Simple Summary
Oocyte maturation is a complex process in which the oocyte acquires the capacity to be fertilized and to support embryo development. Although oocyte maturation in vitro has undergone significant advancements, the systems that are currently in place still need to be further optimized. This study aimed to examine alternative culture systems for bovine oocyte in vitro maturation. Specifically, we used liquid marbles and differentially shaped 96-well plates (flat, v-shaped, and round-bottom). Both systems were able to support oocyte nuclear maturation, but embryo development was reduced when in vitro maturation was performed in liquid marbles. Differently shaped wells during maturation did not affect embryo yield but presented a reduction in blastocyst quality. Hence, 96-well plates might be an alternative culture system to mature oocytes in small groups, but further analyses to test possible toxic effects are needed.
Abstract
In vivo-matured oocytes exhibit higher developmental competence than those matured in vitro but mimicking the in vivo environment by in vitro conditions has been challenging. Until now, conventional two-dimensional (2D) systems have been used for in vitro maturation of bovine cumulus-oocytes-complexes (COCs). However, using such systems present certain limitations. Therefore, alternative low-cost methodologies may help to optimize oocyte in vitro maturation. Here, we used two different systems to culture COCs and evaluate their potential influence on embryo development and quality. In the first system, we used treated fumed silica particles to create a 3D microenvironment (liquid marbles; LM) to mature COCs. In the second system, we cultured COCs in 96-well plates with different dimensions (flat, ultra-low attachment round-bottom, and v-shaped 96-well plates). In both systems, the nuclear maturation rate remained similar to the control in 2D, showing that most oocytes reached metaphase II. However, the subsequent blastocyst rate remained lower in the liquid marble system compared with the 96-well plates and control 2D systems. Interestingly, a lower total cell number was found in the resulting embryos from both systems (LM and 96-well plates) compared with the control. In conclusion, oocytes matured in liquid marbles or 96-well plates showed no remarkable change in terms of meiotic resumption. None of the surface geometries influenced embryo development while oocyte maturation in liquid marbles led to reduced embryo development. These findings show that different geometry during maturation did not have a large impact on oocyte and embryo development. Lower embryo production after in vitro maturation in liquid marbles was probably detected because in vitro maturation was performed in serum-free medium, which makes oocytes more sensitive to possible toxic effects from the environment.
1. Introduction
Although extensive research has been conducted in the last decades to improve the conditions for in vitro maturation (IVM) of bovine oocytes, the developmental competence of in vitro-matured oocytes is still suboptimal when compared with in vivo-matured oocytes [1,2]. One of the reasons why in vitro maturation is not always successful is that this process is extremely sensitive to environmental factors such as pH [3], temperature variations [4], oxygen tension [5], media composition [6], or type of culture (group or individual) [7]. If these factors are kept under control, another major difference is the lack of continuous nutrient supply or dynamic removal of waste; this can be mimicked in vitro by microfluidics culture, but such systems are tedious to develop and less user-friendly [8,9,10]. A third factor which is different during routine in vitro maturation is that oocytes are not cultured in a follicular shape, while in vivo, the oocyte is maturing inside the preovulatory follicle.
Traditional methods for in vitro maturation rely on two-dimensional (2D) culturing techniques using plastic culture plates, which have been used for decades to propagate monolayer cell cultures [11]. In such conventional 2D systems, cells are grown on flat, firm culture substrates, which are economic and relatively easy to handle. However, in such culture conditions, cells can adhere and spread freely in the horizontal plane but they have limited possibility for spreading in the vertical dimension [12]. Hence, the major drawback of 2D-culture systems is that they do not fully imitate the in vivo microenvironment where cells are grown in a complex three-dimensional (3D) matrix, which has an impact on cell–cell and cell–extracellular matrix interactions, and consequently on cell responses (differentiation, proliferation, apoptosis, gene, and protein expression) [13,14,15,16,17,18]. Furthermore, the cell morphology in 2D systems is different from that in the natural structures of tissues, which might affect their functionality, secretion of growth factors, organization of internal structures, and cell signaling [19,20].
There is growing evidence suggesting that 3D cell culture models reflect more precisely the actual microenvironment in which cells grow in native tissues [21]. These models allow cell adhesion in all three dimensions, whereas in the 2D system, it is restricted to the x-y plan. Likewise, 3D systems improve cell communication and soluble factors are more stable in those systems compared with 2D systems [11,22]. In terms of in vitro embryo production, studies on 3D systems were designed to support oocyte IVM in several species using alginate microbeads [23], alginate hydrogels [24], glass scaffolds [25], agarose matrix [26], or the hanging drop method [27]. Recently, so-called liquid marbles (LM) have also become interesting as 3D-shaped bioreactors. Liquid marbles are culture medium droplets encapsulated with hydrophobic particles which prevent direct contact between the liquid inside and the surrounding environment, thus reducing the risk of contamination, while the hydrophobic shell of the LM remains permeable for gases [28]. These properties make LM a promising alternative as 3D microbioreactors for cell culture. This system has been used for culturing microorganisms [29], embryoid bodies [30,31], or olfactory ensheathing cell spheroids [32]. The use of liquid marbles during ovine IVM showed slightly better blastocyst rates using polytetrafluoroethylene [33] and silica powder [34], while in porcine, fluorinated ethylene propylene liquid marbles were able to maintain the 3D organization of the COCs [24]. However, liquid marbles have not been validated yet in the bovine model.
Cellular responses are influenced by topographical surface features. For instance, human epithelial cells presented differences in orientation, migration, and morphology when culturing them on pillar or pit surfaces [35]. Similarly, concave and convex surfaces have been found to influence stem cells’ differentiation into osteoblasts [36], and a v-shaped surface has been related to changes in cell shape and mRNA expression in fibroblasts [37,38] and osteoblast-like cells [39]. However, all these studies used complex systems to recreate the different surface geometries. Thus, a simpler alternative could be to use 96-well plates with different shapes, which are available on the market and are easy to use and standardize. V-shaped 96-well plates have been applied in ovarian follicle culture, allowing growth and differentiation of bovine [40] and human follicles [41]. Similarly, round-bottomed, ultra-low attachment plates have been used in mouse follicle culture in order to prevent the attachment and flattening of the follicles to the surface of the plate, showing that non-attachment culture conditions have an impact on cumulus cells gene expression and oocyte developmental competence [42]. Besides, the effect of three differentially shaped 96-well plates have been tested during culture of several human cell lines (retinal epithelial, alveolar epithelial, and dermal fibroblastic) [43]; nevertheless, to our knowledge, no studies have been performed with these plates to evaluate the potential influence of different surface topographies during oocyte IVM on embryo development.
In the present work, we evaluated the potential effects of using two alternative culture systems during oocyte IVM: (1) encapsulation in liquid marble microbioreactors and (2) differently shaped culture substrates (flat, round, and v-shaped 96-well plates).
2. Materials and Methods
2.1. Experimental Design
2.1.1. Experiment 1: Evaluation of Liquid Marbles as 3-D Model for In Vitro Maturation
Liquid marbles were tested as microbioreactors for in vitro maturation of bovine oocytes. To do so, a total of 941 cumulus-oocyte complexes (COCs) in seven replicates were used in three different maturation systems: liquid marbles (LM) (n = 301 COCs) as the 3D model, 2D droplets (n = 309 COCs) as the flat culture with similar oocyte/medium ratio to LM (5 COCs/30 μL), and the control group (n = 331 COCs) as our standard condition (60 COCs/500 μL). After maturation, COCs from all groups were randomly distributed for in vitro fertilization (IVF) and in vitro culture (IVC; LM = 241, 2D droplets = 256, and Control = 262 COCs) or nuclear maturation assessment (LM = 60, 2D droplets = 53, and Control = 69 COCs).
2.1.2. Experiment 2: Evaluation of Different Surface Geometries for In Vitro Maturation
A comparative study was carried out to evaluate the effect of three different bottom-shaped multi-well plates during oocyte IVM on embryo development. Firstly, we conducted a pilot study including 3 replicates (n = 1414 COCs) to select the ideal work volume of maturation medium per well and to analyze the effect of paraffin oil overlay in oocytes matured in multi-well plates. Cumulus-oocyte complexes were matured in v-shaped 96-well plates under the following conditions: five COCs in 30 μL maturation medium with 30 μL oil overlay (Oil V-shaped-30 (OV-30); n = 160 COCs) or without oil (V-shaped-30 (V-30); n = 120 COCs), ten COCs in 60 μL maturation medium with 30 μL oil overlay (OV-60; n = 163 COCs) or without oil (V-60; n = 155 COCs), and twenty COCs in 120 μL maturation medium with 30 μL oil overlay (OV-120; n = 143 COCs) or without oil (V-120; n = 148 COCs). A control group (n = 252 COCs) was also included. After maturation, IVF and IVC were performed routinely.
Subsequently, based on the results of the pilot study, different surface geometries were compared during maturation using three differentially shaped 96-well plates: flat (F), which can be represented as a cylindrical structure; ultra-low attachment round-bottom (R), with the appearance of a cylinder on the top and a semi-hemisphere on the bottom; and v-shaped (V); which can be represented as a cylinder on the top and a cone on the bottom. Besides evaluating the effect of closer interactions between COCs enhanced by the geometry of round and v-shaped 96-well plates (Figure S1), these plates also allow comparison of the impact of cell adhesion (i.e., the round-bottom plates present a covalently bonded hydrogel that minimizes cell attachment, while v-shaped plates are not coated). To do so, six replicates were performed (n = 1992 COCs) using the conditions described as for the OV-60, resulting in 4 groups: F-60 (n = 427 COCs), R-60 (n = 549 COCs), V-60 (n = 491 COCs), and control group (n = 525 COCs). After maturation, COCs from all groups were randomly assigned to IVF and IVC (F-60 = 374, R-60 = 504, V-60 = 440 and control group = 487 COCs) or nuclear maturation assessment (F-60 = 53, R-60 = 45, V-60 = 51 and control group = 38 COCs). The experimental design is depicted in Figure 1.
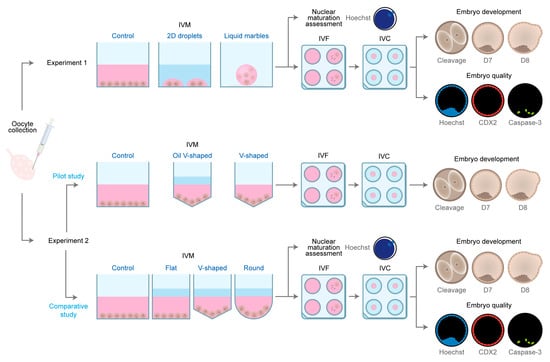
Figure 1.
Schematic representation of the experimental design.
2.2. Media and Reagents
Tissue culture media (TCM)-199 and antibiotics (gentamycin and kanamycin) were obtained from Life Technologies Europe (Ghent, Belgium). Phosphate-Buffered Saline (PBS) was purchased from Gibco™ 20012019, Thermo Fisher Scientific (Waltham, MA, USA). All other products not indicated otherwise were provided by Sigma-Aldrich (Diegem, Belgium). Before use, every media was filtered (0.22 μM; GE Healthcare-Whatman, Diegem, Belgium).
2.3. Source of Oocytes and In Vitro Maturation
Bovine ovaries were obtained from a local slaughterhouse, transported to the laboratory, and prepared for further processing within 2 h after collection. The ovaries were disinfected with 96% ethanol and cleaned three times in physiological saline (37 °C) containing 50 mg/mL of kanamycin. Cumulus-oocyte complexes were recovered with an 18-gauge needle and a 10 mL syringe from 4–8-mm-diameter follicles. Oocytes surrounded by three or more layers of compact cumulus cells and a uniformly granulated cytoplasm were selected, washed in warm HEPES—Tyrode’s Albumin Lactate Pyruvate media (HEPES-TALP), and randomly assigned to different IVM systems. Four IVM systems, as described below, were evaluated according to the experimental group (see Section 2.1). All treatment groups were cultured for 22 h in 5% CO2 in the air at 38.5 °C.
Control. Sixty COCs were cultured in 500 μL maturation medium (TCM-199 Earle’s salts supplemented with 20 ng/mL epidermal growth factor and 50 μg/mL gentamicin) in flat-bottom 4-well dishes (Thermo Fisher®, Merelbeke, Belgium) without oil covering.
Encapsulation in liquid marbles. A single droplet of 30 μL maturation medium containing five COCs was carefully placed on top of a layer of approximately 1 cm treated fumed silica powder (Cabot Corp, Cab-O-Sil, TS-530), which was equally distributed in a 6 cm Petri dish (Figure 2A). The Petri dish was mildly shaken in circular motions to ensure that the surface of the droplet was completely and uniformly coated with the hydrophobic particles. To manipulate the LM, the edge of a 1000 µL micropipette tip was cut to make its diameter to a small extent of the LM diameter in order to ensure a proper grip, but big enough to avoid collapse. Before transferring the marbles, the modified tip was coated with some powder to prevent its adhesion to the tip. Then, the LM was picked up slowly (Figure 2B) and placed on a well of a 24-well plate (Thermo Scientific) whose surface was previously covered with a small quantity of silica powder (Figure 2C). To avoid evaporation, the central space of the 24-well plate was filled with 5 mL sterile HEPES-TALP medium. After IVM, the LM (Figure 2D,E) was placed in maturation medium to disrupt the silica powder’s hydrophobicity, causing the marble’s dissolution (Figure 2F). The released COCs were washed three times in maturation medium to remove silica particles before proceeding to the next step.
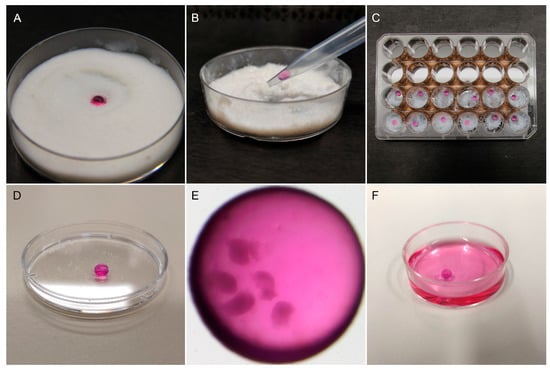
Figure 2.
Oocyte encapsulation in liquid marbles. (A) One droplet of maturation medium containing the oocytes was placed in treated fumed silica powder on a Petri dish. The Petri dish was gently shaken to form the liquid marble. (B) A modified 1000 µL micropipette tip was used to manipulate the liquid marbles. (C) Liquid marbles were placed individually in the wells of a 24-well plate containing a small amount of silica powder. The central space of the plate was filled with 5 mL HEPES-TALP to prevent evaporation. (D) Resulting liquid marble drop. (E) Five COCs encapsulated in a liquid marble drop before IVM, observed under a stereomicroscope. (F) After maturation, liquid marbles were dissolved in maturation medium.
2D droplets. Droplets of 30 μL maturation medium were prepared in a Petri dish (60 × 15 mm; Thermo Fisher Scientific, Waltham, MA USA) and covered with 7.5 mL paraffin oil (SAGE, CooperSurgical, Trumbull, CT, USA). Five COCs were matured in each droplet of maturation medium.
Shaped culture in 96-well plates. Firstly, oocytes were matured in v-shaped 96-well plates under the following conditions: five COCs in 30 μL of maturation medium with or without paraffin oil overlay of 30 μL, ten COCs in 60 μL of maturation medium with or without a paraffin oil overlay of 30 μL, and twenty COCs in 120 μL of maturation medium with or without a paraffin oil overlay of 30 μL. Secondly, ten COCs were matured in 60 μL of maturation medium with paraffin oil overlay in flat, ultra-low attachment round-bottom, and v-shaped Corning® 96-well plates (Avantor, VWR, Leuven, Belgium; CatNo. 734-1793, 444-1020, 734-1798, respectively).
2.4. In Vitro Fertilization and Embryo Culture
Standard in vitro methods were used to generate bovine embryos, as previously described by Wydooghe et al. [44]. Briefly, using a discontinuous 45/90% Percoll® gradient (GE Healthcare Biosciences, Uppsala, Sweden), sperm capacitation of frozen-thawed straws from a known fertile bull was performed. Consequently, the sperm pellet was washed in IVF–TALP medium and a final concentration of 1 × 106 spermatozoa/mL was adjusted using IVF–TALP medium enriched with BSA (Sigma A8806; 6 mg/mL) and heparin (20 μg/mL).
After 22 h of IVM, oocytes from each treatment group in Experiments 1 and 2 were pooled to reach groups of 60 COCs. Then, oocytes were washed in IVF-TALP and subsequently co-incubated in 500 μL IVF-TALP with Percoll-purified spermatozoa for 21 h at 38.5 °C in 5% CO2 in humidified air. After fertilization, zona attached sperm and cumulus cells were removed by vortexing for 3 min in 2.5 mL Hepes-TALP. The presumed zygotes were randomly selected and cultured in groups of 25 in 50 μL droplets of synthetic oviductal fluid (SOF), 0.4% (w/v) BSA (Sigma A9647), and ITS (5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL selenium). Each droplet was covered with 900 μL paraffin oil and incubated at 38.5 °C for 8 days in 5% CO2, 5% O2, and 90% N2.
Cleavage was evaluated 45 h post insemination and blastocyst yield was recorded on day 7 and day 8 post insemination. Both rates were calculated as a percentage over the presumed zygotes.
2.5. Evaluation of Oocyte Nuclear Stage (Maturation Assessment)
After maturation, oocytes were denuded by vortexing for 8 min in 2.5 mL Hepes-TALP and fixed with 4% paraformaldehyde (w/v). Then, oocytes were transferred to 0.1% (w/v) polyvinylpyrrolidone (PVP) in PBS containing 10 μg/mL of Hoechst 33,342 (Life Technologies, Ghent, Belgium) for 10 min. Nuclear morphology was evaluated using a fluorescence microscope (BRESSER Science ADL 601 F LED). The proportion of oocytes in each meiotic stage—germinal vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), metaphase II (MII), or degenerated—was recorded (Figure 3).
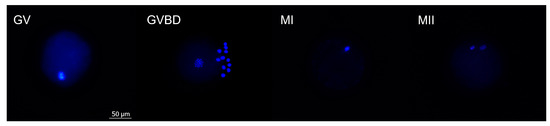
Figure 3.
Representative images of Hoechst-stained oocytes in different nuclear maturation stages. (GV) Germinal vesicle. (GVBD) Germinal vesicle breakdown. (MI) Metaphase I. (MII) Metaphase II. The scale bar applies to all the images.
2.6. Embryo Quality Assessment
Embryo quality was determined by differential apoptotic staining for CDX2, a transcription factor only expressed by trophectoderm cells, and caspase-3, a cysteine-aspartic acid protease involved in the signaling pathways of cell apoptosis. The protocol was performed according to Wydooghe et al. [45]. Briefly, day-8 blastocysts were fixed in 4% paraformaldehyde (w/v) at room temperature for at least 20 min and then stored in PBS supplemented with 0.5% BSA at 4 °C until the staining was performed (Figures S2 and S3). Firstly, blastocysts were incubated with ready-to-use anti-CDX2 primary antibodies (Biogenex, San Ramon, CA, USA). Embryos were next incubated with rabbit active caspase-3 primary antibody (0.768 ng/mL, Cell Signaling Technology, Leiden, The Netherlands), followed by incubation in goat anti-mouse Texas Red secondary antibody (20 μg/mL in blocking solution, Molecular Probes, Merelbeke, Belgium) and then in goat antirabbit FITC secondary antibody (10 μg/mL in blocking solution, Molecular Probes). Finally, the embryos were transferred to nuclear stain, Hoechst 33,342 (50 μg/mL in PBS/BSA). A negative control was also included in which embryos were not incubated with CDX2 and active caspase-3 antibodies. Samples were examined by a single observer using fluorescence microscopy (Leica DM 5500 B) with a triple bandpass filter. With this staining protocol, the number of trophectoderm (TE) cells, inner cell mass number (ICM), total cell number (TCN = TE + ICM), ICM/TCN ratio, the total number of apoptotic cells (AC), and the ratio of apoptotic cells (ACR; AC/TCN) were estimated.
2.7. Statistical Analyses
The statistical analyses were performed using R-core (version 4.2.1; R Core Team, Vienna, Austria). The oocyte/zygote/embryo was considered as the unit of interest. Generalized mixed-effects models were used to test the effect of IVM conditions on oocyte nuclear maturation, cleavage, and embryo development rates. The effect of IVM conditions on blastocyst differential staining parameters was fitted in mixed linear regression models. For all the models, the replicate was set as random. Results are expressed as least square means and standard errors. The differences between treatment groups were assessed using Tukey’s post hoc test. The significance and tendency levels were set at p < 0.05 and p < 0.1, respectively.
3. Results
3.1. Experiment 1: Evaluation of Liquid Marbles as a 3D Model for In Vitro Maturation
3.1.1. Effect of Liquid Marbles on Oocyte Nuclear Maturation
Nuclear maturation assessment by Hoechst staining demonstrated that oocytes in the three groups resumed meiosis (i.e., no germinal vesicles were found). Most oocytes in all treatment groups reached the metaphase II stage with no significant differences among groups (p > 0.05; Table 1). Likewise, the proportion of oocytes that reached germinal vesicle breakdown, metaphase I, or degenerated was similar among groups (p > 0.05).

Table 1.
Nuclear maturation assessment of oocytes matured in: (A) control group, (B) 2D droplets, and (C) liquid marbles (LM).
3.1.2. Effect of Liquid Marbles on Embryo Development and Embryo Quality
Firstly, we determine the effect of oocyte maturation conditions on cleavage and blastocyst rates. Although there were no significant differences in the cleavage rates between groups (LM: 79.9 ± 3.1%; 2D droplets: 85.7 ± 2.9%; control group: 86.0 ± 3.1%; p > 0.05; Figure 4A), oocytes matured in LM showed lower day 7 (17.6 ± 3.4%) and day 8 (26.1 ± 3.7%) blastocyst rates compared with 2D droplets (26.4 ± 4.2%, p = 0.048, and 38.8 ± 4.2%, p = 0.008, respectively) and control (29.8 ± 4.9%, p = 0.01, and 40.1 ± 4.6%, p = 0.007, respectively).
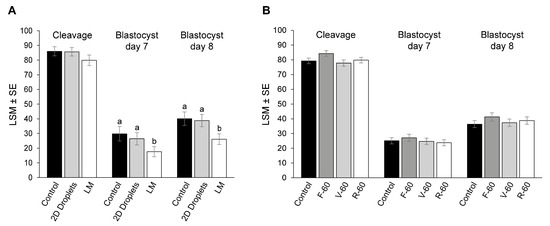
Figure 4.
Cleavage, day 7, and day 8 blastocyst rates are expressed as a percentage of presumed zygotes. (A) Experiment 1. Oocytes were in vitro matured in liquid marbles (LM), 2D droplets, and a control group. (B) Experiment 2. Oocytes were in vitro matured in flat, v-shaped, and ultra-low attachment round-bottom 96-well plates, and a control group. Different superscripts (a and b) represent statistical differences (p < 0.05) among groups. Results are expressed as least square means ± standard error (LSM ± SE).
Further on, we determined the effect of maturation conditions on embryo quality by differential apoptotic staining of blastocysts. Differences among IVM culture systems in blastocyst quality parameters are shown in Table 2. Maturation in LM produced blastocysts with lower TCN and TE than in control (p < 0.01). Maturation in 2D droplets reduced the TCN, ICM, and TE (p < 0.01) and increased the AC/TCN ratio compared with control (p = 0.03).

Table 2.
Effect of the oocyte in vitro maturation in LM on embryo quality.
3.2. Experiment 2: Evaluation of Different Surface Geometries for In Vitro Maturation
3.2.1. Effect of Volume of Medium and Oil Overlay on 96-Well Plates Culture
Initially, a pilot study was performed to establish the optimum volume of maturation medium and the effect of paraffin oil overlay during oocyte IVM in 96-well plates on embryo development. The V-30 group was excluded from the treatment groups due to excessive maturation medium evaporation after IVM. Although there were no significant differences in the cleavage, blastocyst day 7, and blastocyst day 8 rates for all the treatment groups compared with the control (p > 0.05), OV-60 had a numerically higher blastocyst rate at day 8. Therefore, we selected IVM culture conditions as described for this group for follow-up experiments. For detailed information on the pilot study results, see Supporting Information (Table S1).
3.2.2. Effect of Surface Geometry on Oocyte Nuclear Maturation, Embryo Development, and Embryo Quality
Hoechst staining was used to analyze the meiotic progression of the oocytes after IVM in flat, v-shaped, and ultra-low attachment round-bottom 96-well plates. No differences were found in the proportion of mature, immature, or degenerated oocytes in the three tested geometries and the control group (p > 0.05; Table 3).

Table 3.
Nuclear maturation assessment of oocytes matured in: (A) control group, (B) F-60, (C) V-60, and (D) R-60.
Cleavage, day 7, and day 8 blastocysts rates were similar in the three 96-well plates and the control group (p > 0.05; Figure 4B). Differences among treatments in blastocyst quality parameters determined by differential apoptotic staining are shown in Table 4. In vitro maturation in flat, ultra-low attachment round-bottom and v-shaped 96-well plates resulted in blastocysts with lower TCN and TE than control (p < 0.05). Blastocysts in the R-60 group also presented lower ICM compared with control (p = 0.01). However, there were no differences in the ICM/TCN or AC/TCN ratios among treatments and control (p > 0.05).

Table 4.
Effect of the oocyte in vitro maturation in three different surface geometries on embryo quality.
Main results from both culture systems (LM and 96-well plates) are summarized in Table S2.
4. Discussion
Currently, most standard oocyte IVM is performed in two-dimensional culture systems, which are both economical and practical. However, these systems have some limitations such as cell flattening and/or decreased interaction among cells that might affect their developmental potential. Therefore, novel alternative systems to mature oocytes might enhance the interaction between the oocyte, the cumulus cells, and different factors within the culture medium, and consequently improve the developmental competence of the oocyte. In this study, we used liquid marbles as microreactors to perform serum-free IVM for the first time in the bovine model. Moreover, we also tested a simple and practical system using differently shaped and coated 96-well plates. We found that both liquid marbles and 96-well plates had a similar effect as 2D-control culture systems in terms of oocyte nuclear maturation, while embryo development was similar after oocyte maturation in the 96-well plates but lower in LM.
We proved that meiotic resumption is not affected in bovine oocytes by the use of LM, since the proportion of oocytes that reached metaphase II was similar in both 2D-controls and liquid marbles systems, which concord with previous studies in cats [41] and sheep [33,34]. In our study, although oocytes matured in LM were able to reach the blastocyst stage, the blastocyst yield was reduced compared with oocytes matured in 2D-controls. Moreover, embryos derived from oocytes matured in LM exhibited a lower total cell number count than those matured in a standard 2D system. It has been shown that oocyte/medium ratios ranging between 1:1 and 1:10 during in vitro maturation do not influence embryo development in cattle [46], hence the slightly different ratio between the control group (1:8) and the 2D droplets and LM (1:6) provides no explanation for the lower total cell number observed in these last two groups. On the other hand, it has been demonstrated that material toxicity can decrease the total cell number and affect embryo rates [47,48]. Still, the toxicity of treated fumed silica particles has not been tested in oocytes. Therefore, we hypothesize that treated fumed silica particles could exhibit some toxicity in bovine oocytes since our findings differ from the results obtained by Bebbere et al., who matured ovine oocytes in LM formed with the same particles, showing only a tendency of the LM to improve the blastocyst rate compared with those matured in 2D conditions [34]. In a previous study of the same group, a similar blastocyst yield was obtained after maturation of ovine oocytes in polytetrafluoroethylene marbles compared with group culture in 2D [33]. On the other hand, in pig oocytes, although embryo development has not been assessed, Gorczyca et al. (2020) [24] performed ultrastructural evaluation of COCs after IVM in fluorinated ethylene propylene marbles, and interestingly, liquid marbles were able to maintain the 3D organization by preventing their flattening and consequent disruption of gap junctions. It is noteworthy that both ovine and porcine results were obtained in serum-containing maturation medium. Although our blastocyst rates in a serum-free system are within the range of serum-containing bovine in vitro embryo culture systems (20–40% [49]), serum-free systems are more prone to toxic influences [50,51,52] and the absence of serum during maturation might be more relevant in the LM system. Moreover, additional manipulation during the LM preparation compared with the standard system may also have affected the developmental capacity of the oocytes.
Besides the LM system, in the current study, we evaluated the effect of different geometry surfaces on oocyte developmental competence. Although we tested a range of medium volumes and evidenced the importance of paraffin oil overlay when low volumes are used, we did not find differences in oocyte nuclear maturation, embryo development, or embryo quality among the three surface geometries tested. Despite the fact that blastocysts derived from oocytes matured in 96-well plates presented similar ICM/TCM and AC/TCN ratios than 2D-controls, the TCN count was lower than those derived from oocytes matured in standard conditions. These results may be due to certain toxicity, resulting in an impact on embryo quality. However, trophectoderm growth in in vitro embryos was clearly not adversely affected by culture conditions and this fact might give the false impression of good-quality blastocyst production when solely morphological criteria or total cell numbers are used to evaluate embryo quality [53]. The only previous study we found that compared flat, round bottom and v-shaped 96-well plates was not performed on embryos but on cell lines [43]. In this study, different human cell lines, namely A549 (alveolar epithelial), ARPE-19 (retinal epithelial), and Malme-3M (dermal fibroblast), were used, of which the cells attached and spread differently in each plate, but the phenotype and functionality of the cells were not affected by the surface topography [43]. Apart from the different surface geometries, in our study, we investigated both non-treated and ultra-low attachment plates; here, the latter are used to prevent protein adsorption to the culture surface, helping to minimize monolayer cell adhesion to the culture vessel. Therefore, initially, we hypothesized that ultra-low attachment 96-well plates might limit COCs flattening and, consequently, improve developmental rates. However, although ultra-low attachment plates facilitated handling of COCs, we did not find any effect on the evaluated outcomes compared with the non-treated plates.
In order to implement new systems, it is necessary to first study their impact on fundamental cellular processes. In in vitro embryo production, oocyte maturation and embryo development and quality are the main outcome parameters to evaluate culture systems. In our study, we showed that oocyte meiotic resumption was not affected by any of the culture systems. However, only nuclear maturation was assessed and further studies to evaluate differences in cytoplasmic maturation may be performed. On the other hand, despite finding inferior blastocyst quality in both systems and a lower blastocyst rate in LM, we were unable to identify the reason for these declines. Therefore, additional toxicity testing or molecular analyses may further explain our findings. Besides, we thought that our systems would help to preserve the cells’ three-dimensional structure, which would have been translated into an increase in our outcome parameters, thus no ultrastructural evaluation was performed. Furthermore, although LM, v-shaped, and round-bottom 96-well plates enhance the convergence of cumulus-oocyte complexes and secreted factors on the bottom of the marble or well, consequently improving cell interaction (Figure S1); it must be acknowledged that they are still static systems.
Interestingly, our experiments with different surface topographies exhibited a similar blastocyst rate in comparison with traditional culture but LM reduced it. Yet, alternative culture systems entail higher costs and complexity than conventional IVM (Table 5). Liquid marbles demonstrated the highest level of complexity and risk of loss of oocytes during the elaboration of the marble, with the longest handling time, which might be an additional explanation for the lower blastocyst yield. On the other hand, 96-well dishes made the handling of COCs during preparation and recovery more difficult due to the smaller diameter of the wells.

Table 5.
Characteristics of in vitro maturation systems.
5. Conclusions
For the first time, liquid marbles and shaped 96-well plates were tested in bovine IVM. We showed that there are differences in development and quality in embryos resulting from oocytes cultured in the 2D control system compared with 3D LM and 96-well plate systems. Importantly, no adverse results were observed in terms of embryo development when cultured in 96-well plates. The decrease that we observed in embryo yield after IVM in LM stresses the importance of using serum-free culture media, as we did, when testing novel recipients for bovine oocyte and embryo culture in order to control for possible toxic effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13101635/s1, Table S1: Developmental results of the pilot study in v-shaped 96-well plates; Figure S1: Organization of oocytes in three differentially shaped 96-well plates; Table S2: Summary of main results of the tested systems; Figure S2. Representative images of bovine day 8 blastocysts; Figure S3: Differential apoptotic staining of day 8 blastocyst.
Author Contributions
Conceptualization, A.F.-M., A.V.S. and K.C.P.; methodology, A.F.-M., A.V.S. and K.C.P.; validation, A.F.-M., D.A.-V., A.V.S. and K.C.P.; formal analysis, A.F.-M., O.B.P. and D.A.-V.; investigation, A.F.-M., D.A.-V., N.A.-D. and C.B.; visualization, A.F.-M.; writing—original draft preparation, A.F.-M.; writing—review and editing, A.F.-M., D.A.-V., O.B.P., A.V.S. and K.C.P.; supervision, A.V.S. and K.C.P.; funding acquisition, A.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860960.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that no ethical review is needed when working with slaughterhouse material and embryos of less than 2 weeks old.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study were included in the manuscript and its Supplementary Materials. Raw data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Petra Van Damme for her valuable technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tšuiko, O.; Catteeuw, M.; Esteki, M.Z.; Destouni, A.; Pascottini, O.B.; Besenfelder, U.; Havlicek, V.; Smits, K.; Kurg, A.; Salumets, A.; et al. Genome Stability of Bovine in Vivo-Conceived Cleavage-Stage Embryos Is Higher Compared to in Vitro-Produced Embryos. Hum. Reprod. 2017, 32, 2348–2357. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ko, D.-S.; Lee, H.-C.; Lee, H.-J.; Park, W.-I.; Kim, S.S.; Park, J.-K.; Yang, B.-C.; Park, S.-B.; Chang, W.-K.; et al. Comparison of Maturation, Fertilization, Development, and Gene Expression of Mouse Oocytes Grown In Vitro and In Vivo. J. Assist. Reprod. Genet. 2004, 21, 233–240. [Google Scholar] [CrossRef]
- Downs, S.M.; Mastropolo, A.M. Culture Conditions Affect Meiotic Regulation in Cumulus Cell-enclosed Mouse Oocytes. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/%28SICI%291098-2795%28199704%2946%3A4%3C551%3A%3AAID-MRD13%3E3.0.CO%3B2-Z (accessed on 5 July 2022).
- Edwards, J.L.; Saxton, A.M.; Lawrence, J.L.; Payton, R.R.; Dunlap, J.R. Exposure to a Physiologically Relevant Elevated Temperature Hastens In Vitro Maturation in Bovine Oocytes. J. Dairy Sci. 2005, 88, 4326–4333. [Google Scholar] [CrossRef] [PubMed]
- Whitty, A.; Kind, K.L.; Dunning, K.R.; Thompson, J.G. Effect of Oxygen and Glucose Availability during in Vitro Maturation of Bovine Oocytes on Development and Gene Expression. J. Assist Reprod. Genet. 2021, 38, 1349–1362. [Google Scholar] [CrossRef]
- Fesahat, F.; Firouzabadi, R.D.; Faramarzi, A.; Khalili, M.A. The Effects of Different Types of Media on in Vitro Maturation Outcomes of Human Germinal Vesicle Oocytes Retrieved in Intracytoplasmic Sperm Injection Cycles. Clin. Exp. Reprod. Med. 2017, 44, 79–84. [Google Scholar] [CrossRef]
- Doherty, E.M.O.; Wade, M.G.; Hill, J.L.; Boland, M.P. Effects of Culturing Bovine Oocytes Either Singly or in Groups on Development to Blastocysts. Theriogenology 1997, 48, 161–169. [Google Scholar] [CrossRef]
- Weng, L. IVF-on-a-Chip: Recent Advances in Microfluidics Technology for In Vitro Fertilization. SLAS Technol. 2019, 24, 373–385. [Google Scholar] [CrossRef]
- Weng, L.; Lee, G.Y.; Liu, J.; Kapur, R.; Toth, T.L.; Toner, M. On-Chip Oocyte Denudation from Cumulus–Oocyte Complexes for Assisted Reproductive Therapy. Lab Chip 2018, 18, 3892–3902. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, W.; Yamanaka, K.; Sugiyama, D.; Teshima, Y.; Briones-Nagata, M.P.; Maeki, M.; Yamashita, K.; Takahashi, M.; Miyazaki, M. Simple Separation of Good Quality Bovine Oocytes Using a Microfluidic Device. Sci. Rep. 2018, 8, 14273. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Elsdale, T.; Bard, J. Collagen Substrata for Studies on Cell Behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Bates, R.C.; Buret, A.; van Helden, D.E.; Horton, M.A.; Burns, G.E. Apoptosis Induced by Inhibition of Intercellular Contact. J. Cell Biol. 1994, 125, 403–415. [Google Scholar] [CrossRef]
- Bell, S.E.; Mavila, A.; Salazar, R.; Bayless, K.J.; Kanagala, S.; Maxwell, S.A.; Davis, G.E. Differential Gene Expression during Capillary Morphogenesis in 3D Collagen Matrices: Regulated Expression of Genes Involved in Basement Membrane Matrix Assembly, Cell Cycle Progression, Cellular Differentiation and G-Protein Signaling. J. Cell Sci. 2001, 114, 2755–2773. [Google Scholar] [CrossRef]
- Ghosh, S.; Spagnoli, G.C.; Martin, I.; Ploegert, S.; Demougin, P.; Heberer, M.; Reschner, A. Three-Dimensional Culture of Melanoma Cells Profoundly Affects Gene Expression Profile: A High Density Oligonucleotide Array Study. J. Cell Physiol. 2005, 204, 522–531. [Google Scholar] [CrossRef]
- Kirshner, J.; Chen, C.J.; Liu, P.; Huang, J.; Shively, J.E. CEACAM1-4S, a Cell-Cell Adhesion Molecule, Mediates Apoptosis and Reverts Mammary Carcinoma Cells to a Normal Morphogenic Phenotype in a 3D Culture. Proc. Natl. Acad. Sci. USA 2003, 100, 521–526. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Philp, D.; Hoffman, M.P. Role of the Extracellular Matrix in Morphogenesis. Curr. Opin. Biotechnol. 2003, 14, 526–532. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between Cell Shape and Type of Collagen Synthesised as Chondrocytes Lose Their Cartilage Phenotype in Culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric Cues for Directing the Differentiation of Mesenchymal Stem Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Anton, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension—How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015. [Google Scholar] [CrossRef] [PubMed]
- Mastrorocco, A.; Cacopardo, L.; Martino, N.A.; Fanelli, D.; Camillo, F.; Ciani, E.; Roelen, B.A.J.; Ahluwalia, A.; Dell’Aquila, M.E. One-Step Automated Bioprinting-Based Method for Cumulus-Oocyte Complex Microencapsulation for 3D in Vitro Maturation. PLoS ONE 2020, 15, e0238812. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Wartalski, K.; Tabarowski, Z.; Duda, M. Proteolytically Degraded Alginate Hydrogels and Hydrophobic Microbioreactors for Porcine Oocyte Encapsulation. J. Vis. Exp. 2020, 2020, e61325. [Google Scholar] [CrossRef]
- Shen, P.; Xu, J.; Wang, P.; Zhao, X.; Huang, B.; Wu, F.; Wang, L.; Chen, W.; Feng, Y.; Guo, Z.; et al. A New Three-Dimensional Glass Scaffold Increases the in Vitro Maturation Efficiency of Buffalo (Bubalus Bubalis) Oocyte via Remodelling the Extracellular Matrix and Cell Connection of Cumulus Cells. Reprod. Domest. Anim. 2020, 55, 170–180. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, M.S.; Lee, E.; Lee, S.T. In Vitro Maturation Using an Agarose Matrix with Incorporated Extracellular Matrix Proteins Improves Porcine Oocyte Developmental Competence by Enhancing Cytoplasmic Maturation. J. Tissue Eng. Regen. Med. 2021, 15, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Machida, R.; Hiraga, K.; Hiradate, Y.; Suda, Y.; Tanemura, K. Hanging Drop Monoculture for Selection of Optimal Antioxidants During In Vitro Maturation of Porcine Oocytes. Reprod. Domest. Anim. 2014, 49, e26–e30. [Google Scholar] [CrossRef]
- Tian, J.; Arbatan, T.; Li, X.; Shen, W. Liquid Marble for Gas Sensing. Chem. Commun. 2010, 46, 4734–4736. [Google Scholar] [CrossRef]
- Tian, J.; Fu, N.; Chen, X.D.; Shen, W. Respirable Liquid Marble for the Cultivation of Microorganisms. Colloids Surf. B Biointerfaces 2013, 106, 187–190. [Google Scholar] [CrossRef]
- Sarvi, F.; Arbatan, T.; Chan, P.P.Y.; Shen, W. A Novel Technique for the Formation of Embryoid Bodies inside Liquid Marbles. RSC Adv. 2013, 3, 14501–14508. [Google Scholar] [CrossRef]
- Lin, K.; Chen, R.; Zhang, L.; Zang, D.; Geng, X.; Shen, W. Transparent Bioreactors Based on Nanoparticle-Coated Liquid Marbles for in Situ Observation of Suspending Embryonic Body Formation and Differentiation. ACS Appl. Mater. Interfaces 2019, 11, 8789–8796. [Google Scholar] [CrossRef]
- Vadivelu, R.K.; Ooi, C.H.; Yao, R.Q.; Tello Velasquez, J.; Pastrana, E.; Diaz-Nido, J.; Lim, F.; Ekberg, J.A.K.; Nguyen, N.T.; St John, J.A. Generation of Three-Dimensional Multiple Spheroid Model of Olfactory Ensheathing Cells Using Floating Liquid Marbles. Sci. Rep. 2015, 5, 15083. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Idda, A.; Kelly, J.; Ariu, F.; Bogliolo, L.; Bebbere, D. A Novel Technique for in Vitro Maturation of Sheep Oocytes in a Liquid Marble Microbioreactor. J. Assist. Reprod. Genet. 2016, 33, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bebbere, D.; Nieddu, S.M.; Ariu, F.; Piras, D.; Ledda, S. 3d Liquid Marble Microbioreactors Support in Vitro Maturation of Prepubertal Ovine Oocytes and Affect Expression of Oocyte-Specific Factors. Biology 2021, 10, 1101. [Google Scholar] [CrossRef]
- Kim, M.H.; Sawada, Y.; Taya, M.; Kino-oka, M. Influence of Surface Topography on the Human Epithelial Cell Response to Micropatterned Substrates with Convex and Concave Architectures. J. Biol. Eng. 2014, 8, 13. [Google Scholar] [CrossRef]
- Graziano, A.; D’Aquino, R.; Cusella-De Angelis, M.G.; de Francesco, F.; Giordano, A.; Laino, G.; Piattelli, A.; Traini, T.; de Rosa, A.; Papaccio, G. Scaffold’s Surface Geometry Significantly Affects Human Stem Cell Bone Tissue Engineering. J. Cell Physiol. 2008, 214, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.; Firth, J.D.; Uitto, V.J.; Brunette, D.M. Substratum Surface Topography Alters Cell Shape and Regulates Fibronectin MRNA Level, MRNA Stability, Secretion and Assembly in Human Fibroblasts. J. Cell Sci. 1995, 108, 1563–1573. [Google Scholar] [CrossRef]
- Chou, L.; Firth, J.D.; Uitto, V.J.; Brunette, D.M. Effects of Titanium Substratum and Grooved Surface Topography on Metalloproteinase-2 Expression in Human Fibroblasts. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/%28SICI%291097-4636%2819980305%2939%3A3%3C437%3A%3AAID-JBM13%3E3.0.CO%3B2-7 (accessed on 18 March 2022).
- Matsuzaka, K.; Yoshinari, M.; Shimono, M.; Inoue, T. Effects of Multigrooved Surfaces on Osteoblast-like Cells in Vitro: Scanning Electron Microscopic Observation and MRNA Expression of Osteopontin and Osteocalcin. J. Biomed. Mater. Res. A 2004, 68, 227–234. [Google Scholar] [CrossRef]
- Walters, K.A.; Binnie, J.P.; Campbell, B.K.; Armstrong, D.G.; Telfer, E.E. The Effects of IGF-I on Bovine Follicle Development and IGFBP-2 Expression Are Dose and Stage Dependent. Reproduction 2006, 131, 515–523. [Google Scholar] [CrossRef]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A Two-Step Serum-Free Culture System Supports Development of Human Oocytes from Primordial Follicles in the Presence of Activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef]
- Sánchez, F.; Romero, S.; Albuz, F.K.; Smitz, J. In Vitro Follicle Growth under Non-Attachment Conditions and Decreased FSH Levels Reduces Lhcgr Expression in Cumulus Cells and Promotes Oocyte Developmental Competence. J. Assist. Reprod. Genet. 2012, 29, 141–152. [Google Scholar] [CrossRef]
- Shafaie, S.; Hutter, V.; Brown, M.B.; Cook, M.T.; Chau, D.Y.S. Influence of Surface Geometry on the Culture of Human Cell Lines: A Comparative Study Using Flat, Round-Bottom and v-Shaped 96 Well Plates. PLoS ONE 2017, 12, e0186799. [Google Scholar] [CrossRef]
- Wydooghe, E.; Vandaele, L.; Heras, S.; de Sutter, P.; Deforce, D.; Peelman, L.; de Schauwer, C.; van Soom, A. Autocrine Embryotropins Revisited: How Do Embryos Communicate with Each Other in Vitro When Cultured in Groups? Biol. Rev. 2017, 92, 505–520. [Google Scholar] [CrossRef]
- Wydooghe, E.; Vandaele, L.; Beek, J.; Favoreel, H.; Heindryckx, B.; de Sutter, P.; van Soom, A. Differential Apoptotic Staining of Mammalian Blastocysts Based on Double Immunofluorescent CDX2 and Active Caspase-3 Staining. Anal. Biochem. 2011, 416, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Brum, D.S.; Leivas, F.G.; Silva, C.A.M.; Rubin, M.I.B.; Rauber, L.P.; Fialho, S.S.; Pilla, L.F.C.; Bernardi, M.L.; Brum, D.S.; Leivas, F.G.; et al. The Effects of the Number of Oocytes and the Volume of Maturation Medium in Bovine in Vitro Embryo Production. Anim. Reprod. 2018, 2, 70–73. [Google Scholar]
- Celá, P.; Veselá, B.; Matalová, E.; Večeřa, Z.; Buchtová, M. Embryonic Toxicity of Nanoparticles. Cells Tissues Organs 2014, 199, 1–23. [Google Scholar] [CrossRef]
- Ferraz, M.D.A.M.M.; Henning, H.H.W.; da Costa, P.F.; Malda, J.; Le Gac, S.; Bray, F.; van Duursen, M.B.M.; Brouwers, J.F.; van de Lest, C.H.A.; Bertijn, I.; et al. Potential Health and Environmental Risks of Three-Dimensional Engineered Polymers. Environ. Sci. Technol. Lett. 2018, 5, 80–85. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent Advances in Bovine in Vitro Embryo Production: Reproductive Biotechnology History and Methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Biswas, D.; Hyun, S.H. Supplementation of Fetal Bovine Serum Increased the Quality of in Vitro Fertilized Porcine Embryo. J. Adv. Vet Anim. Res. 2021, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.L.; Huang, Y.J. Effects of Serum on Cytotoxicity of Nano- and Micro-Sized ZnO Particles. J. Nanoparticle Res. 2013, 15, 1829. [Google Scholar] [CrossRef]
- Pavani, K.C.; Hendrix, A.; van den Broeck, W.; Couck, L.; Szymanska, K.; Lin, X.; de Koster, J.; van Soom, A.; Leemans, B. Isolation and Characterization of Functionally Active Extracellular Vesicles from Culture Medium Conditioned by Bovine Embryos In Vitro. Int. J. Mol. Sci. 2018, 20, 38. [Google Scholar] [CrossRef]
- van Soom, A.; Ysebaert, M.T.; de Kruif, A. Relationship between timing of development, morula morphology, and cell allocation to inner cell mass and trophectoderm in in vitro-produced bovine embryos. Mol. Reprod. Dev. 2017, 47, 47–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).