First Description of Sarcoptic Mange in a Free-Ranging European Wildcat (Felis silvestris silvestris) from Spain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
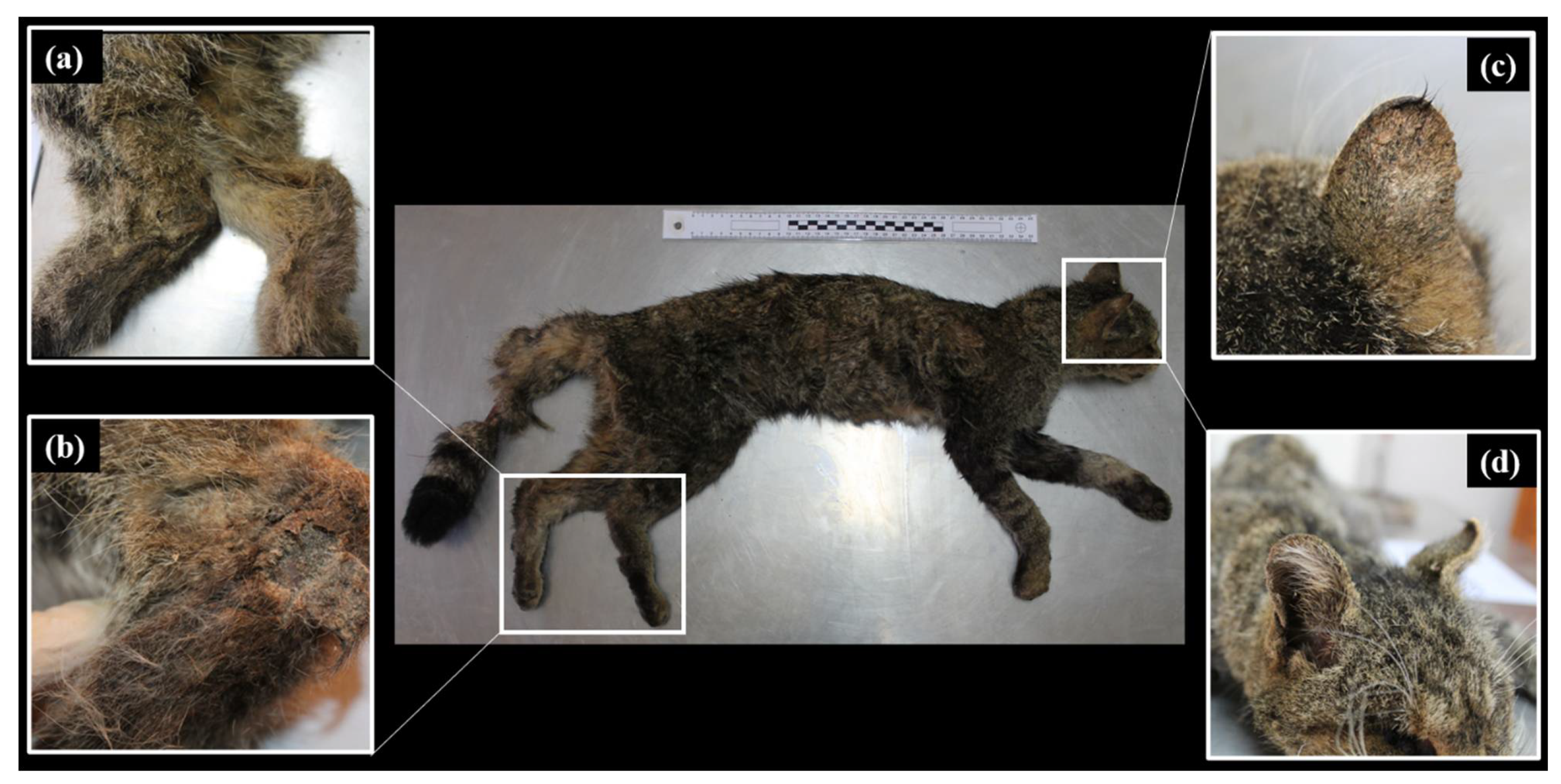
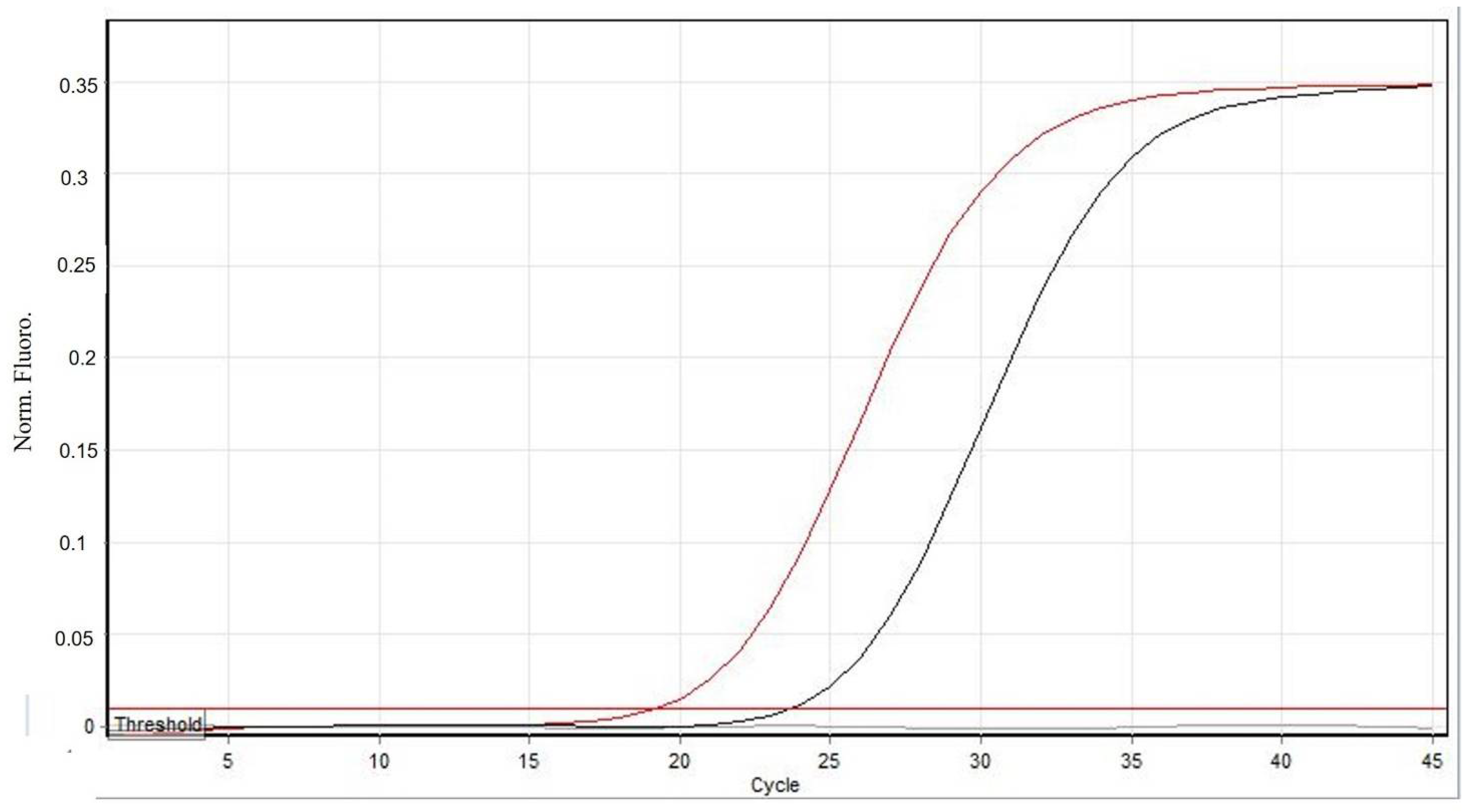
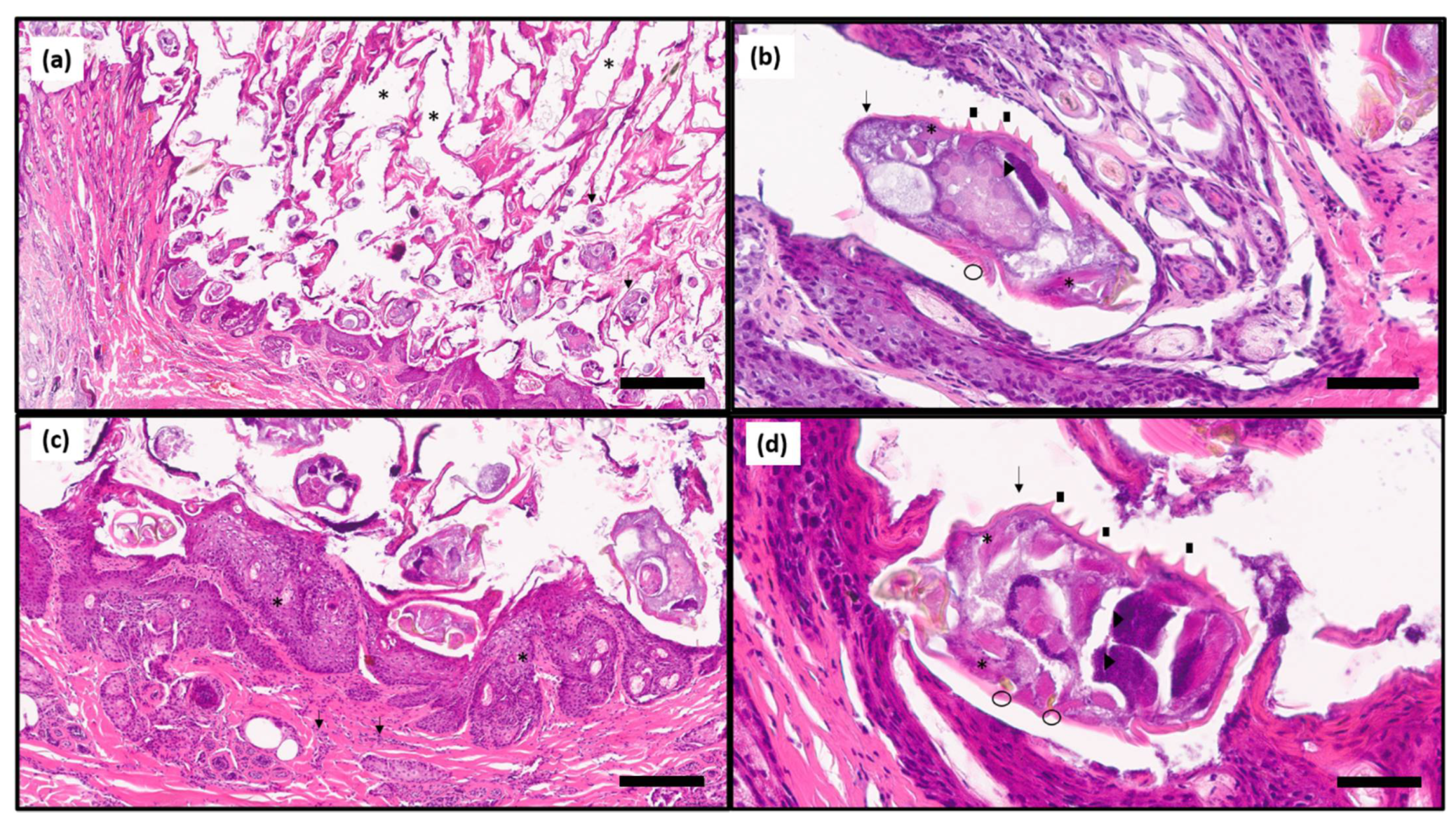
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bornstein, S.; Mörner, T.; Samuel, W.M. Parasitic diseases of wild mammals. Sarcoptes scabiei and sarcoptic mange. In Parasitic Diseases of Wild Mammals, 2nd ed.; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Iowa State University Press: Ames, IO, USA, 2001; pp. 107–119. [Google Scholar]
- Escobar, L.E.; Carver, S.; Cross, P.C.; Rossi, L.; Almberg, E.S.; Yabsley, M.J.; Niedringhaus, K.D.; Van Wick, P.; Dominguez-Villegas, E.; Gakuya, F.; et al. Sarcoptic mange: An emerging panzootic in wildlife. Transbound. Emerg. Dis. 2021, 1–16. [Google Scholar] [CrossRef]
- Mörner, T. Sarcoptic mange in Swedish wildlife. Rev. Sci. Tech. 1992, 11, 1115–1121. [Google Scholar] [CrossRef]
- Martin, R.W.; Handasyde, K.A.; Skerratt, L.F. Current distribution of sarcoptic mange in wombats. Aust. Vet. J. 1998, 76, 411–414. [Google Scholar] [CrossRef]
- Kalema-Zikusoka, G.; Koch, R.A.; Macfie, E.J. Scabies in freeranging mountain gorillas (Gorilla berengei berengei) in Bwindi impenetrable National Park, Uganda. Vet. Rec. 2002, 150, 12. [Google Scholar] [CrossRef]
- Munson, L.; Terio, K.A.; Ryser-Degiorgis, M.P.; Lane, E.P.; Courchamp, F. Wild felid diseases: Conservation implications and management strategies. In Biology and Conservation of Wild Felids, 1st ed.; Macdonald, D.W., Loveridge, A.J., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 237–259. [Google Scholar]
- Oleaga, A.; García, A.; Balseiro, A.; Casais, R.; Mata, E.; Crespo, E. First description of sarcoptic mange in the endangered Iberian lynx (Lynx pardinus): Clinical and epidemiological features. Eur. J. Wildl. Res. 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Lozano, J.; Malo, A.F. Conservation of the European wildcat (Felis silvestris) in Mediterranean environments: A reassessment of current threats. In Mediterranean Ecosystems: Dynamics, Management and Conservation, 1st ed.; Williams, G.S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 1–31. [Google Scholar]
- Gil-Sánchez, J.M.; Barea-Azcón, J.M.; Jaramillo, J.; Herrera-Sánchez, F.J.; Jiménez, J.; Virgós, E. Fragmentation and low density as major conservation challenges for the southernmost populations of the European wildcat. PLoS ONE 2020, 15, e0227708. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Duperón, E.; Virgós, E.; Moleón, M.; Barea-Azcón, J.M.; Gil-Sánchez, J.M. How accurate are coat traits for discriminating wild and hybrid forms of Felis silvestris? Mammalia 2014, 79, 101–110. [Google Scholar] [CrossRef]
- Krüger, M.; Hertwig, S.T.; Jetschke, G.; Fischer, M.S. Evaluation of anatomical characters and the question of hybridization with domestic cats in the wildcat population of thuringia, Germany. J. Zoolog. Syst. Evol. Res. 2009, 47, 268–282. [Google Scholar] [CrossRef]
- Kitchener, A.C.; Yamaguchi, N.; Ward, J.M.; Macdonald, D.W. A diagnosis for the Scottish wildcat (Felis silvestris): A tool for conservation action for a critically-endangered felid. Anim. Conserv. 2005, 8, 223–237. [Google Scholar] [CrossRef]
- Gentil, M.; Gruber, A.D.; Müller, E. Prevalence of Dog Circovirus in healthy and diarrhoeic dogs. Tierärztl. Prax 2017, 45, 89–94. [Google Scholar]
- Gut, M.; Leutenegger, C.M.; Huder, J.B.; Pedersen, N.C.; Lutz, H. One-Tube fluorogenic reverse transcription- polymerase chain reaction for the quantitation of feline coronaviruses. J. Virol. Meth. 1999, 77, 37–46. [Google Scholar] [CrossRef]
- Brunner, C.; Kanellos, T.; Meli, M.L.; Sutton, D.J.; Gisler, R.; Gomes-Keller, M.A.; Hofmann-Lehmann, R.; Lutz, H. Antibody induction after combined application of an adjuvanted recombinant FeLV vaccine and a multivalent modified live virus vaccine with a chlamydial component. Vaccine 2006, 24, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, S.; Hübner, J.; Kurth, R.; Denner, J. Antibodies neutralizing feline leukaemia virus (FeLV) in cats immunized with the transmembrane envelope protein p15E. Immunology 2006, 117, 229–237. [Google Scholar] [CrossRef]
- Klein, D.; Musil, C.; Hirt, R.; Gold, P.; Thalhammer, J.G.; Günzburg, W.H. Möglichkeiten und Grenzen neuer molekularer Untersuchungsmethoden in der klinischen Mikrobiologie: Dargestellt am Beispiel des Felinen Immundefizienzvirus. Wien. Tierärztl. Mschr. 2000, 87, 269–277. [Google Scholar]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Di Trani, L.; Buonavoglia, C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 2006, 136, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Francino, O.; Altet, L.; Sánchez-Robert, E.; Rodriguez, A.; Solano-Gallego, L.; Alberola, J.; Ferrer, L.; Sánchez, A.; Roura, X. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet. Parasitol. 2006, 137, 214–221. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Micr. Infec. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Wall, R.; Shearer, D. Mite (Acari). In Veterinary Entomology: Arthropod Ectoparasites of Veterinary Importance, 1st ed.; Wall, R., Shearer, D., Eds.; Chapman and Hall: London, UK, 1997; pp. 59–64. [Google Scholar]
- Casais, R.; Prieto, M.; Balseiro, A.; Solano, P.; Parra, F.; Martín Alonso, J.M. Identification and heterologous expression of a Sarcoptes scabiei cDNA encoding a structural antigen with immunodiagnostic potential. Vet. Res. 2007, 38, 435–450. [Google Scholar] [CrossRef]
- Oleaga, A.; Casais, R.; González-Quirós, P.; Prieto, M.; Gortázar, C. Sarcoptic mange in red deer from Spain: Improved surveillance or disease emergence? Vet. Parasitol. 2008, 154, 103–113. [Google Scholar] [CrossRef]
- Falconi, C.; Oleaga, A.; López-Olvera, J.; Casais, R.; Prieto, M.; Gortázar, C. Prevalence of antibodies against selected agents shared between Cantabrian chamois (Rupicapra pyrenaica parva) and domestic goats. Eur J. Wildl. Res. 2010, 56, 319–332. [Google Scholar] [CrossRef]
- Casais, R.; Goyena, E.; Martínez-Carrasco, C.; Ruiz de Ybáñez, R.; Alonso de Vega, F.; Ramis, G.; Prieto, J.M.; Berriatua, E. Variable performance of a human derived Sarcoptes scabiei recombinant antigen ELISA in swine mange diagnosis. Vet. Parasitol. 2013, 197, 397–403. [Google Scholar] [CrossRef]
- Casais, R.; Millán, J.; Rosell, J.M.; Dalton, K.P.; Prieto, J.M. Evaluation of an ELISA using recombinant Ssλ20ΔB3 antigen for the serological diagnosis of Sarcoptes scabiei infestation in domestic and wild rabbits. Vet. Parasitol. 2015, 214, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; Villafuerte, R.; Blanco, J.C.; Fernández de Luco, D. Enzootic sarcoptic mange in red foxes in Spain. Z. Jagdwiss. 1998, 44, 251–256. [Google Scholar] [CrossRef]
- Millán, J.; Ruiz-Fons, F.; Márquez, F.J.; Viota, M.; López-Bao, J.V.; Martín-Mateo, M.P. Ectoparasites of the endangered Iberian lynx Lynx pardinus and sympatric wild and domestic carnivores in Spain. Med. Vet. Entomol. 2007, 21, 248–254. [Google Scholar] [CrossRef]
- Millán, J. First description of sarcoptic mange in wild European rabbit (Oryctolagus cuniculus). Eur. J. Wildl. Res. 2010, 56, 455–457. [Google Scholar] [CrossRef]
- Andrews, J.R.H. The origin and evolution of host associations of Sarcoptes scabiei and the subfamily Sarcoptinae Murray. Acarologia 1983, 24, 85–94. [Google Scholar]
- Linnell, J.; Odden, J.; Pedersen, V.; Andersen, R. Records of intra-guild predation by Eurasian Lynx, Lynx lynx. Can. Field-Nat. 1998, 112, 707–708. [Google Scholar]
- Gakuya, F.; Ombui, J.; Heukelbach, J.; Maingi, N.; Muchemi, G.; Ogara, W.; Mijele, D.; Alasaad, S. Knowledge of mange among Masai pastoralists in Kenya. PLoS ONE 2012, 7, e43342. [Google Scholar] [CrossRef]
- Alasaad, S.; Permunian, R.; Gakuya, F.; Mutinda, M.; Soriguer, R.C.; Rossi, L. Sarcoptic-mange detector dogs used to identify infected animals during outbreaks in wildlife. BMC Vet. Res. 2012, 8, 110. [Google Scholar] [CrossRef]
- Kolodziej-Sobocinska, M.; Zalewski, A.; Kowalczyk, R. Sarcoptic mange vulnerability in carnivores of the Białowieza Primeval Forest, Poland: Underlying determinant factors. Ecol. Res. 2014, 29, 237–244. [Google Scholar] [CrossRef]
- Nájera, F.; Sánchez-Cuerda, S.; López, G.; Del Rey-Wamba, T.; Rueda, C.; Vallverdú-Coll, N.; Panadero, J.; Palacios, M.J.; López-Bao, J.V.; Jiménez, J. Lynx eats cat: Disease risk assessment during an Iberian lynx intraguild predation. Eur. J. Wildl. Res. 2019, 65, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kraabøl, M.; Gundersen, V.; Fangel, K.; Olstad, K. The taxonomy, life cycle and pathology of Sarcoptes scabiei and Notoedres cati (Acarina, Sarcoptidae): A review in a fennoscandian wildlife perspective. Fauna. Norv. 2015, 35, 21–33. [Google Scholar] [CrossRef]
- Montecino-Latorre, D.; Cypher, B.L.; Rudd, J.L.; Clifford, D.L.; Mazet, J.A.K.; Foley, J.E. Assessing the role of dens in the spread, establishment and persistence of sarcoptic mange in an endangered canid. Epidemics 2019, 27, 28–40. [Google Scholar] [CrossRef]
- Serieys, L.E.; Lea, A.J.; Epeldegui, M.; Armenta, T.C.; Moriarty, J.; VandeWoude, S.; Carver, S.; Foley, J.; Wayne, R.K.; Riley, S.P.; et al. Urbanization and anticoagulant poisons promote immune dysfunction in bobcats. Proc. Royal. Soc. B 2018, 285, 20172533. [Google Scholar] [CrossRef]
- Huffam, S.E.; Currie, B.J. Ivermectin for Sarcoptes scabiei hyperinfestation. Int. J. Infect. Dis. 1998, 2, 152–154. [Google Scholar] [CrossRef]
- Walton, S.F.; Currie, B.J. Problems in diagnosing scabies: A global disease in human and animal populations. Clin. Microbiol. Rev. 2007, 20, 268–279. [Google Scholar] [CrossRef]
- Nakagawa, T.L.D.R.; Takai, Y.; Kubo, M.; Sakai, H.; Masegi, T.; Yanai, T. A pathological study of Sepsis associated with Sarcoptic mange in raccoon dogs (Nyctereutes procyonoides) in Japan. J. Comp. Pathol. 2009, 141, 177–181. [Google Scholar] [CrossRef]
- Espinosa, J.; Ráez-Bravo, A.; López-Olvera, J.R.; Pérez, J.M.; Lavín, S.; Tvarijonaviciute, A.; Cano-Manuel, F.J.; Fandos, P.; Soriguer, R.C.; Granados, J.E.; et al. Histopathology, microbiology and the inflammatory process associated with Sarcoptes scabiei infection in the Iberian ibex, Capra pyrenaica. Parasites Vectors 2017, 10, 596. [Google Scholar] [CrossRef]
- Beaumont, M.; Barratt, E.M.; Gottelli, D.; Kitchener, A.C.; Daniels, M.J.; Pritchard, J.K.; Bruford, M.W. Genetic diversity and introgression in the Scottish wildcat. Mol. Ecol. 2001, 10, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.W.; Yamaguchi, N.; Kitchener, A.C.; Daniels, M.; Kilshaw, K.; Driscoll, C. Reversing cryptic extinction: The history, present and future of the Scottish wildcat. In Biology and Conservation of Wild Felids, 1st ed.; Macdonald, D.W., Loveridge, A.J., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 471–492. [Google Scholar]
- Yamaguchi, N.; Kitchener, A.; Driscoll, C.; Nussberger, B.; Silvestris, F. The IUCN Red List of Threatened Species; 2015; p. e.T60354712A50652361. Available online: https://doi.org/10.2305/IUCN.UK.2015-2.RLTS.T60354712A50652361.en (accessed on 1 August 2021).
- Gil-Sanchez, J.M.; Valenzuela, G.; Sanchez, J.F. Iberian wild cat Felis silvestris tartessia predation on rabbit Oryctolagus cuniculus: Functional response and age selection. Acta Theriol. 1999, 44, 421–428. [Google Scholar] [CrossRef]
- Soto, C.A.; Palomares, F. Surprising low abundance of European wildcats in a Mediterranean protected area of Southwestern Spain. Mammalia 2014, 78, 57–65. [Google Scholar] [CrossRef]
- Tompkins, D.M.; Carver, S.; Jones, M.E.; Krkos, M.; Skerratt, L.F. Emerging infectious diseases of wildlife: A critical perspective. Trends Parasitol. 2015, 31, 149–159. [Google Scholar] [CrossRef] [PubMed]
- González-Astudillo, V.; León-Alvarado, O.D.; Ossa-López, P.A.; Rivera-Páez, F.A.; Ramírez-Chaves, H.E. Sarcoptic mange in wild quichua porcupines (Coendou quichua Thomas, 1899) in Colombia. IJP Int. J. Parasitol. 2018, 7, 95–98. [Google Scholar] [CrossRef] [PubMed]
| Analyte 1 | Tissue Sample | Result | Test | Laboratory and/or Reference |
|---|---|---|---|---|
| FPV | Mesenteric ganglia | Negative | PCR | Laboklin [13] |
| FCoV | Intestinal scraping samples | Negative | PCR | Laboklin [14] |
| FHV-1 | Clot, spleen | Negative | PCR | Laboklin |
| FCV | Clot | Negative | PCR | Laboklin [15] |
| FeLV provirus | Clot, mesenteric ganglia, bone marrow | Negative | PCR | Laboklin [16] |
| FIV | Clot | Negative | PCR | Laboklin [17] |
| P27 Ag FeLV | Blood | Negative | ELISA | IDEXX SNAP® Combo Plus, IDEXX Laboratories, ME, USA |
| FIV Ab | Blood | Negative | ELISA | IDEXX SNAP® Combo Plus, IDEXX Laboratories, ME, USA |
| CDV | Clot | Negative | PCR | Laboklin [18] |
| Leishmania spp. | Spleen | Negative | PCR | Laboklin [19] |
| Mycobacterium spp. | Lung | Negative | Culture | Regional Agricultural Laboratory-LARAGA |
| Leptospira spp. | Kidney | Negative | PCR | Laboklin [20] |
| Anticoagulant rodenticide | Liver | Positive | HPLC-MS/MS | Regional Agricultural Laboratory-LARAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera, F.; Crespo, E.; García-Talens, A.; Grande-Gómez, R.; Herrera-Sánchez, F.J.; Gentil, M.; Cortés-García, C.; Müller, E.; Calero-Bernal, R.; Revuelta, L. First Description of Sarcoptic Mange in a Free-Ranging European Wildcat (Felis silvestris silvestris) from Spain. Animals 2021, 11, 2494. https://doi.org/10.3390/ani11092494
Nájera F, Crespo E, García-Talens A, Grande-Gómez R, Herrera-Sánchez FJ, Gentil M, Cortés-García C, Müller E, Calero-Bernal R, Revuelta L. First Description of Sarcoptic Mange in a Free-Ranging European Wildcat (Felis silvestris silvestris) from Spain. Animals. 2021; 11(9):2494. https://doi.org/10.3390/ani11092494
Chicago/Turabian StyleNájera, Fernando, Elena Crespo, Amalia García-Talens, Rebeca Grande-Gómez, Francisco Javier Herrera-Sánchez, Michaela Gentil, Carmen Cortés-García, Elisabeth Müller, Rafael Calero-Bernal, and Luis Revuelta. 2021. "First Description of Sarcoptic Mange in a Free-Ranging European Wildcat (Felis silvestris silvestris) from Spain" Animals 11, no. 9: 2494. https://doi.org/10.3390/ani11092494
APA StyleNájera, F., Crespo, E., García-Talens, A., Grande-Gómez, R., Herrera-Sánchez, F. J., Gentil, M., Cortés-García, C., Müller, E., Calero-Bernal, R., & Revuelta, L. (2021). First Description of Sarcoptic Mange in a Free-Ranging European Wildcat (Felis silvestris silvestris) from Spain. Animals, 11(9), 2494. https://doi.org/10.3390/ani11092494








