Simple Summary
The mechanism of follicular cyst formation is largely unknown but changes in follicular composition are known to be involved. In particular, there is abnormal hormone secretion in cystic follicles. Here, we found there was disruption of hormone secretion in the fluid of cystic follicles in sows. The glucocorticoid receptor was highly expressed, and the melatonin receptor was weakly expressed in cystic follicles compared with control follicles. Thus, secretion of steroid hormones in cystic follicles is disrupted and disturbances in signaling via cortisol and melatonin are involved in the development of follicular cysts in sows.
Abstract
(1) Background: Cortisol and melatonin (MT) act in regulating follicular development. We hypothesized that abnormal levels of cortisol, MT, and steroids in theca interna cells might be involved in the development of follicular cysts in sows. (2) Methods: To test this hypothesis, we measured the mRNA levels of enzymes involved in steroid hormone synthesis, the glucocorticoid receptor (GR), and melatonin receptors (MTRs) in theca interna cells of cystic and normal porcine follicles. (3) Results: The concentrations of estradiol, progesterone, and cortisol were greater in cystic follicles than in control ones (p = 0.034, p = 0.020, p = 0.000), but the concentration of MT was significantly lower (p = 0.045). The levels of GR, 11β-HSD1, and 11β-HSD2 were higher in cystic follicles than in control l follicles. MT types 1 and 2 were significantly lower in cystic follicles (p < 0.05). The mRNA expression levels of genes encoding the steroid hormone synthesis enzymes, steroidogenic acute regulatory protein (StAR), recombinant cytochrome P45011A1 (CYP11A1), and 3β-hydroxysteroid dehydrogenase (3β-HSD) in theca interna cells of cystic follicles were significantly higher than in control follicles. Thus, there was disruption of hormone secretion in the fluid of cystic follicles in sows. (4) Conclusions: The levels of steroid hormones, cortisol and MT are disrupted in porcine cystic follicles.
1. Introduction
A follicular cyst is a kind of ovarian cyst [1] and is a major factor causing infertility in sows, goats, and cattle [2,3,4]. Follicular cysts are associated with 10% of cases of reproductive failure in sows [2,5]. This disease impairs their reproductive performance and causes serious economic losses to pig breeding farms [6]. It is generally believed that animal stress, mismanagement, infectious diseases, and other factors that lead to an abnormal cortisol increase and endocrine disorders are major factors in follicular cyst formation [4,7,8]. The mechanisms are largely unknown, but changes in follicular composition are known to be involved [9,10,11].
The adrenal cortex is the only organ involved in glucocorticoid synthesis, and cortisol is the major glucocorticoid product [12]. This hormone is distributed throughout the body via the bloodstream and enters cells to play its physiological role. Melatonin (MT) is an indoleamine hormone mainly produced by the pineal gland of mammals and is distributed in the pineal gland and in several other organs, such as the ovary and testes [13,14]. The biological functions of MT are mediated by its two high-affinity G-protein-coupled receptors, MT1 and MT2 [15]. MT can act on the hypothalamic–pituitary–ovarian axis (HPO) by regulating the secretion of hypothalamic gonadotropins, which can also directly bind to the ovarian granulosa cells [16,17]. MT in the ovary can be derived from the systemic blood circulation, or synthesized by the granular cells, including the cumulus granulosa cells and oocytes [18]. Circulating MT can be absorbed by the ovaries, but ovarian follicles also have the ability to synthesize and secrete MT [19]. The level of MT is higher in follicular fluid (FF) than in the blood [20], and its concentration in FF rises significantly as follicles mature [21]. MT affects reproductive physiology by modulating sex steroid secretion at various phases of folliculogenesis, mainly mediated via its MT1 and MT2 receptors [22]. Therefore, MT has important paracrine effects in the female reproductive system. A significantly decreased expression was observed for the MT2 receptor in PCOS induced by letrozole in rats [23], and the mRNA expressions of MT1 and MT2 decreased in the theca cells of cystic follicles. Cortisol and MT are involved in regulating follicular development and maintain the follicular microenvironment through glucocorticoid receptors (GRs) and melatonin receptors (MTRs), respectively [24,25].
The cellular levels of the GR and 11β-hydroxysteroid dehydrogenase (11β-HSD) regulate the concentrations and effects of glucocorticoids in tissues. As a major regulator of cortisol metabolism, 11β-HSD is expressed as two isoenzymes, 11β-HSD1 and 11β-HSD2 [26], which regulate follicular development by changing the concentration of cortisol during follicular development and can act in the development of endocrine diseases. 11β-HSD1 converts non-bioactive cortisone to active cortisol, thereby regulating cortisol levels available to intracellular GRs, while 11β-HSD2 converts cortisol to cortisone to protect the mineralocorticoid receptor from undue occupation by cortisol [27]. Under the regulation of 11β-HSD, cortisol in the FF is converted to cortisone and participates in the regulation of follicular development. Blood cortisol levels are abnormally elevated in sows with follicular cyst formation in response to heat stress [28]. The level of cortisol in the FF of cystic bovine follicles is significantly higher than that in normal follicles, and the expression of 11β-HSD1 is significantly increased in the granulosa cells of such follicles [29]. These findings suggest the involvement of cortisol and its metabolic enzymes in the occurrence of follicular cysts in cattle, but there are few studies on spontaneous cystic follicles in sows.
Cortisol and MT levels regulated by the circadian rhythm are out of synchrony. Thus, cortisol secretion peaks during the day, whereas MT peaks at night. MT may play an important role in metabolic diseases, and its absence in pinealectomized animals causes the development of ovarian cysts via the altered synthesis of luteinizing hormone (LH) and follicle stimulating hormone [13]. The acute lowering of cortisol secretion stimulates MT secretion. When MT is low in the serum, it leads to increased cortisol secretion, and the administration of exogenous prolonged-release MT can rectify cortisol production.
Follicular theca interna cells provide structural support for follicles and secrete precursors for steroid synthesis by the granulosa cells. Here, we speculated that porcine theca interna cells might show abnormal expression of steroid synthetase activities and signaling of cortisol and MT, leading to the formation of cystic follicles.
2. Materials and Methods
2.1. Ethics
The study was conducted at the Beijing Academy of Agriculture and Forestry Sciences, and use of animals in the experiments was approved by the Ethical Committee of Beijing Academy of Agriculture and Forestry Sciences (SYXQ-2012-0034).
2.2. Collection of Ovaries
Gilts (crossbred Landrace × Large white 110–130 kg body weight, aged 200 to 220 days), were used. Ovaries from spontaneous follicular cysts of sows (n = 5) and control follicles (n = 5) were collected from a local abattoir and transported to the laboratory within 2 h in pre-warmed phosphate-buffered saline (PBS; 37 °C) with Pen-Strep antibiotic solution (Biological Industries, Beit HaEmek, Israel). Follicular cysts were diagnosed on the basis of macroscopic characterization (>20 mm diameter, fluid-filled with smooth thin and translucent walls, and the absence of corpora lutea on the ovaries). Normal control follicles (~4–6 mm in diameter) with no gross morphological abnormalities were used as controls.
2.3. Collection of Follicular Fluid and Theca Interna
Ovaries were washed two to three times with PBS in a 100 mm Petri dish. Individual follicles were dissected carefully from the ovarian stroma using forceps and scissors. After making a small incision with a scalpel, two blunt-tipped forceps were used to peel off the outer membrane from the incision, which left the intact theca interna containing FF. This was carefully aspirated from cystic and control isolated follicles with a syringe. The FF was centrifuged at 1000× g for 5 min and stored at −80 °C until hormone measurements. The theca interna was collected by modifying the method of Hatzirodos [30]. Follicles were dissected, and granulosa cells were aspirated and scraped from each follicle with a Pasteur pipette, and washed at least three times to remove other cell types, and the granulosa cells were discarded. The theca interna was then dissected from the follicle wall under a stereomicroscope in PBS. The theca interna was then frozen in liquid nitrogen and stored at −80 °C for RNA extraction and mRNA analysis.
2.4. Steroids, Cortisol, and Melatonin Assays
Estrogen (GEL4598-A) was measured in FF using enzyme-linked immunosorbent assays (ELISAs) for pigs (Gene Lab Biotechnology Co., Ltd., Beijing, China), according to the manufacturer’s instructions; the intra- and inter-assay coefficients of variation for serum were 4.1–6.8% and 6.7–9.4%, respectively. Progesterone (GEL4686-A) was measured in FF using ELISA for pigs (Gene Lab Biotechnology Co., Ltd., Beijing, China), according to the manufacturer’s instructions. The intra- and inter-assay coefficients of variation for the serums were 2.9–4.8% and 6.8–9.2%, respectively. The level of MT in FF was measured using a specific ELISA kit (RE54021, IBL International Gmbh, Hamburg, Germany). For the assays, the sensitivity was 1 ng/mL, and the intra- and inter-assay coefficients of variation were 5.2–12.2% and 5.1–14.9%, respectively. The concentration of cortisol was measured using 125I-labeled radioimmunoassay kits (S10940097, Beijing North Biotechnology Institute, Beijing, China), according to the manufacturer’s instructions. For the assays, the sensitivity was 2 ng/mL, and the intra- and inter-assay coefficients of variation were <10% and <15%, respectively.
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated using RNAzol reagent (RNAzol RT reagent, rn190; Molecular Research Center, Cincinnati, OH, USA). A NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA) was used for qualitative analysis. The PCR primers for genes were designed by NCBI. All primer sequences, accession numbers, product length and primer positions for qPCR are listed in Table 1. Primer sequences were synthesized by Shanghai Bioengineering Co., Ltd. Quantitative amplification of cDNA was performed in 0.2 mL PCR tubes using iScript advanced cDNA synthesis kits (Bio-Rad Laboratories, Hercules, CA, USA). Amplification efficiencies of the primer set candidates can then be verified experimentally and their specificity confirmed by melt-curve analysis and agarose gel electrophoresis of RT-qPCR amplification products. The qPCR was performed using a Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA) Chrome 4 Real-Time qPCR System. The qPCR mix (10 μL) included 5 μL of SYBR green premix, 0.3 μL of each forward and reverse primer (10 μmol/L), 4 μL of cDNA and 0.4 μL of dH2O. The qPCR conditions were as follows: 2 min denaturation at 95 °C, 40 cycles of PCR for the quantitative analysis (95 °C for 5 s and 60 °C for 30 s), one cycle for the melting curve analysis (95 °C for 5 s, 60 °C for 1 min, 95 °C for 1 s) and cooling at 4 °C. The relative expression level for each gene was calculated using the 2−ΔΔCT method. The qPCR analysis was performed three times for each group sample. We defined the gene expression cut-off as a mean Ct value of 35. GAPDH was used as the reference gene for GR, 11β-HSD, MT1, MT2, LHCGR, StAR, 3β-HSD, CYP11A1.

Table 1.
Primers for PCR amplification.
2.6. Data Analysis and Statistics
Data from control and cystic follicle groups were analyzed using two-tailed Student’s t tests with IBM SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY, USA). All data are presented as the mean ± standard deviation. A p value of <0.05 was considered statistically significant. Statistical significance was evaluated using data from at least three independent experiments.
3. Results
3.1. Hormone Concentrations in Follicular Fluid
The concentrations of estradiol, progesterone, cortisol, and MT in the FF of cystic and control follicles are shown in Figure 1. The estradiol concentration was significantly higher in the cystic follicles (p = 0.034). The progesterone concentration was higher in cystic follicles than in control follicles (p = 0.020). The concentration of cortisol in cystic FF was much higher than that in control follicles (p = 0.000). However, the concentration of MT was significantly lower (p = 0.045).
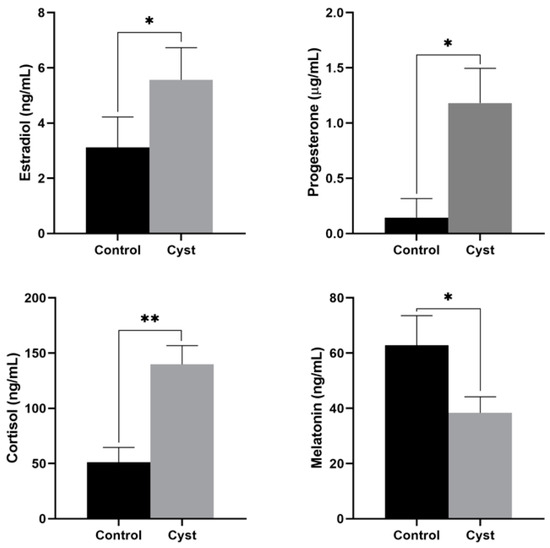
Figure 1.
Concentrations of estradiol, progesterone, cortisol, and melatonin in the follicular fluid of cystic and control follicles. * Indicates statistically significant (p < 0.05); ** Indicates statistically extremely significant (p < 0.01).
3.2. Relative mRNA Levels of GR, 11β-HSD, MT Receptor and Steroidogenic Enzymes
The expression levels of GR mRNA in the theca interna of cystic follicles were significantly higher than those in control follicles (p = 0.016). Moreover, 11β-HSD1 and 11β-HSD2 mRNA levels were higher in cystic follicles than in control follicles (p = 0.011; p = 0.026; Figure 2a). The transcription levels of MT1 and MT2 were lower in cystic follicles than in control follicles (p = 0.025; p = 0.011; Figure 2b). Next, we measured the mRNA levels of StAR and steroid hormone synthase genes by RT-qPCR (Figure 2c). The mRNA expressions of StAR, CYP11A1 and 3β-HSD in theca interna cells of cystic follicles were significantly higher than in control follicles (p = 0.000; p = 0.005; p = 0.001). Expression of LHCGR in the theca interna of cystic follicles was significantly lower than that in control follicles (p = 0.005).
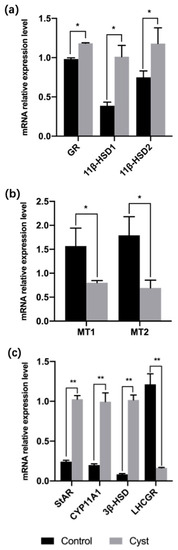
Figure 2.
(a) mRNA levels for GR and 11β-HSD1/2 in the theca interna of cystic and control follicles. (b) mRNA levels of MT receptors in the theca interna of cystic and control follicles. (c) mRNA levels of steroidogenic enzymes and LHCGR in theca interna of cystic and control follicles. * Indicates statistically significant (p < 0.05); ** Indicates statistically extremely significant (p < 0.01).
4. Discussion
As the basic unit of ovarian structure and function, a follicle is not only the site of oogenesis but also the site of steroid hormone synthesis and secretion. Normal follicular development requires subtle and precise regulation by interactions between these hormones and a complex signaling network [31]. Disruption of hormone secretion can lead to ovarian diseases and impair the reproductive performance of animals [32]. Cortisol and MT are involved in regulating follicle development and maintaining the follicular microenvironment [33,34,35]. The abnormal change of concentration of these molecules causes ovarian dysfunction [13,29].
We found that the concentration of cortisol in the FF of cystic follicles was significantly higher than in control follicles. Moreover, sows with low-quality cumulus-oocytes complex have FF with a higher concentration of cortisol [36]. Cortisol is higher in the FF of spontaneous or adrenocorticotropic hormone-induced follicular cysts in cattle [29], which is consistent with these results. Stressors induce elevated cortisol levels and suppress the HPO axis activity [37]. Stress-induced increases in adrenal glucocorticoids cause an increase that contributes to the hypothalamic suppression of reproductive function [38]. Cortisol affects follicular function as determined by the amount of GR, the intracellular concentration of glucocorticoids, and the activity of 11β-HSD during follicular development [39]. In this study, the mRNA expressions for GR and 11β-HSD1/2 were higher than in normal follicles. Cortisol concentrations in the FF and 11-HSD1 mRNA are significantly elevated in human patients with polycystic ovarian syndrome (PCOS); increased 11-HSD1 expression is the major cause of increased cortisol concentrations in the FF of such patients [40]. Additionally, there might be disruption of the internal follicular environment, which could also be a factor in the high expression of 11β-HSD2 [41]. In theca interna cells of cattle, GR expression was higher in spontaneous cystic ovarian follicles than in normal tertiary follicles [42]. The increase in GR and 11-HSD expression in cystic follicles could be related to the formation of follicle cysts in sows.
High concentrations of cortisol affect the synthesis and secretion of MT [43], but also influence the physiological function of MT in follicles by suppressing the expression of MT receptors. MT can act on the HPO axis by regulating the production of hypothalamic gonadotropins, which can also directly bind to ovarian granulosa cells to exert effects on the HPO axis [15]. Low MT levels are linked to ovarian problems, MT levels in the FF of women with PCOS are notably lower than in healthy women [13]. Consistent with the results of this study, MT levels in the cystic follicles of gilts were lower than in control follicles. A significantly decreased expression was observed for the MT2 receptor in PCOS induced by letrozole in rats [23], and the mRNA expressions of MT1 and MT2 decreased in the theca cells of cystic follicles in the present study.
The levels of estrogen, progesterone and steroidogenic enzymes expression increased. Estrogen excretion by sows with large cystic follicles was relatively high [44], and cold stress increased progesterone and cortisol levels [45]. The inhibited gene expression of steroidogenic enzymes (Cyp11a1, StAR and 3β-HSD) reduced the production of progesterone and 17β-estradiol [46]. Glucocorticoids regulate the expression of StAR through the GR and affect the synthesis of steroid hormones [47]. Acute stress induced by capture, short confinement, or anesthesia results in significant elevation of plasma cortisol and increased mRNA post-stress levels of StAR and CYP11A1 [48]. Here, during the formation of porcine follicular cysts, excess cortisol might have affected the mRNA expressions of StAR, CYP11A1, and 3β-HSD, resulting in abnormally elevated levels of progesterone in FF. LH is a necessary factor that triggers ovulation via the LHCGR [42]. Glucocorticoids influence the gonadal responsiveness to LH and the expression of LHCGR [49]. Enhanced secretion of cortisol decreases LHCGR content in follicles [50]. Here, the mRNA expression level of LHCGR was significantly decreased in the theca interna cells of cystic follicles, consistent with findings that the concentration of corticosterone in rat increased under constraining stress and that the expression of LHCGR decreased significantly [48]. How elevated cortisol induces follicular cysts remains to be determined.
5. Conclusions
The levels of steroid hormones, cortisol and MT were clearly disrupted in the cystic follicles of gilts. Molecular alterations of steroid hormone synthases, GR, LHCGR, and the MTR might be involved in this pathology.
Author Contributions
Writing—review &editing; Methodology, Y.Q.; Writing—original draft, J.B.; Investigation; Data curation, J.D.; Investigation, J.Z.; Validation; Investigation; Formal analysis, T.Z.; Validation, S.Z.; Supervision, X.X.; Resources; Project administration; Funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Natural Science Foundation of Beijing (6204037), The Scientific & Technological Innovation Ability Construction Project of the Beijing Academy of Agriculture and Forestry Sciences (KJCX20200209), and Youth Research Fund of Beijing Academy of Agriculture and Forestry Sciences (QNJJ202029).
Institutional Review Board Statement
All experiments were approved by the Ethical Committee of Beijing Academy of Agriculture and Forestry Sciences (SYXQ-2012-0034).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data are still being processed to produce other papers.
Acknowledgments
We thank James M. Cummins, for editing the language of a draft of this manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Braw-Tal, R.; Pen, S.; Roth, Z. Ovarian cysts in high-yielding dairy cows. Theriogenology 2009, 72, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Castagna, C.D.; Peixoto, C.H.; Bortolozzo, F.P.; Wentz, I.; Neto, G.B.; Ruschel, F. Ovarian cysts and their consequences on the reproductive performance of swine herds. Anim. Reprod. Sci. 2004, 81, 115–123. [Google Scholar] [CrossRef]
- Medan, M.S.; Watanabe, G.; Sasaki, K.; Taya, K. Transrectal ultrasonic diagnosis of ovarian follicular cysts in goats and treatment with GnRH. Domest. Anim. Endocrinol. 2004, 27, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Talukder, S.; Ingenhoff, L.; Kerrisk, K.L.; Celi, P. Plasma oxidative stress biomarkers and progesterone profiles in a dairy cow diagnosed with an ovarian follicular cyst. Vet. Q. 2014, 34, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.; Leppavuori, A.; Pyorala, S. Evaluation of reproductive failure of female pigs based on slaughterhouse material and herd record survey. Anim. Reprod. sci. 1998, 52, 235–244. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Jiang, Y.; Gao, S.; Chen, S.; Zheng, X.; Liu, Z.; Zhao, Y.; Li, H.; Yu, J.; et al. Association of gene polymorphisms of estrogen receptor, follicle-stimulating hormone beta and leptin with follicular cysts in Large White sows. Theriogenology 2017, 103, 143–148. [Google Scholar] [CrossRef]
- Einarsson, S.; Brandt, Y.; Lundeheim, N.; Madej, A. Stress and its influence on reproduction in pigs: A review. Acta Vet. Scand. 2008, 50, 48. [Google Scholar] [CrossRef] [Green Version]
- Kesler, D.J.; Garverick, H.A. Ovarian cysts in dairy cattle: A review. J. Anim. Sci. 1982, 55, 1147–1159. [Google Scholar] [CrossRef]
- Marelli, B.E.; Diaz, P.U.; Salvetti, N.R.; Rey, F.; Ortega, H.H. mRNA expression pattern of gonadotropin receptors in bovine follicular cysts. Reprod. Biol. 2014, 14, 276–281. [Google Scholar] [CrossRef]
- Mendes, M.H.; Pinto, M.H.; Gimeno, E.J.; Barbeito, C.G.; Sant’Ana, F.J.D. Lectin histochemical pattern on the normal and cystic ovaries of sows. Reprod. Domest. Anim. 2019, 54, 1366–1374. [Google Scholar] [CrossRef]
- Ortega, H.H.; Salvetti, N.R.; Muller, L.A.; Amable, P.; Lorente, J.A.; Barbeito, C.G.; Gimeno, E.J. Characterization of cytoskeletal proteins in follicular structures of cows with cystic ovarian disease. J. Comp. Pathol. 2007, 136, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Buijs, R.; Swaab, D. Glucocorticoid hormone (cortisol) affects axonal transport in human cortex neurons but shows resistance in Alzheimer’s disease. Br. J. Pharmacol. 2004, 143, 606–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojaverrostami, S.; Asghari, N.; Khamisabadi, M.; Khoei, H.H. The role of melatonin in polycystic ovary syndrome: A review. Int. J. Reprod. BioMed. 2019, 17, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Tamura, H.; Cruz, M.H.; Fuentes-Broto, L. Clinical relevance of melatonin in ovarian and placental physiology: A review. Gynecol. Endocrinol. 2014, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, G.; Robeva, R.; Konakchieva, R. Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int. J. Mol. Sci. 2021, 23, 471. [Google Scholar] [CrossRef]
- Kang, J.T.; Koo, O.J.; Kwon, D.K.; Park, H.J.; Jang, G.; Kang, S.K.; Lee, B.C. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 2009, 46, 22–28. [Google Scholar] [CrossRef]
- Dodi, A.; Bussolati, S.; Grolli, S.; Grasselli, F.; Di Lecce, R.; Basini, G. Melatonin modulates swine luteal and adipose stromal cell functions. Reprod. Fertil. Dev. 2021, 33, 198–208. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.T.; Ishizuka, B.; Kudo, Y.; Fusama, S.; Amemiya, A.; Sumi, Y. Detection of melatonin and serotonin N-acetyltransferase and hydroxyindole-O-methyltransferase activities in rat ovary. Mol. Cell. Endocrinol. 1997, 136, 7–13. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.Y. Melatonin and the circadian system: Contributions to successful female reproduction. Fertil. Steril. 2014, 102, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Sun, T.C.; Wang, H.P.; Chen, X. Research progress of melatonin (MT) in improving ovarian function: A review of the current status. Aging 2021, 13, 17930–17947. [Google Scholar] [CrossRef] [PubMed]
- Basheer, M.; Rai, S.; Ghosh, H.; Hajam, Y.A. Therapeutic Efficacy of Melatonin Against Polycystic Ovary Syndrome (PCOS) Induced by Letrozole in Wistar Rat. Pak. J. Biol. Sci. 2018, 21, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolla-Neto, J.; Amaral, F.G.; Soares, J.M., Jr.; Gallo, C.C.; Furtado, A.; Cavaco, J.E.; Goncalves, I.; Santos, C.; Quintela, T. The Crosstalk between Melatonin and Sex Steroid Hormones. Neuroendocrinology 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.T.; Maside, C.; Lima, L.F.; Magalhaes-Padilha, D.M.; Padilha, R.T.; Matos, M.; Figueiredo, J.R.; Campello, C.C. Immunolocalization for glucocorticoid receptor and effect of cortisol on in vitro development of preantral follicles. Vet. Anim. Sci. 2019, 7, 100060. [Google Scholar] [CrossRef]
- Krozowski, Z.; Li, K.X.; Koyama, K.; Smith, R.E.; Obeyesekere, V.R.; Stein-Oakley, A.; Sasano, H.; Coulter, C.; Cole, T.; Sheppard, K.E. The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J. Steroid Biochem. Mol. Biol. 1999, 69, 391–401. [Google Scholar] [CrossRef]
- Tetsuka, M.; Yamamoto, S.; Hayashida, N.; Hayashi, K.G.; Hayashi, M.; Acosta, T.J.; Miyamoto, A. Expression of 11beta-hydroxysteroid dehydrogenases in bovine follicle and corpus luteum. J. Endocrinol. 2003, 177, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Scholten, J.A.; Liptrap, R.M. A role for the adrenal cortex in the onset of cystic ovarian follicles in the sow. Can. J. Comp. Med. 1978, 42, 525–533. [Google Scholar]
- Amweg, A.N.; Salvetti, N.R.; Stangaferro, M.L.; Paredes, A.H.; Lara, H.H.; Rodriguez, F.M.; Ortega, H.H. Ovarian localization of 11beta-hydroxysteroid dehydrogenase (11betaHSD): Effects of ACTH stimulation and its relationship with bovine cystic ovarian disease. Domest. Anim. Endocrinol. 2013, 45, 126–140. [Google Scholar] [CrossRef]
- Hatzirodos, N.; Irving-Rodgers, H.F.; Hummitzsch, K.; Rodgers, R.J. Transcriptome profiling of the theca interna from bovine ovarian follicles during atresia. PLoS ONE 2014, 9, e99706. [Google Scholar] [CrossRef] [Green Version]
- Salvetti, N.R.; Alfaro, N.S.; Velazquez, M.M.; Amweg, A.N.; Matiller, V.; Diaz, P.U.; Ortega, H.H. Alteration in localization of steroid hormone receptors and coregulatory proteins in follicles from cows with induced ovarian follicular cysts. Reproduction 2012, 144, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruddenklau, A.; Campbell, R.E. Neuroendocrine Impairments of Polycystic Ovary Syndrome. Endocrinology 2019, 160, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Hamidovic, A.; Karapetyan, K.; Serdarevic, F.; Choi, S.H.; Eisenlohr-Moul, T.; Pinna, G. Higher Circulating Cortisol in the Follicular vs. Luteal Phase of the Menstrual Cycle: A Meta-Analysis. Front. Endocrinol. 2020, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Okamoto, A.; Ikeda, M.; Tate, S.; Sumita, M.; Kawamoto, R.; Tonai, S.; Lee, J.Y.; Shimada, M.; Yamashita, Y. Cortisol induces follicle regression, while FSH prevents cortisol-induced follicle regression in pigs. Mol. Hum. Reprod. 2021, 27. [Google Scholar] [CrossRef] [PubMed]
- Acosta, T.J.; Tetsuka, M.; Matsui, M.; Shimizu, T.; Berisha, B.; Schams, D.; Miyamoto, A. In vivo evidence that local cortisol production increases in the preovulatory follicle of the cow. J. Reprod. Dev. 2005, 51, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costermans, N.; Soede, N.M.; van Tricht, F.; Blokland, M.; Kemp, B.; Keijer, J.; Teerds, K.J. Follicular fluid steroid profile in sows: Relationship to follicle size and oocyte qualitydagger. Biol. Reprod. 2020, 102, 740–749. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Irahara, M. Gonadotropin-Inhibitory Hormone Plays Roles in Stress-Induced Reproductive Dysfunction. Front. Endocrinol 2017, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. USA 2009, 106, 11324–11329. [Google Scholar] [CrossRef] [Green Version]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar]
- Zhu, Q.; Zuo, R.; He, Y.; Wang, Y.; Chen, Z.J.; Sun, Y.; Sun, K. Local Regeneration of Cortisol by 11beta-HSD1 Contributes to Insulin Resistance of the Granulosa Cells in PCOS. J. Clin. Endocrinol. Metab. 2016, 101, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Tetsuka, M.; Nishimoto, H.; Miyamoto, A.; Okuda, K.; Hamano, S. Gene expression of 11beta-HSD and glucocorticoid receptor in the bovine (Bos taurus) follicle during follicular maturation and atresia: The role of follicular stimulating hormone. J. Reprod. Dev. 2010, 56, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amweg, A.N.; Rodriguez, F.M.; Huber, E.; Marelli, B.E.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Role of Glucocorticoids in Cystic Ovarian Disease: Expression of Glucocorticoid Receptor in the Bovine Ovary. Cells Tissues Organs 2016, 201, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Aluru, N.; McGuire, A.; Park, Y.J.; Vijayan, M.M.; Takemura, A. Effect of cortisol on melatonin production by the pineal organ of tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Liptrap, R.M. Oestrogen excretion by sows with induced cystic ovarian follicles. Res. Vet. Sci. 1973, 15, 215–219. [Google Scholar] [CrossRef]
- Herrera, A.Y.; Nielsen, S.E.; Mather, M. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol. Stress 2016, 3, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Bakhshalizadeh, S.; Amidi, F.; Shirazi, R.; Shabani, N.M. Vitamin D3 regulates steroidogenesis in granulosa cells through AMP-activated protein kinase (AMPK) activation in a mouse model of polycystic ovary syndrome. Cell Biochem. Funct. 2018, 36, 183–193. [Google Scholar] [CrossRef]
- Anuka, E.; Gal, M.; Stocco, D.M.; Orly, J. Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Mol. Cell. Endocrinol. 2013, 371, 47–61. [Google Scholar] [CrossRef]
- Lin, H.; Yuan, K.M.; Zhou, H.Y.; Bu, T.; Su, H.; Liu, S.; Zhu, Q.; Wang, Y.; Hu, Y.; Shan, Y.; et al. Time-course changes of steroidogenic gene expression and steroidogenesis of rat Leydig cells after acute immobilization stress. Int. J. Mol. Sci. 2014, 15, 21028–21044. [Google Scholar] [CrossRef] [Green Version]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Ortega, H.H.; Marelli, B.E.; Rey, F.; Amweg, A.N.; Diaz, P.U.; Stangaferro, M.L.; Salvetti, N.R. Molecular aspects of bovine cystic ovarian disease pathogenesis. Reproduction 2015, 149, R251–R264. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).