Complex Organisms Must Deal with Complex Threats: How Does Amphibian Conservation Deal with Biphasic Life Cycles?
Abstract
Simple Summary
Abstract
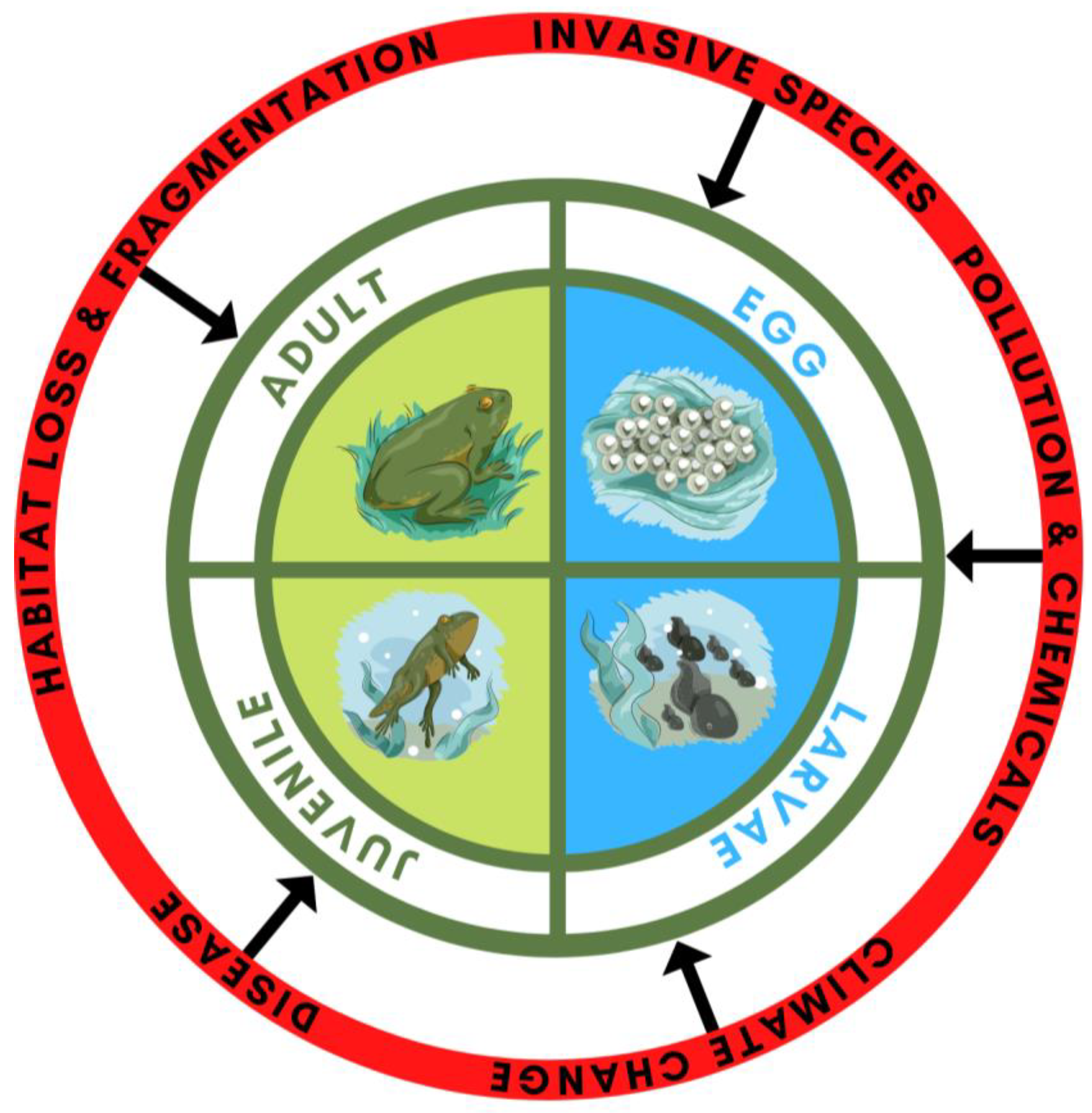
1. Introduction
2. Methods
3. Threats to the Aquatic Stage of the Amphibian Life Cycle: Embryos and Larvae
3.1. Climate Change
3.2. Invasive Species
3.3. Diseases: Chytridiomycosis
3.4. Pollution and Chemical Contamination
4. Mitigating Threats at the Aquatic Stage of the Amphibian Life Cycle
4.1. Mitigating Climate Change
4.2. Mitigating Aquatic Invasives
4.3. Mitigating Chytrid in the Aquatic Environment
4.4. Mitigating Pollution and Chemical Contamination
5. Threats at the Terrestrial Stage of the Amphibian Life Cycle: Juveniles and Adults
5.1. Disease: Chytridiomycosis
5.2. Habitat Loss and Fragmentation
6. Mitigating Threats at the Terrestrial Stage of the Amphibian Life Cycle
6.1. Mitigating Chytrid within the Terrestrial Environment
6.2. Mitigating Habitat Fragmentation
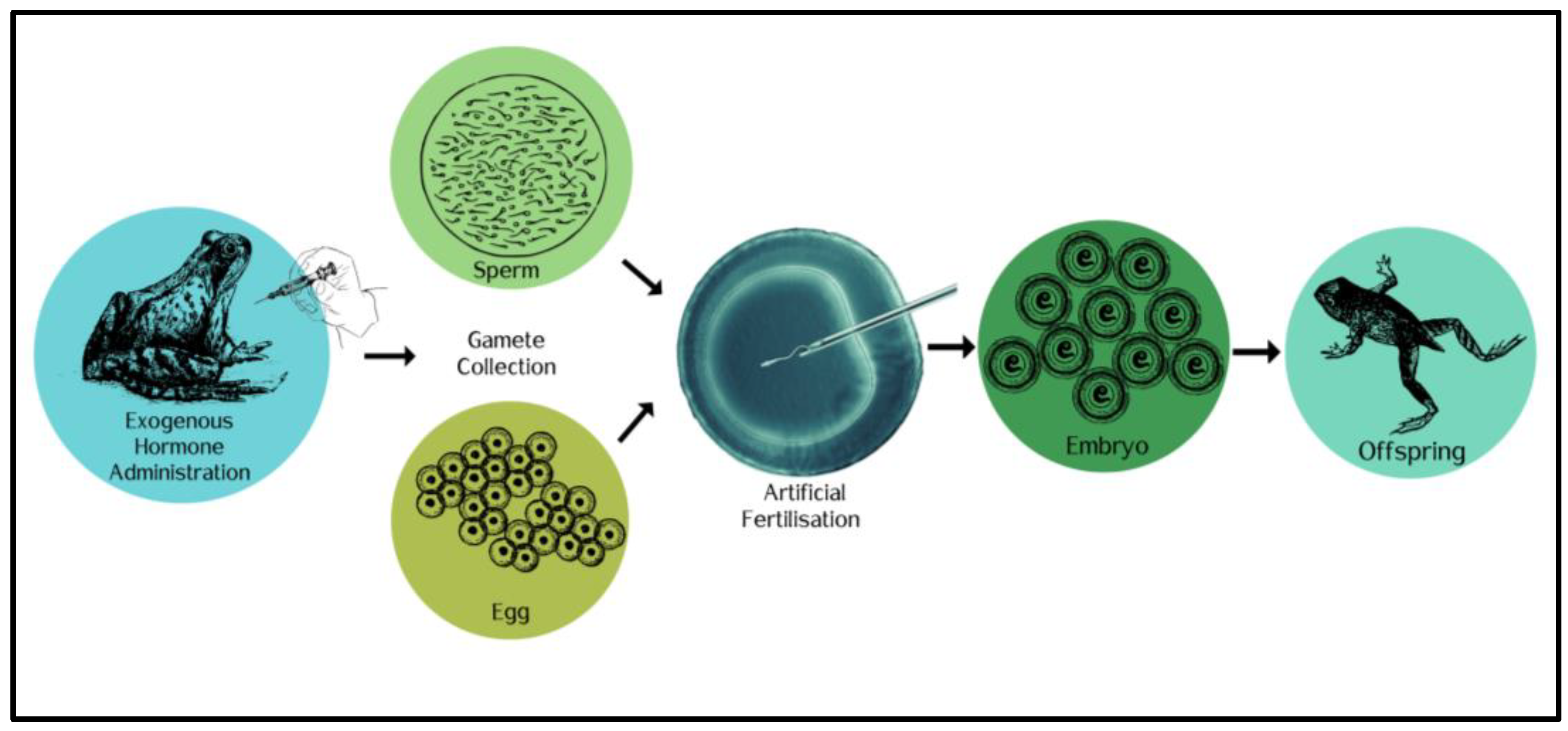
7. Ex Situ Conservation Actions: Managing the Frog, Not the Threat
8. Evidence of Effect: Successful Studies That Consider Multiple Actions to Mitigate Threats at Multiple Life Stages
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Ehrlich, P.R. Conservation Biology for All; Oxford University Press: Oxford, UK, 2010; p. 360. [Google Scholar]
- Burdon, F.J. Understanding the connectivity of ecosystems in the Anthropocene. J. Anim. Ecol. 2021, 90, 1600–1604. [Google Scholar] [CrossRef]
- Brook, B.; Sodhi, N.; Bradshaw, C. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Souza, D.; Verburg, P.H.; Dobrovolski, R. Habitat loss, extinction predictability and conservation efforts in the terrestrial ecoregions. Biol. Conserv. 2020, 246, 108579. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Munstermann, M.J.; Heim, N.A.; McCauley, D.J.; Payne, J.L.; Upham, N.S.; Wang, S.C.; Knope, M.L. A global ecological signal of extinction risk in terrestrial vertebrates. Conserv. Biol. 2022, 36, e13852. [Google Scholar] [CrossRef]
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef]
- Banerjee, P.; Dey, G.; Antognazza, C.M.; Sharma, R.K.; Maity, J.P.; Chan, M.Y.; Huang, Y.-H.; Lin, P.-Y.; Chao, H.-C.; Lu, C.-M.; et al. Reinforcement of Environmental DNA Based Methods (Sensu Stricto) in Biodiversity Monitoring and Conservation: A Review. Biology 2021, 10, 1223. [Google Scholar] [CrossRef]
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar] [CrossRef]
- IUCN. Conservation Actions Classification Scheme (Version 2.0). 2022. Available online: https://www.iucnredlist.org/resources/conservation-actions-classification-scheme (accessed on 25 May 2022).
- Klein, C.J.; Beher, J.; Chaloupka, M.; Hamann, M.; Limpus, C.; Possingham, H.P. Prioritization of Marine Turtle Management Projects: A Protocol That Accounts for Threats to Different Life History Stages. Conserv. Lett. 2017, 10, 547–554. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Klop-Toker, K.; Wallace, S.; Stock, S.; Hayward, M.; Mahony, M. The decline of Australian heath frogs and summary of current threats. In Imperiled: The Encyclopedia of Conservation; Berryman, R., Carol, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Burns, T.J.; Scheele, B.C.; Brannelly, L.A.; Clemann, N.; Gilbert, D.; Driscoll, D.A. Indirect terrestrial transmission of amphibian chytrid fungus from reservoir to susceptible host species leads to fatal chytridiomycosis. Anim. Conserv. 2021, 24, 602–612. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Urbina, J.; Snyder, P.W.; Reynolds, E.; Dang, T.; Hoverman, J.T.; Han, B.; Olson, D.H.; Searle, C.; Hambalek, N.M. Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies. Diversity 2018, 10, 81. [Google Scholar] [CrossRef]
- Lindenmayer, D. Fire, forests and fauna (The 2020 Krebs Lecture). Pac. Conserv. Biol. 2021, 27, 118–125. [Google Scholar] [CrossRef]
- Saumure, R.A.; Rivera, R.; Jaeger, J.R.; O’Toole, T.; Ambos, A.; Guadelupe, K.; Bennett, A.R.; Marshall, Z. Leaping from extinction: Rewilding the relict leopard frog in Las Vegas, Nevarda, USA. In Global Conservation Translocation Perspectives: 2021. Case Studies from around the Globe; Soorae, P.S., Ed.; IUCN SSC Conservation Translocation Specialist Group, Environment Agency—Abu Dhabi and Calgary Zoo, Canada: Gland, Switzerland, 2021; pp. 76–81. [Google Scholar]
- Beebee, T.J.C. Amphibian breeding and climate change. Nature 2002, 374, 219–220. [Google Scholar] [CrossRef]
- Reading, C.J. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 2007, 151, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Segan, D.B.; Murray, K.A.; Watson, J.E.M. A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob. Ecol. Conserv. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- Rowan, L.; Arbogast, B.; Kamel, S.J. Assessing the genetic consequences of habitat fragmentation on the federally threatened cheat mountain salamander (Plethodon nettingi): A comparative, multi-locus approach. Conserv. Genet. 2022, 23, 699–711. [Google Scholar] [CrossRef]
- Potvin, D.A.; Parris, K.M.; Date, K.L.S.; Keely, C.C.; Bray, R.D.; Hale, J.; Hunjan, S.; Austin, J.J.; Melville, J. Genetic erosion and escalating extinction risk in frogs with increasing wildfire frequency. J. Appl. Ecol. 2017, 54, 945–954. [Google Scholar] [CrossRef]
- Stock, S.; Klop-Toker, K.; Wallace, S.; Kelly, O.; Callen, A.; Seeto, R.; Mahony, S.V.; Hayward, M.W.; Mahony, M. Uncovering inbreeding, small popu-lations, and strong genetic isolation in an Australian threatened frog, Litoria littlejohni. Conserv. Genet. 2023. [Google Scholar] [CrossRef]
- Calef, G.W. Natural mortality of tadpoles in a population of Rana aurora. Ecology 1973, 54, 741–758. [Google Scholar] [CrossRef]
- Melvin, S.D.; Houlahan, J.E. Tadpole mortality varies across experimental venues: Do laboratory populations predict responses in nature? Oecologia 2012, 169, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R. Decline of a Tropical Montane Amphibian Fauna. Conserv. Biol. 1998, 12, 106–117. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Dausmann, K.H.; Paesler, K.; Babos, P.; Sabatino, N.M.; Peck, M.A.; Glos, J. Shifts in sensitivity of amphibian metamorphosis to endocrine disruption: The common frog (Rana temporaria) as a case study. Conserv. Physiol. 2020, 8, coaa100. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, H.; Arsovski, D.; Bonnaire, E.; Duguet, R.; Joly, P.; Besnard, A. The impact of severe drought on survival, fecundity, and population persistence in an endangered amphibian. Ecosphere 2016, 7, e01246. [Google Scholar] [CrossRef]
- Parris, M.J.; Storfer, A.; Collins, J.P.; Davidson, E.W. Life-History Responses to Pathogens in Tiger Salamander (Ambystoma tigrinum) Larvae. J. Herpetol. 2005, 39, 366–372. [Google Scholar] [CrossRef]
- Earl, J.E.; Chaney, J.C.; Sutton, W.B.; Lillard, C.E.; Kouba, A.J.; Langhorne, C.; Krebs, J.; Wilkes, R.P.; Hill, R.D.; Miller, D.L.; et al. Ranavirus could facilitate local extinction of rare amphibian species. Oecologia 2016, 182, 611–623. [Google Scholar]
- Watling, J.I.; Hickman, C.R.; Orrock, J.L. Predators and invasive plants affect performance of amphibian larvae. Oikos 2011, 120, 735–739. [Google Scholar] [CrossRef]
- Ficetola, F.G.; Siesa, M.E.; Manenti, R.; Bottoni, L.; De Bernardi, F.; Padoa-Schioppa, E. Early assessment of the impact of alien species: Differential consequences of an invasive crayfish on adult and larval amphibians. Divers. Distrib. 2011, 17, 1141–1151. [Google Scholar]
- Haggerty, C.J.E.; Crisman, T.L.; Rohr, J.R. Effects of forestry-driven changes to groundcover and soil moisture on amphibian desiccation, dispersal, and survival. Ecol. Appl. 2019, 29, e01870. [Google Scholar] [CrossRef] [PubMed]
- Child, T.; Phillips, B.L.; Brown, G.P.; Shine, R. The spatial ecology of cane toads (Bufo marinus) in tropical Australia: Why do metamorph toads stay near the water? Austral Ecol. 2008, 33, 630–640. [Google Scholar] [CrossRef]
- Suriyamongkol, T.; Forks, K.; Villamizar-Gomez, A.; Wang, H.-H.; Grant, W.E.; Forstner, M.R.J.; Mali, I. A Simple Conservation Tool to Aid Restoration of Amphibians following High-Severity Wildfires: Use of PVC Pipes by Green Tree Frogs (Hyla cinerea) in Central Texas, USA. Diversity 2021, 13, 649. [Google Scholar] [CrossRef]
- Harper, E.B.; Rittenhouse, T.A.G.; Semlitsch, R.D. Demographic Consequences of Terrestrial Habitat Loss for Pool-Breeding Amphibians: Predicting Extinction Risks Associated with Inadequate Size of Buffer Zones. Conserv. Biol. 2008, 22, 1205–1215. [Google Scholar] [CrossRef]
- Van Rooij, P.; Martel, A.; Haesebrouck, F.; Pasmans, F. Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Vet. Res. 2015, 46, 137. [Google Scholar] [CrossRef]
- Sunderland, T.; Sunderland-Groves, J.; Shanley, P.; Campbell, B. Bridging the Gap: How Can Information Access and Exchange between Conservation Biologists and Field Practitioners Be Improved for Better Conservation Outcomes? Biotropica 2009, 41, 549–554. [Google Scholar] [CrossRef]
- Ferraro, P.J.; Pattanayak, S.K. Money for Nothing? A Call for Empirical Evaluation of Biodiversity Conservation Investments. PLoS Biol. 2006, 4, e105. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Blumstein, D.T.; Swaisgood, R.R. Conservation translocations: A review of common difficulties and promising directions. Anim. Conserv. 2020, 23, 121–131. [Google Scholar] [CrossRef]
- Watson, A.S.; Castillo, L. Are protected areas working for endangered frogs in the Peruvian Andes? Biodivers. Conserv. 2022, 31, 1847–1866. [Google Scholar] [CrossRef]
- Hamer, A.J.; Lane, S.J.; Mahony, M.J. Management of freshwater wetlands for the endangered green and golden bell frog (Litoria aurea): Roles of habitat determinants and space. Biol. Conserv. 2002, 106, 413–424. [Google Scholar] [CrossRef]
- Canessa, S.; Spitzen–van der Sluijs, A.; Martel, A.; Psmans, F. Mitigation of amphibian disease requires a stronger connection between research and management. Biol. Conserv. 2019, 236, 236–242. [Google Scholar] [CrossRef]
- Grant, E.H.C.; Muths, E.; Schmidt, B.R.; Petrovan, S.O. Amphibian conservation in the Anthropocene. Biol. Conserv. 2019, 236, 543–547. [Google Scholar] [CrossRef]
- Meredith, H.M.R.; St. John, F.A.V.; Collen, B.; Black, S.A.; Griffiths, R.A. Practitioner and scientist perceptions of successful amphibian conservation. Conserv. Biol. 2018, 32, 366–375. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. 2023. Available online: https://www.iucnredlist.org (accessed on 13 March 2023).
- Stephenson, P.; Workman, C.; Grace, M.; Long, B. Testing the IUCN Green List of Species. Oryx 2020, 54, 10–11. [Google Scholar] [CrossRef]
- Wilbur, H.M. Complex life cycles. Annu. Rev. Ecol. Syst. 1980, 11, 67–93. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Wassersug, R.J. The Adaptive Significance of the Tadpole Stage with Comments on the Maintenance of Complex Life Cycles in Anurans. Am. Zool. 1975, 15, 405–417. [Google Scholar] [CrossRef]
- Anstis, M. Frogs and Tadpoles of Australia; New Holland Publishers: Chatswood, NSW, Australia, 2018. [Google Scholar]
- Brooks, G.C.; Kindsvater, H.K. Early Development Drives Variation in Amphibian Vulnerability to Global Change. Front. Ecol. Evol. 2022, 10, 813414. [Google Scholar] [CrossRef]
- Biek, R.; Funk, W.C.; Maxell, B.A.; Mills, L.S. What Is Missing in Amphibian Decline Research: Insights from Ecological Sensitivity Analysis. Conserv. Biol. 2002, 16, 728–734. [Google Scholar] [CrossRef]
- Székely, D.; Cogălniceanu, D.; Székely, P.; Armijos-Ojeda, D.; Espinosa-Mogrovejo, V.; Denoël, M. How to recover from a bad start: Size at metamorphosis affects growth and survival in a tropical amphibian. BMC Ecol. 2020, 20, 24. [Google Scholar] [CrossRef]
- Anderson, T.L.; Ousterhout, B.H.; Peterman, W.E.; Drake, D.L.; Semlitsch, R.D. Life history differences influence the impacts of drought on two pond-breeding salamanders. Ecol. Appl. 2015, 25, 1896–1910. [Google Scholar] [CrossRef]
- Dodd, C.K. The effects of drought on population-structure, activity, and orientation of toads (Bufo quercicus and B. terrestris) at a temporary pond. Ethol. Ecol. Evol. 1994, 6, 331–349. [Google Scholar] [CrossRef]
- Gervasi, S.S.; Foufopoulos, J. Costs of plasticity: Responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct. Ecol. 2008, 22, 100–108. [Google Scholar] [CrossRef]
- Lawler, J.J.; Shafer, S.L.; Bancroft, B.A.; Blaustein, A.R. Projected Climate Impacts for the Amphibians of the Western Hemisphere. Conserv. Biol. 2010, 24, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Ruthsatz, K.; Dausmann, K.H.; Peck, M.A.; Glos, J. Thermal tolerance and acclimation capacity in the European common frog (Rana temporaria) change throughout ontogeny. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Blossey, B.; Maerz, J.C.; Joule, S.J. Invasive plant and experimental venue affect tadpole performance. Biol. Invasions 2006, 8, 327–338. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Population differences in responses of red-legged frogs (Rana aurora) to introduce bullfrogs (Rana catesbeiana). Ecology 1997, 78, 1752–1760. [Google Scholar] [CrossRef]
- Kupferberg, S.J. Bullfrog (Rana catesbeiana) invasion of a California river: The role of larval competition. Ecology 1997, 78, 1736–1751. [Google Scholar] [CrossRef]
- Calfee, R.D.; Little, E.E. Toxicity of cadmium, copper, and zinc to the threatened Chiricahua leopard frog (Lithobates [Rana] chiricahuensis). Bull. Environ. Contam. Toxicol. 2017, 99, 679–683. [Google Scholar] [CrossRef]
- Salice, C.J.; Rowe, C.L.; Pechmann, J.H.K.; Hopkins, W.A. Multiple stressors and complex life cycles: Insights from a population-level assessment of breeding site contamination and terrestrial habitat loss in an amphibian. Environ. Toxicol. Chem. 2011, 30, 2874–2882. [Google Scholar] [CrossRef]
- Rudolf, V.H.W.; Rödel, M.-O. Phenotypic plasticity and optimal timing of metamorphosis under uncertain time constraints. Evol. Ecol. 2007, 21, 121–142. [Google Scholar] [CrossRef]
- Laurila, A.; Pakkasmaa, S.; Merilä, J. Influence of seasonal time constraints on growth and development of common frog tadpoles: A photoperiod experiment. Oikos 2001, 95, 451–460. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Peck, M.A.; Dausmann, K.H.; Sabatino, N.M.; Glos, J. Patterns of temperature induced developmental plasticity in anuran larvae. J. Therm. Biol. 2018, 74, 123–132. [Google Scholar] [CrossRef]
- Tejedo, M.; Marangoni, F.; Pertoldi, C.; Richter-Boix, A.; Laurila, A.; Orizaola, G.; Nicieza, A.G.; Álvarez, D.; Gomez-Mestre, I. Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Clim. Res. 2010, 43, 31–39. [Google Scholar] [CrossRef]
- Brady, L.D.; Griffiths, R.A. Developmental responses to pond desiccation in tadpoles of the British anuran amphibians (Bufo bufo, B. calamita and Rana temporaria). J. Zool. 2000, 252, 61–69. [Google Scholar] [CrossRef]
- Amburgey, S.; Funk, C.W.; Murphy, M.; Muths, E. Effects of hydroperiod duration on survival, development rate, and size at metamorphosis in boreal chorus frog tadpoles (Pseudacris maculata). Herpetologica 2012, 68, 456–467. [Google Scholar] [CrossRef]
- Mansoor, S.; Farooq, I.; Kachroo, M.M.; Mahmoud, A.E.D.; Fawzy, M.; Popescu, S.M.; Alyemeni, M.N.; Sonne, C.; Rinklebe, J.; Ahmad, P. Elevation in wildfire frequencies with respect to the climate change. J. Environ. Manag. 2022, 301, 113769. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.; Amraoui, M.; Menezes, I.; Pereira, M.G. Drought in Portugal: Current regime, comparison of indices and impacts on extreme wildfires. Sci. Total Environ. 2019, 685, 150–173. [Google Scholar] [CrossRef]
- Manasee, W.A.; Weerathunga, T.; Rajapaksa, G. The impact of elevated temperature and CO2 on growth, physiological and immune responses of Polypedates cruciger (common hourglass tree frog). Front. Zool. 2020, 17, 3. [Google Scholar]
- Von May, R.; Catenazzi, A.; Santa-Cruz, R.; Gutierrez, A.S.; Moritz, C.; Rabosky, D.L. Thermal physiological traits in tropical lowland amphibians: Vulnerability to climate warming and cooling. PLoS ONE 2019, 14, e0219759. [Google Scholar] [CrossRef]
- Delgado-Suazo, P.; Burrowes, P.A. Response to thermal and hydric regimes point to differential inter- and intraspecific vulnerability of tropical amphibians to climate warming. J. Therm. Biol. 2022, 103, 103148. [Google Scholar] [CrossRef]
- Díaz-Ricaurte, J.C.; Serrano, F.C.; Martins, M. VTMaxHerp: A data set of voluntary thermal maximum temperatures of amphibians and reptiles from two Brazilian hotspots. Ecology 2022, 103, e3602. [Google Scholar] [CrossRef]
- Mathwin, R.; Wassens, S.; Young, J.; Ye, Q.; Bradshaw, C.J.A. Manipulating water for amphibian conservation. Conserv. Biol. 2021, 35, 24–34. [Google Scholar] [CrossRef]
- Chandler, H.C.; Rypel, A.L.; Jiao, Y.; Haas, C.A.; Gorman, T.A. Hindcasting historical breeding conditions for an endangered salamander in ephemeral wetlands of the Southeastern USA: Implications of climate change. PLoS ONE 2016, 11, e0150169. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.C.; Young, J.E.; Johnson, G.N.; Seigel, R.A. Stochastic variation in reproductive success of a rare frog, Rana sevosa: Implications for conservation and for monitoring amphibian populations. Biol. Conserv. 2003, 111, 171–177. [Google Scholar] [CrossRef]
- Rowe, C.L.; Dunson, W.A. Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of Central Pennsylvania, USA. Oecologia 1995, 102, 397–403. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Scott, D.E.; Gibbons, J.W.; Semlitsch, R.D. Influence of wetland hydroperiod on diversity and abundance of metamorphosing juvenile amphibians. Wetl. Ecol. Manag. 1989, 1, 3–11. [Google Scholar] [CrossRef]
- Ashpole, S.L.; Bishop, C.A.; Murphy, S.D. Reconnecting Amphibian Habitat through Small Pond Construction and Enhancement, South Okanagan River Valley, British Columbia, Canada. Diversity 2018, 10, 108. [Google Scholar] [CrossRef]
- Goldspiel, H.B.; Cohen, J.B.; McGee, G.G.; Gibbs, J.P. Forest land-use history affects outcomes of habitat augmentation for amphibian conservation. Glob. Ecol. Conserv. 2019, 19, e00686. [Google Scholar] [CrossRef]
- Simpkins, C.A.; Castley, J.G.; Shuker, J.D.; Morrison, C.; Hero, J.-M. Battling habitat loss: Suitability of anthropogenic waterbodies for amphibians associated with naturally acidic, oligotrophic environments. Pac. Conserv. Biol. 2022, 28, 174–183. [Google Scholar] [CrossRef]
- Simpkins, C.A.; Shuker, J.D.; Lollback, G.W.; Castley, J.G.; Hero, J.-M. Environmental variables associated with the distribution and occupancy of habitat specialist tadpoles in naturally acidic, oligotrophic waterbodies. Austral Ecol. 2014, 39, 95–105. [Google Scholar] [CrossRef]
- Babbitt, K.J.; Baber, M.J.; Tarr, T.L. Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Can. J. Zool. 2003, 81, 1539–1552. [Google Scholar] [CrossRef]
- Jocqué, M.; Graham, T.; Brendonck, L. Local structuring factors of invertebrate communities in ephemeral freshwater rock pools and the influence of more permanent water bodies in the region. Hydrobiologia 2007, 592, 271–280. [Google Scholar] [CrossRef]
- Ujszegi, J.; Bertalan, R.; Ujhegyi, N.; Verebélyi, V.; Nemesházi, E.; Mikó, Z.; Kásler, A.; Herczeg, D.; Szederkényi, M.; Vili, N.; et al. “Heat waves” experienced during larval life have species-specific consequences on life-history traits and sexual development in anuran amphibians. Sci. Total Environ. 2022, 835, 155297. [Google Scholar] [CrossRef]
- Carey, C.; Alexander, M.A. Climate change and amphibian declines: Is there a link? Divers. Distrib. 2003, 9, 111–121. [Google Scholar] [CrossRef]
- Ren, C.; Teng, Y.; Shen, Y.; Yao, Q.; Wang, H. Altered temperature affect body condition and endochondral ossification in Bufo gargarizans tadpoles. J. Therm. Biol. 2021, 99, 103020. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Stefan, H.G. Simulations of climate effects on water temperature, dissolved oxygen, and ice and snow covers in lakes of the contiguous United States under past and future climate scenarios. Limnol. Ocean. 2009, 54, 2359–2370. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Lima, I.B.; Da Silva, N.G.; Machado, J.R.; Machado, J.F.F.; Rivaroli, L. Histological changes in the bullfrog (Lithobates catesbeianus) myocardium induced by severe hypoxia during embryonic development. Biológia 2021, 76, 1529–1534. [Google Scholar] [CrossRef]
- Su, R.C.; Meyers, C.M.; Warner, E.A.; Garcia, J.A.; Refsnider, J.M.; Lad, A.; Breidenbach, J.D.; Modyanov, N.; Malhotra, D.; Haller, S.T.; et al. Harmful Algal Bloom Toxicity in Lithobates catesbeiana Tadpoles. Toxins 2020, 12, 378. [Google Scholar] [CrossRef]
- Raffel, T.R.; Rohr, J.R.; Kiesecker, J.M.; Hudson, P.J. Negative effects of changing temperature on amphibian immunity under field conditions. Funct. Ecol. 2006, 20, 819–828. [Google Scholar] [CrossRef]
- Turner, A.; Wassens, S.; Heard, G.; Peters, A. Temperature as a driver of the pathogenicity and virulence of amphibian chytrid fungus Batrachochytrium dendrobatidis: A systematic review. J. Wildl. Dis. 2021, 57, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.N. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2004, 52, 273–288. [Google Scholar] [CrossRef]
- D’Amore, A.; Kirby, E.; McNicholas, M. Invasive species shifts ontogenetic resource partitioning and microhabitat use of a threatened native amphibian. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 534–541. [Google Scholar] [CrossRef]
- Rowe, J.C.; Garcia, T.S. Impacts of Wetland Restoration Efforts on an Amphibian Assemblage in a Multi-invader Community. Wetlands 2014, 34, 141–153. [Google Scholar] [CrossRef]
- Pinero-Rodríguez, M.J.; Fernández-Zamudio, R.; Arribas, R.; Gomez-Mestre, I.; Díaz-Paniagua, C. The invasive aquatic fern Azolla filiculoides negatively impacts water quality, aquatic vegetation and amphibian larvae in Mediterranean environments. Biol. Invasions 2021, 23, 755–769. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Díaz-Paniagua, C. Invasive predatory crayfish do not trigger inducible defences in tadpoles. Proc. R. Soc. B Biol. Sci. 2011, 278, 3364–3370. [Google Scholar] [CrossRef]
- Hamer, A.J. Exotic predatory fish reduce amphibian reproduction at wetlands in an urbanising landscape. Hydrobiologia 2022, 849, 121–139. [Google Scholar] [CrossRef]
- Klop-Toker, K.; Valdez, J.; Stockwell, M.; Clulow, S.; Clulow, J.; Mahony, M. Community level impacts of invasive mosquitofish may exacerbate the impact to a threatened amphibian. Austral Ecol. 2018, 43, 213–224. [Google Scholar] [CrossRef]
- Wake, D.B. Action on amphibians. Trends Ecol. Evol. 1998, 13, 379–380. [Google Scholar] [CrossRef]
- Speare, R.; Field, K.; Koehler, J.; McDonald, K.R. “Disapperaing” Australian rainforest frogs: Have we found the answer? In Proceedings of the Second World Congress of Herpetology, Adelaide, SA, Australia, 29 December 1993–6 January 1994. [Google Scholar]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Marantelli, G.; Berger, L.; Speare, R.; Keegan, L. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac. Conserv. Biol. 2004, 10, 173–179. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Romansic, J.M.; Scheessele, E.A.; Han, B.A.; Pessier, A.P.; Longcore, J.E. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv. Biol. 2005, 19, 1460–1468. [Google Scholar] [CrossRef]
- Cashins, S.D. Epidemiology of Chytridiomycosis in Rainforest Stream Tadpoles; James Cook University: Townsville, QLD, Australia, 2009. [Google Scholar]
- Venesky, M.D.; Parris, M.J.; Storfer, A. Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. EcoHealth 2009, 6, 565–575. [Google Scholar] [CrossRef]
- Parris, M.J.; Baud, D.R. Interactive Effects of a Heavy Metal and Chytridiomycosis on Gray Treefrog Larvae (Hyla chrysoscelis). Copeia 2004, 2004, 344–350. [Google Scholar] [CrossRef]
- Rachowicz, L.J.; Vredenburg, V.T. Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Dis. Aquat. Org. 2004, 61, 75–83. [Google Scholar] [CrossRef]
- Robert, J.; Ohta, Y. Comparative and developmental study of the immune system in Xenopus. Dev. Dyn. 2009, 238, 1249–1270. [Google Scholar] [CrossRef]
- Humphries, J.E.; Lanctôt, C.M.; Robert, J.; McCallum, H.I.; Newell, D.A.; Grogan, L.F. Do immune system changes at metamorphosis predict vulnerability to chytridiomycosis? An update. Dev. Comp. Immunol. 2022, 136, 104510. [Google Scholar] [CrossRef]
- Abu Bakar, A.; Bower, D.S.; Stockwell, M.P.; Clulow, S.; Clulow, J.; Mahony, M.J. Susceptibility to disease varies with ontogeny and immunocompetence in a threatened amphibian. Oecologia 2016, 181, 997–1009. [Google Scholar] [CrossRef]
- Sauer, E.L.; Cohen, J.M.; Lajeunesse, M.J.; McMahon, T.A.; Civitello, D.J.; Knutie, S.A.; Nguyen, K.; Roznik, E.A.; Sears, B.F.; Bessler, S.; et al. A meta-analysis reveals temperature, dose, life stage, and taxonomy influence host susceptibility to a fungal parasite. Ecology 2020, 101, e02979. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, C.; Wepener, V.; Smit, N.J. Acute and chronic effects of acidic pH on four subtropical frog species. Water SA 2016, 42, 52–62. [Google Scholar] [CrossRef]
- Porter, K.R.; Hakanson, D.E. Toxicity of mine drainage to embryonic and larval boreal toads (Bufonidae: Bufo boreas). Copeia 1976, 1976, 327–331. [Google Scholar] [CrossRef]
- Mphepya, J.N.; Pienaar, J.J.; Galy-Lacaux, C.; Held, G.; Turner, C.R. Precipitation Chemistry in Semi-Arid Areas of Southern Africa: A Case Study of a Rural and an Industrial Site. J. Atmos. Chem. 2004, 47, 1–24. [Google Scholar] [CrossRef]
- Leuven, R.S.E.W.; den Hartog, C.; Christiaans, M.M.C.; Heijligers, W.H.C. Effects of water acidification on the distribution pattern and the reproductive success of amphibians. Experientia 1986, 42, 495–503. [Google Scholar] [CrossRef]
- Dolmen, D.; Finstad, A.G.; Skei, J.K. Amphibian recovery after a decrease in acidic precipitation. Ambio 2018, 47, 355–367. [Google Scholar] [CrossRef]
- Gorissen, S.; Greenlees, M.; Shine, R. A skink out of water: Impacts of anthropogenic disturbance on an Endangered reptile in Australian highland swamps. Oryx 2017, 51, 610–618. [Google Scholar] [CrossRef]
- Holla, L.; Barclay, E. Mine Subsidence on the Southern Coalfield New South Wales; New South Wales Department of Mineral Resources: Sydney, NSW, Australia, 2000; 118p.
- Shuhaimi-Othman, M.; Nadzifah, Y.; Umirah, N.S.; Ahmad, A.K. Toxicity of metals to tadpoles of the common Sunda toad, Duttaphrynus melanostictus. Toxicol. Environ. Chem. 2012, 94, 364–376. [Google Scholar] [CrossRef]
- Lakhani, L. How to reduce impact of pesticides in aquatic environment. Soc. Issues Environ. Probl. 2015, 3, 29–38. [Google Scholar] [CrossRef]
- Boudh, S.; Singh, J.S. Pesticide Contamination: Environmental Problems and Remediation Strategies. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R.N., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 245–269. [Google Scholar]
- Relyea, R.A. The leathal impacts of roundup on aquatic and terrestrial amphibians. Ecol. Appl. 2005, 15, 1118–1124. [Google Scholar] [CrossRef]
- Rohr, J.R.; Schotthoefer, A.M.; Raffel, T.R.; Carrick, H.J.; Halstead, N.; Hoverman, J.T.; Johnson, C.M.; Johnson, L.B.; Lieske, C.; Piwoni, M.D.; et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature 2008, 455, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.; Hall, J.E.; Miró, A.; O’Brien, K.; Falaschi, M.; Jehle, R. Reversing a downward trend in threatened peripheral amphibian (Triturus cristatus) populations through interventions combining species, habitat and genetic information. J. Nat. Conserv. 2021, 64, 126077. [Google Scholar] [CrossRef]
- Miró, A.; O’Brien, D.; Tomàs, J.; Buchaca, T.; Sabás, I.; Osorio, V.; Lucati, F.; Pou-Rovira, Q.; Ventura, M. Rapid amphibian community recovery following removal of non-native fish from high mountain lakes. Biol. Conserv. 2020, 251, 108783. [Google Scholar] [CrossRef]
- Walston, L.J.; Mullin, S.J. Responses of a Pond-breeding Amphibian Community to the Experimental Removal of Predatory Fish. Am. Midl. Nat. 2007, 157, 63–73. [Google Scholar] [CrossRef]
- Simberloff, D. We can eliminate invasions or live with them. Successful management projects. Biol. Invasions 2009, 11, 149–157. [Google Scholar] [CrossRef]
- West, M.; Todd, C.R.; Gillespie, G.R.; McCarthy, M. Recruitment is key to understanding amphibian’s different population-level responses to chytrid fungus infection. Biol. Conserv. 2020, 241, 108247. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hunter, D.A.; Grogan, L.F.; Berger, L.; Kolby, J.E.; McFadden, M.S.; Marantelli, G.; Skerratt, L.F.; Driscoll, D.A. Interventions for Reducing Extinction Risk in Chytridiomycosis-Threatened Amphibians. Conserv. Biol. 2014, 28, 1195–1205. [Google Scholar] [CrossRef]
- Stice, M.J.; Briggs, C.J. Immunization is ineffective at preventing infection and mortality due to the amphibian chytrid fungus Batrachochytrium dendrobatidis. J. Wildl. Dis. 2010, 46, 70–77. [Google Scholar] [CrossRef]
- Cashins, S.D.; Grogan, L.F.; McFadden, M.; Hunter, D.; Harlow, P.S.; Berger, L.; Skerratt, L.F. Prior Infection Does Not Improve Survival against the Amphibian Disease Chytridiomycosis. PLoS ONE 2013, 8, e56747. [Google Scholar] [CrossRef]
- Fernández-Loras, A.; Martín-Beyer, B.; Garner, T.W.J.; Bosch, J. Itraconazole and thiophanate-methyl fail to clear tadpoles naturally infected with the hypervirulent lineage of Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2018, 131, 73–78. [Google Scholar] [CrossRef]
- Knapp, R.A.; Joseph, M.B.; Smith, T.C.; Hegeman, E.E.; Vredenburg, V.T.; Erdman, J.E., Jr.; Boiano, D.M.; Jani, A.J.; Briggs, C.J. Effectiveness of antifungal treatments during chytridiomycosis epizootics in populations of an endangered frog. Peer J. 2022, 10, e12712. [Google Scholar] [CrossRef]
- Bosch, J.; Sanchez-Tomé, E.; Fernández-Loras, A.; Oliver, J.A.; Fisher, M.C.; Garner, T.W.J. Successful elimination of a lethal wildlife infectious disease in nature. Biol. Lett. 2015, 11, 20150874. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, M.P.; Clulow, J.; Mahony, M.J. Sodium chloride inhibits the growth and infective capacity of the amphibian chytrid fungus and increases host survival rates. PLoS ONE 2012, 7, e36942. [Google Scholar] [CrossRef] [PubMed]
- Beranek, C.T.; Maynard, C.; McHenry, C.; Clulow, J.; Mahony, M. Rapid population increase of the threatened Australian amphibian Litoria aurea in response to wetlands constructed as a refuge from chytrid-induced disease and introduced fish. J. Environ. Manag. 2021, 291, 112638. [Google Scholar] [CrossRef] [PubMed]
- Callen, A.; Pizzatto, L.; Stockwell, M.P.; Clulow, S.; Clulow, J.; Mahony, M.J. The effect of salt dosing for chytrid mitigation on tadpoles of a threatened frog, Litoria aurea. J. Comp. Physiol. B 2023, 193, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nordheim, C.L.; Detmering, S.E.; Civitello, D.J.; Johnson, P.T.J.; Rohr, J.R.; McMahon, T.A. Metabolites from the fungal pathogen Batrachochytrium dendrobatidis (bd) reduce Bd load in Cuban treefrog tadpoles. J. Appl. Ecol. 2021, 59, 2398–2403. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, J.S. Managing Environmental Pollution. In Cyanoremediation: A Green-Clean Tool for Decontamination of Synthetic Pesticides from Agro- and Aquatic Ecosystems, in Agro-Environmental Sustainability; Singh, J.S., Seneviratne, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 59–83. [Google Scholar]
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Nazir, M.S.; Tahir, Z.; Hassan, S.U.; Ali, Z.; Akhtar, M.N.; Azam, K.; Abdullah, M.A. Remediation of Pesticide in Water. In Sustainable Agriculture Reviews 47: Pesticide Occurrence, Analysis and Remediation Vol. 1 Biological Systems; Ahamed Inamuddin, M.I., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 271–307. [Google Scholar]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Boamah, P.O.; Huang, Y.; Hua, M.; Zhang, Q.; Wu, J.; Onumah, J.; Sam-Amoah, L.K.; Boamah, P.O. Sorption of heavy metal ions onto carboxylate chitosan derivatives—A mini-review. Ecotoxicol. Environ. Saf. 2015, 116, 113–120. [Google Scholar] [CrossRef]
- Guieysse, B.; Norvill, Z.N. Sequential chemical–biological processes for the treatment of industrial wastewaters: Review of recent progresses and critical assessment. J. Hazard. Mater. 2014, 267, 142–152. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, C.; Li, J.; Zhao, G.; Ren, X.; Wang, X. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Adv. 2012, 2, 8821–8826. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.; Sahu, A.; Shukla, R.; Singh, B.; Rai, J.; Sharma, P.; Lade, H. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Griffiths, R.A. Temporary ponds as amphibian habitats. Aquat. Conserv. Mar. Freshw. Ecosyst. 1997, 7, 119–126. [Google Scholar] [CrossRef]
- Hillman, S.S.; Withers, P.C.; Drewes, R.C.; Hillyard, S.D. Ecology and Environmental Physiology of Amphibians; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Brannelly, L.A.; Webb, R.J.; Hunter, D.A.; Clemann, N.; Howard, K.; Skerratt, L.F.; Berger, L.; Scheele, B.C. Non-declining amphibians can be important reservoir hosts for amphibian chytrid fungus. Anim. Conserv. 2018, 21, 91–101. [Google Scholar] [CrossRef]
- Langhammer, P.F.; Burrowes, P.A.; Lips, K.R.; Bryant, A.B.; Collins, J.P. Susceptability to the Amphibian Chytrid funggus Varies with Ontogeny in the Direct-Developing Frog, Eleutherodactylus Coqui. J. Wildl. Dis. 2014, 50, 438–446. [Google Scholar] [CrossRef]
- Fisher, M.C.; Garner, T.W.J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 2020, 18, 332–343. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef]
- Pessier, A.P.; Nichols, D.K.; Longcore, J.E.; Fuller, M.S. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea). J. Vet. Diagn. Investig. 1999, 11, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.J.; Schlaepfer, M.A. Nothing a hot bath won’t cure: Infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLoS ONE 2011, 6, e28444. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.M.; Kerby, J.; Gentry, L.R.; Donnelly, M.A. Temporal Variation in Infection Prevalence by the Amphibian Chytrid Fungus in Three Species of Frogs at La Selva, Costa Rica. Biotropica 2012, 44, 779–784. [Google Scholar] [CrossRef]
- Kriger, K.M.; Pereoglou, F.; Hero, J.M. Latitudinal Variation in the Prevalence and Intensity of Chytrid (Batrachochytrium dendrobatidis) Infection in Eastern Australia. Conserv. Biol. 2007, 21, 1280–1290. [Google Scholar] [CrossRef]
- Sapsford, S.J.; Alford, R.A.; Schwarzkopf, L. Elevation, Temperature, and Aquatic Connectivity All Influence the Infection Dynamics of the Amphibian Chytrid Fungus in Adult Frogs. PLoS ONE 2013, 8, e82425. [Google Scholar] [CrossRef]
- Wilk, A.J.; Donlon, K.C.; Peterman, W.E. Effects of habitat fragment size and isolation on the density and genetics of urban red-backed salamanders (Plethodon cinereus). Urban Ecosyst. 2020, 23, 761–773. [Google Scholar] [CrossRef]
- Pough, F.H.; Janis, C.M.; Heiser, J.B. Vertebrate Life, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Rondinini, C.; Bonardi, A.; Baisero, D.; Padoa-Schioppa, E. Habitat availability for amphibians and extinction threat: A global analysis. Divers. Distrib. 2015, 21, 302–311. [Google Scholar] [CrossRef]
- Halstead, B.J.; Rose, J.P.; Clark, D.; Kleeman, P.M.; Fisher, R.N. Multi-scale patterns in the occurrence of an ephemeral pool-breeding amphibian. Ecosphere 2022, 13, e3960. [Google Scholar] [CrossRef]
- Snyder, R.; Mausteller, E.; Matlaga, T.J.H.; Miller, D.A.W. How does landscape permeability affect the movement of eastern red-backed salamanders? J. Wildl. Manag. 2022, 86, e22132. [Google Scholar] [CrossRef]
- Petrovan, S.O.; Schmidt, B.R. Neglected juveniles; a call for integrating all amphibian life stages in assessments of mitigation success (and how to do it). Biol. Conserv. 2019, 236, 252–260. [Google Scholar] [CrossRef]
- Vos, C.C.; Chardon, J. Effects of habitat fragmentation and road density on the distribution pattern of the moor frog Rana arvalis. J. Appl. Ecol. 1998, 35, 44–56. [Google Scholar] [CrossRef]
- Fahrig, L.; Pedlar, J.H.; Pope, S.E.; Taylor, P.D.; Wegner, J.F. Effect of road traffic on amphibian density. Biol. Conserv. 1995, 73, 177–182. [Google Scholar] [CrossRef]
- Cayuela, H.; Bonnaire, É.; Astruc, G.; Besnard, A. Transport infrastructure severely impacts amphibian dispersal regardless of life stage. Sci. Rep. 2019, 9, 8214. [Google Scholar] [CrossRef] [PubMed]
- Sjögren-Gulve, P. Distribution and extinction patterns within a northern metapopulation of the pool frog, Rana Lessonae. Ecology 1994, 75, 1357–1367. [Google Scholar] [CrossRef]
- Klop-Toker, K.; Valdez, J.W.; Stockwell, M.P.; Fardell, L.; Clulow, S.; Clulow, J.; Mahony, M.J. Improving breed-and-release programmes in the face of a threatening pathogen, Batrachochytrium dendrobatidis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2788–2803. [Google Scholar] [CrossRef]
- Stockwell, M.P.; Clulow, J.; Mahony, M.J. Evidence of a salt refuge: Chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 2015, 177, 901–910. [Google Scholar] [CrossRef]
- Clulow, S.; Gould, J.; James, H.; Stockwell, M.; Clulow, J.; Mahony, M. Elevated salinity blocks pathogen transmission and improves host survival from the global amphibian chytrid pandemic: Implications for translocations. J. Appl. Ecol. 2018, 55, 830–840. [Google Scholar] [CrossRef]
- McMahon, T.A.; Sears, B.F.; Venesky, M.D.; Bessler, S.M.; Brown, J.M.; Deutsch, K.; Halstead, N.T.; Lentz, G.; Tenouri, N.; Young, S.; et al. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 2014, 511, 224–227. [Google Scholar]
- Zamudio, K.R.; McDonald, C.A.; Belasen, A.M. High Variability in Infection Mechanisms and Host Responses: A Review of Functional Genomic Studies of Amphibian Chytridiomycosis. Herpetologica 2020, 76, 189–200. [Google Scholar] [CrossRef]
- Kosch, T.A.; Silva, C.N.S.; Brannelly, L.A.; Roberts, A.A.; Lau, Q.; Marantelli, G.; Berger, L.; Skerratt, L. Genetic potential for disease resistance in critically endangered amphibians decimated by chytridiomycosis. Anim. Conserv. 2019, 22, 238–250. [Google Scholar] [CrossRef]
- Petranka, J.W.; Kennedy, C.A.; Murray, S.S. Response of amphibians to restoration of a southern Appalachian wetland: A long-term analysis of community dynamics. Wetlands 2003, 23, 1030–1042. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Critical Elements for Biologically Based Recovery Plans of Aquatic-Breeding Amphibians. Conserv. Biol. 2002, 16, 619–629. [Google Scholar] [CrossRef]
- Becker, C.G.; Fonseca, C.R.; Haddad, C.F.B.; Batista, R.F.; Prado, P.I. Habitat Split and the Global Decline of Amphibians. Science 2007, 318, 1775–1777. [Google Scholar] [CrossRef]
- Gamble, L.R.; McGarigal, K.; Jenkins, C.L.; Timm, B.C. Limitations of regulated “buffer zones” for the conservation of marbled salamanders. Wetlands 2006, 26, 298–306. [Google Scholar] [CrossRef]
- Rothenberger, M.B.; Vera, M.K.; Germanoski, D.; Ramirez, E. Comparing amphibian habitat quality and functional success among natural, restored, and created vernal pools. Restor. Ecol. 2019, 27, 881–891. [Google Scholar] [CrossRef]
- Lambert, M.; Drayer, A.N.; Leuenberger, W.; Price, S.J.; Barton, C. Evaluation of created wetlands as amphibian habitat on a reforested surface mine. Ecol. Eng. 2021, 171, 106386. [Google Scholar] [CrossRef]
- Hossack, B.R. Amphibian dynamics in constructed ponds on a wildlife refuge: Developing expected responses to hydrological restoration. Hydrobiologia 2017, 790, 23–33. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN Species Survival Commission, Reintroduction Specialist Group of the IUCN Species Survival Commission: Gland, Switzerland, 2013; viiii + 57p. [Google Scholar]
- Scheele, B.; Hoffmann, E.; West, M. Recommendations for Conservation Translocations of Australian Frogs; NESP Threatened Sprecies Recovery Hub Project 3.3.6 Report; TSR Hub: Brisbane, QLD, Australia, 2021. [Google Scholar]
- Germano, J.M.; Bishop, P.J. Suitability of amphibians and reptiles for translocation. Conserv. Biol. 2009, 23, 7–15. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hollanders, M.; Hoffmann, E.P.; Newell, D.A.; Lindenmayer, D.B.; McFadden, M.; Gilbert, D.J.; Grogan, L.F. Conservation translocations for amphibian species threatened by chytrid fungus: A review, conceptual framework, and recommendations. Conserv. Sci. Pract. 2021, 3, e524. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Henkanaththegedara, S.M.; Vásconez Duchicela, P.; Vargas Tierras, Y.; Sánchez Capa, M.; Constante Mejía, D.; Jimenez Gutierrez, M.; Charco Guamán, M.; Mestanza Ramón, P. In-Situ and Ex-Situ Biodiversity Conservation in Ecuador: A Review of Policies, Actions and Challenges. Diversity 2020, 12, 315. [Google Scholar] [CrossRef]
- Gillis, A.B.; Guy, E.L.; Kouba, A.J.; Allen, P.J.; Marcec-Greaves, R.M.; Kouba, C.K. Short-term storage of tiger salamander (Ambystoma tigrinum) spermatozoa: The effect of collection type, temperature and time. PLoS ONE 2021, 16, e0245047. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.G.; Silla, A.J. Hormonal induction of gamete release, and in-vitro fertilisation, in the critically endangered Southern Corroboree Frog, Pseudophryne corroboree. Reprod. Biol. Endocrinol. 2010, 8, 144. [Google Scholar] [CrossRef]
- Upton, R.; Clulow, S.; Calatayud, N.E.; Colyvas, K.; Seeto, R.G.Y.; Wong, L.A.M.; Mahony, M.J.; Clulow, J. Generation of reproductively mature offspring from the endangered green and golden bell frog (Litoria aurea) using cryopreserved spermatozoa. Reprod. Fertil. Dev. 2021, 33, 562–572. [Google Scholar] [CrossRef]
- Hunter, D.A. National Recovery Plan for the Southern Corroboree Frog, Pseudophryne corroboree, and the Northern Corroboree Frog, Pseudophryne pengilleyi; Office of Environment and Heritage: Hurstville, NSW, Australia, 2012.
- Skerratt, L.F.; Berger, L.; Clemann, N.; Hunter, D.A.; Marantelli, G.; Newell, D.A.; Philips, A.; McFadden, M.; Hines, H.B.; Scheele, B.; et al. Priorities for management of chytridiomycosis in Australia: Saving frogs from extinction. Wildl. Res. 2016, 43, 105–120. [Google Scholar] [CrossRef]
- Lewis, C.H.R.; Richards-Zawacki, C.L.; Ibáñez, R.; Luedtke, J.; Voyles, J.; Houser, P.; Gratwicke, B. Conserving Panamanian harlequin frogs by integrating captive-breeding and research programs. Biol. Conserv. 2019, 236, 180–187. [Google Scholar] [CrossRef]
- Byrne, P.G.; Silla, A.J. An experimental test of the genetic consequences of population augmentation in an amphibian. Conserv. Sci. Pract. 2020, 2, e194. [Google Scholar] [CrossRef]
- Browne, R.K.; Silla, A.J.; Upton, R.; Della-Togna, G.; Marcec-Greaves, R.; Shishova, N.V.; Uteshev, V.K.; Proaño, B.; Pérez, O.D.; Mansour, N.; et al. Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology 2019, 133, 187–200. [Google Scholar] [CrossRef]
- Howell, L.G.; Mawson, P.R.; Frankham, R.; Rodger, J.C.; Upton, R.M.; Witt, R.R.; Calatayud, N.E.; Clulow, S.; Clulow, J. Integrating biobanking could produce significant cost benefits and minimise inbreeding for Australian amphibian captive breeding programs. Reprod. Fertil. Dev. 2021, 33, 573–587. [Google Scholar] [CrossRef]
- Della Togna, G.; Howell, L.G.; Clulow, J.; Langhorne, C.J.; Marcec-Greaves, R.; Calatayud, N.E. Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives. Theriogenology 2020, 150, 412–431. [Google Scholar] [CrossRef]
- Langhorne, C.J.; Calatayud, N.E.; Kouba, A.J.; Feugang, J.M.; Vance, C.K.; Willard, S.T. Cryoconservation: Successful sperm cryopreservation and develop-mental outcomes using endangered North American amphibians. Cryobiology 2013, 67, 405. [Google Scholar] [CrossRef]
- Peng, L.; Xiao, Y.; Liu, Y. Effect of cryopreservation and short-term storage of Chinese giant salamander sperm. Acta Hydrobiol. Sin. 2011, 35, 325–332. [Google Scholar] [CrossRef]
- Guy, E.L.; Gillis, A.B.; Kouba, A.J.; Barber, D.; Poole, V.; Marcec-Greaves, R.M.; Kouba, C.K. Sperm collection and cryopreservation for threatened newt species. Cryobiology 2020, 94, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, L.; Macale, D.; Pieroni, A.; Bologna, M.A. Restocking of the Apenine yellow-bellied toad in Central Italy. In Global Conservation Translocation Perspectives: 2021; Soorae, P.S., Ed.; IUCN SSC Conservation Translocation Specialist Group, Environmental Agency—Abu Dhabi & Calgary Zoo: Calgary, AB, Canada, 2021. [Google Scholar]
- Cayuela, H.; Gillet, L.; Laudelout, A.; Besnard, A.; Bonnaire, E.; Levionnois, P.; Muths, E.; Dufrêne, M.; Kinet, T. Survival cost to relocation does not reduce population self-sustainability in an amphibian. Ecol. Appl. 2019, 29, e01909. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, H.; Besnard, A.; Quay, L.; Helder, R.; Léna, J.-P.; Joly, P.; Pichenot, J. Demographic response to patch destruction in a spatially structured amphibian population. J. Appl. Ecol. 2018, 55, 2204–2215. [Google Scholar] [CrossRef]
- Hayward, M.W.; Meyer, N.F.V.; Balkenhol, N.; Beranek, C.T.; Bugir, C.K.; Bushell, K.V.; Callen, A.; Dickman, A.J.; Griffin, A.S.; Haswell, P.M.; et al. Intergenerational Inequity: Stealing the Joy and Benefits of Nature from Our Children. Front. Ecol. Evol. 2022, 10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolan, N.; Hayward, M.W.; Klop-Toker, K.; Mahony, M.; Lemckert, F.; Callen, A. Complex Organisms Must Deal with Complex Threats: How Does Amphibian Conservation Deal with Biphasic Life Cycles? Animals 2023, 13, 1634. https://doi.org/10.3390/ani13101634
Nolan N, Hayward MW, Klop-Toker K, Mahony M, Lemckert F, Callen A. Complex Organisms Must Deal with Complex Threats: How Does Amphibian Conservation Deal with Biphasic Life Cycles? Animals. 2023; 13(10):1634. https://doi.org/10.3390/ani13101634
Chicago/Turabian StyleNolan, Nadine, Matthew W. Hayward, Kaya Klop-Toker, Michael Mahony, Frank Lemckert, and Alex Callen. 2023. "Complex Organisms Must Deal with Complex Threats: How Does Amphibian Conservation Deal with Biphasic Life Cycles?" Animals 13, no. 10: 1634. https://doi.org/10.3390/ani13101634
APA StyleNolan, N., Hayward, M. W., Klop-Toker, K., Mahony, M., Lemckert, F., & Callen, A. (2023). Complex Organisms Must Deal with Complex Threats: How Does Amphibian Conservation Deal with Biphasic Life Cycles? Animals, 13(10), 1634. https://doi.org/10.3390/ani13101634




