Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and DNA Extraction
2.3. Ethical Statement
2.4. Molecular Detection of the Tick-Borne Pathogens
2.5. Sequencing of the PCR-Positive Samples
2.6. Phylogenetic Analysis
2.7. Nucleotide Sequence Accession Numbers
2.8. Statistical Analysis
3. Results
3.1. Overall Infection Rate
3.2. Co-Infection Analysis
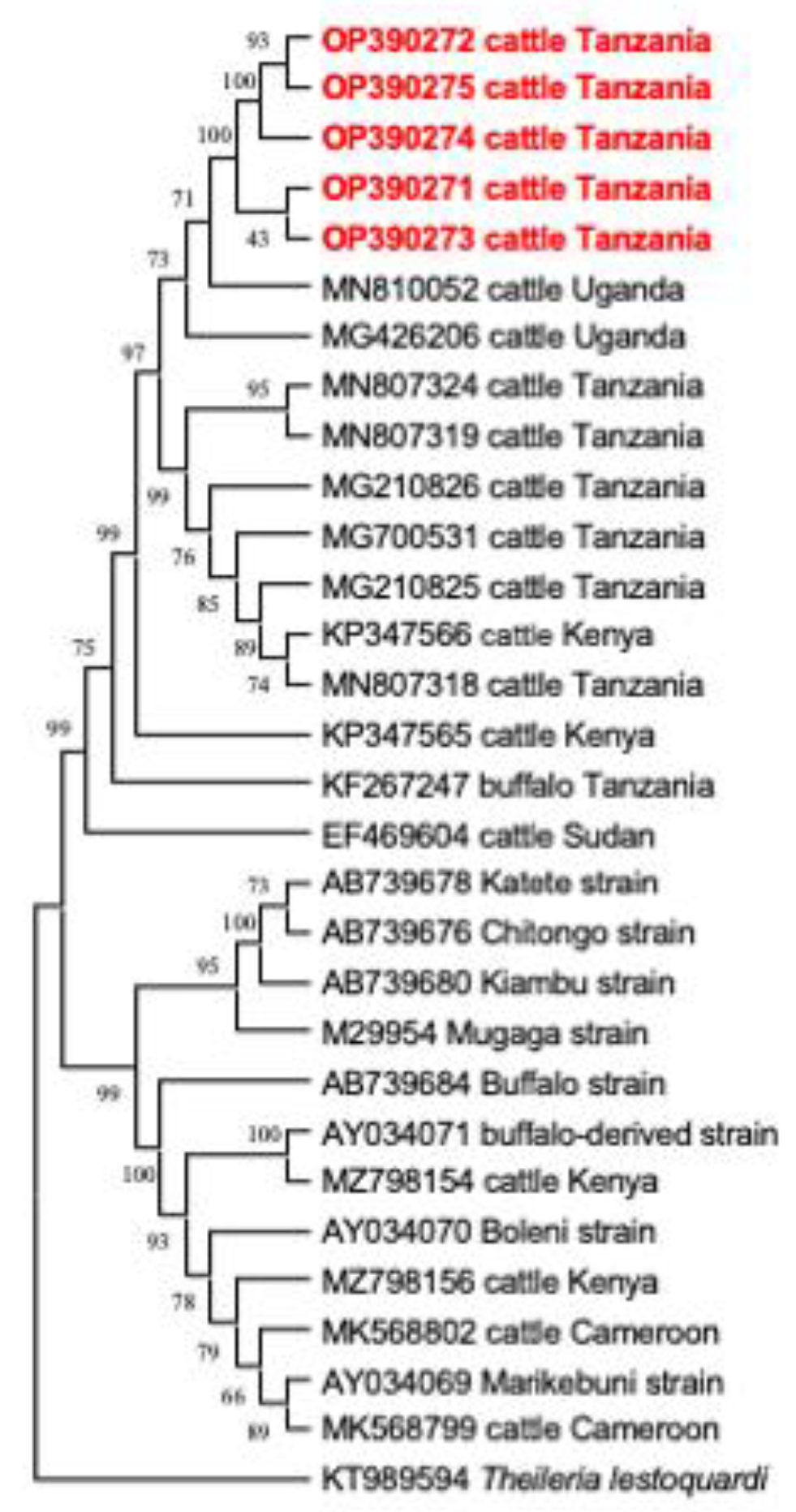
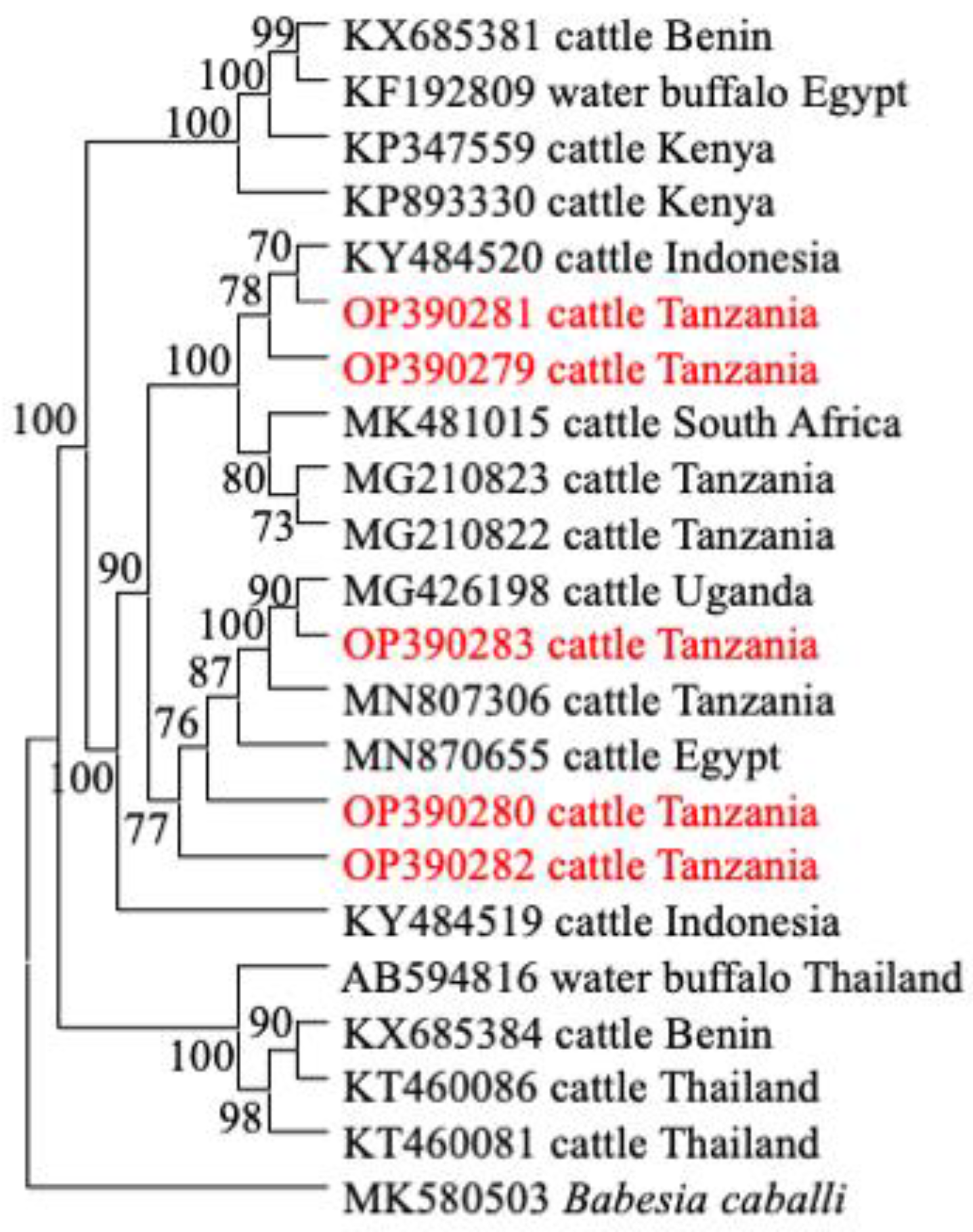
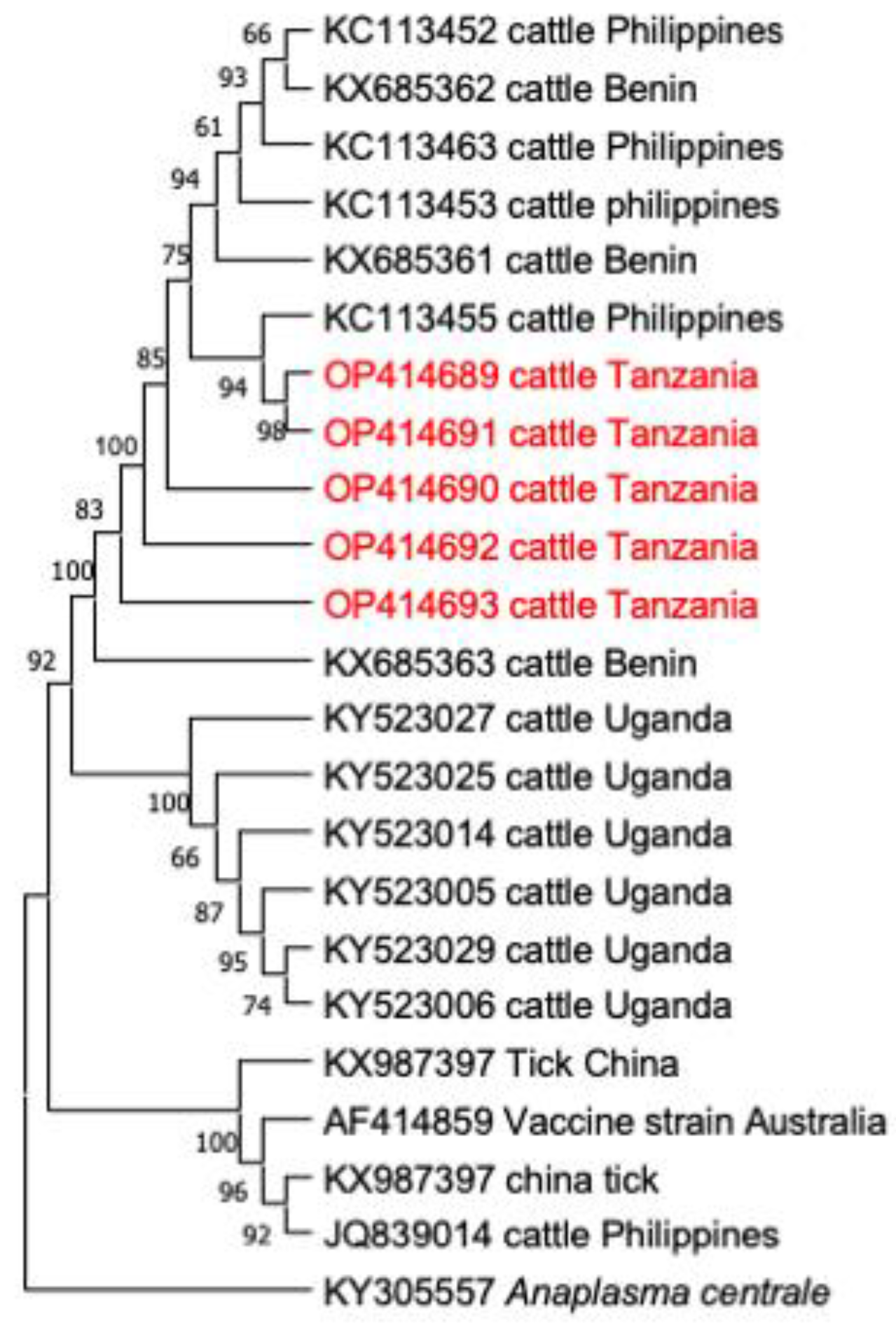
3.3. Comparative Gene Sequence Analyses
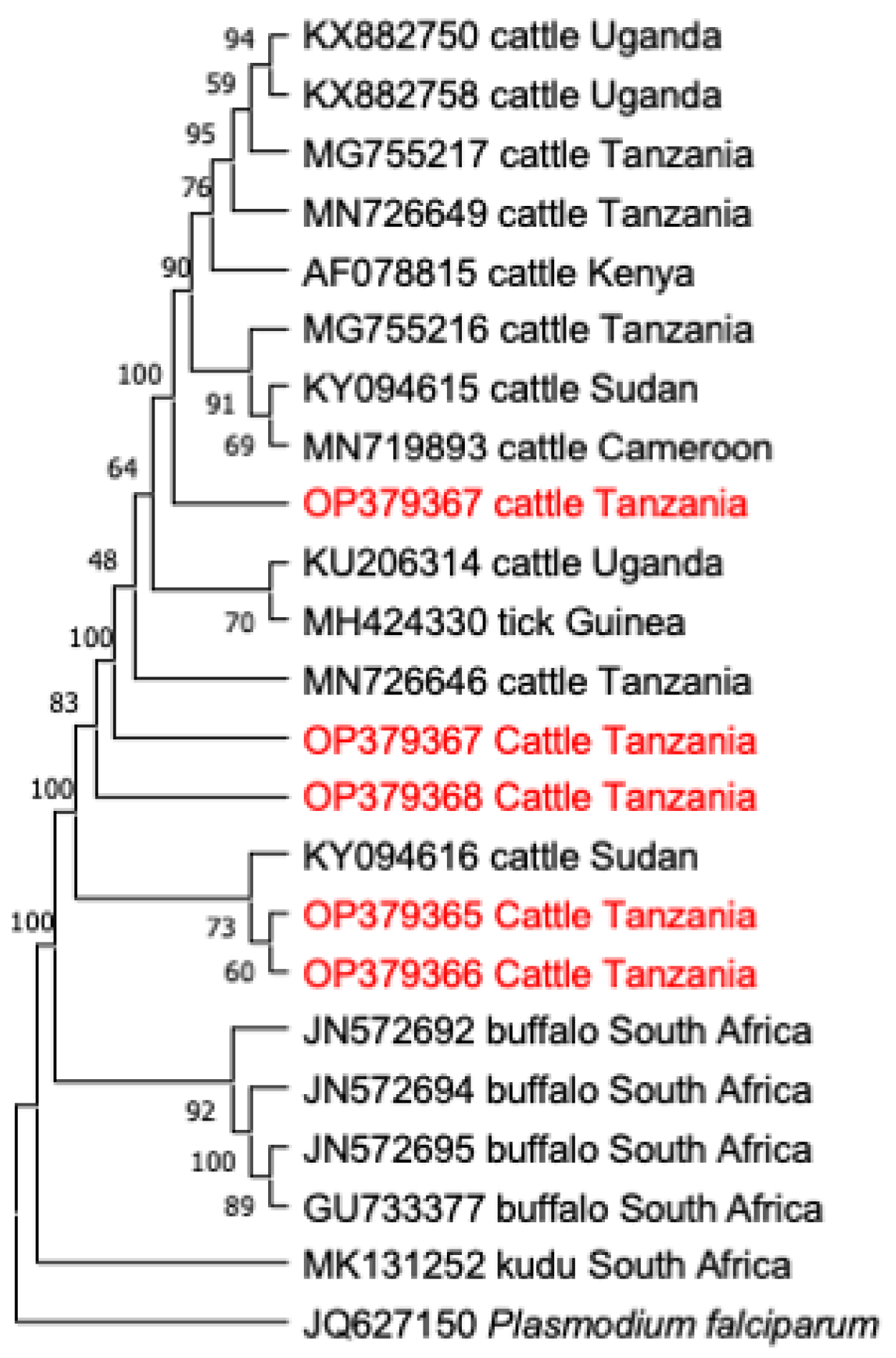
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasozi, K.I.; Matovu, E.; Tayebwa, D.; Natuhwera, J.; Mugezi, I.; Mahero, E. Epidemiology of Increasing Hemo-Parasite Burden in Ugandan Cattle. Open J. Vet. Med. 2014, 4, 220–231. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Collins, N.; Knobel, D.; Chaisi, M.E.; Vorster, I.; Steyn, H.C.; Oosthuizen, M.C. Molecular investigation of tick-borne haemoparasite infections among transhumant zebu cattle in Karamoja Region, Uganda. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kurwijila, L.; Omore, A.; Grace, D. Tanzania Dairy Industry Overview; Sokoine University of Agriculture: Morogoro, Tanzania, 2012. [Google Scholar]
- Kivaria, F.M. Estimated direct economic costs associated with tick-borne diseases on cattle in Tanzania. Trop. Anim. Health Prod. 2006, 38, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Homewood, K.; Trench, P.; Randall, S.; Lynen, G.; Bishop, B. Livestock health and socio-economic impacts of a veterinary intervention in Maasailand: Infection-and-treatment vaccine against East Coast fever. Agric. Syst. 2006, 89, 248–271. [Google Scholar] [CrossRef]
- Ocaido, M.; Muwazi, R.T.; Opuda, J.A. Economic impact of ticks and tick-borne diseases on cattle production systems around Lake Mburo National Park in South Western Uganda. Trop. Anim. Health Prod. 2009, 41, 731–739. [Google Scholar] [CrossRef]
- Engida, E.; Guthiga, P.; Karugia, J. The Role of Livestock in the Tanzanian Economy: Policy Analysis Using a Dynamic Computable General Equilibrium Model for Tanzania. In Proceedings of the International Association of Agricultural Economists 2015 Conference, Milan, Italy, 9–14 August 2015; 15p. Available online: https://ageconsearch.umn.edu/bitstream/212039/2/Legesse-98.pdf (accessed on 13 February 2018).
- Ndagala, D.K. Pastoralists and the State in Tanzania on JSTOR. Nomadic Peoples 1990, 27, 51–64. Available online: https://www.jstor.org/stable/43123307 (accessed on 27 September 2022).
- Swai, E.S.; Karimuribo, E.D.; Kambarage, D.M.; Moshy, W.E. A longitudinal study on morbidity and mortality in youngstock smallholder dairy cattle with special reference to tick borne infections in Tanga region, Tanzania. Vet. Parasitol. 2009, 160, 34–42. [Google Scholar] [CrossRef]
- Ogden, N.H.; Maarouf, A.; Barker, I.K.; Bigras-Poulin, M.; Lindsay, L.R.; Morshed, M.G.; O’Callaghan, C.J.; Ramay, F.; Waltner-Toews, D.; Charron, D.F. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 2005, 35, 375–389. [Google Scholar] [CrossRef]
- Hezron, N.E.; Adrian, M.; Robinson, M.H. Tick infestations in extensively grazed cattle and efficacy trial of high-cis cypermethrin pour-on preparation for control of ticks in Mvomero district in Tanzania. BMC Vet. Res. 2012, 8, 224. [Google Scholar] [CrossRef]
- Mamiro, K.A.; Magwisha, H.B.; Rukambile, E.J.; Ruheta, M.R.; Kimboka, E.J.; Malulu, D.J.; Malele, I.I. Occurrence of Ticks in Cattle in the New Pastoral Farming Areas in Rufiji District, Tanzania. J. Vet. Med. 2016, 2016, 3420245. [Google Scholar] [CrossRef]
- Kerario, I.I.; Muleya, W.; Chenyambuga, S.; Koski, M.; Simuunza, M. Abundance and distribution of Ixodid tick species infesting cattle reared under traditional farming systems in Tanzania. Afr. J. Agric. Res. 2017, 12, 286–299. [Google Scholar]
- Swai, E.S.; Mbise, A.N.; Kessy, V.; Kaaya, E. Farm constraints, cattle disease perception and tick management practices in pastoral Maasai community-Ngorongoro, Tanzania. Livest. Res. Rural Dev. 2005, 17, 2. [Google Scholar]
- Catalano, D.; Biasibetti, E.; Lynen, G.; Di Giulio, G.; De Meneghi, D.; Tomassone, L.; Valenza, F.; Cappuchio, M.T. “Ormilo disease” a disorder of zebu cattle in Tanzania: Bovine cerebral theileriosis or new protozoan disease? Trop. Anim. Health Prod. 2015, 47, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Adjou Moumouni, P.F.; Lee, S.H.; Liu, M.; Khamis, Y.H.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; Galon, E.M.; et al. Molecular detection and characterization of tick-borne protozoan and rickettsial pathogens isolated from cattle on Pemba Island, Tanzania. Ticks Tick Borne Dis. 2018, 9, 1437–1445. [Google Scholar] [CrossRef]
- Laisser, E.L.K.; Chenyambuga, S.W.; Karimuribo, E.D.; Msalya, G.; Kipanyula, M.J.; Mwilawa, A.J.; Mdegela, R.H.; Kusiluka, L.J. A review on prevalence, control measure, and tolerance of Tanzania Shorthorn Zebu cattle to east coast fever in Tanzania. Trop. Anim. Health Prod. 2017, 49, 813–822. [Google Scholar] [CrossRef]
- Binta, M.G.; Losho, T.; Allsopp, B.A.; Mushi, E.Z. Isolation of Theileria taurotragi and Theileria mutans from cattle in Botswana. Vet. Parasitol. 1998, 77, 83–91. [Google Scholar] [CrossRef]
- Moll, G.; Lohding, A.; Young, A.S.; Leitch, B.L. Epidemiology of theileriosis in calves in an endemic area of Kenya. Vet. Parasitol. 1986, 19, 255–273. [Google Scholar] [CrossRef]
- Chaisi, M.E.; Janssens, M.E.; Vermeiren, L.; Oosthuizen, M.C.; Collins, N.E.; Geysen, D. Evaluation of a Real-Time PCR Test for the Detection and Discrimination of Theileria Species in the African Buffalo (Syncerus caffer). PLoS ONE 2013, 8, 10. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Norval, R.A.I.; Uilenberg, G. Rhipicephalus zambeziensis as a vector of bovine theileriae. Trop. Anim. Health Prod. 1983, 15, 39–42. [Google Scholar] [CrossRef]
- Young, A.S.; Purnell, R.E.; Payne, R.C.; Brown, C.G.D.; Kanhai, G.K. Studies on the transmission and course of infection of a Kenyan strain of Theileria mutans. Parasitology 1978, 76, 99. [Google Scholar] [CrossRef]
- De Vos, A.J.; Bessenger, R.; Banting, L.F. Research communication Theileria taurotragi: A probable agent of bovine cerebral theileriosis. Onderstepoort J. Vet. Res. 1981, 48, 177–178. [Google Scholar] [PubMed]
- Pienaar, R.; Latif, A.A.; Mans, B.J. Investigations into the host specificity of Theileria taurotragi. Vet. Parasitol. 2018, 254, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lynen, G.; Zeman, P.; Bakuname, C.; Di Giulio, G.; Mtui, P.; Sanka, P.; Jongejan, F. Cattle ticks of the genera Rhipicephalus and Amblyomma of economic importance in Tanzania: Distribution assessed with GIS based on an extensive Weld survey. Exp. Appl. Acarol. 2007, 43, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Woodford, J.D.; Jones, T.W.; Rae, P.F.; Boid, R.; Bell-Sakyi, L. Seroepidemiological studies of bovine babesiosis on Pemba Island, Tanzania. Vet. Parasitol. 1990, 37, 175–184. [Google Scholar] [CrossRef]
- Lynen, G.; Zeman, P.; Bakuname, C.; Di Giulio, G.; Mtui, P.; Sanka, P.; Jongejan, F. Shifts in the distributional ranges of Boophilus ticks in Tanzania: Evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients Analysis of the Tanzania. Exp. Appl. Acarol. 2008, 44, 147–164. [Google Scholar] [CrossRef]
- Fyumagwa, R.D.; Simmler, P.; Meli, M.L.; Hoare, R.; Hofmann-Lehmann, R.; Lutz, H. Prevalence of Anaplasma marginale in different tick species from Ngorongoro Crater, Tanzania. Vet. Parasitol. 2009, 161, 154–157. [Google Scholar] [CrossRef]
- Swai, E.S.; Karimuribo, E.D.; Kambarage, D.M.; Moshy, E.M.; Mbise, A.N. A comparison of seroprevalence and risk factors for Theileria parva and T. mutans in smallholder dairy cattle in the Tanga and Iringa regions of Tanzania. Vet. J. 2007, 174, 390–396. [Google Scholar] [CrossRef]
- Ringo, A.E.; Rizk, M.A.; Adjou Moumouni, P.F.; Liu, M.; Galon, E.M.; Li, Y.; Ji, S.; Tumwebaze, M.; Byamukama, B.; Thekisoe, O.; et al. Molecular detection and characterization of tick-borne haemoparasites among cattle on Zanzibar Island, Tanzania. Acta Trop. 2020, 211, 105598. [Google Scholar] [CrossRef]
- Cao, S.N.; Zhang, S.; Jia, L.; Xue, S.; Yu, L.; Kamyingkird, K.; Adjou Moumouni, P.F.; Mousa, A.M.; Zhou, M.; Zhang, Y.; et al. Molecular Detection of Theileria species in Sheep from Northern China. J. Vet. Med. Sci. 2013, 75, 1227–1230. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Huyen, N.X.; Cao, S.N.; Tawin, I.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.K.; Yokoyama, N.; Jittapalapong, S.; et al. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- AbouLaila, M.; Yokoyama, N.; Igarashi, I. Development and evaluation of two nested PCR assays for the detection of Babesia bovis from cattle blood. Vet. Parasitol. 2010, 172, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Konnai, S.; Imamura, S.; Nakajima, C.; Witola, W.H.; Yamada, S.; Simuunza, M.; Nambota, A.; Yasuda, J.; Ohashi, K.; Onuma, M. Acquisition and transmission of Theileria parva by vector tick, Rhipicephalus appendiculatus. Acta Trop. 2006, 99, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, A.P.; Sivakumar, T.; Ybanez, R.H.D.; Ratilla, J.C.; Perez, Z.O.; Gabotero, S.R.; Hakimi, H.; Kawazu, S.; Matsumoto, K.; Yokoyama, N.; et al. First Molecular Characterization of Anaplasma marginale in Cattle and Rhipicephalus (Boophilus) microplus Ticks in Cebu, Philippines. J. Vet. Med. Sci. 2012, 75, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Farougou, S.; Adakal, H.; Biguezoton, A.S.; Boko, C. Prévalence de l infection d’ Amblyomma variegatum par Ehrlichia ruminantium dans les élevages extensifs du Bénin. Rev. Med. Vet. 2012, 163, 261–266. [Google Scholar]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F.; Sparagano, O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, O. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chatanga, E.; Maganga, E.; Mohamed, W.M.A.; Ogata, S.; Pandey, G.S.; Abdelbaset, A.E.; Hayashida, K.; Sugimoto, C.; Katakura, K.; Nonaka, N.; et al. High infection rate of tick-borne protozoan and rickettsial pathogens of cattle in Malawi and the development of a multiplex PCR for Babesia and Theileria species identification. Acta Trop. 2022, 231, 106413. [Google Scholar] [CrossRef]
- Simuunza, M.; Weir, M.; Courcier, E.; Tait, A.; Shiels, B. Epidemiological analysis of tick-borne diseases in Zambia. Vet. Parasitol. 2011, 175, 331–342. [Google Scholar] [CrossRef]
- Tembo, S.; Collins, N.E.; Sibeko-Matjila, K.P.; Troskie, M.; Vorster, I.; Byaruhanga, C.; Oosthuizen, M.C. Occurrence of tick-borne haemoparasites in cattle in the Mungwi District, Northern Province, Zambia. Ticks Tick Borne Dis. 2018, 9, 707–717. [Google Scholar] [CrossRef]
- Njiiri, N.E.; Bronvoort, B.M.; Collins, N.E.; Steyn, H.C.; Troskie, M.; Thumbi, S.M.; Sibeko, K.P.; Jennings, A.; Van Wyk, I.C.; Kiara, H.; et al. The epidemiology of tick-borne haemoparasites as determined by the reverse line blot hybridization assay in an intensively studied cohort of calves in western Kenya. Vet. Parasitol. 2015, 210, 69–76. [Google Scholar] [CrossRef]
- Asiimwe, B.B.; Weir, W.; Tait, A.; Lubega, D.W.; Oura, C.A.L. Haemoparasite infection kinetics and the population structure of Theileria parva on a single farm in Uganda. Vet. Parasitol. 2013, 193, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hailemariam, Z.; Krücken, J.; Baumann, M.; Ahmed, J.S.; Clausen, P.H.; Nijhof, A.M. Molecular detection of tick-borne pathogens in cattle from Southwestern Ethiopia. PLoS ONE 2017, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Laisser, E.L.K.; Kipanyula, M.J.; Msalya, G.; Mdegela, R.H.; Karimuribo, E.D.; Mwilawa, A.J.; Mwega, E.D.; Kusiluka, L.; Chenyambuga, S.W. Tick burden and prevalence of Theileria parva infection in Tarime zebu cattle in the lake zone of Tanzania. Trop. Anim. Health Prod. 2014, 46, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Muhanguzi, D.; Picozzi, K.; Hatendorf, J.; Thrusfield, M.; Welburn, C.S.; Kabasa, J.D.; Waiswa, C. Prevalence and spatial distribution of Theileria parva in cattle under crop-livestock farming systems in Tororo District, Eastern Uganda. Parasit. Vectors 2014, 7, 91. [Google Scholar] [CrossRef]
- Gachohi, J.M.; Kitala, P.M.; Ngumi, P.N.; Skilton, R.A. Environment and farm factors associated with exposure to Theileria parva infection in cattle under traditional mixed farming system in Mbeere District, Kenya. Trop. Anim. Health Prod. 2011, 43, 271–277. [Google Scholar] [CrossRef]
- Kazungu, Y.E.M.; Mwega, E.; Kimera, S.I.; Gwakisa, P. Seroprevalence and carrier state of T. parva in cattle under two tick control regimes in small-holder farming system of Tanzania. Livest. Res. Rural. Dev. 2015, 27, 1–13. [Google Scholar]
- Kimaro, E.G.; Chibinga, O. Potential impact of climate change on livestock production and health in East Africa: A review. Livest. Res. Rural Dev. 2013, 25, 8. [Google Scholar]
- Yeoman, G.H.; Walker, J.B.; Ross, J.P.J.; Docker, T.M. The Ixodid Ticks of Tanzania. A Study of the Zoogeography of the Ixodidae of an East African Country; Commonweath Institute of Entomology: London, UK, 1967; 215p. [Google Scholar]
- Atuhaire, D.K.; Muleya, W.; Mbao, V.; Niyongabo, J.; Nyabongo, L.; Nsanganiyumwami, D.; Salt, J.; Namangala, B.; Musoke, A.J. Molecular characterization and population genetics of Theileria parva in Burundi’s unvaccinated cattle: Towards the introduction of east coast fever vaccine. PLoS ONE 2022, 10, 1371. [Google Scholar] [CrossRef]
- Byamukama, B.; Vudricko, P.; Maria, T.; Tayebwa, D.S.; Byaruhanga, J.; Angwe, M.K.; Li, J.; Galon, E.M.; Ringo, A.E.; Liu, M.; et al. Molecular detection of selected tick-borne pathogens infecting cattle at the wildlife-livestock interface of Queen Elizabeth National Park in Kasese District, Uganda. Tick Tick-Borne Dis. 2021, 12, 101772. [Google Scholar] [CrossRef]
- Tayebwa, D.S.; Vudricko, P.; Tuvshintulga, B.; Guswanto, A.; Nugraha, A.B.; Gantuya, S.; El-Saber Batiha, G.; Musinguzi, S.P.; Komugisha, M.; Bbira, J.S.; et al. Molecular epidemiology of Babesia species, Theileria parva, and Anaplasma marginale infecting cattle and the tick control malpractices in Central and Eastern Uganda. Ticks Tick Borne Dis. 2018, 9, 1475–1483. [Google Scholar] [CrossRef]
- Adjou Moumouni, P.F.; Aboge, G.O.; Terkawi, M.A.; Masatani, T.; Cao, S.; Kamyingkird, K.; Jirapattharasate, C.; Zhou, M.; Wang, G.; Liu, M.; et al. Molecular detection and characterization of Babesia bovis, Babesia bigemina, Theileria species and Anaplasma marginale isolated from cattle in Kenya. Parasit. Vectors 2015, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Sungirai, M.; Moyo, D.Z.; de Clercq, P.; Madder, M.; Vanwambeke, S.O.; de Clercq, E.M. Modelling the distribution of Rhipicephalus microplus and R. decoloratus in Zimbabwe. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nyabongo, L.; Kanduma, E.G.; Bishop, R.P.; Machuka, E.; Njeri, A.; Bimenyimana, A.V.; Nkundwanayo, C.; Odongo, D.O.; Pelle, R. Prevalence of tick-transmitted pathogens in cattle reveals that Theileria parva, Babesia bigemina and Anaplasma marginale are endemic in Burundi. Parasit. Vectors 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Magona, J.W.; Walubengo, J.; Olaho-Mukani, W.; Jonsson, N.N.; Welburn, S.W.; Eisler, M.C. Spatial variation of tick abundance and sero-conversion rates of indigenous cattle to Anaplasma marginale, Babesia bigemina and Theileria parva infections in Uganda. Exp. Appl. Acarol. 2011, 55, 203–213. [Google Scholar] [CrossRef]
- Adjou Moumouni, P.F.; Aplogan, G.L.; Katahira, H.; Gao, Y.; Guo, H.; Efstratiou, A.; Jirapattharasate, C.; Wang, G.; Liu, M.; Ringo, A.E.; et al. Prevalence, risk factors, and genetic diversity of veterinary important tick-borne pathogens in cattle from Rhipicephalus microplus-invaded and non-invaded areas of Benin. Tick Tick-Borne Dis. 2018, 8, 450–464. [Google Scholar] [CrossRef]
- Gitau, G.K.; Perry, B.D.; Katende, J.; McDermott, J.J.; Morzaria, S.P.; Young, A. The prevalence of serum antibodies to tick-borne infections in cattle in small-holder dairy farms in Murang’a District, Kenya; A cross-sectional study. Prev. Vet. Med. 1997, 30, 95–107. [Google Scholar] [CrossRef]
- Wesonga, F.D.; Gachohi, J.M.; Kitala, P.M.; Gathuma, J.M.; Njenga, M.J. Theileria parva infection sero-prevalence and associated risk factors in cattle in Machakos County, Kenya. Trop. Anim. Health Prod. 2015, 47, 93–101. [Google Scholar] [CrossRef]
- Miyama, T.; Byaruhanga, J.; Okamura, I.; Uchida, L.; Muramatsu, Y.; Mwebembezi, W.; Vudriko, P.; Makita, K. Effect of chemical tick control practices on tick infestation and Theileria parva infection in an intensive dairy production region of Uganda. Tick Tick-Borne Dis. 2020, 11, 101438. [Google Scholar] [CrossRef]
- Wesonga, F.D.; Gachohi, J.M.; Kitala, P.M.; Gathuma, J.M.; Njenga, M.J. Sero-prevalence of Anaplasma marginale and Babesia bigemina infections and associated risk factors in Machakos County, Kenya. Trop. Anim. Health Prod. 2017, 49, 265–272. [Google Scholar] [CrossRef]
- Kabi, F.; Magona, J.W.; Nasinyama, G.W.; Walubengo, J. Sero-prevalences of Tick-borne infections among the Nkedi Zebu and Ankole cattle in Soroti district, Uganda. J. Protozool. Res. 2008, 18, 61–70. [Google Scholar]
- Kiara, H.; Jennings, A.; Bronsvoort, B.M.; Handel, I.G.; Mwangi, S.T.; Mbole-Kariuki, M.; Van Wyk, I.C.; Poole, E.J.; Hanotte, O.; Coetzer, J.A.W.; et al. A longitudinal assessment of the serological response to Theileria parva and other tick-borne parasites from birth to one year in a cohort of indigenous calves in western Kenya. Parasitology 2014, 141, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.J.; Thumbi, S.M.; Jennings, A.; Chase-Topping, M.; Callaby, R.; Kiara, H.; Oosthuizen, M.C.; Mbole-Kariuki, M.N.; Conraide, I.; Toye, P.G.; et al. Co-infections determine patterns of mortality in a population exposed to parasite infection. Sci. Adv. 2015, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G. Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 2014, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef]

| Target Gene | Assays | Primer Sequences | Annealing | ||
|---|---|---|---|---|---|
| Forward 5′ 🠖 3′ Reverse | temp. (°C) | References | |||
| Theileria spp. (18S rRNA) | PCR | GAAACGGCTACCACATCT | AGTTTCCCCGTGTTGAGT | 55 | [31] |
| nPCR | TTAAACCTCTTCCAGAGT | TCAGCCTTGCGACCATAC | 55 | ||
| B. bigemina (BbigRAP-1a) | PCR | GAGTCTGCCAAATCCTTAC | TCCTCTACAGCTGCTTCG | 55 | [32] |
| nPCR | AGCTTGCTTTCACAACTCGCC | TTGGTGCTTTGACCGACGACAT | 55 | ||
| B. bovis (BboSBP-2) | PCR | CTGGAAGTGGATCTCATGCAACC | TCACGAGCACTCTACGGCTTTGCAG | 64 | [33] |
| nPCR | GAATCTAGGCATATAAGGCAT | ATCCCCTCCTAAGGTTGGCTAC | 58 | ||
| T. parva (p104) | PCR | ATTTAAGGAACCTGACGTGACTGC | TAAGATGCCGACTATTAATGACACC | 65 | [34] |
| nPCR | GGCCAAGGTCTCCTTCAGATTACG | TGGGTGTGTTTCCTCGTCATCTGC | 60 | ||
| A. marginale (groEL) | PCR | GACTACCACATGCTCCATACTGACTG | GACGTCCACAACTACTGCATTCAAG | 74–65 | [35] |
| nPCR | GTCTGAAGATGAGATTGCACAGGTTG | CCTTTGATGCCGTCCAGAGATGCA | 74–68 | ||
| E. ruminantium (pcs20) | PCR | ACTAGTAGAAATTGCACAATCYAT | RCTDGCWGCTTTYTGTTCAGCTAK | 61 | [36] |
| ACTAGTAGAAATTGCACAATCYAT | AACTTGGWGCRRGDARTCCTT | 61 | |||
| T. mutans (18S rRNA) | PCR | GACACAGGGAGGTAGTGACAAG | CTAAGAATTTCACCTCTGACAGT | 60 | [37] |
| nPCR | GACACAGGGAGGTAGTGACAAG | AACATTCGGAGACGCAAGCGAG | 68 | ||
| T. taurotragi (18S rRNA) | PCR | GACACAGGGAGGTAGTGACAAG | CTAAGAATTTCACCTCTGACAGT | 60 | [37] |
| nPCR | GACACAGGGAGGTAGTGACAAG | GAACCGTCCGAAAAAAGCCACG | 68 | ||
| Kwamsisi | Kabuku | Kwamatuku | Kwachaga | Komkonga | Total No. | |
|---|---|---|---|---|---|---|
| Single infection | (n = 51) | (n = 50) | (n = 50) | (n = 50) | (n = 49) | positive (%) |
| T. parva | 2 (4%) | 3 (6%) | 2 (4%) | 3 (6%) | 5 (10%) | 15 (6) |
| T. mutans | 14 (28%) | 11 (22%) | 7 (14%) | 5 (10%) | 9 (18%) | 46 (18) |
| T. taurotragi | 1 (2%) | 2 (4%) | 4 (8%) | 0 (0%) | 2 (4%) | 9 (4) |
| B. bigemina | 1 (2%) | 3 (6%) | 0 (0%) | 3 (6%) | 6 (12%) | 13 (5) |
| A. marginale | 3 (6%) | 1 (2%) | 9 (18%) | 9 (18%) | 1 (2%) | 23 (9) |
| SUB TOTAL | 21 (41%) | 20 (40%) | 22 (44%) | 20 (40%) | 23 (47%) | 106 (42) |
| Double infections | ||||||
| T. parva + T. mutans | 3 (6%) | 5 (10%) | 2 (4%) | 4 (8%) | 3 (6%) | 17 (7) |
| T. parva + T. taurotragi | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | 2 (4%) | 4 (2) |
| T. parva + B. bigemina | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (0) |
| T. parva + A. marginale | 4 (8%) | 2 (4%) | 0 (0%) | 2 (4%) | 4 (8%) | 12 (5) |
| T. mutans + T. taurotragi | 5 (10%) | 1 (2%) | 5 (10%) | 1 (2%) | 2 (4%) | 14 (6) |
| T mutans + B. bigemina | 2 (4%) | 2 (4%) | 0 (0%) | 2 (4%) | 2 (4%) | 8 (3) |
| T. mutans + A. marginale | 2 (4%) | 4 (8%) | 5 (10%) | 4 (8%) | 3 (6%) | 18 (7) |
| T. taurotragi + B. bigemina | 2 (4%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 3 (1) |
| T. taurotragi + A. marginale | 0 (0%) | 2 (4%) | 4 (8%) | 5 (10%) | 1 (2%) | 12 (5) |
| B. bigemina + A. marginale | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0) |
| SUB TOTAL | 18 (35%) | 17 (34%) | 16 (32%) | 21 (42%) | 17 (35%) | 89 (36) |
| Triple infections | ||||||
| T. parva + T. mutans + T. taurotragi | 0 (0%) | 0 (0%) | 1 (1%) | 2 (4%) | 1 (2%) | 4 (2) |
| T. parva + B. bigemina + A. marginale | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0) |
| T. parva + T. mutans + B. bigemina | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 3 (1) |
| T. parva + T. taurotragi + A. marginale | 1 (2%) | 0 (0%) | 1 (2%) | 1 (2%) | 1 (1%) | 4 (2) |
| T. mutans + B. bigemina + A. marginale | 1 (2%) | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | 3 (1) |
| T. taurotragi + B. bigemina + A. marginale | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 2 (1) |
| T. mutans + T. taurotragi + B. bigemina | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0) |
| T. mutans + T. taurotragi + A. marginale | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 1 (2%) | 3 (1) |
| SUB TOTAL | 7 (14%) | 1 (2%) | 3 (6%) | 6 (12%) | 4 (8%) | 21 (8) |
| Quadruple infections | ||||||
| T. parva + T. mutans + T. taurotragi + A. marginale | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1) |
| Total positive samples | 47 (92%) | 39 (78%) | 41 (82%) | 47 (94%) | 44 (90%) | 218 (87) |
| Pathogen | Kwamsisi (n = 51) | Kabuku (n = 50) | Kwamatuku (n = 50) | Kwachaga (n = 50) | Komkonga (n = 49) | Overall (n = 250) |
|---|---|---|---|---|---|---|
| Theileria parva | 14 (27.5%) | 16 (32%) | 5 (10%) | 18 (36%) | 11 (22.5%) | 64 (25.6%) |
| Theileria mutans | 30 (58.8%) | 28 (56%) | 20 (40%) | 22 (44%) | 20 (40.8%) | 120 (48%) |
| Theileria Taurotragi | 11 (21.6%) | 8 (16%) | 14 (28%) | 13 (26%) | 6 (12.3%) | 52 (20.8%) |
| Babesia bigemina | 8 (15.7%) | 7 (14%) | 1 (2%) | 7 (14%) | 10 (20.4%) | 33 (13.2%) |
| Anaplasma marginale | 15 (29.4%) | 13 (26%) | 20 (40%) | 26 (52%) | 7 (14.3%) | 81 (32.4%) |
| Sex | T. parva | T. mutans | T. taurotragi | B. bigemina | A. marginale |
|---|---|---|---|---|---|
| Male (n = 66) | 16 (24.2%) | 37 (56.1%) | 9 (13.6%) | 11 (16.7%) | 20 (30.3%) |
| Female (n = 184) | 48 (26.1%) | 83 (45.1%) | 43 (23.4%) | 22 (11.9%) | 61 (33.2%) |
| Co-Infections | Frequencies | % | Species Combination | Pathogens Involved |
|---|---|---|---|---|
| Double | 89 | 79.4 | 10 | 20 |
| Triple | 21 | 18.8 | 8 | 24 |
| Quadruple | 2 | 1.8 | 1 | 4 |
| Overall | 112 | 100 | 19 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringo, A.E.; Nonga, H.E.; Galon, E.M.; Ji, S.; Rizk, M.A.; El-Sayed, S.A.E.-S.; Mohanta, U.K.; Ma, Z.; Chikufenji, B.; Do, T.T.; et al. Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania. Animals 2022, 12, 3171. https://doi.org/10.3390/ani12223171
Ringo AE, Nonga HE, Galon EM, Ji S, Rizk MA, El-Sayed SAE-S, Mohanta UK, Ma Z, Chikufenji B, Do TT, et al. Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania. Animals. 2022; 12(22):3171. https://doi.org/10.3390/ani12223171
Chicago/Turabian StyleRingo, Aaron Edmond, Hezron Emanuel Nonga, Eloiza May Galon, Shengwei Ji, Mohamed Abdo Rizk, Shimaa Abd El-Salam El-Sayed, Uday Kumar Mohanta, Zhuowei Ma, Boniface Chikufenji, Thanh Thom Do, and et al. 2022. "Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania" Animals 12, no. 22: 3171. https://doi.org/10.3390/ani12223171
APA StyleRingo, A. E., Nonga, H. E., Galon, E. M., Ji, S., Rizk, M. A., El-Sayed, S. A. E.-S., Mohanta, U. K., Ma, Z., Chikufenji, B., Do, T. T., & Xuan, X. (2022). Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania. Animals, 12(22), 3171. https://doi.org/10.3390/ani12223171