pigment granules (<0.5 µm) were disrupted or grouped in the cytoplasm, frequently found along the marginal outline.

Table 2.
Morphometry of Haemoproteus gallinulae de Mello, 1935, in White-breasted Waterhens (Amaurornis phoenicurus) from Salaya suburb, Thailand.
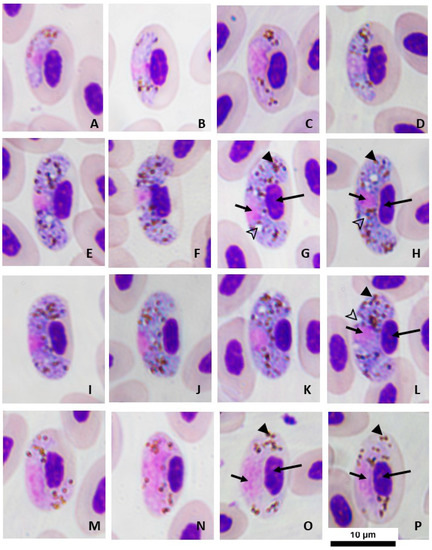
Figure 1.
Haemoproteus (Parahaemoproteus) gallinulae from White-breasted Waterhens (Amaurornis phoenicurus) in Salaya suburb, Thailand. (A–D) Young gametocytes, (E–L) macrogametocytes, (M–P) microgametocytes. Long arrows—nuclei of infected erythrocytes; short arrows—nuclei of the parasite; arrow heads—pigment granules; outline arrow heads—vacuoles. Giemsa’s azure–eosin–methylene blue solution-stained thin blood films. Scale bar = 10 µm.
Macrogametocytes (Table 2; Figure 1E–L): These were usually medium-sized and broadly halteridial, both sides of the marginal entire, usually covering the polar nucleus of erythrocyte infection. The cytoplasm was stained distinctly deep blue with Giemsa. Parasite nucleus was ovoid (almost round), stained pink, normally alongside the erythrocyte nucleus, closer to the erythrocyte membrane than to the erythrocyte nucleus (Figure 1E–L). Infected erythrocytes were significantly hypertrophied in all dimensions (p < 0.01). Erythrocyte nuclei were slightly atrophied (p < 0.05) in terms of the width and area but not in length (p > 0.05). Fully grown gametocytes distinctly laterally displaced the nuclei of the infected erythrocyte (Figure 1I–L; approximate nuclear displacement ratio [NDR] = 0.48). Pigment granules were small- to medium-sized (0.5–1 µm), ovoid, brownish, were approximately 30 in number, were generally scattered throughout the cytoplasm, and were possibly clumped in small group (3–4 granules).
Microgametocytes (Table 2; Figure 1M–P): These exhibited generally characteristic morphology of parasites, similar to macrogametocytes, with a distinct dimorphism in staining; however, fully grown microgametes almost completely covered both sides of the nuclear poles of infected erythrocytes (Figure 1N–P). Pigment granules were usually clumped into a cluster, scattered in the cytoplasm nearly marginal to the parasite nucleus, and were significantly less than those found in macrogametocytes. Erythrocytes infected by microgametocytes were distorted, similar to those infected by macrogametocytes (p > 0.05). The nuclei of infected erythrocytes were occasionally less displaced than those of macrogametocytes (p < 0.05).
3.2. Phylogenetic Relationships between Haemosporidian Parasites
Six out of seventeen White-breasted Waterhens tested positive for haemosporidian parasites. The same cytb sequences belonging to genus Haemoproteus (Figure 2, box A) which correspond morphospecies to H. gallinulae were detected in two of six individuals. This Haemoproteus species was a new lineage, namely, AMPHO01 (GenBank nos.: OQ565584 and OQ565585). Moreover, the other four sequences corresponded to three different lineages of Plasmodium parasites. Two sequences belonged to unidentified Plasmodium lineages (GenBank nos.: OQ565586 and OQ565587) and showed identity with the lineage CXNIG01. The other partial cytb sequence (GenBank no.: OQ565589) had 100% similarity to the P. collidatum lineage FANTAIL01, and the final sequence (GenBank no.: OQ565588) was closely related to P. elongatum. Only unique partial cytb sequences were included in the phylogeny (Figure 2). Coinfections by more than one parasite in the same individual were not detected.
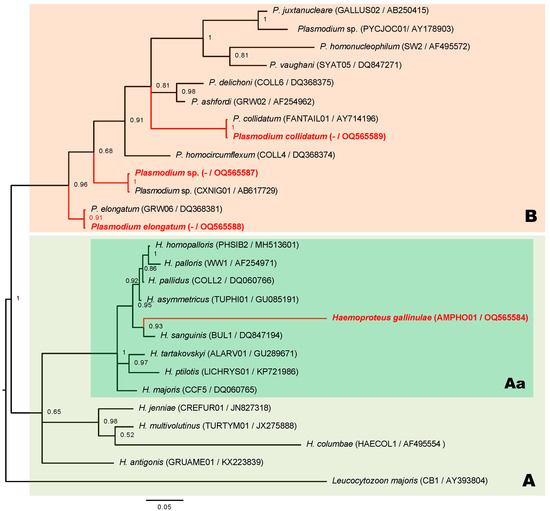
Figure 2.
A Bayesian phylogeny of haemosporidian parasites infecting waterhens (Amaurornis phoenicurus) from Salaya suburb, Thailand. Clusters of genus Haemoproteus parasites (box A), subgenus Parahaemoproteus (box Aa) has H. gallinulae (red font) as member, and genus Plasmodium (box B) including three Plasmodium species (red font) obtained in this study; phylogenetic trees constructed based on partial sequences of cytb gene (27 sequences and 479 bp); values above the branches are posterior probabilities. Leucocytozoon majoris was an outgroup; parentheses show both MalAvi lineages and GenBank accession numbers.
The phylogenetic analysis revealed that the lineage of H. gallinulae AMPHO01 was significantly within the cluster of Haemoproteus-mediated Culicoides midges acting probably as a transmitting insect, indicating that this Haemoproteus species was likely a member of the subgenus Parahaemoproteus (Figure 2, box Aa). The genetic divergence among different lineages and between AMPHO01 and other lineages of the subgenus Parahaemoproteus ranged 9.6–12.7%. The close genetic distance of the cytb sequence-related AMPHO01 lineage was TUPHI01; the COLL2 and BUL1 lineages belonged to H. asymmetricus, H. pallidus, and H. sanguinis, the genetic distances for which were 9.6%, 9.6%, and 9.8%, respectively. However, these parasites were dissimilar in morphological features and host order between Gruiformes (Rallidae family) and Passeriformes. Furthermore, a closely related morphospecies of H. gallinulae was H. antigonis, belonging to the lineage GRUAME01, and described the only available molecular characteristics of Gruiformes birds. The genetic distance was 13.7% and likely higher than the lineage among the subgenus Parahaemoproteus. This indicated that the genetic distance of this cytb gene and the close relationship of Haemoproteus morphospecies found from Gruiformes birds were likely inconsistent. Regarding phylogeny, the Plasmodium lineage obtained in this study was clearly clustered individually (Figure 2, box B). The unidentified Plasmodium lineage (OQ565586) in this study was identical to Plasmodium lineage CXNIG01 and closely related to the P. elongatum GRW06 lineage, with a genetic distance of 4.3%, which was identical to the Plasmodium lineage (OQ565588) currently found. Another lineage of Plasmodium (OQ565589) was identical to P. collidatum FANTAIL01. The genetic divergence of lineage FANTAIL01 was related to P. ashfordi GRW02 with a genetic distance of 7.9%.
4. Discussion
This is the first report on haemosporidian species from White-breasted Waterhens in Thailand; furthermore, we herein report the molecular characteristics of H. gallinulae and describe its morphological characteristics in White-breasted Waterhens. Of the 15 Rallidae species found in Thailand, the White-breasted Waterhen is the only one surveyed for haemosporidian parasites [10]. Moreover, railbirds usually inhabit near wetlands, such as lowland agricultural fields. These locations have an abundance of insect vectors that positively influence haemosporidian infection [30]. Moreover, some Rallidae species were recorded to be residents in this region, including the Black-tailed Crake (Porzana bicolor), Grey-headed Swamphen (Porphyrio poliocephalus), Red-legged Crake (Rallina fasciata), and White-browed Crake (Porzana cinerea) or winter migratory species such as Baillon’s Crake (Porzana pusilla), Band-bellied Crake (Porzana paykullii), Common Moorhen (Gallinula chloropus), Eastern Water Rail (Rallus indicus), Eurasian Coot (Fulica atra), Ruddy-breasted Crake (Porzana fusca), Slaty-breasted Rail (Gallirallus striatus), Slaty-legged Crake (Rallina eurizonoides), Spotted Crake (Porzana porzana), and Watercock (Gallicrex cinerea) [10]. These migratory species can carry this parasite to other areas of their distribution. Therefore, this study provides potential information on haemosporidian parasites in railbirds, particularly White-breasted Waterhens. Furthermore, this study revealed the role of White-breasted Waterhens as a haemosporidian carrier such as a reservoir of avian malaria that involved a susceptible host of multi-avian host species. However, this study examined the haemosporidian parasite in regular adult birds. The pathogen infection possibly stimulated physiological stress [31]. The H:L ratio of differential WBC indicated stress [32,33]. Significantly high results were found in haemosporidian-infected birds (Table 1). This high ratio resulted in an increase in the number of heterophils. Owing to the limited sample size, this heterophil increase was possibly an observation of this parasite infection in this bird species. However, our study revealed haemosporidian infection in White-breasted Waterhens has certain limitations. For instance, the sample size might not fully represent the broader population of these birds. Additionally, potential differences related to sex and age of the birds were not explored in this study due to the limited data collected in these areas. Addressing these limitations in future research could provide more comprehensive insights into the epidemiology of haemosporidian infection in White-breasted Waterhens.
The results of this study revealed four different haemosporidian lineages. Only one was the Haemoproteus parasite for which the characteristic morphology was consistently identified to be Haemoproteus gallinulae. This Haemoproteus species was considered the first record in Thailand. Moreover, there were no data for GenBank accession and lineage code available for H. gallinulae [17]. Although this parasite had been previously characterized either in different host species from Slaty-legged Crake (Rallina eurizonodes) and American Coot (Fulica americana) [15] or type host, Common Moorhen (Gallinula chloropus) [34] exhibited a different characteristic morphology. This different morphology of H. gallinulae was hypothesized to depend on the other genera of hosts in the Rallidae family. In this study, the morphological features of the parasite were characterized in another host species, Amaurornis phoenicurus (Table 1). The effect of macro- and microgametocytes on erythrocyte distortion was consistent with previously reported findings [15]. Furthermore, H. gallinulae from White-breasted Waterhens presented the following key characteristics: (1) broadly halteridial form in fully grown gametocytes (Figure 1E–P); (2) markedly lateral displacement of the infected erythrocyte nuclei with an NDR of <0.5 (approximately 0.48 shown in macrogametocytes in the present study); and (3) number of pigment granules in macrogametocytes was >25 (about 29.89 in this study). These key features were consistent with the key characteristics of Haemoproteus parasite from Gruiformes birds [17]. However, a high pleomorphic form of H. gallinulae is likely represented in full-growing gametocytes, similar to those found in other Haemoproteus parasites such as H. sacharovi [35]. Therefore, the characteristic morphology of the Haemoproteus parasite presents the unique features of individual species, at least for all the key characteristic features that should be found in fully grown gametocytes.
The phylogeny of the haemosporidian parasite in White-breasted Waterhens revealed that H. gallinulae was distinctly classified into the subgenus Parahaemoproteus. Although the morphological characteristics between H. gallinulae and H. antigonis were indistinguishable, the details of pigment granules differed [17,36]. However, the genetic distance between these two morphospecies (13.7%) was higher than that between H. asymmetricus (9.6%) and H. sanguinis (9.8%), indicating that close genetic relationships exist between distinguishable morphospecies found in different hosts at the order level [37,38]. Similarly, there was a close genetic distance of the cytb sequence as well between H. janniae and H. iwa, but the morphospecies of these two Haemoproteus species are distinguishable [39,40]. Examination of only the genetic differences in the partial cytb sequence was not sufficient for distinguishing between some species of Haemoproteus parasites. Therefore, Haemoproteus species identification was performed based on morphological and molecular features.
Furthermore, the three obtained lineages of Plasmodium parasite in this study should be discussed. First, the P. collidatum (FANTAIL01) lineage was considered a generalist lineage that infects a wide range of hosts and had previously infected 16 bird species, including Rufous Fantail (Rhipidura rufifrons) [41,42]. However, this Plasmodium lineage had never been reported in White-breasted Waterhens. Subsequently, this Waterhen was added to one of several host species of the FANTAIL01 lineage in south and southeast Asia and the Oceania region [41,43]. Moreover, this lineage caused 25% mortality in experimental infection of Eurasian Siskin (Spinus spinus) [43]. This indicated that this lineage of Plasmodium parasite caused significantly virulent avian malaria.
Another Plasmodium lineage was the well-known P. elongatum GRW06 lineage, which is also presently found to infect White-breasted Waterhens. Although there are previous reports of infection in other species of the Rallidae family, such as Corncrake (Crex crex) and Purple Gallinule (Porphyrio martinica), this Plasmodium lineage was distributed worldwide in resident and migratory birds such as Great Reed-warbler (Acrocephalus arundinaceus), Madagascar Swamp Warbler (Acrocephalus newtoni), Eurasian Marsh-Harrier (Circus aeruginosus), and Carrion Crow (Corvus corone) [44,45,46,47], together with marine birds such as penguin (Spheniscus magellanicus) [48]. Furthermore, infection with P. elongatum GRW06 was a cause of severe avian malaria [49]. Another Plasmodium lineage had not been identified at the species level belonging to the CXNIG01 lineage that was isolated from Culex nigropunctatus mosquitoes in Japan [50]. Moreover, this unidentified Plasmodium (GenBank no.: AB601445) was previously reported to infect White-breasted Waterhens. This lineage of Plasmodium parasite was rarely recorded in islands of Japan. Therefore, to the best of our knowledge, this is the first report of this Plasmodium lineage in additional geographic regions in Salaya suburb, Thailand.
5. Conclusions
This is the first study of haemosporidian infection in White-breasted Waterhen resident birds. The findings present a new record of haemosporidian parasites in Thailand, and the H. gallinulae AMPHO01 lineage was characterized morphologically from different host species in Rallidae. Phylogenetic analysis revealed close lineages of Haemoproteus species classified into the subgenus Parahaemoproteus. Although morphospecies between H. gallinulae and H. antigonis (Haemoproteus of Gruiformes host) were difficult to distinguish, the genetic distance of partial cytb sequences was probably distinctly different. Moreover, this study found three different lineages of Plasmodium parasite. Although one of them was not identified as a species found in Culex mosquitoes, it was found in the avian host White-breasted Waterhen. Furthermore, two lineages, namely, P. collidatum and P. elongatum, were identified for the first time in this avian host. These findings reveal the role of White-breasted Waterhens in possibly harboring multiple species or serving as a reservoir host of the haemosporidian parasite. Knowledge of the relationship between the host and haemosporidian parasites can provide a principle for disease management.
Author Contributions
Conceptualization, P.P. and S.R.; methodology, P.P., K.P., N.C., W.C., S.B. and S.R.; formal analysis, P.P. and S.R.; investigation, P.P., K.P., N.C., W.C., S.B. and S.R.; writing—original draft, P.P.; writing—review and editing the manuscript, P.P. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Mahidol University (Basic Research Fund: fiscal year 2022).
Institutional Review Board Statement
This study was reviewed and approved by the Faculty of Veterinary Science—Animal Care and Use Committee (FVS-ACUC) of Mahidol University (protocol no. MUVS-2020-11-55).
Data Availability Statement
The partial cytochrome (cytb) gene sequences have been deposited in GenBank under the accession numbers OQ565584 to OQ565589. Voucher specimens (accession no. G466274) of H. gallinulae from White-breasted Waterhens were deposited in the Queensland Museum, Queensland, Australia.
Acknowledgments
The authors would like to express special thanks to The Monitoring and Surveillance Center for Zoonotic Diseases in Wildlife and Exotic Animals (MoZWE), Faculty of Veterinary Science, Mahidol University, for supporting our research fieldwork and providing a laboratory facility and to Nawapol Udpuay and Pornphawit Mo-mai, scientists of Mahidol University-Frontier Research Facility (MU-FRF) for their assistance and support on the high-resolution inverted microscope.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valkiunas, G. Avian Malaria Parasites and Other Haemosporidia, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Sol, D.; Jovani, R.; Torres, J. Parasite mediated mortality and host immune response explain age-related differences in blood parasitism in birds. Oecologia 2003, 135, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Longoria, L.; Palinauskas, V.; Ilgūnas, M.; Valkiūnas, G.; Hellgren, O. Differential gene expression of Plasmodium homocircumflexum (lineage pCOLL4) across two experimentally infected passerine bird species. Genomics 2020, 112, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Reviriego, M.; Hermosell, I.G.; Balbontín, J.; Bensch, S.; Relinque, C.; Rodríguez, L.; Garcia-Longoria, L.; de Lope, F. Malaria infection and feather growth rate predict reproductive success in house martins. Oecologia 2013, 171, 853–861. [Google Scholar] [CrossRef]
- Coon, C.A.C.; Garcia-Longoria, L.; Martin, L.B.; Magallanes, S.; de Lope, F.; Marzal, A. Malaria infection negatively affects feather growth rate in the house sparrow Passer domesticus. J. Avian Biol. 2016, 47, 779–787. [Google Scholar] [CrossRef]
- Palinauskas, V.; Žiegytė, R.; Ilgūnas, M.; Iezhova, T.A.; Bernotienė, R.; Bolshakov, C.; Valkiūnas, G. Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int. J. Parasitol. 2015, 45, 51–62. [Google Scholar] [CrossRef]
- Gulliver, E.; Hunter, S.; Howe, L.; Castillo-Alcala, F. The pathology of fatal avian malaria due to Plasmodium elongatum (GRW6) and Plasmodium matutinum (LINN1) infection in New Zealand kiwi (Apteryx spp.). Animals 2022, 12, 3376. [Google Scholar] [CrossRef]
- Schumm, Y.R.; Metzger, B.; Neuling, E.; Austad, M.; Galea, N.; Barbara, N.; Quillfeldt, P. Year-round spatial distribution and migration phenology of a rapidly declining trans-Saharan migrant—Evidence of winter movements and breeding site fidelity in European turtle doves. Behav. Ecol. Sociobiol. 2021, 75, 152. [Google Scholar] [CrossRef]
- Zehtindjiev, P.; Križanauskienė, A.; Bensch, S.; Palinauskas, V.; Asghar, M.; Dimitrov, D.; Scebba, S.; Valkiūnas, G. A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction-based protocols for amplification of the cytochrome B gene. J. Parasitol. 2012, 98, 657–665. [Google Scholar] [CrossRef]
- Round, P. Checklist of the Birds of Thailand. Available online: https://www.thaibirding.com/book_reviews/roundlist.htm (accessed on 13 July 2020).
- Kumar, P.; Gupta, S.K. Diversity and abundance of wetland birds around Kurukshetra, India. Our Nat. 2009, 7, 212–217. [Google Scholar] [CrossRef]
- Bhaibulaya, M.; Indra-Ngarm, S.; Ananthapruti, M. Freshwater fishes of Thailand as experimental intermediate hosts for Capillaria philippinensis. Int. J. Parasitol. 1979, 9, 105–108. [Google Scholar] [CrossRef]
- Yoshino, T.; Uemura, J.; Endoh, D.; Kaneko, M.; Osa, Y.; Asakawa, M. Parasitic nematodes of anseriform birds in Hokkaido, Japan. Helminthologia 2009, 46, 117–122. [Google Scholar] [CrossRef]
- Jaafar, N.; Babjee, M.A. Parasites of the White-Breasted Waterhen (Amaurornis phoenicurus); Universiti Putra Malaysia: Serdang, Malaysia, 2011. [Google Scholar]
- Bennett, G.F. Avian Haemoproteidae. 14. The haemoproteids of the avian family Rallidae. Can. J. Zool. 1980, 58, 321–325. [Google Scholar] [CrossRef]
- Prompiram, P.; Kaewviyudth, S.; Sukthana, Y.; Rattanakorn, P. Study of morphological characteristic and prevalence of haemoproteus blood parasite in passerines in bung Boraphet. Thai J. Vet. Med. 2015, 45, 399–409. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae). Malar. J. 2022, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, R.D., Jr.; Fedynich, A.M.; Pence, D.B. Quantification of hematozoa in blood smears. J Wildl. Dis. 1987, 23, 558–565. [Google Scholar] [CrossRef]
- Clark, P.; Boardman, W.; Raidal, S. Atlas of Clinical Avian Hematology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Waldenström, J.; Bensch, S.; Hasselquist, D.; Ostman, O. A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J. Parasitol. 2004, 90, 191–194. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT; Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; pp. 95–98. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. Malavi MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModelTest v2. Program Distributed by the Author: Evolutionary Biology Centre; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rambout, A. FigTree: Tree Figure Drawing Tool, Version 1.4.0. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 18 September 2020).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Van Hoesel, W.; Marzal, A.; Magallanes, S.; Santiago-Alarcon, D.; Ibáñez-Bernal, S.; Renner, S.C. Management of ecosystems alters vector dynamics and haemosporidian infections. Sci. Rep. 2019, 9, 8779. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Gross, W.B.; Siegel, H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983, 27, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.H. Avian blood leucocyte responses to stress. Worlds Poult. Sci. J. 1993, 49, 34–43. [Google Scholar] [CrossRef]
- Sacchi, L.; Prigioni, C. Haematozoa of Italian birds. II. First European record of Haemoproteus gallinulae de Mello, 1935. In Gallinula chloropus and Redescription (Apicomplexa Haemosporina); Atti della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale: Milano, Italy, 1986; Volume 127, pp. 27–32. [Google Scholar]
- Valkiūnas, G.; Santiago-Alarcon, D.; Levin, I.I.; Iezhova, T.A.; Parker, P.G. A new Haemoproteus species (Haemosporida: Haemoproteidae) from the endemic Galapagos dove Zenaida galapagoensis, with remarks on the parasite distribution, vectors, and molecular diagnostics. J. Parasitol. 2010, 96, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.R.; Hamer, S.A.; Hartup, B.K.; Snowden, K.F.; Medeiros, M.C.; Outlaw, D.C.; Hamer, G.L. A novel Haemosporida clade at the rank of genus in North American cranes (Aves: Gruiformes). Mol. Phylogenet. Evol. 2017, 109, 73–79. [Google Scholar] [CrossRef]
- Musa, S.; Mackenstedt, U.; Woog, F.; Dinkel, A. Untangling the actual infection status: Detection of avian haemosporidian parasites of three Malagasy bird species using microscopy, multiplex PCR, and nested PCR methods. Parasitol. Res. 2022, 121, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Duc, M.; Iezhova, T.A. Description of Haemoproteus asymmetricus n. sp. (Haemoproteidae), with remarks on predictability of the DNA haplotype networks in haemosporidian parasite taxonomy research. Acta Trop. 2021, 218, 105905. [Google Scholar] [CrossRef]
- Levin, I.I.; Valkiūnas, G.; Iezhova, T.A.; O’Brien, S.L.; Parker, P.G. Novel Haemoproteus species (Haemosporida: Haemoproteidae) from the swallow-tailed gull (Lariidae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. J. Parasitol. 2012, 98, 847–854. [Google Scholar] [CrossRef]
- Levin, I.I.; Valkiūnas, G.; Santiago-Alarcon, D.; Cruz, L.L.; Iezhova, T.A.; O’Brien, S.L.; Hailer, F.; Dearborn, D.; Schreiber, E.A.; Fleischer, R.C.; et al. Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos Pelecaniform birds: Evidence from molecular and morphological studies, with a description of Haemoproteus iwa. Int. J. Parasitol. 2011, 41, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Beadell, J.S.; Gering, E.; Austin, J.; Dumbacher, J.P.; Peirce, M.A.; Pratt, T.K.; Atkinson, C.T.; Fleischer, R.C. Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol. Ecol. 2004, 13, 3829–3844. [Google Scholar] [CrossRef]
- Silva-Iturriza, A.; Ketmaier, V.; Tiedemann, R. Profound population structure in the Philippine Bulbul Hypsipetes philippinus (Pycnonotidae, Aves) is not reflected in its Haemoproteus haemosporidian parasite. Infect. Genet. Evol. 2012, 12, 127–136. [Google Scholar] [CrossRef]
- Platonova, E.; Aželytė, J.; Iezhova, T.; Ilgūnas, M.; Mukhin, A.; Palinauskas, V. Experimental study of newly described avian malaria parasite Plasmodium (Novyella) collidatum n. sp., genetic lineage pFANTAIL01 obtained from South Asian migrant bird. Malar. J. 2021, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tris, J.; Hellgren, O.; Krizanauskiene, A.; Waldenström, J.; Secondi, J.; Bonneaud, C.; Fjeldså, J.; Hasselquist, D.; Bensch, S. Within-host speciation of malaria parasites. PLoS ONE 2007, 2, e235. [Google Scholar] [CrossRef]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Nedorost, N.; Matt, J.; Kübber-Heiss, A.; Alic, A.; Konicek, C.; Weissenböck, H. Avian haemosporidian parasites of accipitriform raptors. Malar. J. 2022, 21, 14. [Google Scholar] [CrossRef]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Weissenböck, H. The nuclear 18S ribosomal DNAs of avian haemosporidian parasites. Malar. J. 2019, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.; Mackenstedt, U.; Woog, F.; Dinkel, A. Avian malaria on Madagascar: Prevalence, biodiversity and specialization of haemosporidian parasites. Int. J. Parasitol. 2019, 49, 199–210. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Gardiner, C.H.; Yabsley, M.J.; Swanepoel, L.; Kolesnikovas, C.K.M.; Silva-Filho, R.P.; Ewbank, A.C.; Catão-Dias, J.L. Schistosomes and microfilarial parasites in Magellanic penguins. J. Parasitol. 2018, 104, 322–328. [Google Scholar] [CrossRef]
- Palinauskas, V.; Žiegytė, R.; Iezhova, T.A.; Ilgūnas, M.; Bernotienė, R.; Valkiūnas, G. Description, molecular characterisation, diagnostics and life cycle of Plasmodium elongatum (lineage pERIRUB01), the virulent avian malaria parasite. Int. J. Parasitol. 2016, 46, 697–707. [Google Scholar] [CrossRef]
- Ejiri, H.; Sato, Y.; Kim, K.S.; Tamashiro, M.; Tsuda, Y.; Toma, T.; Miyagi, I.; Murata, K.; Yukawa, M. First record of avian Plasmodium DNA from mosquitoes collected in the Yaeyama Archipelago, southwestern border of Japan. J. Vet. Med. Sci. 2011, 73, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).