Simple Summary
Aquaporins are membrane channels that allow for the movement of water and solutes in cells. They have been reported to play crucial roles in mammalian early development and cryopreservation processes. However, there are few studies focused on the characterization of aquaporins in cumulus oocyte and embryo complexes of cattle. Moreover, no studies have been carried out on Brahman, Holstein, Gir and Romosinuano, important bovine breeds in milk and meat production. Therefore, the objective of this study was to evaluate their presence, transcript level and possible functions in the cumulus oocyte complex of the Brahman, Holstein, Gir and Romosinuano breeds and in embryos from five bovine crosses. Aquaporins 1–12 were found in both cumulus oocyte complexes and embryos, and we found possible parental effects on the expression of aquaporins 6 and 12b in cumulus oocyte complexes and aquaporins 4, 8 and 9 in embryos. This allows one to evidence possible functions in the early development of the Brahman, Holstein, Gir and Romosinuano bovine breeds.
Abstract
Aquaporins (AQPs) are proteins with various functions related to proper cell function and early development in mammals. The aim of this study was to evaluate the presence of AQPs and determine their mRNA levels in the cumulus oocyte complex (COC) of four bovine breeds and in blastocysts of five bovine crosses. Grade I, II and III COCs were collected by ovum pick up from non-lactating heifers of the Brahaman, Holstein, Gir and Romosinuano breeds. Embryos were produced in vitro up to the blastocyst stage of the bovine ♀Gir × ♂Holstein, ♀Holstein × ♂Gir, ♀Brahman × ♂Holstein, ♀Holstein × ♂Brahman, and ♀Romosinuano × ♂Holstein crosses. mRNA expression of AQP1-AQP12b was estimated in COC and embryos by real-time-PCR. The presence of the twelve AQPs in the COCs and bovine embryos was established. Additionally, significant differences were determined in the expression of AQP6 and AQP12b in COCs, as well as in transcripts levels of AQP4, AQP8 and AQP9 from bovine embryos. Gene expression of AQPs in COCs and bovine embryos is consistent with the previously described biological functions. This is the first report of AQPs in COC of Gir, Brahman, Holstein and Romosinuano and embryos of five crossbreeds between Bos indicus and B. taurus.
1. Introduction
Aquaporins (AQPs) are a family of conserved integral transmembrane proteins considered fundamental for the correct function of cells such as water transport (i.e., fluid secretion, fluid absorption and cell volume regulation), cell adhesion, cell migration, cell proliferation and cell differentiation [1,2,3]. Additionally, AQPs may be involved in inflammatory processes, as regulators of the host’s innate defense at the cell membrane level [4].
In mammals, AQPs are expressed in leukocytes, adipocytes, kidneys, reproductive system tissues, lungs, exocrine glands, eyes and gastrointestinal organs, in which some AQPs are involved in fluid transport [1]. They are classified into three subfamilies according to sequence similarity and substrate preference [3]. The first group is orthodox AQPs, which are water-selective and include AQP0, AQP1, AQP2, AQP4, AQP5, AQP6 and AQP8; the second group is aquaglyceroporins, which are permeable to glycerin, urea and water and include AQP3, AQP7, AQP9 and AQP10; and the third group of super aquaporins is permeable to water and includes AQP11 and AQP12 [3,5]. Furthermore, another category of AQPs, the peroxiporins, have been reported, and they can transport H2O2 across mammalian cell membranes, and this can vary between isoforms [6]. Some of the AQPs that can transport the H2O2 are AQP1, AQP3, AQP5, AQP8, AQP9, and AQP11 [6].
AQPs can be found in the male and female reproductive systems [7]. In the female reproductive system, AQPs participate in cervical dilatation during gestation, early embryonic development, follicular development and the process of cavitation, where they function as conductors for the trans-trophectodermal movement of blastocele fluid (mainly water) and the polarized distribution of Na/KATPase [8,9,10]. In cattle embryos and oocytes, the function of AQPs has been studied during the cryopreservation process where cells dehydrate and rehydrate [11,12].
Differences have been shown regarding gene expression patterns related to reproductive features such as oocyte quality, oocyte recovery, blastocyst production and pregnancy rates, depending on whether the donors belong to Bos taurus taurus or Bos taurus indicus genetic groups [13,14]. Among representative B. indicus breeds, one finds Gir and Brahman livestock. The first is the main dairy cattle in tropical and subtropical regions [13], and the second is a long-life breed reared for meat production with adaptive traits in high ambient temperatures [15]. For B. taurus bovines, Holstein represents a widespread breed for dairy farming [16], while Romosinuano has been used in the meat industry and is the most important Creole Colombian breed, with the best adaptability, rusticity, fertility, meekness and hybrid vigor compared to other breeds [17].
Due to this, the objective of this study was to evaluate the presence of AQP, determine its mRNA levels in cumulus oocyte complexes (COC) of Brahman, Gir, Romosinuano, Holstein breeds and ♀Brahman × ♂Holstein, ♀Gir × ♂Holstein, ♀Holstein × ♂Gir, ♀Romosinuano × ♂Holstein and ♀Holstein × ♂Brahman embryos, and determine the genetic influence of breeds on expression patterns.
2. Materials and Methods
2.1. Follicular Aspiration of Donors
Bovine COC pool (n = 15 each) was taken by Ovum pick up (OPU) from nulliparous and non-lactating heifers aged between 48 to 96 months, belonging to the Brahman (n = 3), Gir (n = 3), Romosinuano (n = 3) and Holstein (n = 3) breeds, in Monteria–Córdoba, with an average temperature of 29 °C and relative humidity between 70% and 85%.
For the transvaginal follicular puncture, an ultrasound machine was used, equipped with a 5–7.5 MHz transvaginal probe and a 60 cm long OPU transvaginal device with a puncture guide and disposable puncture needle (20 G, 0.9 *70 mm) connected to a sterile 50 mL puncture tube through a Teflon hose. The OPU kit is completed with a vacuum pump calibrated at 50–53 mm Hg (20 mL/min). The follicular fluid obtained from the punctures was deposited in the puncture tube with the collection medium (lactated Ringer’s solution supplemented with fetal serum, 1% and sodium heparin, 5000 IU/L). The tubes were kept at 39 °C in a constant-temperature water bath.
After aspiration, the COCs were classified according to Rodrigues et al. [18] in three grades (I, II, III), naked or cumulus-expanded depending on the homogeneity, cytoplasmic morphology, and cumulus cell compactness. For the purposes of this study, COCs of grade I, II, and III were used.
2.2. In Vitro Embryo Production
2.2.1. In Vitro Maturation
COCs were washed four times in TALP-Hepes medium and once in in vitro maturation medium TCM199 supplemented with follicle-stimulating hormone and fetal bovine serum. About ten oocytes were then transferred in microdroplets of 50 µL of TCM199, for a period of 24 h at 38.5 °C, 5% CO2, and 95% humidity in the incubator.
2.2.2. In Vitro Fertilization
Previously matured oocytes were washed four times in TALP-Hepes medium and transferred to TALPFert medium. Straws of 0.25 mL of semen were thawed in a water bath at 37 °C for 35 s, exposed to gradients of Percoll 45/90 in TALP-Sperm, then centrifuged at 500× g for 30 min, and the pellet was resuspended in TALPFert medium. This medium was incubated for 18 hours at 38.5 °C, 5% CO2, and 95% humidity.
2.2.3. Embryo Culture
Zygotes were washed four times in Talp-Hepes medium, and then the embryos were transferred to 50 µL of culture medium SOF-BE1, where they remained in the blastocyst stage at 38.5 °C, 5% CO2, 5% O2, N2 balance and 95% humidity. Three embryo pools (n= 10 each one) were obtained for each ♀Gir × ♂Holstein, ♀Holstein × ♂Gir, ♀Brahman × ♂Holstein, ♀Holstein × ♂Brahman, and ♀Romosinuano × ♂Holstein cross.
2.3. RNA Extraction and cDNA Synthesis
Total RNA extraction was carried out from COC and embryo pools, using the RNA-solv reagent kit (OMEGA, Norcross, GA, USA), following the manufacturer’s instructions with modifications [13], and RNA quality was measured by spectrophotometry with the NanoDrop One (Thermo Scientific, Wilmington, DE, USA). cDNA synthesis was performed using the GoScriptTM Reverse Transcription System kit (Promega, Madison, WI, USA), and cDNA quality was determined through Endpoint PCR and agarose gel electrophoresis.
2.4. RT-PCR and Quantitative Polymerase Chain Reaction (qPCR)
RT-PCR amplification from cDNA was carried out using GoTaq® Flexi DNA polymerase (Promega, Madison, WI, USA) and AQP primer sets designed in Geneious Prime software (Table 1), following the manufacturer’s instructions for identifying the putative transcript expression of water channels in COC from four breeds and embryos from crosses. Amplification was realized in a ProFlexTM PCR System (Applied Biosystems, Carlsbad, CA, USA) with modifications at annealing temperature for each set of primers (Table 1). Amplicons were revealed on 2% agarose gel electrophoresis (PowerPac™ HC, Bio-Rad, Hercules, CA, USA) stained with HydraGreen™ (ACTGene, Piscataway, NJ, USA) by using GeneRuler 100 bp DNA Ladder (Thermo Fisher Scientific, Waltham, MA, USA). Gel was visualized under UV light, in the ENDUROTM GDS gel documentation system (Labnet International, Inc, Woodbridge, NJ, USA).

Table 1.
Primer sequences for aquaporin (AQP) genes in cattle.
qPCR assays (n = 324) were run in duplicate with Luna® Universal qPCR Master Mix (New England BioLabs Inc.; Beverly, MA, USA) following the manufacturer’s instructions, in a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) by Fast ramp program. Each primer product was validated by melt-curve analysis and gel electrophoresis imaging to ensure amplification from genomic DNA was not present. Relative gene expression was calculated using the 2−∆∆Ct method [20], and actb was set as a normalization gene [19]. Data were expressed as fold change.
2.5. Statistical Analysis
Data were analyzed using descriptive statistics and the Shapiro–Wilk normality test on GraphPad Prism v9 (La Jolla, CA, USA). In the case of parametric data, an ANOVA test using Tukey’s test as post hoc was carried out. Otherwise, when the data were non-parametric, the Kruskal–Wallis test was performed using the Dunn post hoc. Differences were considered statistically significant with a p-value < 0.05.
3. Results
3.1. AQPs mRNA Level in COC
In the case of COCs, statistical differences in the transcribed expression of AQP6 and AQP12b were found. Regarding AQP6, there are no differences in the level of transcripts between Romosinuano and Gir; however, AQP6 mRNA expression was higher in Romosinuano compared to Brahman (p = 0.010) and Holstein (p = 0.004); likewise, Gir has a higher expression than Brahman (p = 0.004) and Holstein (p = 0.002) (Figure 1). In addition, the AQP12b mRNA level was higher in Gir compared to Holstein (p = 0.039), but this AQP was expressed similarly between Romosinuano, Brahman and Gir, and Romosinuano, Brahman and Holstein (Figure 1). Moreover, the expressions of AQP1, AQP2, AQP3, AQP4, AQP5, AQP7, AQP8, AQP9, AQP10, AQP11 and AQP12b show no statistical differences in the COCs of the four breeds.
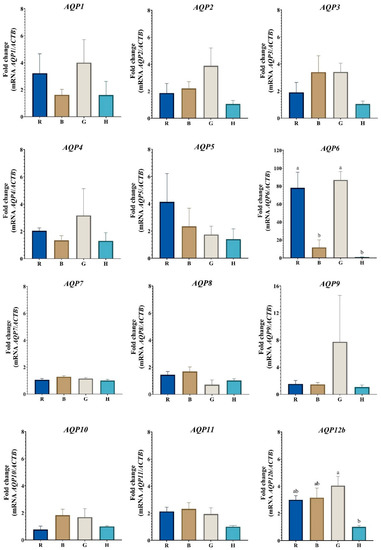
Figure 1.
Relative expression of aquaporin gene transcripts in cattle COC from Romosinuano (R), Brahman (B), Gir (G), and Holstein (H) breeds. Different letters (a and b) denote statistical differences (p < 0.05). AQPs names in italics denote its mRNA expression levels.
On the other hand, despite the fact that no significant differences were determined in the expression of all the AQPs in the COCs, it is possible to see in Figure 1 that the Gir COCs show an upward numerical trend of the transcripts for all the AQPs (with the exception of AQP5) compared to the COCs of the other species, where the mRNA levels of AQP2, AQP3, AQP7, AQP8, AQP10, AQP11 and AQP12b are higher in Gir and Brahman (B. indicus species), respectively, in contrast to those obtained by Romosinuano and Holstein, and the AQP1, AQP4, AQP6 and AQP9 transcripts have a higher expression in Gir and Romosinuano, respectively, unlike Brahman and Holstein.
3.2. AQPs mRNA Level in Cattle Embryos
The expression of AQP4 and AQP9 in the blastocysts is influenced by the parental effect of Romosinuano as an oocyte donor breed, since the expression of these AQPs is similar in the crosses between ♀Gir × ♂Holstein and ♀Holstein × ♂Gir and between ♀Brahman × ♂Holstein and ♀Holstein × ♂Brahman, but differs between ♀Gir × ♂Holstein and ♀Romosinuano × ♂Holstein (AQP4, p = 0.04; AQP9, p = 0.01), with the mRNA level being lower in the latter.
Moreover, AQP8 transcripts are related by the parental effect of Brahman as sire, since its expression differs in ♀Holstein × ♂Brahman compared to ♀Brahman × ♂Holstein (p = 0.0015), ♀Gir × ♂Holstein (p = 0.0016), ♀Holstein × ♂Gir (p = 0.0011) and ♀Romosinuano × ♂Brahman (p < 0.0001), but is similar to ♀Gir × ♂Holstein (p > 0.9999), ♀Holstein × ♂Gir (p = 0.9986) and ♀Romosinuano × ♂Brahman (p = 0.1818) when Holstein is the sire (♀Brahman × ♂Holstein).
On the other hand, in the expression of AQPs in the blastocysts of the five bovine crosses between the B. indicus and B. taurus breeds, it is possible to see, in Figure 2, the upward numerical trend in the AQP1, AQP2, AQP5, AQP7, AQP10 and AQP12b transcripts when Brahman is the oocyte donor breed (♀Brahman × ♂Holstein), and a downward trend in the expression of all AQPs (except for AQP3) when Romosinuano is the oocyte donor breed (♀Romosinuano × ♂Holstein).
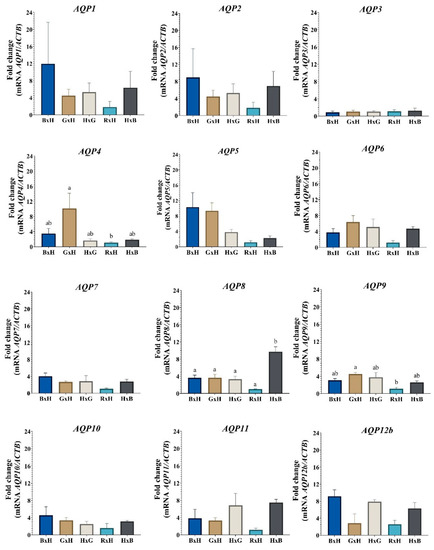
Figure 2.
Relative expression of aquaporin gene transcripts in cattle embryos. BxH: ♀Brahman × ♂Holstein, GxH: ♀Gir × ♂Holstein, HxG: ♀Holstein × ♂Gir, RxH: ♀Romosinuano × ♂Holstein, and HxB: ♀Holstein × ♂Brahman. Different letters (a and b) denote statistical differences (p < 0.05). AQPs names in italics denote its mRNA expression levels.
4. Discussion
4.1. AQPs mRNA Level in COC
In accordance with Jin et al. [12], bovine oocyte permeability occurs mainly by simple diffusion and, to a lesser extent, by AQPs. Therefore, we propose the importance of AQP1, AQP10 and AQP11 in the regulation of the flow of water, CO2, NH3 and glycerol through the cell membranes of the COCs of the Brahman, Holstein, Gir and Romosinuano breeds [21,22,23,24]. Since our study determined the expression of all the AQPs in the COCs of the Brahman, Holstein, Gir and Romosinuano breeds, and several studies have found the expression of AQP1, AQP3, AQP4, AQP5, AQP7 and AQP9 in bovine oocytes [12,25,26,27,28], their role in the development should be clarified.
Moreover, AQP3 expression in the COC of B. indicus and B. taurus may play a role in water movement and cytoplasmic maturity in immature oocytes [12], influencing oocyte quality and its survival in cryopreservation [28,29]. On the other hand, the cryoprotectant ethylene glycol has been shown to stimulate the expression of AQP7 in bovine oocytes [28,30]; thus, its presence in the COC of Gir, Romosinuano, Brahman and Holstein could be involved in tolerance to hyperosmotic stress during cryopreservation and a greater ease in the diffusion of water [28,30].
COCs are formed by undifferentiated granulosa cells (GC), and previous studies have characterized the presence of AQP1, AQP3, AQP4, AQP5, AQP7 and AQP9 in theca cells as GC of bovine ovarian follicles [25,31,32], this being consistent with AQPs’ expression in COCs of B. indicus and B. taurus. AQP1, AQP5, AQP7 and AQP9 may play a key role in the formation of the antrum in the Gir, Brahman, Holstein and Romosinuano breeds, since the transport of water and glycerol has been reported during the formation of the antrum mediated by AQP1, AQP7 and AQP9 [9,31], the involvement of AQP3, AQP4, and AQP5 in antral follicular fluid flow [31,32], and the inhibition of GC apoptosis by all AQPs except AQP4 and AQP7 [9,33,34].
On the other hand, exposure to high temperatures affects folliculogenesis [35]. In this way, the adaptations to heat stress presented in B. indicus and B. taurus [36,37] may explain the absence of significant differences in the expression of the majority of the AQPs. The significant differences in the expression of AQP6 and AQP12b may be related to the genetic diversity provided by migration, natural selection and geographical separation [38]. In addition, the higher expression levels of AQP2, AQP3, AQP7, AQP10, and AQP12b in Gir and Brahman may be due to their high adaptive capacity to harsh environments, given their tolerance to heat, and to both internal and external parasites, in comparison to Holstein and Romosinuano breeds [39,40].
To the authors’ knowledge, this study is the first report of AQPs’ gene expression in the COC of Gir, Brahman, Romosinuano and Holstein, and of AQP2, AQP6, AQP10 and AQP12b in bovine COC.
4.2. AQPs’ mRNA Level in Embryos
In agreement with our study, the presence of AQP3, AQP7, AQP9 and AQP11 has been reported in bovine embryos by qPCR [11,13,41], and several bovine embryo transcriptome studies have found mRNA expression of all AQPs, except for AQP10 [23,42,43,44,45,46]. Thus, AQPs’ expression in blastocysts of these breed crosses may be a heat-protection measure during embryonic development.
The function of AQP1, AQP2, AQP8 and AQP9 in the embryonic development of the five bovine crosses evaluated could be the homeostasis of the maternal-fetal fluid [47], since these AQPs have been reported in the fetal membrane, blastocysts and trophectoderm of different mammals, respectively [8,48,49,50,51]. Furthermore, it has been suggested that AQP8 and AQP9 could play a role in the preservation of cytoplasmic osmolarity during glycerol consumption in embryonic cells [52]. Moreover, AQP9 and AQP10 may be involved in the movement of other solutes, such as urea, purines and pyrimidines, during the embryo development of theses bovine crosses [3,8].
According to Wohlres–Viana et al. [13], no significant differences were found in the AQP3 mRNA expression of the different embryo crosses between B. indicus and B. taurus breeds. Therefore, the presence of AQP3 in these blastocysts could be related to fundamental developmental processes, such as the movement of water through the trophectoderm [8,53] and the maturation of the zygote to the morula stage by the facilitated diffusion of glycerol and ethylene glycol together with AQP7 [49,54].
On the other hand, AQP4 and AQP5 have been found, as in this study, in B. indicus blastocysts and in 8-cell blastomeres and the blastocoel cavity of mice [50,55,56,57]. Therefore, their presence in the five bovine crosses between B. indicus and B. taurus could be related to water transport, given their high solute selectivity [55,58,59]. In addition, it has been found that AQP5 is phosphorylated in the blastocyst stage, leading to its displacement to the cytoplasmic membrane in embryos in vivo [59], so it could play an important role in the general fluid homeostasis and blastocoel formation of the bovine crosses studied [57,58,59]. Likewise, AQP6 may be involved in water transport and blastocoel formation of the bovine crosses given its high permeability to nitrate [57,60].
5. Conclusions
This is the first report on the twelve AQPs in the COCs of Gir, Brahman, Holstein and Romosinuano and in embryos of five crosses between B. indicus and B. taurus. Expression of the AQP mRNA level in COC and bovine embryos is consistent with previously described biological functions in other mammals. Our findings provide the basis for identifying key roles for AQPs in COCs and bovine embryonic development; however, further studies are required to characterize and elucidate their functions, as well as their possible use in cryopreservation.
Author Contributions
Conceptualization, J.M.P.-D. and I.S.R.-B.; methodology, J.M.P.-D., R.E.C.-V., J.S.C.-M., K.J.L.-V., M.P.H.-S., H.F.U.-G. and J.S.N.-G.; validation, J.M.P.-D., R.E.C.-V. and J.S.C.-M.; formal analysis, J.M.P.-D., R.E.C.-V., J.S.C.-M. and I.S.R.-B.; investigation, J.M.P.-D., R.E.C.-V. and J.S.C.-M.; resources, R.J.O.-A. and I.S.R.-B.; data curation, J.M.P.-D., R.E.C.-V. and J.S.C.-M.; writing—original draft preparation, J.M.P.-D., K.J.L.-V. and J.S.N.-G.; writing—review and editing, J.M.P.-D. and I.S.R.-B.; visualization, J.M.P.-D. and I.S.R.-B.; supervision, I.S.R.-B.; project administration, R.J.O.-A. and I.S.R.-B.; funding acquisition, R.J.O.-A. and I.S.R.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Sistema General de Regalias de Colombia, under the framework of the project identified with the code: BPIN 2016000100026.
Institutional Review Board Statement
Procedures followed the guidelines for the care of animals and their use in research established by the Ethics Committee of the University of Tolima under framework project No. 50618 (Law 84/1989 and Resolution 8430/1993) [61,62].
Informed Consent Statement
Study was carried out with the written informed consent of the owners of the donors and the sires.
Data Availability Statement
The corresponding author will share the data provided in this study if requested.
Acknowledgments
We would like to thank the members of Union Temporal Embriotecno y Embriovet for their support with sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef] [PubMed]
- Laloux, T.; Junqueira, B.; Maistriaux, L.C.; Ahmed, J.; Jurkiewicz, A.; Chaumont, F. Plant and Mammal Aquaporins: Same but Different. Int. J. Mol. Sci. 2018, 19, 521. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.; Soveral, G. Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development. Int. J. Mol. Sci. 2021, 22, 1845. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; Lobo, C.; Carvalho, A.; Moura, A.; Rodrigues, A. Review Structure, function, and localization of aquaporins: Their possible implications on gamete cryopreservation. Genet. Mol. Res. 2013, 12, 6718–6732. [Google Scholar] [CrossRef] [PubMed]
- Erudaitius, D.; Huang, A.; Kazmi, S.; Buettner, G.R.; Rodgers, V.G.J. Peroxiporin Expression Is an Important Factor for Cancer Cell Susceptibility to Therapeutic H2O2: Implications for Pharmacological Ascorbate Therapy. PLoS ONE 2017, 12, e0170442. [Google Scholar] [CrossRef]
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.; Huet-Hudson, Y.M. Estrogen Regulation of Aquaporins in the Mouse Uterus: Potential Roles in Uterine Water Movement. Biol. Reprod. 2003, 69, 1481–1487. [Google Scholar] [CrossRef]
- Barcroft, L.C.; Offenberg, H.; Thomsen, P.; Watson, A.J. Aquaporin proteins in murine trophectoderm mediate transepithelial water movements during cavitation. Dev. Biol. 2003, 256, 342–354. [Google Scholar] [CrossRef]
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F. Water Permeability of an Ovarian Antral Follicle Is Predominantly Transcellular and Mediated by Aquaporins. Endocrinology 2002, 143, 2905–2912. [Google Scholar] [CrossRef]
- Andrew, J.W.; Watson, A.J.; Barcroft, L.C. Regulation of blastocyst formation. Front. Biosci. 2001, 6, 708. [Google Scholar] [CrossRef]
- Camargo, L.S.A.; Boite, M.C.; Wohlres-Viana, S.; Mota, G.B.; Serapiao, R.V.; Sa, W.F.; Viana, J.H.M.; Nogueira, L.A.G. Osmotic challenge and expression of aquaporin 3 and Na/K ATPase genes in bovine embryos produced in vitro. Cryobiology 2011, 63, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Kawai, Y.; Hara, T.; Takeda, S.; Seki, S.; Nakata, Y.-I.; Matsukawa, K.; Koshimoto, C.; Kasai, M.; Edashige, K. Pathway for the Movement of Water and Cryoprotectants in Bovine Oocytes and Embryos1. Biol. Reprod. 2011, 85, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Wohlres-Viana, S.; Pereira, M.M.; Viana, J.H.M.; Machado, M.A.; Camargo, L.S.D.A. Comparison of gene expression in Bos indicus and Bos taurus embryos produced in vivo or in vitro. Livest. Sci. 2011, 140, 62–67. [Google Scholar] [CrossRef]
- Moschini, G.A.D.L.; Gaitkoski, D.; de Almeida, A.B.M.; Hidalgo, M.M.T.; Martins, M.I.M.; Blaschi, W.; Barreiros, T.R.R. Comparison between in vitro embryo production in Bos indicus and Bos taurus cows. Res. Soc. Dev. 2021, 10, e38810716712. [Google Scholar] [CrossRef]
- Randel, R. Unique Reproductive Traits of Brahman and Brahman Based Cows. In Factors Affecting Calf Crop; CRC Press: Boca Raton, FL, USA, 2021; pp. 23–44. [Google Scholar] [CrossRef]
- Vásquez, R.; Martínez, R.; Ballesteros, H.; Grajales, H.; Pérez, G.J.E.; Abuabara, Y.; Barrera, C.G.P. El Ganado Romosinuano en la Producción de Carne en Colombia; CORPOICA: Bogotá, Colombia, 2006. [Google Scholar]
- Camargo, L.; Viana, J.; Ramos, A.; Serapião, R.; de Sa, W.; Ferreira, A.; Guimarães, M.; Filho, V.D.V. Developmental competence and expression of the Hsp 70.1 gene in oocytes obtained from Bos indicus and Bos taurus dairy cows in a tropical environment. Theriogenology 2007, 68, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Domiciano, L.F.; Borges, R.M.G.D.S.; Rondon, G.M.; Da Rocha, N.T.; De Souza, G.A.N.; Júnior, V.B.D.S.; Xavier, M.F.N. Qualitative aspects of oocytes from nelore and senepol breeds reared in a tropical region. Nucl. Anim. 2020, 12, 111–121. [Google Scholar] [CrossRef]
- Lozano-Villegas, K.J.; Rodríguez-Hernández, R.; Herrera-Sánchez, M.P.; Uribe-García, H.F.; Naranjo-Gómez, J.S.; Otero-Arroyo, R.J.; Rondón-Barragán, I.S. Identification of Reference Genes for Expression Studies in the Whole-Blood from Three Cattle Breeds under Two States of Livestock Weather Safety. Animals 2021, 11, 3073. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Geyer, R.R.; Musa-Aziz, R.; Qin, X.; Boron, W.F. Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. Am. J. Physiol. Cell. Physiol. 2013, 304, C985–C994. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef]
- Ishii, M.; Ohta, K.; Katano, T.; Urano, K.; Watanabe, J.; Miyamoto, A.; Inoue, K.; Yuasa, H. Cellular Physiology Biochemistry and Biochemistr y Dual Functional Characteristic of Human Aquaporin 10 for Solute Transport. Cell. Physiol. Biochem. 2011, 27, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Cheon, Y.-P. Temporal Aquaporin 11 Expression and Localization during Preimplantation Embryo Development. Dev. Reprod. 2015, 19, 53–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, P.; Meng, J.; Liu, W.; Smith, G.W.; Yao, J.; Lyu, L. Transcriptome Analysis of Bovine Ovarian Follicles at Predeviation and Onset of Deviation Stages of a Follicular Wave. J. Genom. 2016, 2016, 3472748. [Google Scholar] [CrossRef]
- Katz-Jaffe, M.; McCallie, B.; Preis, K.; Filipovits, J.; Gardner, D. Transcriptome analysis of in vivo and in vitro matured bovine MII oocytes. Theriogenology 2009, 71, 939–946. [Google Scholar] [CrossRef]
- Halstead, M.M.; Ma, X.; Zhou, C.; Schultz, R.M.; Ross, P.J. Chromatin remodeling in bovine embryos indicates species-specific regulation of genome activation. Nat. Commun. 2020, 11, 4654. [Google Scholar] [CrossRef]
- Martínez, T.; Vendrell-Flotats, M.; López-Béjar, M.; Mogas, T. Exposure to hyperosmotic solutions modifies expression of AQP3 and AQP7 on bovine oocytes. Cryobiology 2018, 85, 143. [Google Scholar] [CrossRef]
- Edashige, K.; Yamaji, Y.; Kleinhans, F.W.; Kasai, M. Artificial expression of aquaporin-3 improves the survival of mouse oocytes after cryopreservation. Biol. Reprod. 2003, 68, 87–94. [Google Scholar] [CrossRef]
- Tan, Y.-J.; Zhang, X.-Y.; Ding, G.-L.; Li, R.; Wang, L.; Jin, L.; Lin, X.-H.; Gao, L.; Sheng, J.-Z.; Huang, H.-F. Aquaporin7 plays a crucial role in tolerance to hyperosmotic stress and in the survival of oocytes during cryopreservation. Sci. Rep. 2015, 5, 17741. [Google Scholar] [CrossRef]
- Williams, L. The Role of Aquaporins in the Developing Ovarian Follicle. Doctoral Dissertation, University of Nottingham, Nottingham, UK, 2012. [Google Scholar]
- Kim, C.-W.; Choi, E.-J.; Kim, E.-J.; Siregar, A.S.; Han, J.; Kang, J.H.A.D. Aquaporin 4 expression is downregulated in large bovine ovarian follicles. J. Anim. Reprod. Biotechnol. 2020, 35, 315–322. [Google Scholar] [CrossRef]
- Verkman, A. Applications of aquaporin inhibitors. Drug News Perspect. 2001, 14, 412–420. [Google Scholar] [CrossRef]
- Wang, D.; Di, X.; Wang, J.; Li, M.; Zhang, D.; Hou, Y.; Hu, J.; Zhang, G.; Zhang, H.; Sun, M.; et al. Increased Formation of Follicular Antrum in Aquaporin-8-Deficient Mice Is Due to Defective Proliferation and Migration, and Not Steroidogenesis of Granulosa Cells. Front. Physiol. 2018, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, J.; Vernunft, A.; Koczan, D.; Spitschak, M.; Kuhla, B. Exposure of Lactating Dairy Cows to Acute Pre-Ovulatory Heat Stress Affects Granulosa Cell-Specific Gene Expression Profiles in Dominant Follicles. PLoS ONE 2016, 11, e0160600. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.F.; Daigle, C.L.; Moriel, P.; Smith, S.B.; Tedeschi, L.O.; Vendramini, J.M.B. Cattle adapted to tropical and subtropical environments: Social, nutritional, and carcass quality considerations. J. Anim. Sci. 2020, 98, skaa014. [Google Scholar] [CrossRef]
- Porto-Neto, L.; Reverter-Gomez, T.; Prayaga, K.C.; Chan, E.; Johnston, D.J.; Hawken, R.J.; Fordyce, G.; Garcia, J.F.; Sonstegard, T.S.; Bolormaa, S.; et al. The Genetic Architecture of Climatic Adaptation of Tropical Cattle. PLoS ONE 2014, 9, e113284. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, C.; Schönherz, A.; Guldbrandtsen, B.; Marjanovic, J.; Calus, M.; Hinrichs, D.; Thaller, G. Assessing the genetic background and genomic relatedness of red cattle populations originating from Northern Europe. Genet. Sel. Evol. 2021, 53, 23. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Azevedo, A.; Verneque, R.; Gasparini, K.; Peixoto, M.; da Silva, M.; Lopes, P.; Guimarães, S.; Machado, M. Quantitative trait loci affecting milk production traits on bovine chromosome 6 in zebuine Gyr breed. J. Dairy Sci. 2011, 94, 971–980. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; Lima, R.S.; Satrapa, R.A.; Barros, C.M. Physiology and endocrinology symposium: Influence of cattle genotype (Bos indicus vs. Bos taurus) on oocyte and preimplantation embryo resistance to increased temperature. J. Anim. Sci. 2013, 91, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Lloreda, V.; Coy, P.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Rizos, D. Effect of bovine oviductal fluid on development and quality of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2017, 29, 621–629. [Google Scholar] [CrossRef]
- Ishibashi, K.; Morinaga, T.; Kuwahara, M.; Sasaki, S.; Imai, M. Cloning and identification of a new member of water channel (AQP10) as an aquaglyceroporin. Biochim. Biophys. Acta 2002, 1576, 335–340. [Google Scholar] [CrossRef]
- Graf, A.; Krebs, S.; Zakhartchenko, V.; Schwalb, B.; Blum, H.; Wolf, E. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, 4139–4144. [Google Scholar] [CrossRef]
- Min, B.; Cho, S.; Park, J.S.; Lee, Y.-G.; Kim, N.; Kang, Y.-K. Transcriptomic Features of Bovine Blastocysts Derived by Somatic Cell Nuclear Transfer. G3 (Bethesda) 2015, 5, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Park, J.S.; Kang, Y.-K. Determination of Oocyte-Manipulation, Zygote-Manipulation, and Genome-Reprogramming Effects on the Transcriptomes of Bovine Blastocysts. Front. Genet. 2018, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Charpigny, G.; Guienne, B.M.-L.; Richard, C.; Adenot, P.; Dubois, O.; Gélin, V.; Peynot, N.; Daniel, N.; Brochard, V.; Nuttinck, F. PGE2 Supplementation of Oocyte Culture Media Improves the Developmental and Cryotolerance Performance of Bovine Blastocysts Derived From a Serum-Free in vitro Production System, Mirroring the Inner Cell Mass Transcriptome. Front. Cell Dev. Biol. 2021, 9, 672948. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wintour, E.M. Aquaporins in development—A review. Reprod. Biol. Endocrinol. 2005, 3, 18. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, H.; Beall, M.; Ma, T.; Hao, R.; Ross, M.G. Role of aquaporin 1 in fetal fluid homeostasis. J. Matern. Neonatal Med. 2014, 27, 505–510. [Google Scholar] [CrossRef]
- Xiong, Y.; Tan, Y.-J.; Xiong, Y.-M.; Huang, Y.-T.; Hu, X.-L.; Lu, Y.-C.; Ye, Y.-H.; Wang, T.-T.; Zhang, D.; Jin, F.; et al. Expression of Aquaporins in Human Embryos and Potential Role of AQP3 and AQP7 in Preimplantation Mouse Embryo Development. Cell. Physiol. Biochem. 2013, 31, 649–658. [Google Scholar] [CrossRef]
- Richard, C.; Gao, J.; Brown, N.; Reese, J. Aquaporin Water Channel Genes Are Differentially Expressed and Regulated by Ovarian Steroids during the Periimplantation Period in the Mouse. Endocrinology 2003, 144, 1533–1541. [Google Scholar] [CrossRef]
- Wang, S.; Su, Y.; Fang, R.; Ramierez, B.; Ross, M.G. Cloning and expression of aquaporin 8 water channel in ovine and human chorioamniotic membranes: Molecular mechanism of intramembranous pathway for amniotic fluid reabsorption. J. Soc. Gynecol. Investig. 2000, 7, 183A. [Google Scholar]
- Offenberg, H.; Thomsen, P.D. Functional challenge affects aquaporin mRNA abundance in mouse blastocysts. Mol. Reprod. Dev. 2005, 71, 422–430. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Carrageta, D.F.; Bernardino, R.L.; Alves, M.G.; Oliveira, P.F. Aquaporins and Animal Gamete Cryopreservation: Advances and Future Challenges. Animals 2022, 12, 359. [Google Scholar] [CrossRef]
- Edashige, K.; Ohta, S.; Tanaka, M.; Kuwano, T.; Valdez, D.M., Jr.; Hara, T.; Jin, B.; Takahashi, S.-I.; Seki, S.; Koshimoto, C.; et al. The Role of Aquaporin 3 in the Movement of Water and Cryoprotectants in Mouse Morulae. Biol. Reprod. 2007, 77, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sponchiado, M.; Gomes, N.S.; Fontes, P.K.; Martins, T.; del Collado, M.; Pastore, A.D.A.; Pugliesi, G.; Nogueira, M.F.G.; Binelli, M. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS ONE 2017, 12, e0175954. [Google Scholar] [CrossRef]
- Passaro, C.; Tutt, D.; Mathew, D.J.; Sanchez, J.M.; Browne, J.A.; Boe-Hansen, G.B.; Fair, T.; Lonergan, P. Blastocyst-induced changes in the bovine endometrial transcriptome. Reproduction 2018, 156, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Offenberg, H.; Barcroft, L.C.; Caveney, A.; Viuff, D.; Thomsen, P.D.; Watson, A.J. mRNAs encoding aquaporins are present during murine preimplantation development. Mol. Reprod. Dev. 2000, 57, 323–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Cui, Y.; Fan, Z.; Cook, G.A.; Nishimura, H. Two distinct aquaporin-4 cDNAs isolated from medullary cone of quail kidney. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 84–93. [Google Scholar] [CrossRef][Green Version]
- Park, J.-W.; Shin, Y.K.; Choen, Y.-P. Adaptive Transition of Aquaporin 5 Expression and Localization during Preimplantation Embryo Development by In Vitro Culture. Dev. Reprod. 2014, 18, 153–160. [Google Scholar] [CrossRef]
- Ikeda, M.; Beitz, E.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Characterization of Aquaporin-6 as a Nitrate Channel in Mammalian Cells. Requirement of pore-lining residue threonine 63. J. Biol. Chem. 2002, 277, 39873–39879. [Google Scholar] [CrossRef]
- Adams, D.C.; Nielsen, M.K.; Schacht, W.H.; Clark, R.T. Designing and conducting experiments for range beef cows. J. Anim. Sci. 2000, 77, 510–528. [Google Scholar] [CrossRef]
- Clark, J.D.; Gebhart, G.F.; Gonder, J.C.; Keeling, M.E.; Kohn, D.F. The 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997, 38, 41–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).