Simple Summary
Garlic juice is one of the most popular health drinks in Asia. Garlic juice processing waste (GJPW) is a potential feed ingredient for aquaculture, because it is rich in bioactive chemicals, which makes it an attractive alternative to synthetic antibiotics and/or antioxidants. This study investigates the effects of various dietary levels of garlic juice processing waste on the growth performance and health status of juvenile black (Sebastes schlegelii). On the basis of the results, rockfish fed diets supplemented with GJPW show improvement in growth performance, digestive enzyme activity, and growth and antioxidant-related gene expression. Therefore, considering the effects on the overall performance of juvenile rockfish, GJPW is a promising useful additive, and 2.5 g kg−1 dietary GJPW was found to be a suitable dietary level for juvenile rockfish black rockfish.
Abstract
An 8-week feeding trial was conducted to evaluate the effects of various dietary levels of garlic juice processing waste (GJPW) on the growth, feed utilization, digestive and antioxidant enzyme activity, growth- and antioxidant-related gene expression, and resistance to Streptococcus iniae infection of juvenile black rockfish (Sebastes schlegelii). A total of 450 juvenile rockfish were randomly distributed into 30 L rectangular tanks (30 fish per tank). Five experimental diets were prepared in triplicate. The fish were fed experimental diets supplemented with GJPW at concentrations of 0 (GJPW0, control), 2.5 (GJPW2.5), 5 (GJPW5), 7.5 (GJPW7.5), and 10 g kg−1 (GJPW10) diet. All of the GJPW-supplemented treatments (2.5, 5, 7.5, and 10 g kg−1) significantly enhanced weight gain (WG), specific growth rate (SGR), feed efficiency (FE), protein efficiency ratio (PER), and digestive enzyme activity (amylase, trypsin, and lipase). A decreasing trend was seen in plasma aspartate aminotransferase (ALT), alanine aminotransferase (AST), and glucose (GLU) content with increasing dietary levels of GJPW. In contrast, plasma lysozyme and antioxidant enzyme activities were significantly increased with increasing dietary GJPW levels. Furthermore, GJPW administration significantly upregulated the expression of insulin-like growth factor-1 (IGF-1), superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST) in the liver of rockfish. A challenge test with S. iniae showed significantly higher resistance in the GJPW-supplemented treatments than in the control. In short, dietary supplementation GJPW enhanced growth performance and antioxidant response in juvenile black rockfish, with suitable effects in fish fed with 2.5 g kg−1 GJPW for 8 weeks.
1. Introduction
Aquaculture, which will have a population of 9.7 billion by 2050, is unquestionably the fastest growing animal protein source in the world [1]. Intensification of aquaculture has led to stressful conditions and has been the consequence of the outbreak of disease [2,3]. While synthetic antibiotics have been widely employed to resolve this concern for years, antibiotics in aquaculture can have a negative influence on the environment and contribute to the development of bacterial resistance to numerous drugs [4]. As a result, several recent research studies on the use of plant-based products as a feed additive in the aqua-feed industry have concentrated on improving growth performance, digestive system function, immunological parameters, and bacterial resistance [5,6,7]. Due to their active biomolecules such as alkaloids, phenolic compounds, sterols, flavonoids, glycosides, essential oils, saponins, and terpenes, phyto-additives enhance the health and growth of fish and also provide antioxidant activity [8,9]. Garlic (Allium sativum L.) has been used for culinary and medicinal purposes throughout history [10,11]. Garlic components contain a diverse array of bioactive compounds, including allicin (diallyl thiosulfate), which imparts garlic’s characteristic pungent aroma and medicinal properties [12], as well as vitamins (ascorbic acid, thiamine and riboflavin), minerals (potassium, phosphorus, calcium, magnesium, sodium, iron, selenium, and germanium), flavonoids (phenolic acids) [13], amino acids [14], steroidal saponins [15] and phytosterols [15], which present antibacterial, antiviral, antiparasitic, immunostimulatory and antioxidant properties [16,17,18]. Previous studies have shown that dietary supplementation of garlic contributed to growth performance [19,20], immune responses [21,22,23], antioxidant status [24] and disease resistance [16,25,26] in fish.
Garlic juice is one of the most popular health drinks in Asia [27]. In Korea, garlic juice is commonly commercially available for more than 2,000 won (KRW, $1 USD) per 100 mL, and there are many garlic juice producers [28]. However, garlic juice processing waste (GJPW) are considered garbage or used as fertilizer [29]. In addition to contributing to the sustainable development of the aquaculture industry due to the use of waste from garlic juice production that can be discarded from the environment, contaminated soil, and water course, it can be a viable alternative in a practical fish formulated diet [30].
Black rockfish (Sebastes schlegelii) is one of the most important mariculture fish in the Wester North Pacific due to its high economic and ecological values [31,32]. In particular, its annual aquaculture production in Korea was 17,473 tons, the second largest production among the marine finfish aquaculture in 2021 [33]. However, various diseases, such as lymphocystis disease virus, Streptococcus iniae, Vibrio anguillarum, Edwardsiella tarda, and Aeromonas salmonicida, etc., have threatened sustainable production, causing enormous economic losses to the culture industry of S. schlegelii [34,35,36].
In a recent study, growth performance, non-specific immunity, and resistance against V. harveyi infection in juvenile S. schlegelii were notably improved by dietary 1% addition of GJPW [37]. Although applicability of GJPW as the functional feed additive in S. schlegelii feed was successfully reported [37], their suitable inclusion levels in formulated diet on rockfish must be proved before a practical application. To the best of our knowledge, no dietary information is available about the different GJPW levels on growth performance and health status of S. schlegelii. Thus, the aim of this study was to obtain a GJPW that could be used as a source of feed additives for black rockfish juveniles and its graded level of effects on growth, feed utilization, antioxidant and digestive enzyme activities, growth- and antioxidant-related gene expression, and disease resistance against S. iniae.
2. Materials and Methods
2.1. Preparation of Experimental Diet
GJPW were provided from Youngjin Healthy Juice Store (Daegu, Korea). GJPW were dried for 72 h at 20 °C in an agricultural product dryer (KED-M07D1, Kiturami Co., Ltd., Seoul, Korea) for 72 h, then crushed into a fine powder, and kept at −20 °C. After processing, the composition of GJPW were analyzed in laboratory and the result indicated that the GJPW retained certain functional compounds, such as total phenolic (27.3 mg gallic acid 100 g sample−1), flavonoids (36.8 mg 100 g sample−1). Additionally, GJPW clearly displays a dose-dependent DPPH and ABTS radical scavenging activities (Table 1).

Table 1.
Total phenolic and flavonoid content and antioxidant activities of ethanol extract from garlic juice processing waste (GJPW).
Five isonitrogenous and isolipidic experimental diets were formulated with the supplementation of 0 g kg−1 diet (GJPW0), 2.5 g kg−1 diet (GJPW2.5), 5 g kg−1 diet (GJPW5), 7.5 g kg−1 diet (GJPW7.5) and 10 g kg−1 diet (GJPW10) GJPW (Table 2). The main protein sources were pollock meal and fermented soybean meal. The fish and soybean oils, and wheat flour provided as sources of lipid and carbohydrate, respectively. Dry ingredients of all experimental diets were mechanically mixed to insure homogeneity. Then, to form dough, fish and soybean oils and distilled water were added to the mixture. The moist dough was then chopped (3.0 mm diameter, SL Machinery, Incheon, Korea) to prepare pellets. The pellets were dried at 20 °C for 48 h in an agricultural product dryer (KED-M07D1, Kiturami Co., Ltd., Seoul, Korea) and then stored at −20 °C in a freezer until use.

Table 2.
Experimental diet formulation (g kg−1, dry matter basis).
2.2. Feeding Trial
Black rockfish were acquired from a commercial hatchery (Namhae-gun, Gyeongsangnam-do, Korea) and taken to the Marine Bio-Education and Research Center (Gyeongsang National University, Tongyeong-si, Gyeongsangnam-do, Korea). Fish were kept in the experimental conditions for two weeks and fed a commercial diet (Jeil Feed Co., Haman-gun, Gyeongsangnam-do, Korea; 52% crude protein and 10% crude lipids) two times a day before feeding trial. There were 450 juvenile rockfish with an average body weight of 2.2 g that were starved for 24 h before being randomly distributed in 15 flow-through rectangular tanks (30 L), each tank held 30 fish. For eight weeks, three replicate groups of fish were fed the five experimental diets twice daily at 09:00 and 17:00. During the feeding trial, the mean water temperature, dissolved oxygen and salinity were 21.2 ± 0.22 °C, 7.0 ± 0.06 mg/L and 30.13 ± 0.12 psu, respectively. The photoperiod was set to follow natural conditions, each tank had constant aeration.
2.3. Sample Collection
At the end of the feeding trial, all fish were deprived of feed for a day and were anesthetized with tricaine methane sulphonate (MS-222) at 200 ppm before sampling and counted and individual weight and length were recorded to measure growth parameters. Five fish were randomly taken from each tank (15 fish per group) for chemical composition analysis. Blood samples were obtained from the caudal veins of ten anesthetized fish in each tank using heparinized syringes and gently transferred to 1.5 mL Eppendorf tubes. Plasma was collected by centrifugation for 10 min at 7,500 rpm. Plasma samples were stored at −80 °C in a freezer until analysis. Fish were dissected immediately after blood sampling to collect the liver and viscera for calculating condition parameters. Then, intestine and liver were rinsed with cold distilled water and stored at −80 °C until further digestive enzyme and gene expression analysis, respectively.
2.4. Challenge Test
After sampling, ten fish were randomly selected for the challenge test from each tank. The S. iniae strain was provided by the Korean Culture Collection of Aquatic Microorganisms, National Institute of Fisheries Science (Busan, Korea). All fish were artificially injected via intraperitoneal injection with 0.1 mL of pathogenic S. iniae culture suspension at a concentration of 5.0 × 106 CFU/mL. The injection concentration was based on our preliminary results (LD50 4.96 × 106 CFU/mL). The water temperature was maintained at 20.5 ± 0.02 °C (mean ± SE) with dissolved oxygen (7.1 ± 0.03 mg/L) and, fish survival was monitored daily for six days throughout the challenge test. Dead fish were removed every 12 h throughout the observation period.
2.5. Analysis
2.5.1. Chemical Analysis
The total phenolic content of GJPW was determined using a Folin-Ciocalteu regent [38]. The 50 μL of the sample was vortexed with 1 mL Folin-Ciocalteu reagent. After 3 min reaction time, the 1 mL of 10% sodium carbonate solution was added to the mixture. After 60 min of incubation at room temperature, the absorbance at 700 nm was determined. The standard was gallic acid and quercetin (Sigma-Aldrich Co., St Louis, MO, USA).
The flavonoids content of GJPW was determined following the method described by Moreno et al. [39]. The 1 mL of sample was diluted with 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 mol/L aqueous potassium acetate, and 4.3 mL of 80% ethanol. The absorbance at 415 nm was determined After 40 min at room temperature in a dark room. The quercetin (Sigma-Aldrich Co., St Louis, MO, USA) was used as the standard.
The DPPH scavenging activity was assessed using the method of Blois [40]. To summarize, 80 μL of sample or ascorbic acid as positive control was mixed with 100 μL of 150 μM DPPH in methanol, and the mixture was kept in the room temperature for 10 min. The absorbance at 525 nm was evaluated using a microplate reader (SpectraMax® M2/M2e, Sunnycale, CA, USA).
The radical scavenging activity of ABTS was determined using the method reported by Re et al. [41]. Briefly, ABTS radical was created by incubating 7 mM ABTS with 2.4 mM potassium persulfate in the dark for 16 h, and then the working solution was prepared by diluting with distilled water to absorbance of 1.5 at 414 nm. A 50 μL of sample or ascorbic acid as positive control and 100 μL of the working solution were mixed, allowed to stand for 5 min at the room temperature, and the absorbance at 414 nm was measured.
The proximate composition of the experimental diet and whole-body samples were determined using the procedures recommended by the Association of Official Agricultural Chemists [42]. The crude protein and crude lipid contents were determined by the Kjeldahl method and Soxhlet extraction methods using a KD310–A–1015 KjelROC Analyzer (OPSIS Liquid LINE, Skytteskogsvägen, Furulund, Sweden) and Soxtec extractor (ST 243 Soxtec™; FOSS, Hillerod, Denmark), respectively. Moisture content was determined by oven drying at 105 °C for 24 h, and ash was determined using a muffle furnace at 600 °C for 4 h.
2.5.2. Plasma Chemistry Analysis
The activity of aspartate aminotransferase activity (AST) and alanine aminotransferase activity (ALT), total cholesterol (T-CHO), total protein (TP) and glucose (GLU) were determined using an automatic chemistry system (Fuji Dri-Chem NX500i; Fujifilm, Tokyo, Japan).
2.5.3. Digestive Enzymes Measurements
Stored intestine tissue were homogenized in 10 volumes (v/w) of ice-cold, 0.86% physiological saline in an ice bath with a TissueLyser II (QIAGEN, Venlo, Netherlands), then centrifuged at 13,000 rpm for 10 min at 4 °C to obtain the supernatant. Amylase, trypsin and lipase activities were measured using a commercial kit (Abcam, Trumpington, Cambridge, UK). All digestive enzymes activity were determined according to the manufacturer guidelines with a spectrophotometer (Thermo Scientific MULTISKAN GO, Vantaa, Finland).
2.5.4. Lysozyme and Antioxidant Enzyme Activities Analysis
The activity levels of plasma lysozyme were measured using a commercial kit (EnzChek™ Lysozyme Assay Kit, Thermo Fisher Scientific, Waltham, MA, USA) in accordance with Galagarza et al. [43]. Fluorescence intensity was determined with a fluorescence reader (1420 Multilabel Counter Victor3, Perkin Elmer, Waltham, MA, USA) at excitation/emission wavelengths of 485/535 nm.
Superoxide dismutase (SOD) activity was measured using a Cayman’s Superoxide Dismutase Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) following the manufacturer’s instructions. Briefly, 10 μL of plasma was added to 200 μL of the radical detector. The reaction was initiated by adding 20 μL of xanthine oxidase, and the resultant mixture was incubated at room temperature on a shaker for 20 min. The absorbance was measured at 440 nm using a spectrophotometer (Thermo Scientific MULTISKAN GO, Vantaa, Finland). Catalase (CAT) activity was analyzed using a Cayman’s Catalase Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) following the manufacturer’s instructions. Briefly, 20 μL of plasma was added to 30 μL of methanol and 100 μL of assay buffer. The reaction was initiated by adding 20 μL of H2O2, and the mixture was incubated at room temperature for 20 min. To terminate the reaction, 30 μL of potassium hydroxide and purpald chromagen were added, and the mixture was incubated at room temperature for 10 min. Finally, 10 μL of potassium periodate was added and incubated at room temperature on a shaker for 5 min. The solution absorbance was measured at 540 nm (Thermo Scientific MULTISKAN GO, Vantaa, Finland). Glutathione (GSH) concentration was measured using a Cayman’s GSH Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) following the manufacturer’s instructions. Briefly, 150 μL of freshly prepared assay cocktail containing MES buffer [2-(N-morpholino)ethanesulfonic acid], cofactor mixture, enzyme mixture, water, and DTNB [5,5′-dithio-bis-(2-nitrobenzoic acid)] was added to 50 μL plasma samples in a 96-well plate. The absorbance was measured at 405 nm at 5 min intervals for 30 min (Thermo Scientific MULTISKAN GO, Vantaa, Finland).
2.6. Expression of Growth and Antioxidant-Related Genes
2.6.1. Primer Design
For quantitative PCR (qPCR) assay the specific primer pairs were designed using the NCBI Genbank to investigate the effects of GJPW on the expression of insulin-like growth factor (IGF-1), SOD, glutathione S-transferase (GST) and CAT (Table 3). In this study, β-actin gene was used as a housekeeping gene to normalize the expression levels of the selected genes in black rockfish.

Table 3.
Primer pair sequences of the genes used for quantitative real-time PCR in juvenile black rockfish (Sebastes schlegelii).
2.6.2. Total RNA Extraction and qPCR Assay
The stored liver was used to isolate the total RNA according to the RNAiso Plus (TaKaRa, Kusatsu, Shiga, Japan) manufacturer’s recommendations. Subsequently, genomic DNA contamination was removed by treatment with recombinant DNase I (TaKaRa Bio Inc., Kusatsu, Shiga, Japan), and the quantity and concentration of purified total RNA were measured by a NanoVue (GE Healthcare, Chicago, IL, USA) spectrophotometer. The purified samples were synthesized with cDNA following the protocol using PrimeScript 1st strand cDNA Synthesis Kit (TaKaRa Bio Inc., Japan).
The expression level of IGF-1, SOD, GST, and CAT was measured using specific primer, B Green Premix Ex Taq™ (TaKaRa Bio Inc., Japan) and Thermal Cycler Dice Real Time System III (TaKaRa Bio Inc., Japan). A melting curve was run at the end of the 45 amplification cycles to test for the presence of a unique PCR product. All qPCR data were measured and presented relative to the cycle threshold (Ct) values and then converted to fold changes by 2−∆∆Ct method [44]. The iQ5 optical system (BioRad, Hercules, CA, USA) was used for the data analysis.
2.7. Calculations and Statistical Analyses
To calculate fish performance parameters using the following formula:
Survival (SR, %) = (number of fish at the end of the trial/number of fish at the beginning of the trial) × 100
Weight gain (WG, g/fish) = final body weight − initial body weight
Specific growth rate (SGR, %/day) = [ln final weight of fish − ln initial weight of fish)/days of feeding] × 100
Feed consumption (FC, g/fish) = total dry feed intake/number of surviving fish
Feed efficiency (FE) = WG of fish/feed consumed
Protein efficiency ratio (PER) = WG of fish/protein consumed
Protein retention (PR, %) = Protein gain/protein consumed × 100
Condition factor (CF) = Fish weight/total length3 × 100
Hepatosomatic index (HSI, %) = (liver weight/whole-body weight) × 100
Viscerosomatic index (VSI, %) = (viscera weight/whole-body weight) × 100
All percentage values were arcsine-transformed before analysis. The results were expressed as the mean and pooled standard error of the means. The Levene’s test was used to test the homogeneity of variances among treatments. After that, the data were subjected to one-way analysis of variance (ANOVA) by Duncan’s multiple range test [45] were analyzed at significant differences (p < 0.05). Also, orthogonal polynomial contrasts (linear, quadratic, and cubic) were used to evaluate the response for all dependent variables [46], and the data were subjected to regression analysis to fit the best model when the statistical significance was detected. Fish survival during the 6-days post-observation period after artificial S. iniae injection was analyzed using Kaplan–Meier survival curve, Log-rank and Wilcoxon tests. All statistical analyses were carried out using SPSS version 25.0 program statistical software package (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Growth, Feed Utilization, and Biological Parameters
After 8-weeks of the feeding trial, growth performance and biological parameters of rockfish fed the experimental diets were shown in Table 4.

Table 4.
Growth performance of juvenile rockfish fed with different levels of garlic juice processing waste (GJPW) inclusion in diets.
The WG and SGR of rockfish exhibited an increase with increasing GJPW content in a linear model, both of which were shown to be significantly higher in the GJPW2.5, GJPW5, GJPW7.5 and GJPW10 treatments than in the GJPW0 treatment (p < 0.05). However, graded levels of dietary GJPW presented no significant effects on SR, CF, VSI and HSI of juvenile rockfish. With the gradual increase of dietary GJPW level, the FE showed a linear trend (p < 0.05). The FE of fish fed the GJPW0 was significantly lower than that of fish fed the GJPW2.5, GJPW5, GJPW7.5 and GJPW10 treatments. As dietary GJPW level increased gradually, the PER and PR significantly increased linearly (p < 0.05). The PR was significantly higher in the GJPW supplemented treatments than that in the control (GJPW0) treatment (p < 0.05). However, the PR of fish fed GJPW7.5 and GJPW10 was significantly higher than that in GJPW0, but did not significantly differ from that in GJPW2.5, GJPW5 and GJPW7.5 treatments. No relationship was found for FC among treatments.
3.2. Whole-Body Proximate Composition
As shown in Table 5, the whole-body moisture, crude protein, crude lipid, and ash contents of fish fed the experimental diet for 8-weeks showed no significant difference among treatments.

Table 5.
Proximate composition (%, wet weight basis) of whole-body of juvenile rockfish fed with different levels of garlic juice processing waste (GJPW) inclusion in diets.
3.3. Hematological Parameters
As dietary GJPW level increased gradually, the AST, ALT, and GLU contents showed a linearly decreasing trend (p < 0.05), while T-CHO and TP showed no significant difference among groups (Table 6). The AST, ALT, and GLU were significantly lower in the GJPW2.5, GJPW5, GJPW7.5 and GJPW10 treatments than in the GJPW0 treatment (p < 0.05). The activities of lysozyme, SOD and CAT, and GSH content in plasma in fish all showed significantly increasing linear model in response to the dietary GJPW level (p < 0.05). All treatment groups showed a higher lysozyme, SOD and CAT activity, and GSH content than that of the control treatment group with a significant difference (p < 0.05).

Table 6.
Hematologic parameters of juvenile rockfish fed with different levels of inclusion of garlic juice processing waste (GJPW) in diets.
3.4. Digestive Enzyme Activities
Amylase, trypsin, and lipase activities of fish fed the experimental diet for 8 weeks were listed in Table 7. The activities of amylase, trypsin, and lipase enzyme in the intestine of rockfish all showed a positive linear trend in response to dietary GJPW level (p < 0.05). The amylase, lipase and trypsin enzyme activity were significant improvement among all fish fed with GJPW supplemented diets compared to the control group (p < 0.05).

Table 7.
Digestive enzyme activities of juvenile rockfish fed with different levels of garlic juice processing waste (GJPW) inclusion in diets.
3.5. Genes Expression by Quantitative Real Time PCR
The mRNA levels of the growth (IGF-1) and antioxidant (SOD, GST, and CAT) genes in each treatment in comparison with the GJPW0 treatment were shown in Figure 1. IGF-1 mRNA transcription level of fish fed the GJPW2.5, GJPW5, GJPW7.5, and GJPW10 diets significantly increased compared to GJPW0 diets (p < 0.05). The highest expression level of IGF-1 gene was shown in the fish fed with GJPW10 diet compared to other treatments. SOD, GST, and CAT gene expressions were significantly upregulated in the GJPW2.5, GJPW5, GJPW7.5 and GJPW10 treatments compared to the GJPW0 treatment (p < 0.05).
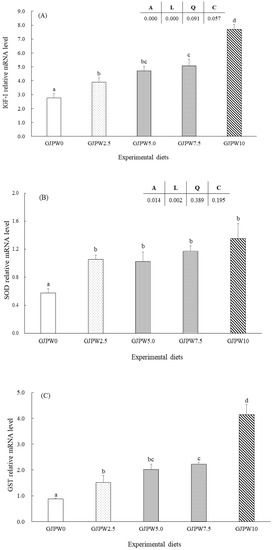
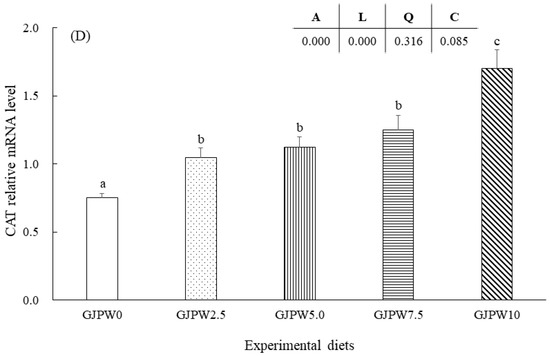
Figure 1.
Relative expression [insulin-like growth factor (IGF-1) (A), superoxide dismutase (SOD) (B), glutathione S-transferase (GST) (C), and catalase (CAT) (D)] in liver samples of juvenile black rockfish fed with different levels of garlic juice processing waste (GJPW) for 8 weeks. All data are shown as mean ± SE of three replicates. Bars with different letters show statistically significant differences (p < 0.05).
3.6. Challenge Test
The Figure 2 shown that survival of fish artificially infected with S. iniae was observed for 6 days post-infection. Survival of fish fed the GJPW0 diet was significantly (p < 0.05) lower than that of fish fed the GJPW2.5, GJPW5, GJPW7.5, and GJPW10 diets.
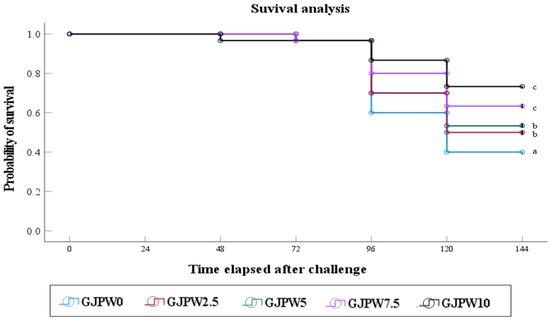
Figure 2.
The survival of juvenile black rockfish fed experimental diets with different levels of inclusion of garlic juice processing waste (GJPW) for eight weeks and then infected by Streptococcus iniae. Values are means of triplicate groups. Different letters indicate significant differences (p < 0.001; log-rank and Wilcoxon tests). GJPW: garlic juice processing waste.
4. Discussion
After the process of manufacturing healthy juice of garlic, known as medicinal plants, wastes also contained a large number of functional compounds derived from many studies, such as phenols and flavonoids [18,47,48,49]. Phenolic compounds and flavonoids are widely known for their beneficial effects on the growth, feed utilization, antioxidant activities, immunity, and disease resistance of fish, thus enhancing their health [50,51,52]. In this study, GJPW used as feed additives also showed sufficient phenols and flavonoids content as well as antioxidant activities even after the juice process of the raw materials. These bioactive compounds can attribute to improve the health of fish, as found in this study for juvenile rockfish fed the dietary supplements with GJPW. In addition to its bioactive compounds, garlic is rich in organosulfur compounds [53,54]. The most common organosulfur compounds include S-allyl cysteine, S-allyl-mercaptocysteine, and allicin, which were abundant in garlic extracts, maceration, and juice [55,56]. Garlic also has other bioactive compounds such as polysaccharides and fructans [57,58]. In fish culture, these bioactive compounds present antibacterial, antiparasitic, antioxidant, immunostimulatory, and growth promoting activities [17]. The presence of these compounds and antioxidant activities (DPPH and ABTS) in GJPW may have contributed to the performance of juvenile black rockfish.
In this study, the growth (WG and SGR) parameters and feed utilization (FE, PER and PR) indices at 2.5–10 g kg−1 in treated fish with dietary GJPW improved after 8-weeks feeding trial. These results means that the GJPW supplementation improved feed utilization, thus increasing WG and SGR. These results were similar to other studies on the addition of garlic in fish feed. Aly and Atti [59] found that tilapia (Oreochromis niloticus) fed with a diet supplemented with 10 and 20 g kg−1 of the crushed garlic diet for 8-weeks showed increased growth by improving feed utilization. Thanikachalam et al. [60] reported that catfish (Clarias gariepinus) fed diets containing garlic husk powder of 5, 10 and 15 g kg−1 diet for 20 days showed a significant effect in growth and feed utilization compared to the control diet. Especially, the supplementation of garlic in fish feed can improves growth performance due to allicin, which is a potent stimulant for the chemoreception, which increases the feed intake and/or feed utilization in fish [17]. In addition, the active components, such as phenolic compounds from plant additive could improve the digestibility and the availability of nutrients resulting in an increase in feed utilization [61,62,63]. Furthermore, this study showed that the alteration in digestive enzyme activities in intestine, which significantly increased in fish fed the supplemented GJPW diet, resulting in improved utilization of nutrients. Hassaan et al. [64] showed that inducing the secretion of intestinal digestive enzymes by the fish may increase the digestion and absorption of the nutrients of feed. This evidence indicated that the use of GJPW in feed has a positive effect on growth performance by maximizing feed utilization by activating the action of digestive enzymes in fish.
Additionally, there was high correlation between IGF-1 gene expression and growth performance in this study. In general, IGF-1 is mainly secreted from the liver after releasing the growth hormone [65]. In this vein, nutritional status can affect the growth hormone-IGF axis, and this may influence the acceleration of somatic growth in fish [2,66,67]. The higher growth performance was recorded by fish fed diet supplemented with GJPW compared to the control (GJPW0) treatment, and the relative expression of IGF-1 gene mRNA exhibited the same trend. This finding indicated that the supplementation of 2.5 to 10 g of GJPW in diet can have a positive regulatory effect on the transcription of the IGF-1, which can eventually involve hyperplastic and hypertrophic muscular growth. Our result was similar to that of Mostafavi et al. [1], Ramezani et al. [68] and Safari et al. [69] who reported that the relative expression of growth-related gene was significantly up-regulated in fish fed diet supplemented with plant additives. In addition, Fuentes et al. [70] noted that fish have been made transgenic for the growth-related gene showed significantly improved growth by muscle hypertrophy and hyperplasia. Moreover, in this study, GJPW enhanced growth factors by feed utilization and stimulated growth-related gene expression. However, further study on the dietary GJPW supplementation effect on the physiological properties of fish is needed in the future.
Our results revealed that dietary GJPW supplementation did not significantly affect the whole-body composition of fish. Previous studies reported that the whole-body composition could reflect fish quality and is influenced by several factors including feed composition and feeding strategy [71]. Several studies recorded significant influence in the whole-body composition after being fed a garlic-supplemented diet such as feeding trials in in rainbow trout, Oncorhynchus mykiss [19,23]; African catfish, C. gariepinus [72]; red belly tilapia, Tillapia zillii [73]; Nile tilapia, O. niloticus [59]; Asian sea bass, Lates calcarifer (Bloch) [74]; and starlet sturgeon, Acipenser ruthenus [75]. The reason for this discrepancy in result can be attributed to different fish species and culture conditions or different ingredients of diets.
The hematologic parameters indicated the health and nutritional status in fish [76,77]. Some studies showed that hematological parameters were not always altered by fish diet [78]. In this study, hematological parameters (AST, ALT and GLU) showed significant differences among the hematologic values of fish fed with or without GJPW. Generally, AST exists in hepatocyte mitochondria, while ALT is spread around the hepatic cells and bile duct [79]. Increased serum AST and ALT activity in fish indicates an enzyme leak in the damaged plasma membrane and/or an increase in enzyme synthesis by liver tissue [80]. Therefore, the activity of AST and ALT is used as important indicators of liver health and their functions [81]. In addition, it can also be used to asses fish health status and stress indicators [82]. In this study, plasma ALT and AST activities decreased with increasing dietary level of GJPW, which indicated that a better tendency occurred in the hepatocytes of rockfish. In addition, the lowest levels of AST and ALT may be associated with liver protection properties in which antioxidants such as flavonoids. Phenols inhibit the release of liver damage enzymes into plasma through antioxidant activity and lipid peroxidation prevention of cell membranes [83]. In this context, natural antioxidant flavonoids improved the liver function and enhanced its ability against oxidative stress and tissue damage [84,85,86].
Generally, an increased immune response is affected by nutrients in fish diet [87]. In line with this study, the increase in plasma lysozyme activity was observed with the addition of 2.5 to 10.0 g GJPW per kg diet. In fish, the non-specific immune system was improved by increasing plasma lysozyme activity [6,78]. In particular, previous studies presented that garlic-added feed enhanced lysozyme, then improved immune capability [21,74]. Moreover, fish antioxidant systems were made of non-enzymatic compounds and antioxidant enzymes including SOD and CAT [1,88]. In this study, fish fed the addition of 2.5 g GJPW per kg diet showed a significant improvement in plasma SOD and CAT values, as well as fish fed supplements with GJPW higher than GJPW0 diet (control diet). The phenols and saponin contained in garlic were known to provide antioxidant activity [18,47,48]. Previous study has also demonstrated that plant flavonoids, such as ferns [88], orange peel [89], and A. mongolicum Regel [90] flavonoids, have strong antioxidant activity. These compounds can inhibit the formation of free radicals, enhance the absorption mechanism of endogenous radicals, and increase cellular antioxidant enzymes, such as SOD, CAT and glutathione peroxidase, and hemeoxygenase-1 [90]. Similar to this study, Nile tilapia (O. niloticus) fed with diets containing different garlic sources; natural garlic (40 g kg−1 diet), capsule of garlic oil (250 mg kg−1 diet), and garlic powder (32 g kg−1 diet) revealed improved activities of SOD and CAT [91]. Also, dietary garlic powder could significantly improve SOD and CAT activities in common carp (C. carpio) [91]. Furthermore, the expression of genes (SOD, GSH and CAT) related to the antioxidant defense system were examined to investigate further the molecular mechanisms of GJPW improved antioxidant responses in this study. Results showed that all tested genes encoding antioxidant enzymes in liver were associated with significant upregulation of fish fed with GJPW. The upregulation of antioxidant related genes may contribute to an increase in their enzyme activities [92].
Currently, bacterial challenge tests were often used as a final indicator of fish health status after nutrition trials [93], since the methodology to comprehensively investigate immunity and disease resistance of fish is still limited, and an effective biomarker for disease resistance of fish has been difficult to identify [94]. In this study, dietary 2.5 to 10 g kg−1 GJPW had a significantly enhanced survival rate of rockfish compared to the challenge with S. iniae. is a Gram-positive bacterium that is known to cause serious Streptococcosis to aquaculture worldwide [95]. Previous studies reported that the various types of garlic additives can enhance resistance of fish to bacterial pathogens [23,60,74]. Particularly, Lee et al. [37] presented that a diet supplemented with 1% garlic juice extraction waste improved the survival rate of S. schlegelii against Gram-negative V. harveyi. This promotion of disease resistance against Gram-negative and Gram-positive bacteria of juvenile rockfish contributed to the administration of bioactive compounds in GJPW and to the regulation of lysozyme activity and antioxidant capacity.
5. Conclusions
This study indicates that juvenile rockfish fed diets supplemented with GJPW show improvement in growth performance and hemato-physiological variables. In the meantime, the digestive enzyme activity, growth, and antioxidant-related gene expression were also increased by the supplementation of feed with GJPW. In conclusion, considering the effects on the overall performance of juvenile rockfish, GJPW is a promising function additive and 2.5 g kg−1 dietary GJPW was found to be a suitable dietary level for black rockfish.
Author Contributions
Conceptualization, H.S.K.; methodology, H.S.K.; investigation, T.H.L., H.Y.O., D.-Y.L., K.-D.K., R.-W.K., J.-G.K., M.-Y.S. and C.-H.L.; writing—original draft preparation, H.S.K.; writing—review and editing, H.S.K.; supervision, J.-G.K., K.-D.K. and H.S.K.; project administration, K.-D.K. and H.S.K.; funding acquisition, H.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government Ministry of Science and ICT (MSIT) (2020R1G1A1006483). This research was also funded by National Institute of Fisheries Science (R2022017).
Institutional Review Board Statement
All experiments were performed following the guidelines of the International Animal Care and Use Committee of Gyeongsang National University, Korea (approval no. GNU-211230-E0107).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mostafavi, Z.S.M.; Shekarabi, S.P.H.; Mehrgan, M.S.; Islami, H.R. Amelioration of growth performance, physio-metabolic responses, and antioxidant defense system in rainbow trout, Oncorhynchus mykiss, using dietary dandelion, Taraxacum officinale, flower extract. Aquaculture 2022, 546, 737296. [Google Scholar] [CrossRef]
- Paknejad, H.; Shekarabi, S.P.H.; Mehrgan, M.S.; Hajimoradloo, A.; Khorshidi, Z.; Rastegari, S. Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus). Fish Physiol. Biochem. 2020, 46, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Srichaiyo, N.; Tonhsiri, S.; Hoseinifar, S.H.; Dawood, M.A.O.; Jaturasitha, S.; Esteba, M.Á.; Ringø, E.; Van Ooan, H. The effects gotu kola (Centella asiatica) powder on growth performance, skin mucus, and serum immunity of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Rep. 2020, 16, 100239. [Google Scholar] [CrossRef]
- Garcia-Migura, L.; Hendriksen, R.S.; Fraile, L.; Aarestrup, F.M. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 2014, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Ribeiro, K.; Melo, J.F.B.; Teixeira, D.V.; Vidal, L.V.O.; Copatti, C.E. Essential oil from ginger influences the growth, haematological and biochemical variables and histomorphometry of intestine and liver of Nile tilapia juveniles. Aquaculture 2021, 534, 36325. [Google Scholar] [CrossRef]
- De Souza, E.M.; De Souza, R.C.; Melo, J.F.B.; Da Costa, M.M.; De Souza, S.A.; De Souza, A.M.; Copatti, C.E. Cymbopogon flexuosus essential oil as an additive improves growth, biochemical and physiological responses and survival against Aeromonas hydrophila infection in Nile tilapia. An. Acad. Bras. Cienc. 2020, 92, e20190140. [Google Scholar] [CrossRef] [PubMed]
- Felix e Silva, A.; Copatti, C.E.; De Oliveira, E.P.; Bonfá, H.C.; De Melo, F.V.S.T.; Camargo, A.C.S.; Melo, J.F.B. Effects of whole banana meal inclusion as replacement for corn meal on digestibility, growth performance, haematological and biochemical variables in practical diets for tambaqui juveniles (Colossoma macropomum). Aquac. Rep. 2020, 17, 100307. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Hancz, C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev. Aquac. 2011, 3, 103–119. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Kallel, F.; Driss, D.; Chaari, F.; Belghith, L.; Bouaziz, F.; Ghorbel, R.; Ellouz, C. Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: Influence of extracting solvents on its antimicrobial and antioxidant properties. Ind. Crops Prod. 2014, 62, 34–41. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [PubMed]
- Santhosha, S.G.; Jamuna, P.; Prabhavathi, S.N. Bioactive components of garlic and their physiological role in health maintenance: A review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Lawson, L.D. Garlic: A review of its medicinal effects and indicated active compounds. In Phytomedicines of Europe: Their Chemistry and Biological Activity; Lawson, L.D., Bauer, R., Eds.; ASC Press: Washington, DC, USA, 1998; pp. 176–209. [Google Scholar]
- Sasaki, J.; Lu, C.; Machiya, E.; Tanahashi, M.; Hamada, K. Processed black garlic (Allium sativum) extracts enhance anti-tumor potency against mouse tumors. Med. Aromat. Plant Sci. Biotechnol. 2007, 1, 278–281. [Google Scholar]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Kovačević, D.B. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Kuo, C.M.; Hong, J.W.; Chou, R.L.; Lee, Y.H.; Chen, T.I. The effects of garlic-supplemented diets on antibacterial activities against Photobacterium damselae subsp. piscicida and Streptococcus iniae and on growth in Cobia, Rachycentron canadum. Aquaculture 2015, 435, 111–115. [Google Scholar] [CrossRef]
- Lee, J.Y.; Gao, Y. Review of the application of garlic, Allium sativum, in aquaculture. J. World Aquac. Soc. 2012, 43, 447–458. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.; Xu, X.; Gan, R.; Tang, G.; Corke, H.; Mavumengwana, V.; Li, H. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Büyükdeveci, M.E.; Balcázar, J.L.; Demirkale, I.; Dikel, S. Effects of garlic-supplemented diet on growth performance and intestinal microbiota of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 486, 170–174. [Google Scholar] [CrossRef]
- Esmaeili, M.; Kenari, A.A.; Rombenso, A.N. Effects of fish meal replacement with meat and bone meal using garlic (Allium sativum) powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquac. Nutr. 2017, 23, 1225–1234. [Google Scholar] [CrossRef]
- Adineh, H.; Harsij, M.; Jafaryan, H.; Asadi, M. The effects of microencapsulated garlic (Allium sativum) extract on growth performance, body composition, immune response and antioxidant status of rainbow trout (Oncorhynchus mykiss) juveniles. J. Appl. Anim. Res. 2020, 48, 372–378. [Google Scholar] [CrossRef]
- Diab, A.M.; Sake, O.A.; Eldakroury, M.F.; Elseify, M.M. Effects of garlic (Allium sativum) and curcumin (Turmeric, Curcuma longa Linn) on Nile tilapia immunity. Vet. Med. J. 2014, 60, C1–C19. [Google Scholar]
- Nya, E.J.; Austin, B. Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2009, 32, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.A.A. Effects of garlic (Allium sativum) on some antioxidant activities in tilapia nilotica (Oreochromis niloticus). World J. Fish Marine Sci. 2009, 1, 56–64. [Google Scholar]
- Fridman, S.; Sinai, T.; Zilberg, D. Efficacy of garlic based treatments against monogenean parasites infecting the guppy (Poecilia reticulata (Peters)). Vet. Parasitol. 2014, 203, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Militz, T.A.; Southgate, P.C.; Carton, A.G.; Hutson, K.S. Dietary supplementation of garlic (Allium sativum) to prevent monogenean infection in aquaculture. Aquaculture 2013, 408–409, 95–99. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Butt, M.S.; Khalid, N.; Sultan, S.; Raza, A.; Aleem, M.; Abbas, M. Garlic (Allium sativum): Diet based therapy of 21st century—A review. Asian Pac. J. Trop. Dis. 2018, 5, 271–278. [Google Scholar] [CrossRef]
- The Information on Garlic Juice Products and Producer. Available online: http://www.pulmaru.com/ (accessed on 6 October 2022).
- Kim, Y.J. Effects of dietary supplementation of garlic by-products on performance and carcass characteristic of chicken meat. Korean J. Poult. Sci. 2010, 37, 221–228. [Google Scholar] [CrossRef]
- Kang, J.H.; Son, H.J.; Min, S.C.; Oh, D.H.; Song, K.B. Antimicrobial activity of black garlic pomace extract and its application to cleansing of fresh spinach leaves for microbial control. J. Korean Soc. Food Sci. Nutr. 2017, 46, 450–458. [Google Scholar] [CrossRef]
- Hwang, H.K.; Son, M.H.; Myeong, J.I.; Kim, C.W.; Min, B.H. Effects of stocking density on the cage culture of Korean rockfish (Sebastes schlegeli). Aquaculture 2014, 434, 303–306. [Google Scholar] [CrossRef]
- Kai, Y.; Soes, D.M. A record of Sebastes schlegelii Hilgendorf, 1880 from Dutch coastal waters. Aquat. Invasions 2009, 4, 417–419. [Google Scholar] [CrossRef]
- KOSIS (Korea Statistical Information Service). Survey on the Status of Aquaculture. 2022. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1EW0004&vw_cd=MT_ZTITLE&list_id=K2_7&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=MT_ZTITLE&path=%252FstatisticsList%252FstatisticsListIndex.do (accessed on 6 October 2022).
- Kang, Y.; Kim, A.; Lee, Y.; Kim, N.; Roh, H.; Kim, D. Complete genome sequence of Aeromonas salmonicida subsp. masoucida Strain BR19001YR, Isolated from Diseased Korean Rockfish (Sebastes schlegelii). Microbiol. Resour. Announc. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.I.; Ko, J.Y.; Lee, W.L.; Kim, S.R.; Song, J.Y.; Kim, D.K. A new genotype of lymphocystivirus, LCDV-RF, from lymphocystis diseased rockfish. Arch. Virol. 2006, 151, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kitani, Y.; Kikuchi, N.; Zhang, G.; Ishizaki, S.; Shimakura, K.; Shiomi, K.; Nagashima, Y. Antibacterial action of L-amino acid oxidase from the skin mucus of rockfish Sebastes schlegelii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, C.; Kim, K.; Lim, H.J.; Kim, H.S. Effects of diet supplementation with plant juice processing by-products on juvenile black rockfish (Sebastes schlegelii) growth performance, feed utilization, non-specific immunity, and disease resistance against Vibrio harveyi. Aquac. Rep. 2021, 21, 100831. [Google Scholar] [CrossRef]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil. Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vatuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Method of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Galagarza, O.A.; Smith, S.A.; Drahos, D.J.; Eifert, J.D.; Williams, R.C.; Kuhn, D.D. Modulation of innate immunity in Nile tilapia (Oreochromis niloticus) by dietary supplementation of Bacillus subtilis endospores. Fish Shellfish Immunol. 2018, 3, 171–179. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Davis, M.J. Contrast coding in multiple regression analysis: Strengths, weaknesses, and utility of popular coding structures. J. Data Sci. 2010, 8, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, C.B.; Reid, A.; Lall, N. Garlic (Allium sativum) and its associated molecules, as medicine. In Medicinal Plants for Holistic Health and Well-Being; Lall, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–295. [Google Scholar]
- Szychowski, K.A.; Rybczynska-Tkaczyk, K.; Gawel-Beben, K.; Swieca, M.; Karas, M.; Jakuczyk, A.; Matysiak, M.; Binduga, U.E.; Gminski, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the garlic (Allium sativum) properties for fish aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Raieni, R.F.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Habotta, O.A.; Dawood, M.A.; Kari, Z.A.; Tapingkae, W.; Van Doan, H. Antioxidative and immunostimulant potential of fruit derived biomolecules in aquaculture. Fish Shellfish Immunol. 2022, 130, 317–322. [Google Scholar] [CrossRef]
- Jahazi, M.A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Van Doan, H.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Darbyshire, B.; Henry, R.J. Differences in fructan content and synthesis in some allium species. N. Phytol. 1981, 87, 249–256. [Google Scholar] [CrossRef]
- Gambogou, B.; Anani, K.; Karou, S.D.; Ameyapoh, Y.A.; Simpore, J. Effect of Aqueous garlic extract on biofilm formation and antibiotic susceptibility of multidrug-resistant uropathogenic Escherichia coli clinical isolates in Togo. Int. J. Adv. Multidiscip. Res. 2018, 5, 23–33. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; López, M.L.; Saínz Espuñes, T.R. Bioactive components of functional foods from vegetable origin. Rev. Mex. Cienc. Farm. 2006, 37, 58–68. [Google Scholar]
- Portz, D.; Koch, E.; Slusarenko, A.J. Effects of garlic (Allium sativum L.) juice containing allicin on Phytophthora infestans and on downy mildew of cucumber caused by Pseudoperonospora cubensis. Eur. J. Plant Pathol. 2008, 122, 197–206. [Google Scholar] [CrossRef]
- Chandrashekar, P.M.; Venkatesh, Y.P. Fructans from aged garlic extract produce a delayed immunoadjuvant response to ovalbumin antigen in BALB/c mice. Immunopharmacol. Immunotoxicol. 2012, 34, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Hanif, M.A.; Rehman, R.; Riaz, M. Garlic. In Medicinal plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Academic Press; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–315. [Google Scholar] [CrossRef]
- Aly, S.M.; Atti, N.M.A.; Mohamed, M.F. Effect of garlic on the survival, growth, resistance and quality of Oreochromis niloticus. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 277–296. [Google Scholar]
- Thanikachalam, K.; Kasi, M.; Rathinam, X. Effect of garlic peel on growth, hematological parameters and disease resistance against Aeromonas hydrophila in African catfish Clarias gariepinus (Bloch) fingerlings. Asian Pac. Trop. Med. 2010, 3, 614–618. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- De Souza, E.M.; De Souza, R.C.; Melo, J.F.B.; Da Costa, M.M.; De Souza, A.M.; Copatti, C.E. Evaluation of the effects of Ocimum basilicum essential oil in Nile tilapia diet: Growth, biochemical, intestinal enzymes, haematology, lysozyme and antimicrobial challenges. Aquaculture 2019, 504, 7–12. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Abdel Rahman, A.A.S. Exogenous xylanase improves growth, protein digestibility and digestive enzymes activities in Nile tilapia, Oreochromis niloticus, fed different ratios of fish meal to sunflower meal. Aquac. Nutr. 2019, 25, 841–853. [Google Scholar] [CrossRef]
- Duan, C. Nutritional and developmental regulation of insulin-like growth factors in fish. J. Nutr. 1998, 128, 306S–314S. [Google Scholar] [CrossRef]
- Kumar, V.; Khalil, W.K.B.; Becker, K. Influences of incorporating detoxified Jatropha curcas kernel meal in common carp (Cyprinus carpio L.) diet on the expression of growth hormone- and insulin-like growth factor-1-encoding genes. J. Anim. Physiol. Anim. Nutr. 2011, 97, 97–108. [Google Scholar] [CrossRef]
- Rashmeei, M.; Shekarabi, S.P.H.; Mehrgan, M.S.; Paknejad, H. Assessment of dietary chaste tree (Vitex agnus-castus) fruit extract on growth performance, hemato-biochemical parameters, and mRNA levels of growth and appetite-related genes in goldfish (Carassius auratus). Aquac. Fish. 2022, 7, 296–303. [Google Scholar] [CrossRef]
- Ramezani, F.; Shekarabi, S.P.H.; Mehrgan, M.S.; Foroudi, F.; Islami, H.R. Supplementation of Siberian sturgeon (Acipenser baerii) diet with barberry (Berberis vulgaris) fruit extract: Growth performance, hemato-biochemical parameters, digestive enzyme activity, and growth-related gene expression. Aquaculture 2021, 540, 736750. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Nejadmoghadam, S.; Jafar, A. Transciptomic study of mucosal immune, antioxidant and growth related genes and non-specific immune response of common carp (Cyprinus carpio) fed dietary Ferula (Ferula assafoetida). Fish Shellfish Immunol. 2016, 55, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.N.; Safian, D.; Valdés, J.A.; Molina, A. Isolation and selection of suitable reference genes for real-time PCR analyses in the skeletal muscle of the fine flounder in response to nutritional status: Assessment and normalization of gene expression of growth-related genes. Fish Physi. Biochem. 2013, 39, 765–777. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.; Parisi, G.; Médale, F.; Lupi, P.; Kaushik, S.J.; Poli, B.M. Effect of long-term feeding with a plant protein mixture based diet on growth and body/fillet quality traits of large rainbow trout (Oncorhynchus mykiss). Aquaculture 2004, 236, 413–429. [Google Scholar] [CrossRef]
- Agbebi, O.T.; Ogunmuyiwa, T.G.; Herbert, S.M. Effect of dietary garlic source on feed utilization, growth and Histopathology of the African catfish (Clarias gariepinus). J. Agric. Sci. 2013, 5, 26–34. [Google Scholar] [CrossRef][Green Version]
- Jegede, T. Effect of garlic (Allium sativum) on growth, nutrient utilization, resistance and survival of Tilapia zillii (Gervais 1852) fingerlings. J. Agric. Sci. 2012, 4, 269–274. [Google Scholar] [CrossRef]
- Talpur, A.D.; Ikhwanuddin, M. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 2012, 364–365, 6–12. [Google Scholar] [CrossRef]
- Lee, D.; Lim, S.; Han, J.; Lee, S.; Ra, C.; Kim, J. Effects of dietary garlic powder on growth, feed utilization and whole body composition changes in fingerling sterlet sturgeon, Acipenser ruthenus. Asian-Australas. J. Anim. Sci. 2014, 27, 1303–1310. [Google Scholar] [CrossRef]
- Lemos, C.H.P.; Ribeiro, C.V.M.; De Oliveira, C.P.B.; Couto, R.D.; Copatti, C.E. Effects of interaction between pH and stocking density on the growth, haematological and biochemical responses of Nile tilapia juveniles. Aquaculture 2018, 495, 62–67. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2018, 10, 334–350. [Google Scholar] [CrossRef]
- Morante, V.H.P.; Copatti, C.E.; Souza, A.R.L.; Da Costa, M.M.; Braga, L.G.T.; Souza, A.M.; De Melo, F.V.S.T.; Camargo, A.C.S.; Melo, J.F.B. Assessment the crude grape extract as feed additive for tambaqui (Colossoma macropomum), an omnivorous fish. Aquaculture 2021, 544, 737068. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; El-Garhy, H.A.S.; Moustafa, M.M.A.; Mohamed, S.A.; El-Haroun, E.R. Effect of Silybum marianum seeds as a feed additive on growth performance, serum biochemical indices, antioxidant status, and gene expression of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Aquaculture 2019, 509, 178–187. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H. Serum metabolic enzyme activities and hepatocyte ultrastructure of common carp after gallium exposure. Zool. Stud. 2003, 42, 455–461. [Google Scholar]
- Zhai, S.; Lu, J.; Chen, X. Effects of dietary grape seed Proanthocyanidins on growth performance, some serum biochemical parameters and body composition of tilapia (Oreochromis niloticus) fingerlings. Ital. J. Anim. Sci. 2014, 13, 3357. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Ananthan, G.; Senthilkumar, D.; Khan, A.B.; Jeevanantham, K. Comparative investigation on haematological and biochemical studies on wild marine teleost fishes from Vellar estuary, southeast coast of India. Comp. Clin. Pathol. 2012, 21, 275–281. [Google Scholar] [CrossRef]
- Samavat, Z.; Shamsaie Mehrgan, M.; Jamili, S.; Soltani, M.; Hosseini Shekarabi, S.P. Determination of grapefruit (Citrus paradisi) peel extract bio-active substances and its application in Caspian white fish (Rutilus frisii kutum) diet: Growth, haemato-biochemical parameters and intestinal morphology. Aquac. Res. 2019, 5, 2496–2504. [Google Scholar] [CrossRef]
- Davila, J.C.; Lenherr, A.; Acosta, D. Protective effect of flavonoids on drug-induced hepatotoxicity in vitro. Toxicology 1989, 57, 267–286. [Google Scholar] [CrossRef]
- Qi, S.; Wang, T.; Chen, R.; Wang, C.; Ao, C. Effects of flavonoids from Allium mongolicum Regel on growth performance and growth-related hormones in meat sheep. Anim. Nutr. 2017, 3, 33–38. [Google Scholar] [CrossRef]
- Wu, L.; Hsu, H.; Chen, Y.; Chiu, C.; Lin, Y.; Ho, J.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Waagbø, R. The impact of nutritional factors on the immune system in Atlantic salmon, Salmo salar L.: A review. Aquac. Res. 1994, 25, 175–197. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Cao, J.; Wu, Y.; Xiao, J.; Wang, Q. Analysis of flavonoids and antioxidants in extracts of ferns from Tianmu Mountain in Zhejiang Province (China). Ind. Crops Prod. 2017, 97, 137–145. [Google Scholar] [CrossRef]
- Chen, X.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, X.; Tian, J.; Liu, M.; Wang, G. Dietary flavonoids from Allium mongolicum Regel promotes growth, improves immune, antioxidant status, immune-related signaling molecules and disease resistance in juvenile northern snakehead fish (Channa argus). Aquaculture 2019, 501, 473–481. [Google Scholar] [CrossRef]
- Yousefi, M.; Vatnikov, Y.A.; Kulikov, E.V.; Plushikov, V.G.; Drukovsky, S.G.; Hoseinifar, S.H.; Van Doan, H. The protective effects of dietary garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture 2020, 526, 735400. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Hepatic positive and negative antioxidant responses in zebrafish after intraperitoneal administration of toxic microcystin-LR. Chemosphere 2015, 120, 729–736. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Chi, C.; Kim, H.Y.; Yun, S.; Park, S.C.; Sukumaran, V. Effect of cellular products of potential probiotic bacteria on the immune response of Labeo rohita and susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2015, 46, 716–722. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, S.; Cai, Y.; Wu, Y.; Tian, L.; Wang, S.; Jiang, L.; Guo, W.; Sun, Y.; Zhou, Y. Effects of dietary mannan oligosaccharide supplementation on growth performance, antioxidant capacity, non-specific immunity and immune-related gene expression of juvenile hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Aquaculture 2020, 523, 735195. [Google Scholar] [CrossRef]
- Monir, M.S.; Yusoff, S.B.M.; Zulperi, Z.B.M.; Hassin, H.B.A.; Mohamad, A.; Ngoo, M.S.B.M.H.; Ina-Salwany, M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus × O. niloticus). BMC Vet. Res. 2020, 16, 226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).