Cross Effects of Diets and Rearing Temperatures on Gastrointestinal Evacuation and Growth Performance in Adult Sabah Groupers (Epinephelus fuscoguttatus × E. lanceolatus)
Abstract
Simple Summary
Abstract
1. Introduction
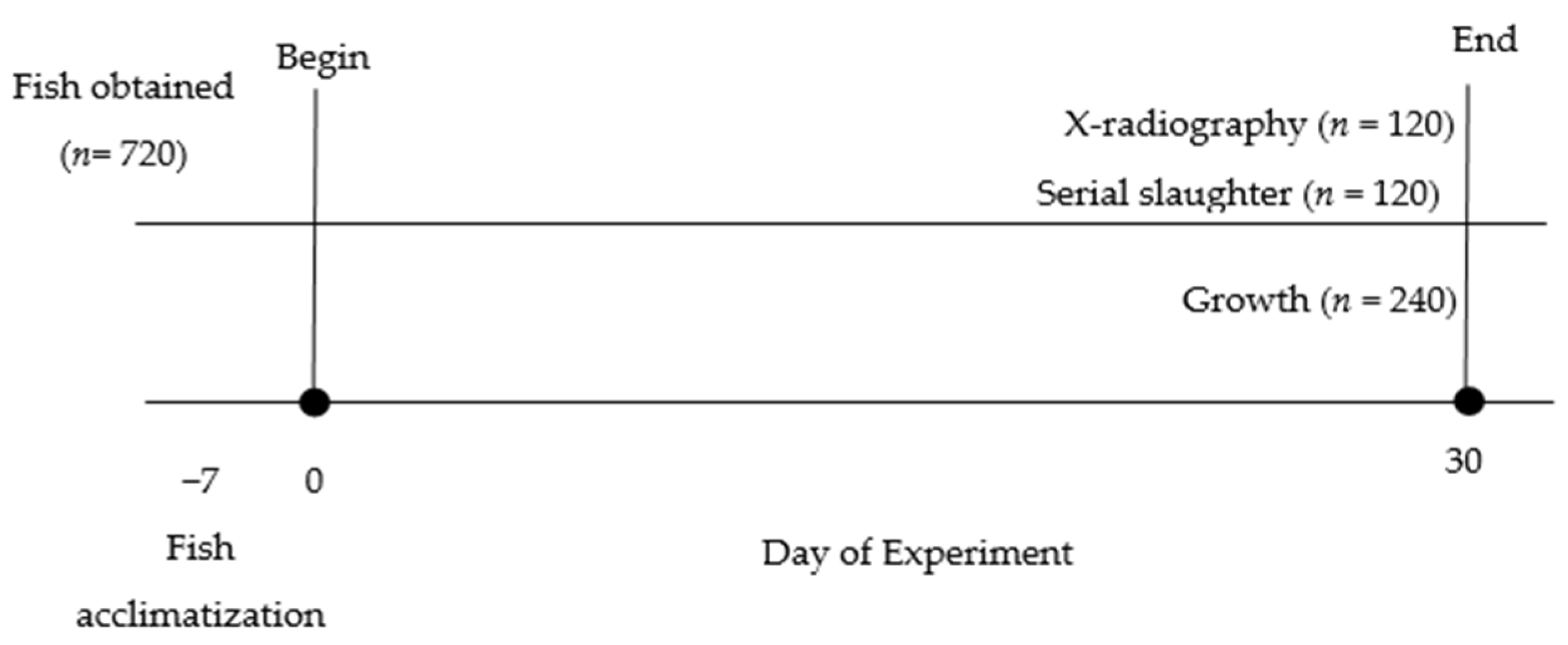
2. Materials and Methods
2.1. Fish Experimental Tank Setup
2.2. Nutrient Composition of Diets
weight with crucible − Crucible weight)/Sample fresh weight × 100
× dry matter %)
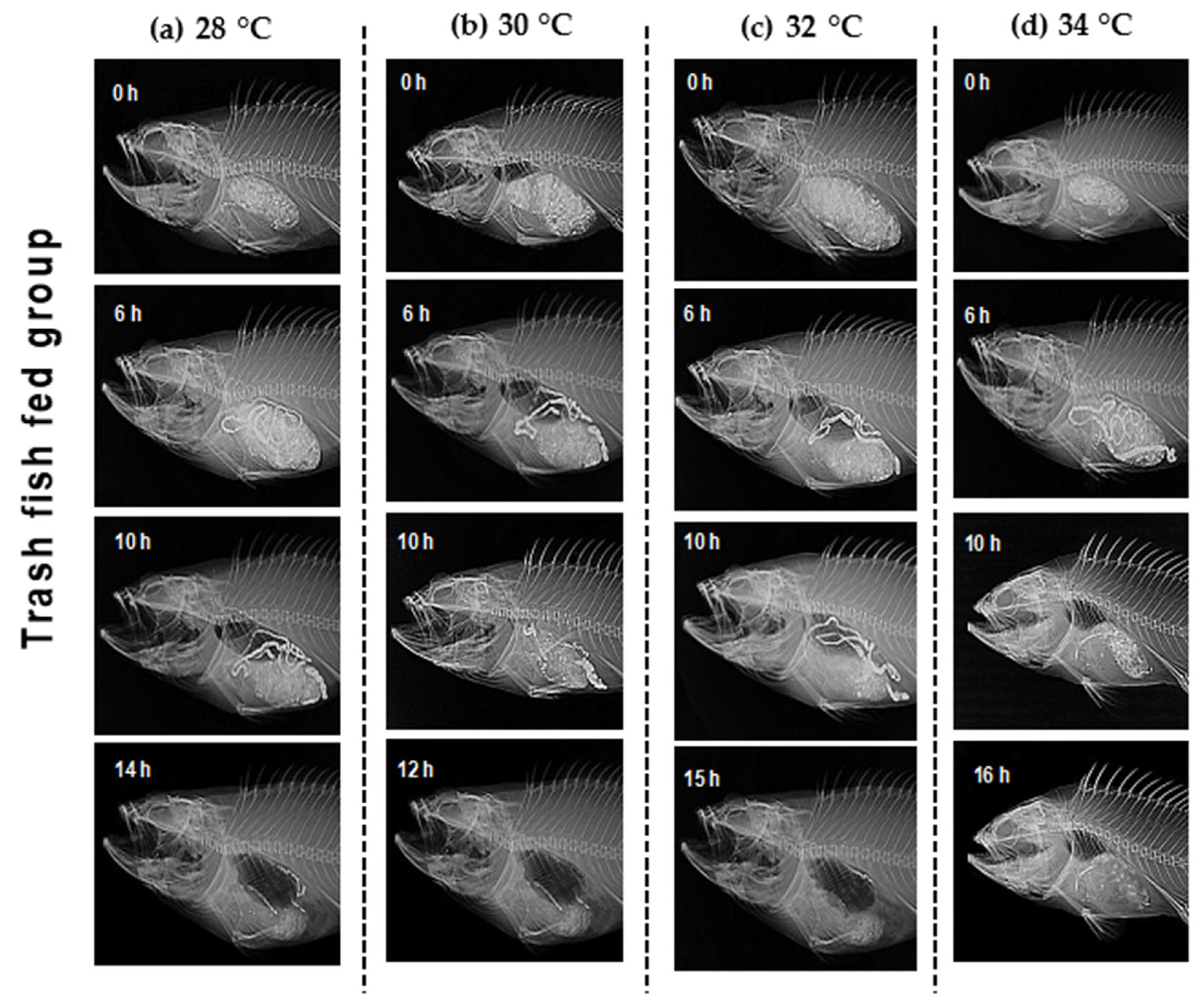
2.3. Gastrointestinal Evacuation Study
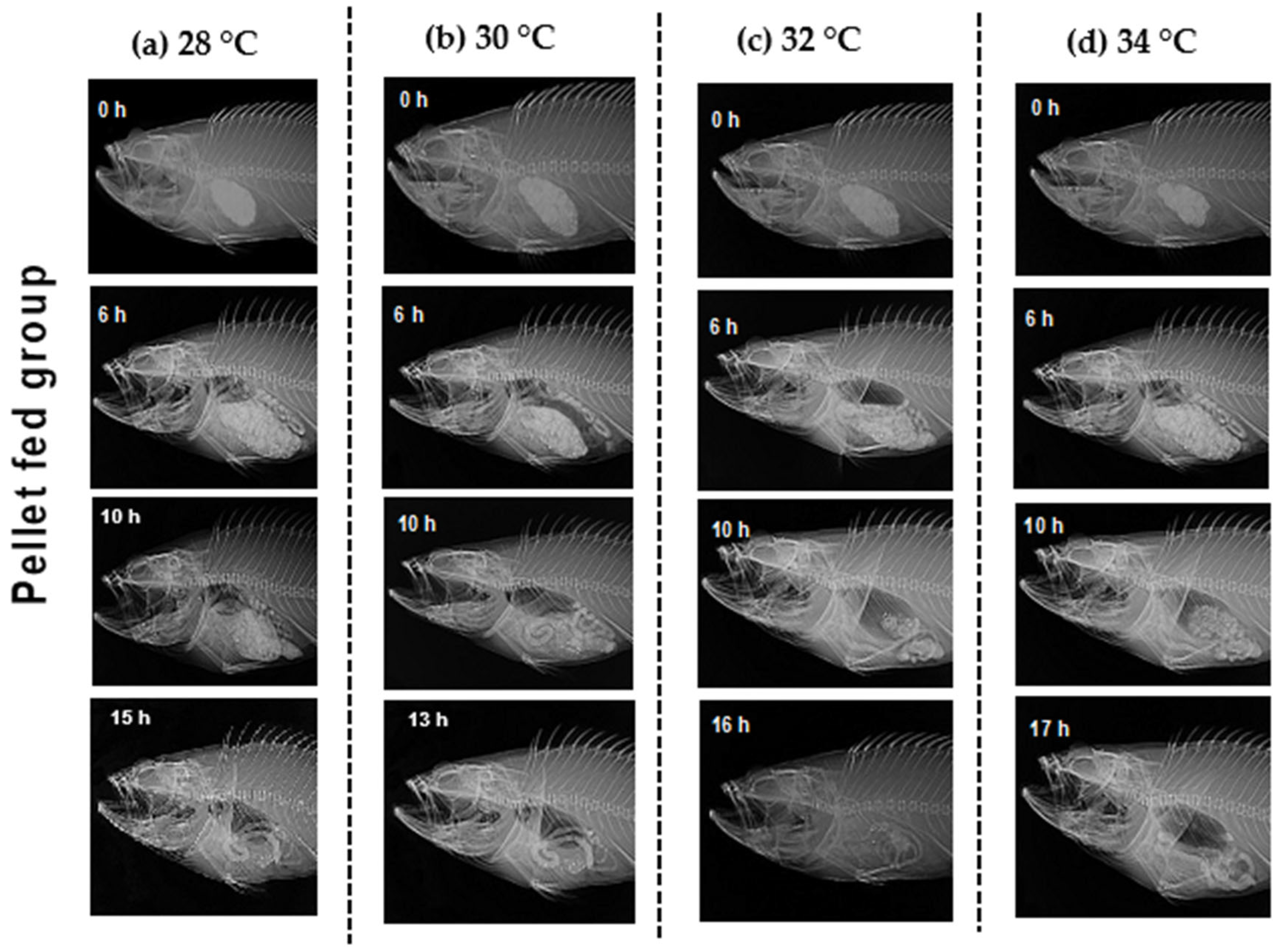
2.3.1. Estimation of Stomach Volume
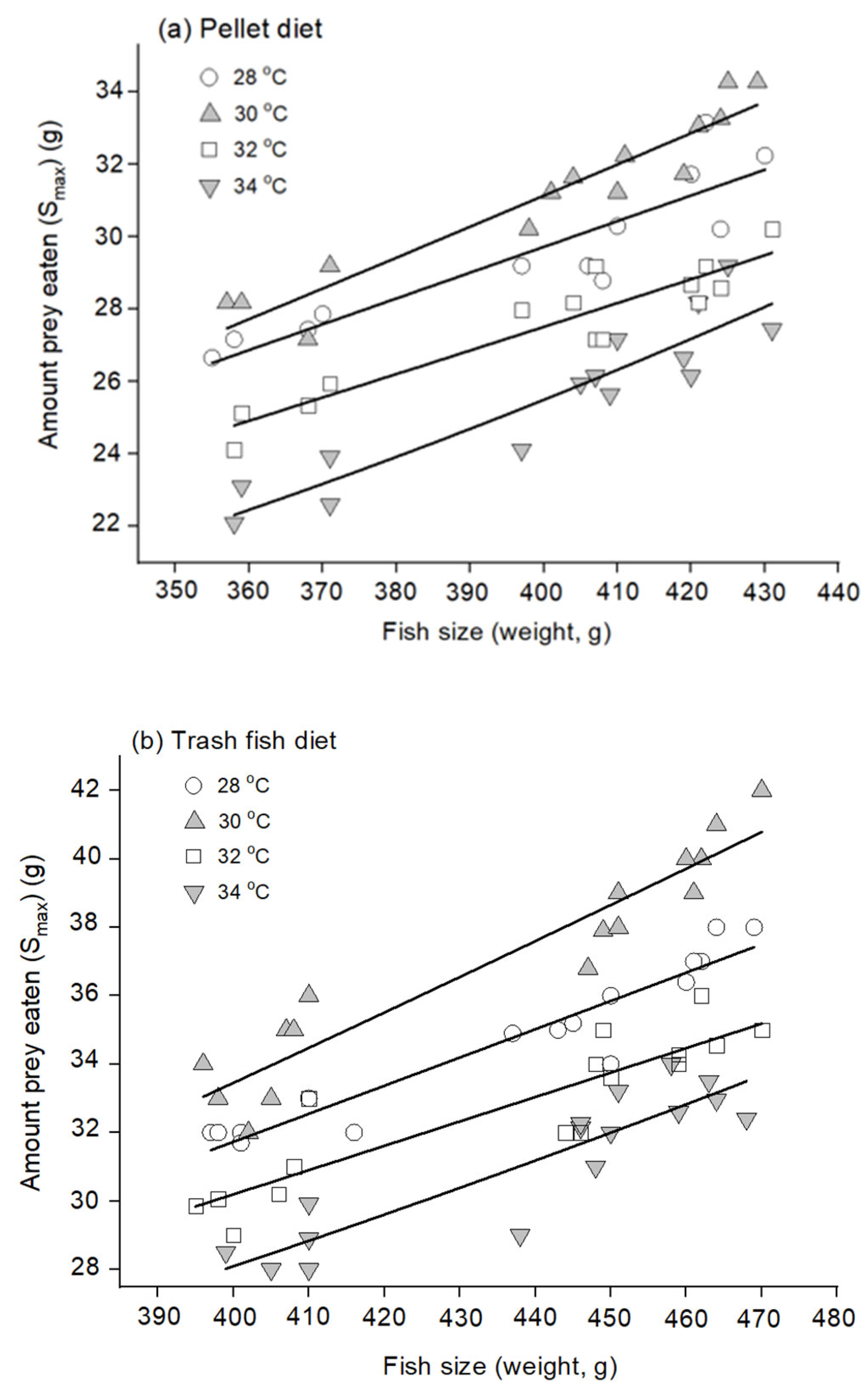
2.3.2. Estimation of Maximum Feed Intake
2.3.3. GET and GER Calculation
2.4. Growth Indices Analysis
weight) × number of fish × number of days/2]
2.5. Statistical Analysis
3. Results
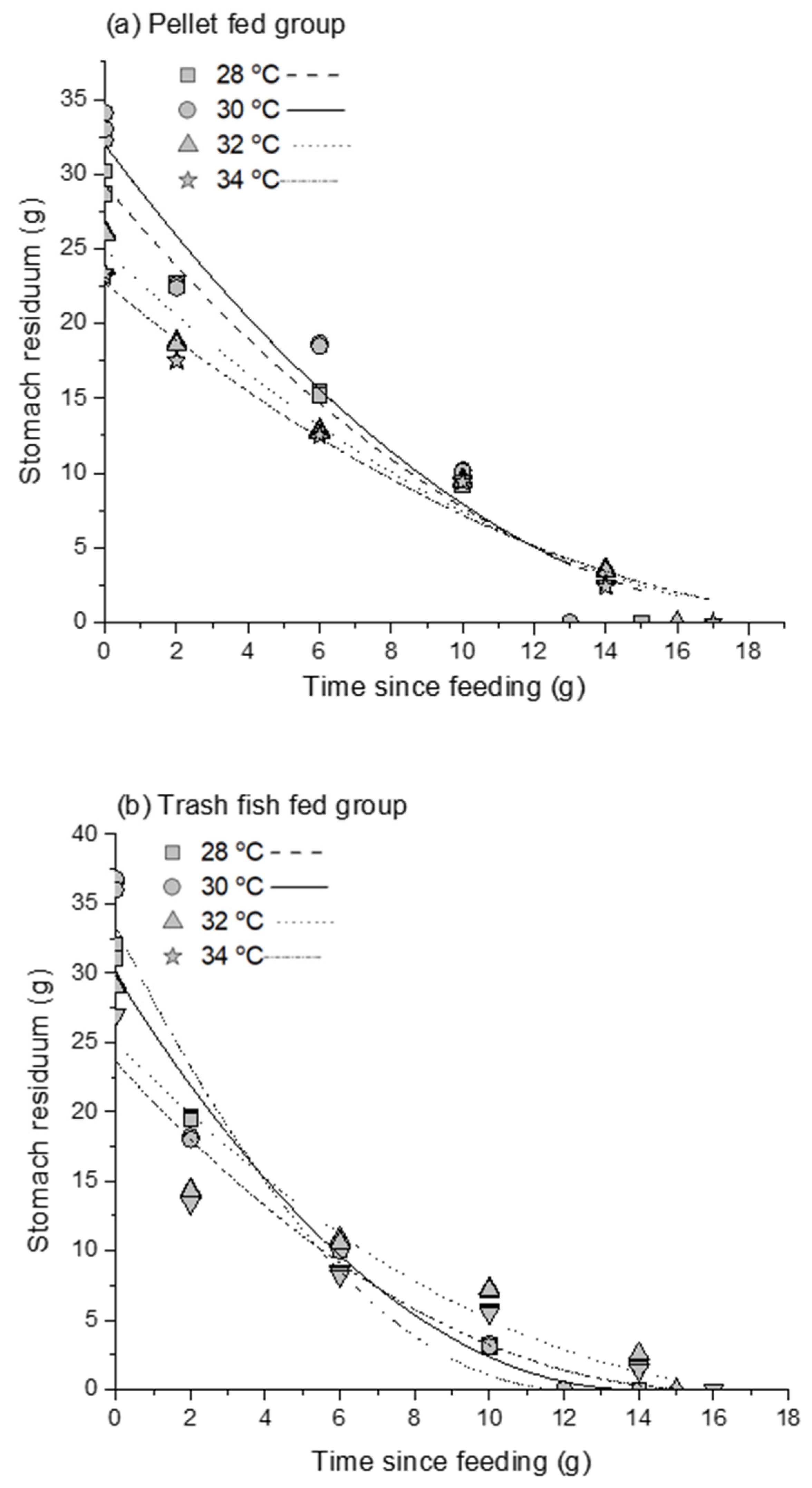
3.1. Gastrointestinal Evacuation
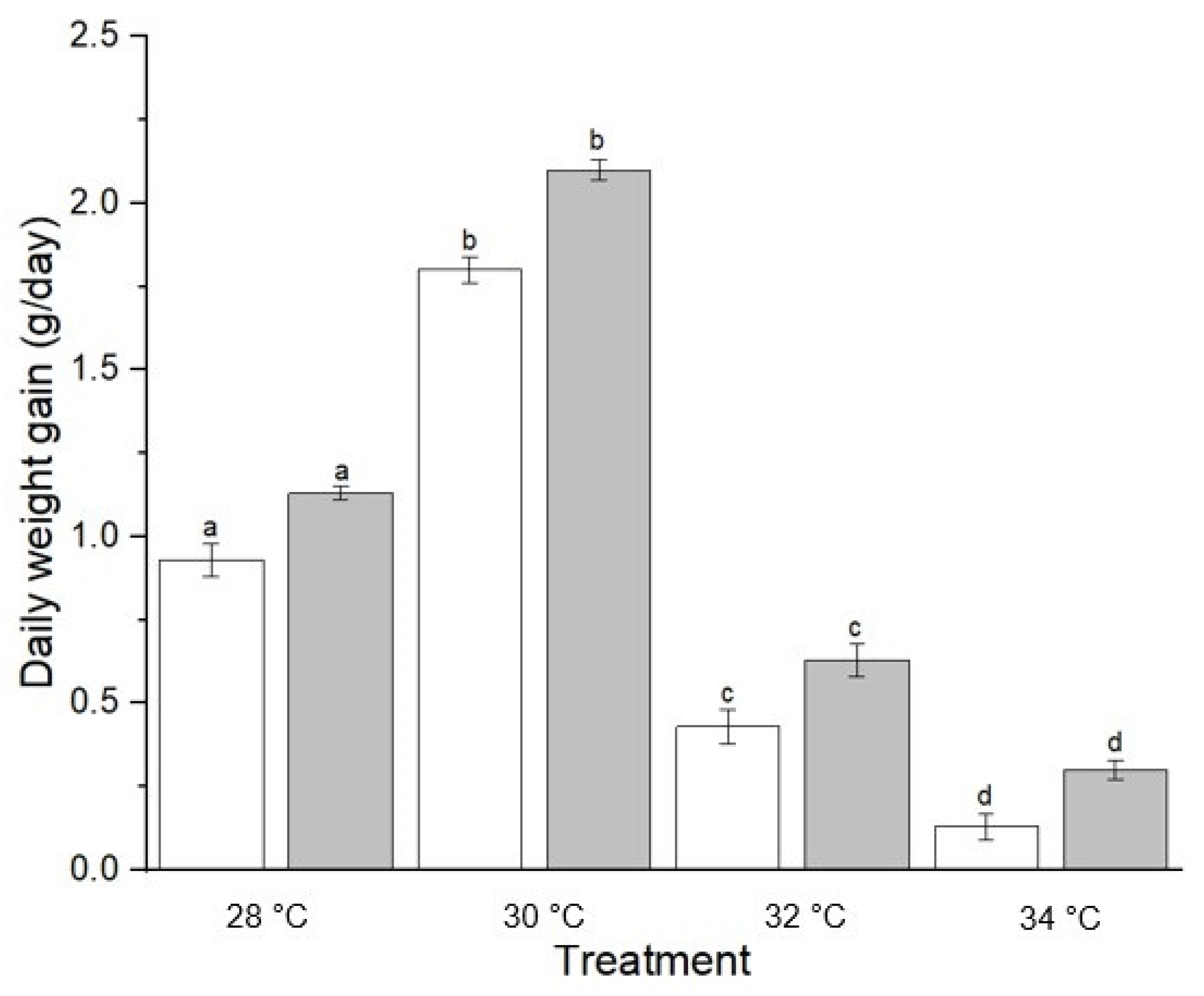
3.2. Growth Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghaffar, M.A.; Bakar, Y.; Das, S.K. Effect of temperature and diet on growth and gastric emptying time of the hybrid. Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂. Aquac. Rep. 2016, 4, 118–124. [Google Scholar]
- Das, S.K.; Ghaffar, M.A.; Bakar, Y.; Brito, M.F.; Mastura, S.S.; Temple, S.E. X-radiographic observations of food passage and nutrient absorption along the alimentary tract of archerfish, Toxotes jaculatrix. Bull. Mar. Sci. 2014, 90, 903–919. [Google Scholar] [CrossRef]
- Noor, N.M.; Das, S.K.; Ghaffar, M.A. Effect of salinities on gastric emptying and nutrient absorption of tiger grouper× giant grouper (Epinephelus fuscoguttatus × E. lanceolatus) hybrid. Sains Malays. 2018, 47, 1077–1084. [Google Scholar] [CrossRef]
- Mazumder, S.K.; Abd Ghaffar, M.; Das, S.K. Exploring the suitable temperature and diet for growth and gastric emptying time of juvenile Malabar Blood Snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Thalassas 2019, 35, 29–41. [Google Scholar]
- Gilannejad, N.; Silva, T.; Martínez-Rodríguez, G.; Yúfera, M. Effect of feeding time and frequency on gut transit and feed digestibility in two fish species with different feeding behaviours, gilthead seabream and Senegalese sole. Aquaculture 2019, 513, 734438. [Google Scholar] [CrossRef]
- Morais, S. The physiology of taste in fish: Potential implications for feeding stimulation and gut chemical sensing. Rev. Fish Sci. Aquac. 2017, 25, 133–149. [Google Scholar] [CrossRef]
- Mazumder, S.K.; Abd Ghaffar, M.; Das, S.K. Effect of temperature and diet on gastrointestinal evacuation of juvenile malabar blood snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Aquaculture 2020, 522, 735114. [Google Scholar]
- Kasihmuddin, S.M.; Ghaffar, M.A.; Das, S.K. Rising Temperature Effects on Growth and Gastric Emptying Time of Freshwater African Catfish (Clarias gariepinus) Fingerlings. Animals 2021, 11, 3497. [Google Scholar] [CrossRef]
- Jobling, M. Fish nutrition research: Past, present and future. Aquac. Int. 2016, 24, 767–786. [Google Scholar] [CrossRef]
- Yokrattanasak, J.; De Gaetano, A.; Panunzi, S.; Satiracoo, P.; Lawton, W.M.; Lenbury, Y. A simple, realistic stochastic model of gastric emptying. PLoS ONE 2016, 11, e0153297. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Llorens, S.; Peruzzi, S.; Falk-Petersen, B.; Godoy-Olmos, S.; Ulleberg, O.; Tomás-Vidal, A.; Jobling, M. Digestive tract morphology and enzyme activities of juvenile diploid and triploid Atlantic salmon (Salmo salar) fed fishmeal-based diets with or without fish protein hydrolysates. PLoS ONE 2021, 16, e0245216. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Ahmad, T.; Sharma, J.; Chakrabarti, R. Effect of temperature on food consumption, immune system, antioxidant enzymes, and heat shock protein 70 of Channa punctata (Bloch, 1793). Fish Physiol. Biochem. 2020, 47, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zanuzzo, F.S.; Bailey, J.A.; Garber, A.F.; Gamperl, A.K. The acute and incremental thermal tolerance of Atlantic cod (Gadus morhua) families under normoxia and mild hypoxia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 233, 30–38. [Google Scholar] [CrossRef]
- Aydın, İ.; Küçük, E.; Polat, H.; Öztürk, R.Ç.; Terzi, Y.; Altınok, İ. Growth and feed conversion ratio of diploid and triploid induced juvenile turbot reared at different water temperatures. Aquaculture 2021, 543, 736981. [Google Scholar] [CrossRef]
- Das, S.K.; Noor, N.M.; Kai, K.S.; Juan, Q.Z.; Iskandar, N.S.; De, M. Effects of temperature on the growth, gastric emptying time, and oxygen consumption rate of mahseer (Tor tambroides) under laboratory conditions. Aquac. Rep. 2018, 12, 20–24. [Google Scholar] [CrossRef]
- Guerreiro, I.; Serra, C.R.; Enes, P.; Couto, A.; Salvador, A.; Costas, B.; Oliva-Teles, A. Effect of short chain fructooligosaccharides (scFOS) on immunological status and gut microbiota of gilthead sea bream (Sparus aurata) reared at two temperatures. Fish Shellfish Immunol. 2016, 49, 122–131. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Mazumder, S.K.; Ghaffar, M.A.; Das, S.K. The effects of temperature on gastric emptying time of Malabar Blood Snapper (Lutjanus malabaricus, Bloch & Schneider 1801) using X-radiography technique. AIP Conf. Proc. 2015, 1678, 020032. [Google Scholar]
- Noor, N.M.; De, M.; Iskandar, A.; Keng, W.L.; Cob, Z.C.; Ghaffar, M.A.; Das, S.K. Effects of elevated carbon dioxide on the growth and welfare of Juvenile tiger grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) hybrid. Aquaculture 2019, 513, 734448. [Google Scholar] [CrossRef]
- Bowzer, J.; Page, M.; Trushenski, J.T. Extrusion Temperature and Pellet Size Interact to Influence Growth Performance of Hybrid Striped Bass Fed Industrially Compounded Aquafeeds. N. Am. J. Aquac. 2016, 78, 284–294. [Google Scholar] [CrossRef]
- Mazlan, A.G.; Grove, D.J. Gastric digestion and nutrient absorption along the alimentary tract of whiting (Merlangius merlangus L.) fed on natural prey. J. Appl. Ichthyol. 2003, 19, 229–238. [Google Scholar] [CrossRef]
- Pirhonen, J.; Muuri, L.; Kalliokoski, S.M.; Puranen, M.; Marjomäki, T.J. Seasonal and ontogenetic variability in stomach size of Eurasian perch (Perca fluviatilis L.). Aquac. Int. 2019, 27, 1125–1135. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Prati, S.; Henriksen, E.H.; Smalås, A.; Knudsen, R.; Klemetsen, A.; Amundsen, P.A. Exploring temporal patterns in fish feeding ecology: Are ontogenetic dietary shifts stable over time? Rev. Fish Biol. Fish 2022, 32, 1141–1155. [Google Scholar] [CrossRef]
- Mazlan, A.G.; Othman, B.H.R.; Grove, D.J. An evaluation of X-radiography studies in estimation of gastric emptying time (GET) in whiting (Merlangius merlangus L.). Online J. Biol. Sci. 2002, 2, 109–115. [Google Scholar]
- Hashim, M.; Abidin, Z.; Das, S.K.; Mazlan, A.G. Gastric emptying and food consumption of Scatophagus argus. Aquac. Aquar. Conserv. Legis. 2018, 11, 278–287. [Google Scholar]
- Bascinar, N.; Bascinar, N.S.; Seyhan, K.; Khan, U. The effect of temperature on the rate of gastric evacuation in brook trout Salvelinus fontinalis fed on commercial pellets: Group-feeding. Pak. J. Zool. 2016, 48, 21–25. [Google Scholar]
- Liu, R.; Zhou, Y.; Li, Z.; Huang, M.; Li, L.; Gao, Q.; Dong, S. Evaluation of the effects of temperature on gastric evacuation and the associated mathematical models in different sizes steelhead trout (Oncorhynchus mykiss). Aquaculture 2021, 549, 737815. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Ajiboye, O.; Zanuzzo, F.S.; Sandrelli, R.M.; Ellen de Fátima, C.P.; Beemelmanns, A. The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic Salmon (Salmo salar). Aquaculture 2020, 519, 734874. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, C.S.; Chen, J.C. Salinity and temperature tolerance of brown-marbled grouper Epinephelus fuscoguttatus. Fish Physiol. Biochem. 2013, 2, 277–286. [Google Scholar] [CrossRef]
- Conde-Sieira, M.; Chivite, M.; Míguez, J.M.; Soengas, J.L. Stress effects on the mechanisms regulating appetite in teleost fish. Front. Endocrinol. 2018, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Pankhurst, N.W.; Pankhurst, P.M. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef]
- Jarrold, M.D.; Munday, P.L. Elevated temperature does not substantially modify the interactive effects between elevated CO2 and diel CO2 cycles on the survival, growth and behavior of a coral reef fish. Front. Mar. Sci. 2018, 5, 458–474. [Google Scholar] [CrossRef]

| Feed | Ash (%) | Moisture (%) | Protein (%) | Lipid (%) |
|---|---|---|---|---|
| Trash fish, Sardinella sp. | 2.3 ± 0.15 | 78.5 ± 0.33 | 55.4 ± 0.62 | 7.3 ± 0.25 |
| Pellet | 11.4 ± 0.08 | 29.0 ± 0.01 | 37.5 ± 0.80 | 15 ± 0.13 |
| Treatment | S0 | ρ | χ2 | r2 |
|---|---|---|---|---|
| 28 °C + pellet | 29.26 ± 0.69 | 0.79 ± 0.02 | 2.24 | 0.98 |
| 30 °C + pellet | 31.95 ± 1.44 | 0.57 ± 0.05 | 9.65 | 0.93 |
| 32 °C + pellet | 24.90 ± 0.70 | 0.61 ± 0.02 | 2.43 | 0.97 |
| 34 °C + pellet | 22.69 ± 0.63 | 0.95 ± 0.02 | 1.99 | 0.97 |
| 28 °C + trash fish | 29.86 ± 0.72 | 0.52 ± 0.04 | 2.09 | 0.98 |
| 30 °C + trash fish | 33.32 ± 1.61 | 0.41 ± 0.10 | 10.01 | 0.95 |
| 32 °C + trash fish | 25.01 ± 1.48 | 0.45 ± 0.06 | 10.11 | 0.90 |
| 34 °C + trash fish | 23.58 ± 1.32 | 0.55 ± 0.07 | 7.49 | 0.91 |
| Factor | Level | Feeding Rate (% day−1) | FCR | SGR (% day−1) | K |
|---|---|---|---|---|---|
| Temperature | |||||
| 28 | 1.30 ± 0.01 ab | 1.48 ± 0.06 ab | 8.33 ± 0.78 b | 1.28 ± 0.01 b | |
| 30 | 1.41 ± 0.02 a | 1.23 ± 0.12 c | 15.18 ± 1.07 a | 1.43 ± 0.02 a | |
| 32 | 1.23 ± 0.02 ab | 1.72 ± 0.06 ab | 4.39 ± 0.81 c | 1.23 ± 0.02 b | |
| 34 | 1.08 ± 0.03 c | 2.17 ± 0.32 a | 1.80 ± 0.69 d | 1.04 ± 0.03 c | |
| Diets | Pellet | 1.24 ± 0.08 a | 1.80 ± 0.24 a | 6.59 ± 2.84 a | 1.22 ± 0.08 a |
| Trash fish | 1.26 ± 0.07 a | 1.51 ± 0.16 a | 8.26 ± 2.99 b | 1.26 ± 0.07 b | |
| Diet × Temperature | 28 °C + pellet | 1.29 ± 0.02 a | 1.55 ± 0.10 a | 7.55 ± 1.61 a | 1.27 ± 0.01 a |
| 30 °C + pellet | 1.39 ± 0.04 a | 1.36 ± 0.02 b | 14.12 ± 1.05 b | 1.41 ± 0.03 b | |
| 32 °C + pellet | 1.21 ± 0.02 a | 1.79 ± 0.02 c | 3.58 ± 1.74 c | 1.21 ± 0.03 c | |
| 34 °C + pellet | 1.01 ± 0.06 a | 2.50 ± 0.65 d | 1.11 ± 1.42 d | 1.01 ± 0.04 d | |
| 28 °C + trash fish | 1.31 ± 0.02 a | 1.42 ± 0.13 a | 9.12 ± 1.01 a | 1.29 ± 0.01 a | |
| 30 °C + trash fish | 1.43 ± 0.01 a | 1.11 ± 0.25 b | 16.25 ± 2.11 b | 1.45 ± 0.04 b | |
| 32 °C + trash fish | 1.26 ± 0.05 a | 1.66 ± 0.13 c | 5.20 ± 0.50 c | 1.26 ± 0.03 c | |
| 34 °C + trash fish | 1.07 ± 0.03 a | 1.85 ± 0.05 d | 2.50 ± 1.55 d | 1.07 ± 0.05 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.K.; De, M.; Noor, N.M.; Bakar, Y.; Cob, Z.C.; Ghaffar, M.A. Cross Effects of Diets and Rearing Temperatures on Gastrointestinal Evacuation and Growth Performance in Adult Sabah Groupers (Epinephelus fuscoguttatus × E. lanceolatus). Animals 2022, 12, 3172. https://doi.org/10.3390/ani12223172
Das SK, De M, Noor NM, Bakar Y, Cob ZC, Ghaffar MA. Cross Effects of Diets and Rearing Temperatures on Gastrointestinal Evacuation and Growth Performance in Adult Sabah Groupers (Epinephelus fuscoguttatus × E. lanceolatus). Animals. 2022; 12(22):3172. https://doi.org/10.3390/ani12223172
Chicago/Turabian StyleDas, Simon Kumar, Moumita De, Noorashikin Md Noor, Yosni Bakar, Zaidi Che Cob, and Mazlan Abd. Ghaffar. 2022. "Cross Effects of Diets and Rearing Temperatures on Gastrointestinal Evacuation and Growth Performance in Adult Sabah Groupers (Epinephelus fuscoguttatus × E. lanceolatus)" Animals 12, no. 22: 3172. https://doi.org/10.3390/ani12223172
APA StyleDas, S. K., De, M., Noor, N. M., Bakar, Y., Cob, Z. C., & Ghaffar, M. A. (2022). Cross Effects of Diets and Rearing Temperatures on Gastrointestinal Evacuation and Growth Performance in Adult Sabah Groupers (Epinephelus fuscoguttatus × E. lanceolatus). Animals, 12(22), 3172. https://doi.org/10.3390/ani12223172









